Erectile Dysfunction in Diabetic Patients: From Etiology to Management
Abstract
1. Introduction
2. Erectile Dysfunction
2.1. Epidemiology
2.2. Pathogenesis
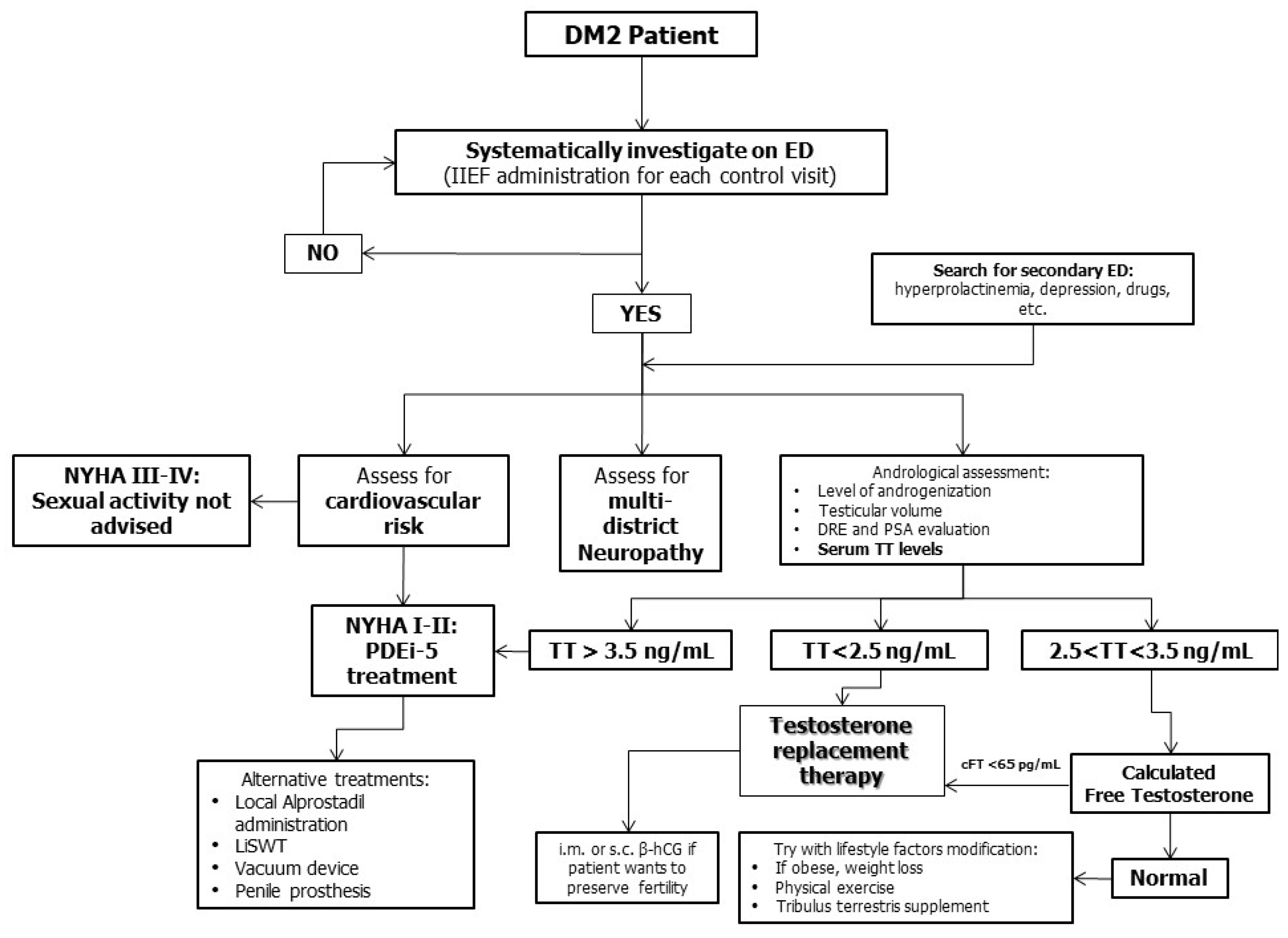
2.3. Diagnosis
2.4. Therapeutic Options
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993, 270, 83–90. [CrossRef]
- Calogero, A.E.; Burgio, G.; Condorelli, R.A.; Cannarella, R.; La Vignera, S. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male 2018, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zvara, P.; Sioufi, R.; Schipper, H.M.; Begin, L.R.; Brock, G.B. Nitric oxide mediated erectile activity is a testosterone dependent event: A rat erection model. Int. J. Impot. Res. 1995, 70, 209–219. [Google Scholar]
- Cannarella, R.; Calogero, A.E.; Aversa, A.; Condorelli, R.A.; La Vignera, S. Differences in Penile Hemodynamic Profiles in Patients with Erectile Dysfunction and Anxiety. J. Clin. Med. 2021, 10, 402. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Chambers, R.; Tang, W.; Stecher, V.; Hassan, T. Real-world observational results from a database of 48 million men in the United States: Relationship of cardiovascular disease, diabetes mellitus and depression with age and erectile dysfunction. Int. J. Clin. Pract. 2018, 72, e13078. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Pizzol, D.; Cosco, T.; Thompson, T.; Carnaghi, M.; Bertoldo, A.; Solmi, M.; Stubbs, B.; Veronese, N. High prevalence of erectile dysfunction in diabetes: A systematic review and meta-analysis of 145 studies. Diabet. Med. 2017, 34, 1185–1192. [Google Scholar] [CrossRef]
- Klein, R.; E Klein, B.; E Lee, K.; E Moss, S.; Cruickshanks, K.J. Prevalence of self-reported erectile dysfunction in people with long-term IDDM. Diabetes Care 1996, 19, 135–141. [Google Scholar] [CrossRef]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Sforza, A.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Type 2 diabetes mellitus and testosterone: A meta-analysis study. Int. J. Androl. 2010, 34, 528–540. [Google Scholar] [CrossRef]
- Evans, M.C.; Hill, J.W.; Anderson, G.M. Role of insulin in the neuroendocrine control of reproduction. J. Neuroendocr. 2021, 33, e12930. [Google Scholar] [CrossRef]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2020, 162. [Google Scholar] [CrossRef]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Dandona, P. Hypogonadotropic Hypogonadism in Men with Diabesity. Diabetes Care 2018, 41, 1516–1525. [Google Scholar] [CrossRef]
- Tariq, K.; Khan, M.A. Asymmetrical dimethyl arginine in type 2 diabetic patients with coronary artery disease. J. Pak. Med. Assoc. 2016, 66, 957–960. [Google Scholar] [PubMed]
- Wierzbicki, A.S.; Solomon, H.; Lumb, P.J.; Lyttle, K.; Lambert-Hammill, M.; Jackson, G. Asymmetric dimethyl arginine levels correlate with cardiovascular risk factors in patients with erectile dysfunction. Atherosclerosis 2006, 185, 421–425. [Google Scholar] [CrossRef]
- Stühlinger, M.C.; Stanger, O. Asymmetric dimethyl-L-arginine (ADMA): A possible link between homocyst(e)ine and endothelial dysfunction. Curr. Drug Metab. 2005, 6, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Sharma, S. Vascular endothelium dysfunction: A conservative target in metabolic disorders. Inflamm. Res. 2018, 67, 391–405. [Google Scholar] [CrossRef]
- Rao, P.M.; Kelly, D.M.; Jones, T.H. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat. Rev. Endocrinol. 2013, 9, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Table of Contents. Available online: https://care.diabetesjournals.org/content/43/Supplement_1 (accessed on 30 July 2021).
- Chmiel, A.; Mizia-Stec, K.; Wierzbicka-Chmiel, J.; Rychlik, S.; Muras, A.; Mizia, M.; Bienkowski, J. Low testosterone and sexual symptoms in men with acute coronary syndrome can be used to predict major adverse cardiovascular events during long-term follow-up. Andrology 2015, 3, 1113–1118. [Google Scholar] [CrossRef]
- Beisswenger, P.J. Glycation and biomarkers of vascular complications of diabetes. Amino Acids 2010, 42, 1171–1183. [Google Scholar] [CrossRef]
- De Nigris, F.; Rienzo, M.; Sessa, M.; Infante, T.; Cesario, E.; Ignarro, L.J.; Al-Omran, M.; Giordano, A.; Palinski, W.; Napoli, C. Glycoxydation promotes vascular damage via MAPK-ERK/JNK pathways. J. Cell Physiol. 2012, 227, 3639–3647. [Google Scholar] [CrossRef]
- Furukawa, S.; Sakai, T.; Niiya, T.; Miyaoka, H.; Miyake, T.; Yamamoto, S.; Maruyama, K.; Ueda, T.; Senba, H.; Todo, Y.; et al. Diabetic peripheral neuropathy and prevalence of erectile dysfunction in Japanese patients aged <65 years with type 2 diabetes mellitus: The Dogo Study. Int. J. Impot. Res. 2016, 29, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Montorsi, P.; Ravagnani, P.M.; Galli, S.; Rotatori, F.; Briganti, A.; Salonia, A.; Rigatti, P.; Montorsi, F. The Artery Size Hypothesis: A Macrovascular Link between Erectile Dysfunction and Coronary Artery Disease. Am. J. Cardiol. 2005, 96, 19–23. [Google Scholar] [CrossRef]
- Zhao, B.; Hong, Z.; Wei, Y.; Yu, D.; Xu, J.; Zhang, W. Erectile Dysfunction Predicts Cardiovascular Events as an Independent Risk Factor: A Systematic Review and Meta-Analysis. J. Sex. Med. 2019, 16, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Terentes-Printzios, D.; Ioakeimidis, N.; Aznaouridis, K.; Rokkas, K.; Synodinos, A.; Christoforatou, E.; Aggelis, A.; Samentzas, A.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: A systematic review and meta-analysis of cohort studies. J. Am. Coll. Cardiol. 2012, 59, E2074. [Google Scholar] [CrossRef][Green Version]
- Yamada, T.; Hara, K.; Umematsu, H.; Suzuki, R.; Kadowaki, T. Erectile Dysfunction and Cardiovascular Events in Diabetic Men: A Meta-analysis of Observational Studies. PLoS ONE 2012, 7, e43673. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Cappelleri, J.C.; Gendrano, N. The International Index of Erectile Function (IIEF): A state-of-the-science review. Int. J. Impot. Res. 2002, 14, 226–244. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; A Yialamas, M. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Pagano, M.; Stahl, P.J. Variation in Penile Hemodynamics by Anatomic Location of Cavernosal Artery Imaging in Penile Duplex Doppler Ultrasound. J. Sex. Med. 2015, 12, 1911–1919. [Google Scholar] [CrossRef]
- Caretta, N.; Ponce, M.D.R.; Minicuci, N.; Palego, P.; Valente, U.; Garolla, A.; Ferlin, A.; Foresta, C. Penile doppler ultrasound predicts cardiovascular events in men with erectile dysfunction. Andrology 2018, 7, 82–87. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Cannarella, R.; Galvano, F.; Grillo, A.; Aversa, A.; Cimino, L.; Magagnini, C.M.; Mongioì, L.M.; Condorelli, R.A.; Calogero, A.E. The ketogenic diet corrects metabolic hypogonadism and preserves pancreatic ß-cell function in overweight/obese men: A single-arm uncontrolled study. Endocrine 2020. [Google Scholar] [CrossRef]
- Haider, K.S.; Haider, A.; Saad, F.; Doros, G.; Hanefeld, M.; Dhindsa, S.; Dandona, P.; Traish, A. Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes, Obes. Metab. 2020, 22, 2055–2068. [Google Scholar] [CrossRef]
- Corona, G.; Goulis, D.G.; Huhtaniemi, I.; Zitzmann, M.; Toppari, J.; Forti, G.; Vanderschueren, D.; Wu, F.C. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: Endorsing organization: European Society of Endocrinology. Andrology 2020, 8, 970–987. [Google Scholar] [CrossRef]
- Sebastianelli, A.; Spatafora, P.; Morselli, S.; Vignozzi, L.; Serni, S.; McVary, K.T.; Kaplan, S.; Gravas, S.; Chapple, C.; Gacci, M. Tadalafil Alone or in Combination with Tamsulosin for the Management for LUTS/BPH and ED. Curr. Urol. Rep. 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Bergandi, L.; Silvagno, F.; Russo, I.; Riganti, C.; Anfossi, G.; Aldieri, E.; Ghigo, D.; Trovati, M.; Bosia, A. Insulin Stimulates Glucose Transport Via Nitric Oxide/Cyclic GMP Pathway in Human Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Mammi, C.; Pastore, D.; Lombardo, M.F.; Ferrelli, F.; Caprio, M.; Consoli, C.; Tesauro, M.; Gatta, L.; Fini, M.; Federici, M.; et al. Sildenafil Reduces Insulin-Resistance in Human Endothelial Cells. PLoS ONE 2011, 6, e14542. [Google Scholar] [CrossRef]
- Poolsup, N.; Suksomboon, N.; Aung, N. Effect of phosphodiesterase-5 inhibitors on glycemic control in person with type 2 diabetes mellitus: A systematic review and meta-analysis. J. Clin. Transl. Endocrinol. 2016, 6, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, S.; Corinaldesi, C.; Colletti, M.; Di Luigi, L.; Antinozzi, C.; Filardi, T.; Scolletta, S.; Basili, S.; Lenzi, A.; Morano, S.; et al. The phosphodiesterase 5 inhibitor sildenafil decreases the proinflammatory chemokine IL-8 in diabetic cardiomyopathy: In vivo and in vitro evidence. J. Endocrinol. Investig. 2018, 42, 715–725. [Google Scholar] [CrossRef]
- Salonia, A.; Bettocchi, C.; Boeri, L.; Capogrosso, P.; Carvalho, J.; Cilesiz, N.C.; Cocci, A.; Corona, G.; Dimitropolous, K.; Gül, M.; et al. EAU Working Group on Male Sexual and Reproductive Health. European Association of Urology Guidelines on Sexual and Re-productive Health-2021 Update: Male Sexual Dysfunction. Eur. Urol. 2021, 25, S0302-2838(21)01813-3. [Google Scholar]
- Beaudreau, S.A.; Van Moorleghem, K.; Dodd, S.M.; Liou-Johnson, V.; Suresh, M.; Gould, C.E. Satisfaction with a Vacuum Constriction Device for Erectile Dysfunction among Middle-Aged and Older Veterans. Clin. Gerontol. 2020, 44, 307–315. [Google Scholar] [CrossRef]
- Sokolakis, I.; Hatzichristodoulou, G. Clinical studies on low intensity extracorporeal shockwave therapy for erectile dysfunction: A systematic review and meta-analysis of randomised controlled trials. Int. J. Impot. Res. 2019, 31, 177–194. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannarella, R.; Barbagallo, F.; Condorelli, R.A.; Gusmano, C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Erectile Dysfunction in Diabetic Patients: From Etiology to Management. Diabetology 2021, 2, 157-164. https://doi.org/10.3390/diabetology2030014
Cannarella R, Barbagallo F, Condorelli RA, Gusmano C, Crafa A, La Vignera S, Calogero AE. Erectile Dysfunction in Diabetic Patients: From Etiology to Management. Diabetology. 2021; 2(3):157-164. https://doi.org/10.3390/diabetology2030014
Chicago/Turabian StyleCannarella, Rossella, Federica Barbagallo, Rosita A. Condorelli, Carmelo Gusmano, Andrea Crafa, Sandro La Vignera, and Aldo E. Calogero. 2021. "Erectile Dysfunction in Diabetic Patients: From Etiology to Management" Diabetology 2, no. 3: 157-164. https://doi.org/10.3390/diabetology2030014
APA StyleCannarella, R., Barbagallo, F., Condorelli, R. A., Gusmano, C., Crafa, A., La Vignera, S., & Calogero, A. E. (2021). Erectile Dysfunction in Diabetic Patients: From Etiology to Management. Diabetology, 2(3), 157-164. https://doi.org/10.3390/diabetology2030014








