Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management
Abstract
:1. Introduction
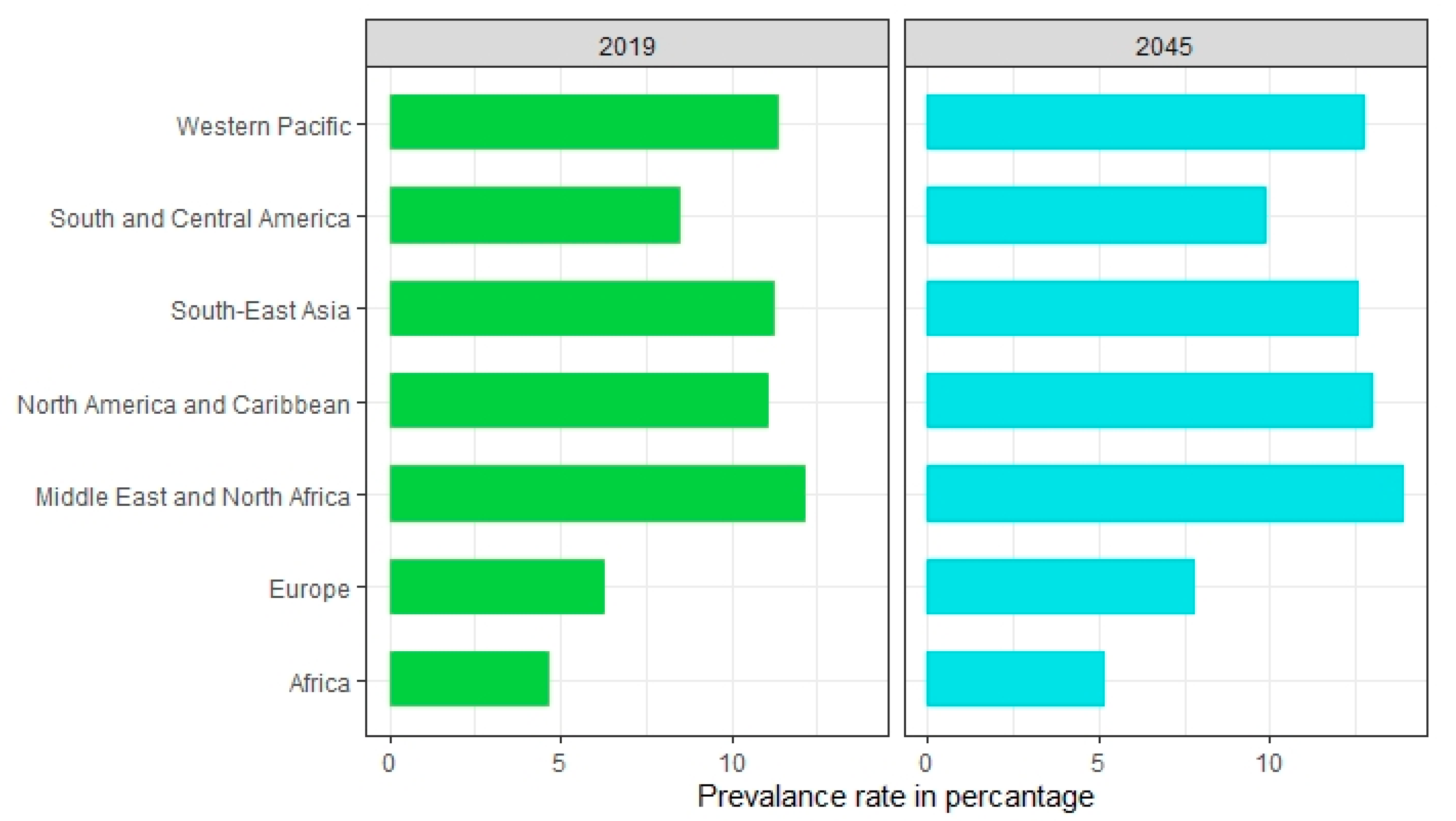
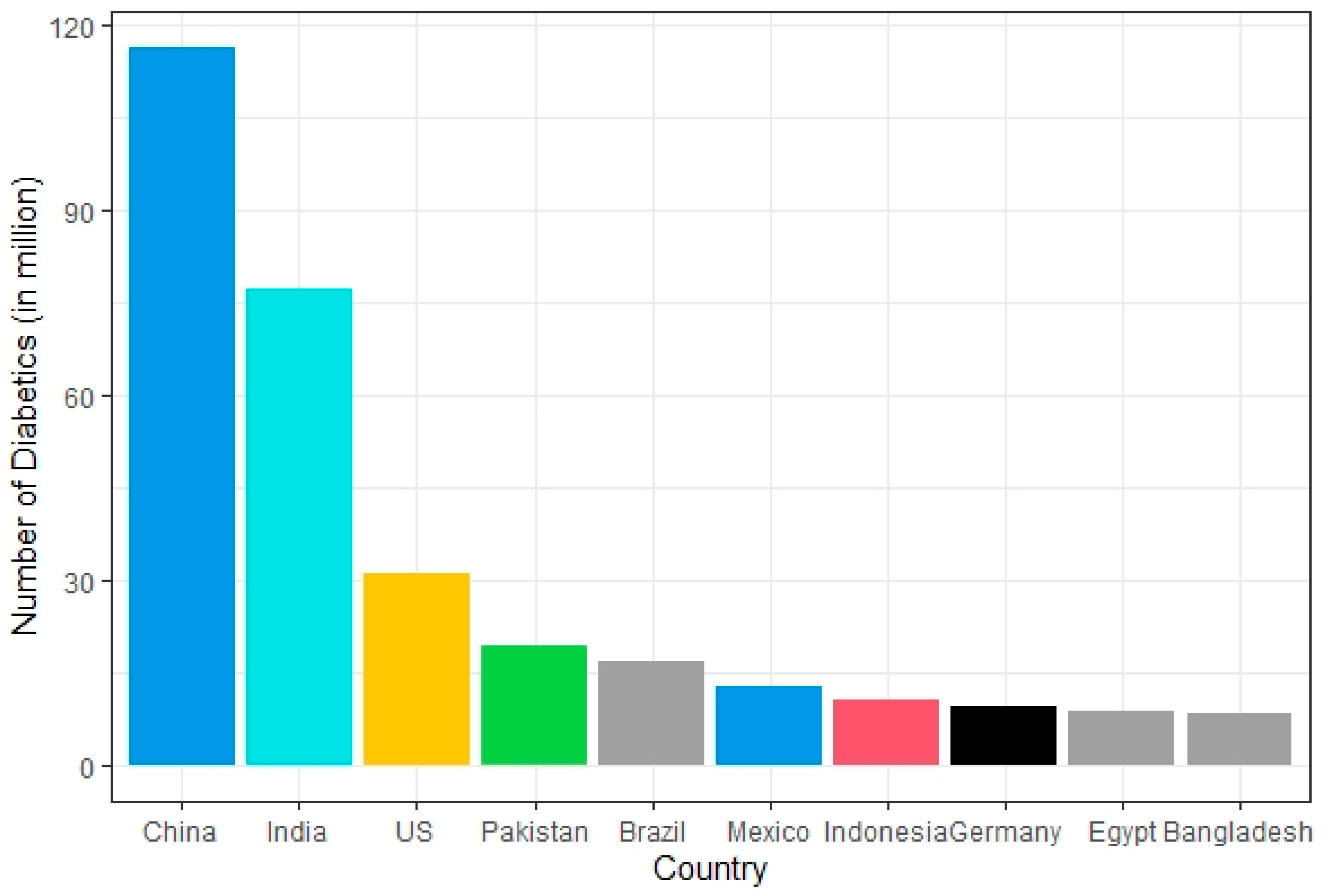
2. Epidemiology of Diabetes
3. Risk Factors for Diabetes
4. Diagnosis of Diabetes
5. Complications of Diabetes
6. Management of Diabetes
7. Recent Technologies for Combating Diabetes
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Global Report on Diabetes; WHO: Geneva, Switzerland, 2017; Available online: http://www.who.int/diabetes/global-report/en/ (accessed on 22 September 2018).
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; WHO: Geneva, Switzerland, 2006. [Google Scholar]
- Morran, M.P.; Vonberg, A.; Khadra, A.; Pietropaolo, M. Immunogenetics of type 1 diabetes mellitus. Mol. Asp. Med. 2015, 42, 42–60. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.C.; Warram, J.H.; Krolewski, A.S.; Soeldner, J.S.; Kahn, C.R.; Bergman, R.N. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: Results of a 25-year follow-up study. Lancet 1992, 340, 925–929. [Google Scholar] [CrossRef]
- Tisch, R.; McDevitt, H. Insulin-dependent diabetes mellitus. Cell 1996, 85, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Reinehr, T. Type 2 diabetes mellitus in children and adolescents. World J. Diabetes 2013, 4, 270–281. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin-independent diabetes mellitus: Metabolic characteristics. Metabolism 1980, 29, 445–454. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nat. Cell Biol. 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Razmaria, A.A. Diabetic neuropathy. JAMA 2015, 314, 2202. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.; Halley, A.; Codd, M.; O’Meara, N.; Firth, R.; Mooney, D.; Acheson, R.W. Prevalence of diabetic retinopathy in patients with diabetes mellitus diagnosed after the age of 70 years. Br. J. Ophthalmol. 1997, 81, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Aronson, D.; Edelman, E.R. Coronary artery disease and diabetes mellitus. Cardiol. Clin. 2014, 32, 439–455. [Google Scholar] [CrossRef] [Green Version]
- Cholesterol Treatment Trialists Collaborators; Reith, C.; Staplin, N.; Herrington, N.G.; Stevens, R.; Emberson, J.; Haynes, R.; Mafham, M.; Armitage, J.; Cass, A.; et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar] [CrossRef]
- Ceriello, A.; Testa, R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care 2009, 32, S232–S236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajans, S.S.; Bell, G.I. MODY. Diabetes Care 2011, 34, 1878–1884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemelman, M.B.; Letourneau, L.; Greeley, S.A.W. Neonatal diabetes mellitus. Clin. Perinatol. 2018, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Jali, M.V.; Kambar, S.; Jali, S.M.; Gowda, S. Familial early onset of type-2 diabetes mellitus and its complications. N. Am. J. Med. Sci. 2009, 1, 377–380. [Google Scholar] [PubMed]
- Alyafei, F.; Soliman, A.; Alkhalaf, F.; Sabt, A.; De Sanctis, V.; Elsayed, N.; Waseef, R. Clinical and biochemical characteristics of familial type 1 diabetes mellitus (FT1DM) compared to non-familial type 1 DM (NFT1DM). Acta Bio Med. Atenei Parm. 2018, 89, 27–31. [Google Scholar]
- Waterhouse, C.; Keilson, J. Cori cycle activity in man. J. Clin. Investig. 1969, 48, 2359–2366. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., III. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, S.K.; Sung, K.M.; Cho, Y.W.; Park, S.W. Management of type 2 diabetes mellitus in older adults. Diabetes Metab. J. 2012, 36, 336–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takebayashi, K.; Inukai, T. Effect of proton pump inhibitors on glycemic control in patients with diabetes. World J. Diabetes 2015, 6, 1122–1131. [Google Scholar] [CrossRef]
- Velho, G.; Froguel, P.; Clement, K.; Pueyo, E.M.; Rakotoambinina, B.; Zouali, H.; Passa, P.; Cohen, D.; Robert, J.J. Primary pancreatic beta-cell secretory defect caused by mutations in glucokinase gene in kindreds of maturity onset diabetes of the young. Lancet 1992, 340, 444–448. [Google Scholar] [CrossRef]
- Bellanné-Chantelot, C.; Carette, C.; Riveline, J.-P.; Valéro, R.; Gautier, J.-F.; Larger, E.; Reznik, Y.; Ducluzeau-Fieloux, P.; Sola-Gazagnes, A.; Hartemann-Heurtier, A.; et al. The type and the position of HNF1A mutation modulate age at diagnosis of diabetes in patients with maturity-onset diabetes of the young (MODY)-3. Diabetes 2007, 57, 503–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staník, J.; Dusatkova, P.; Cinek, O.; Valentínová, L.; Hučková, M.; Škopková, M.; Dusatkova, L.; Stanikova, D.; Pura, M.; Klimeš, I.; et al. De novo mutations of GCK, HNF1A and HNF4A may be more frequent in MODY than previously assumed. Diabetologia 2013, 57, 480–484. [Google Scholar] [CrossRef]
- Haldorsen, I.S.; Vesterhus, M.; Rder, H.; Jensen, D.K.; Svik, O.; Molven, A.; Njlstad, P.R.; Ræder, H.; Søvik, O.; Njølstad, P.R. Lack of pancreatic body and tail inHNF1Bmutation carriers. Diabet. Med. 2008, 25, 782–787. [Google Scholar] [CrossRef]
- Sagen, J.V.; Raeder, H.; Hathout, E.; Shehadeh, N.; Gudmundsson, K.; Baevre, H.; Abuelo, D.; Phornphutkul, C.; Molnes, J.; Bell, G.I.; et al. Permanent neonatal diabetes due to mutations in KCNJ11 encoding Kir6.2: Patient characteristics and initial response to sulfonylurea therapy. Diabetes 2004, 53, 2713–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Støy, J.; Edghill, E.L.; Flanagan, S.E.; Ye, H.; Paz, V.P.; Pluzhnikov, A.; Below, J.E.; Hayes, M.G.; Cox, N.J.; Lipkind, G.M.; et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15040–15044. [Google Scholar] [CrossRef] [Green Version]
- Babenko, A.P.; Polak, M.; Cavé, H.; Busiah, K.; Czernichow, P.; Scharfmann, R.; Bryan, J.; Aguilar-Bryan, L.; Vaxillaire, M.; Froguel, P. Activating Mutations in theABCC8Gene in neonatal diabetes mellitus. N. Engl. J. Med. 2006, 355, 456–466. [Google Scholar] [CrossRef]
- Naylor, R.N.; Greeley, S.A.W.; I Bell, G.; Philipson, L.H. Genetics and pathophysiology of neonatal diabetes mellitus. J. Diabetes Investig. 2011, 2, 158–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catli, G.; Abaci, A.; Flanagan, S.; De Franco, E.; Ellard, S.; Hattersley, A.; Guleryuz, H.; Bober, E. A novel GATA6 mutation leading to congenital heart defects and permanent neonatal diabetes: A case report. Diabetes Metab. 2013, 39, 370–374. [Google Scholar] [CrossRef]
- Brickwood, S.; Bonthron, D.T.; Al-Gazali, I.L.; Piper, K.; Hearn, T.; Wilson, I.D.; Hanley, A.N. Wolcott-Rallison syndrome: Pathogenic insights into neonatal diabetes from new mutation and expression studies of EIF2AK. J. Med. Genet. 2003, 40, 685–689. [Google Scholar] [CrossRef] [Green Version]
- Wildin, R.S.; Ramsdell, F.; Peake, J.; Faravelli, F.; Casanova, J.-L.; Buist, N.; Levy-Lahad, E.; Mazzella, M.; Goulet, O.; Perroni, L.; et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001, 27, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation, IDF Diabetes Atlas, 9th ed.; IDF: Brussels, Belgium, 2019.
- Lee, I.-M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Knott, C.; Bell, S.; Britton, A. Alcohol consumption and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Akter, S.; Goto, A.; Mizoue, T. Smoking and the risk of type 2 diabetes in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 553–561. [Google Scholar] [CrossRef]
- Danaei, G.; Finucane, M.M.; Lu, Y.; Singh, G.M.; Cowan, M.J.; Paciorek, C.J.; Lin, J.K.; Farzadfar, F.; Khang, Y.-H.; Stevens, A.G.; et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet 2011, 378, 31–40. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Donahoo, W.T.; Pereira, R.; Gurevich, I.; Westergren, R.; Enerbäck, S.; Eckel, P.J.; Goalstone, M.L.; Hill, J.O.; Eckel, R.H.; et al. Insulin sensitivity determines the effectiveness of dietary macronutrient composition on weight loss in obese women. Obes. Res. 2005, 13, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Maj, M.; Ilhan, A.; Neziri, D.; Gärtner, W.; Berggård, T.; Attems, J.; Base, W.; Wagner, L. Age related changes in pancreatic beta cells: A putative extra-cerebral site of Alzheimer’s pathology. World J. Diabetes 2011, 2, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; E Manson, J.; Willett, W.C.; Meigs, J.B.; Weikert, C.; Heidemann, C.; A Colditz, G.; Hu, F.B. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am. J. Clin. Nutr. 2005, 82, 675–684. [Google Scholar] [CrossRef]
- Li, D.-W.; Lu, T.-F.; Hua, X.-W.; Dai, H.-J.; Cui, X.-L.; Zhang, J.-J.; Xia, Q. Risk factors for new onset diabetes mellitus after liver transplantation: A meta-analysis. World J. Gastroenterol. 2015, 21, 6329. [Google Scholar] [CrossRef] [PubMed]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.; Faruque, M.O.; Kabir, G.; Hassan, N.; Sikdar, D.; Nahar, Q.; Ali, L. Association of serum TNF-α and IL-6 with insulin secretion and insulin resistance in IFG and IGT subjects in a Bangladeshi population. Int. J. Diabetes Mellit. 2010, 2, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.-D.; Wang, Y.-G.; Li, J. An analysis of islet beta-cell function in hyperuricemia. Zhonghua Nei Ke Za Zhi 2006, 45, 456–458. [Google Scholar]
- Lytvyn, Y.; Perkins, B.A.; Cherney, D.Z. Uric acid as a biomarker and a therapeutic target in diabetes. Can. J. Diabetes 2015, 39, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, A.; Van Hoek, M.; Sijbrands, E.J.; Hofman, A.; Witteman, J.C. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2007, 31, 361–362. [Google Scholar] [CrossRef] [Green Version]
- Kanellis, J.; Kang, D.-H. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin. Nephrol. 2005, 25, 39–42. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Manson, J.E.; Rifai, N.; Buring, J.E.; Ridker, P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 2001, 286, 327–334. [Google Scholar] [CrossRef]
- Nabipour, I.; Vahdat, K.; Jafari, S.; Pazoki, R. Concurrent increased high sensitivity C-reactive protein and chronic infections are associated with coronary artery disease: A population-based study. Indian J. Med. Sci. 2007, 61, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.R.; Tyagi, S.C. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr. Metab. 2004, 1, 10. [Google Scholar] [CrossRef] [Green Version]
- Thorand, B.; Baumert, J.; Chambless, L.; Meisinger, C.; Kolb, H.; Döring, A.; Löwel, H.; Koenig, W. Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middle-aged men and women from the general population. Arter. Thromb. Vasc. Biol. 2006, 26, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Trayhurn, P.; Beattie, J.H. Physiological role of adipose tissue: White adipose tissue as an endocrine and secretory organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.B.; Meigs, J.B.; Li, T.Y.; Rifai, N.; Manson, J.E. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004, 53, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Penfornis, A.; Kury-Paulin, S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006, 32, 539–546. [Google Scholar] [CrossRef]
- Fathallah, N.; Slim, R.; Larif, S.; Hmouda, H.; Ben Salem, C. Drug-induced hyperglycaemia and diabetes. Drug Saf. 2015, 38, 1153–1168. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef]
- Vijan, S. Type 2 diabetes. Ann. Intern. Med. 2010, 152, ITC3-1. [Google Scholar] [CrossRef]
- Simcox, J.A.; McClain, D.A. Iron and diabetes risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, M.A. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb. Perspect. Med. 2012, 2, a007641. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Von Herrath, M.; Powers, A.C.; Clare-Salzler, M. Current concepts on the pathogenesis of type 1 diabetes—Considerations for attempts to prevent and reverse the disease. Diabetes Care 2015, 38, 979–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siu, A.L.; US Preventive Services Task Force. Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2015, 163, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. Summary of revisions for the 2010 clinical practice recommendations. Diabetes Care 2009, 33, S3. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Hodgkinson, A.; Millward, B.A.; Demaine, A.G. High glucose-induced DNA-binding activities of nuclear factor of activated T cells 5 and carbohydrate response element binding protein to the myo-inositol oxygenase gene are inhibited by sorbinil in peripheral blood mononuclear cells from patients with type 1 diabetes mellitus and nephropathy. Int. J. Diabetes Mellit. 2010, 2, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Antonetti, D.A.; Klein, R.; Gardner, T.W. Diabetic retinopathy. N. Engl. J. Med. 2012, 366, 1227–1239. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.K. Diabetic nephropathy—Complications and treatment. Int. J. Nephrol. Renov. Dis. 2014, 7, 361–381. [Google Scholar] [CrossRef] [Green Version]
- Onyiriuka, A.N.; Ifebi, E. Ketoacidosis at diagnosis of type 1 diabetes in children and adolescents: Frequency and clinical characteristics. J. Diabetes Metab. Disord. 2013, 12, 47. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ma, L.; Peng, F.; Wu, Y.; Chen, Y.; Zhan, Y.; Zhang, X. Spontaneous gas gangrene of the scrotum in patient with severe diabetic ketoacidosis. Int. J. Diabetes Mellit. 2010, 2, 196–198. [Google Scholar] [CrossRef] [Green Version]
- Moin, A.S.M.; Butler, P.C.; Butler, A.E. Increased proliferation of the pancreatic duct gland compartment in type 1 diabetes. J. Clin. Endocrinol. Metab. 2016, 102, 200–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satija, A.; Spiegelman, D.; Giovannucci, E.; Hu, F.B. Type 2 diabetes and risk of cancer. BMJ 2014, 350, g7707. [Google Scholar] [CrossRef]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Zoungas, S.; Arima, H.; Gerstein, H.C.; Holman, R.R.; Woodward, M.; Reaven, P.; A Hayward, R.; Craven, T.; Coleman, R.L.; Chalmers, J. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: A meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017, 5, 431–437. [Google Scholar] [CrossRef]
- Leon, B.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef]
- Marseglia, A.; Fratiglioni, L.; Laukka, E.J.; Santoni, G.; Pedersen, N.L.; Bäckman, L.; Xu, W. Early cognitive deficits in type 2 diabetes: A POPULATION-based study. J. Alzheimer’s Dis. 2016, 53, 1069–1078. [Google Scholar] [CrossRef] [Green Version]
- Wojciechowska, J.; Krajewski, W.; Bolanowski, M.; Kręcicki, T.; Zatoński, T. Diabetes and cancer: A review of current knowledge. Exp. Clin. Endocrinol. Diabetes 2016, 124, 263–275. [Google Scholar] [CrossRef]
- Denduluri, S.K.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [Green Version]
- Vigneri, P.; Frasca, F.; Sciacca, L.; Pandini, G.; Vigneri, R. Diabetes and cancer. Endocr. Relat. Cancer 2009, 16, 1103–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, A. Hepatitis C virus infection and type 1 and type 2 diabetes mellitus. World J. Diabetes 2014, 5, 586–600. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, M. Magnesium and type 2 diabetes. World J. Diabetes 2015, 6, 1152–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panunzi, S.; Carlsson, L.M.S.; De Gaetano, A.; Peltonen, M.; Rice, T.; Sjöström, L.; Mingrone, G.; Dixon, J.B. Determinants of diabetes remission and glycemic control after bariatric surgery. Diabetes Care 2016, 39, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, A.-G.; Wallner, M.; Kaiser, I.; Rossbauer, M.; Harsunen, M.H.; Lachmann, L.; Maier, J.; Winkler, C.; Hummel, S. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes 2012, 61, 3167–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.; Barnes, M.; Kessler, N. Case study: Use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. J. Bodyw. Mov. Ther. 2013, 17, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Barnes, M.J.; Kessler, N.J. Case study: Use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. Int. J. Diabetes Mellit. 2015, 3, 72–75. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Hussain, M.E. Obesity and diabetes: An update. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, 73–79. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health benefits of exercise. Cold Spring Harb. Perspect. Med. 2017, 8, a029694. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, M.G.; Grøntved, A.; Blond, K.; Overvad, K.; Tjønneland, A.; Jensen, M.K.; Østergaard, L. Associations between recreational and commuter cycling, changes in cycling, and type 2 diabetes risk: A cohort study of Danish men and women. PLoS Med. 2016, 13, e1002076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riddell, M.C.; Gallen, I.W.; Smart, E.C.; Taplin, E.C.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.S.; Maiya, A.G.; Shastry, B.; Vaishali, K.; Ravishankar, N.; Hazari, A.; Gundmi, S.; Jadhav, R. Exercise and insulin resistance in type 2 diabetes mellitus: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 62, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yue, P.; Wu, X.; Yu, T.; Wang, Y.; Zhou, J.; Kong, D.; Chen, K. Combined intervention of swimming plus metformin ameliorates the insulin resistance and impaired lipid metabolism in murine gestational diabetes mellitus. PLoS ONE 2018, 13, e0195609. [Google Scholar] [CrossRef]
- Ghiasi, R.; Naderi, R.; Sheervalilou, R.; Alipour, M.R. Swimming training by affecting the pancreatic Sirtuin1 (SIRT1) and oxidative stress, improves insulin sensitivity in diabetic male rats. Horm. Mol. Biol. Clin. Investig. 2019, 40, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, D.-C.; Brellenthin, A.G.; Eijsvogels, T.M.; Sui, X.; Church, T.S.; Lavie, C.J.; Blair, S.N. Leisure-time running reduces the risk of incident type 2 diabetes. Am. J. Med. 2019, 132, 1225–1232. [Google Scholar] [CrossRef]
- Riiser, A.; Solbraa, A.; Jenum, A.K.; Birkeland, K.I.; Andersen, L.B. Cycling and walking for transport and their associations with diabetes and risk factors for cardiovascular disease. J. Transp. Health 2018, 11, 193–201. [Google Scholar] [CrossRef]
- Jelleyman, C.; Yates, T.L.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.-W.; Chen, P.-C.; Liao, K.-F.; Muo, C.-H.; Lin, C.-C.; Sung, F.-C. Risk of hepatocellular carcinoma in diabetic patients and risk reduction associated with anti-diabetic therapy: A population-based cohort study. Am. J. Gastroenterol. 2012, 107, 46–52. [Google Scholar] [CrossRef]
- Raptis, S.A.; Dimitriadis, G.D. Oral hypoglycemic agents: Insulin secretagogues, α-glucosidase inhibitors and insulin sensitizers. Exp. Clin. Endocrinol. Diabetes 2001, 109, S265–S287. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Lu, P.; Zhang, J.; Xu, Y.; He, W.; Li, M.; Zhang, S.; Jia, J.; Shao, S.; et al. Incretin-based agents in type 2 diabetic patients at cardiovascular risk: Compare the effect of GLP-1 agonists and DPP-4 inhibitors on cardiovascular and pancreatic outcomes. Cardiovasc. Diabetol. 2017, 16, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef] [Green Version]
- El Mouhayyar, C.; Riachy, R.; Khalil, A.B.; Eid, A.; Azar, S. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: A review. Int. J. Endocrinol. 2020, 2020, 1762164. [Google Scholar] [CrossRef] [PubMed]
- Margonato, D.; Galati, G.; Mazzetti, S.; Cannistraci, R.; Perseghin, G.; Margonato, A.; Mortara, A. Renal protection: A leading mechanism for cardiovascular benefit in patients treated with SGLT2 inhibitors. Heart Fail. Rev. 2021, 26, 337–345. [Google Scholar] [CrossRef]
- Strowig, S.M.; Raskin, P. Combination therapy using metformin or thiazolidinediones and insulin in the treatment of diabetes mellitus. Diabetes Obes. Metab. 2004, 7, 633–641. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Type 2 diabetes and insulin secretagogues. J. Clin. Endocrinol. Metab. 2012, 97, 37A. [CrossRef]
- Garber, A.J. Long-acting glucagon-like peptide 1 receptor agonists: A review of their efficacy and tolerability. Diabetes Care 2011, 34, S279–S284. [Google Scholar] [CrossRef] [Green Version]
- Filippatos, T.D.; Panagiotopoulou, T.V.; Elisaf, M.S. Adverse Effects of GLP-1 Receptor Agonists. Rev. Diabet. Stud. 2014, 11, 202–230. [Google Scholar] [CrossRef] [Green Version]
- Kalra, S.; Bhutani, J.; Mohan, V.; Unnikrishnan, R.; Kunju, P. Alpha-glucosidase inhibitors. Diabetol. Type 2 Diabetes Mellit. 2014, 64, 55. [Google Scholar] [CrossRef]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 24, 1. [Google Scholar] [CrossRef]
- Sebokova, E.; Christ, A.D.; Boehringer, M.; Mizrahi, J. Dipeptidyl peptidase iv inhibitors: The next generation of new promising therapies for the management of type 2 diabetes. Curr. Top. Med. Chem. 2007, 7, 547–555. [Google Scholar] [CrossRef]
- Pathak, R.; Bridgeman, M.B. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. J. Formul. Manag. 2010, 35, 509–513. [Google Scholar]
- Strack, T. Metformin: A review. Drugs Today 2008, 44, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Nanjan, M.; Mohammed, M.; Kumar, B.P.; Chandrasekar, M. Thiazolidinediones as antidiabetic agents: A critical review. Bioorganic Chem. 2018, 77, 548–567. [Google Scholar] [CrossRef] [PubMed]
- Petrovski, G.; Zivkovic, M.; Milenkovic, T.; Ahmeti, I.; Bitovska, I. Successful desensitization in patient with type 2 diabetes with an insulin allergy using insulin pump and glargine. Acta Diabetol. 2014, 51, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Maehr, R.; Chen, S.; Snitow, M.; Ludwig, T.; Yagasaki, L.; Goland, R.; Leibel, R.L.; Melton, D.A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 15768–15773. [Google Scholar] [CrossRef] [Green Version]
- Abdi, R.; Fiorina, P.; Adra, C.N.; Atkinson, M.; Sayegh, M.H. Immunomodulation by mesenchymal stem cells: A potential therapeutic strategy for type 1 diabetes. Diabetes 2008, 57, 1759–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Z.; Sun, H.-S.; Wu, J.; Weisel, R.D.; Keating, A.; Li, Z.-H.; Feng, Z.-P.; Li, R.-K. Intravenously administered bone marrow cells migrate to damaged brain tissue and improve neural function in ischemic rats. Cell Transpl. 2007, 16, 993–1005. [Google Scholar] [CrossRef]
- Tyndall, A.; Walker, A.U.; Cope, A.; Dazzi, F.; De Bari, C.; Fibbe, W.; Guiducci, S.; Jones, S.; Jorgensen, C.; Le Blanc, K.; et al. Immunomodulatory properties of mesenchymal stem cells: A review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res. Ther. 2007, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Ianus, A.; Holz, G.G.; Theise, N.D.; Hussain, M.A. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J. Clin. Investig. 2003, 111, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, K.R.; Moretti, A.; Laugwitz, K.-L. Development: ES cells to the rescue. Science 2004, 306, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Zalzman, M.; Gupta, S.; Giri, R.K.; Berkovich, I.; Sappal, B.S.; Karnieli, O.; Zern, M.A.; Fleischer, N.; Efrat, S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7253–7258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, J.J.; Bhushan, A.; Butler, P.C. The potential for stem cell therapy in diabetes. Pediatr. Res. 2006, 59, 65R–73R. [Google Scholar] [CrossRef] [Green Version]
- Zechmeister-Koss, I.; Huić, M. Vascular endothelial growth factor inhibitors (anti-VEGF) in the management of diabetic macular oedema: A systematic review. Br. J. Ophthalmol. 2011, 96, 167–178. [Google Scholar] [CrossRef]
- Chao, E.C.; Henry, R.R. SGLT2 inhibition—A novel strategy for diabetes treatment. Nat. Rev. Drug Discov. 2010, 9, 551–559. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Wada, J.; Malakauskas, S.M.; Liu, M.; Ren, Y.; Du, C.; Duan, H.; Li, Y.; Li, Y.; et al. In vivo delivery of gremlin siRNA plasmid reveals therapeutic potential against diabetic nephropathy by recovering bone morphogenetic protein-7. PLoS ONE 2010, 5, e11709. [Google Scholar] [CrossRef] [Green Version]
- Kato, M.; Natarajan, R. MicroRNAs in diabetic nephropathy: Functions, biomarkers, and therapeutic targets. Ann. N. Y. Acad. Sci. 2015, 1353, 72–88. [Google Scholar] [CrossRef] [Green Version]
- Ledley, F.D. Pharmaceutical approach to somatic gene therapy. Pharm. Res. 1996, 13, 1595–1614. [Google Scholar] [CrossRef]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. Open Ophthalmol. J. 2013, 7, 4–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randeria, P.S.; Seeger, M.A.; Wang, X.-Q.; Wilson, H.; Shipp, D.; Mirkin, C.A.; Paller, A.S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc. Natl. Acad. Sci. USA 2015, 112, 5573–5578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thattet, U.M.; Dahanukar, S.A. Immunotherapeutic modification of experimental infections by Indian medicinal plants. Phytother. Res. 1989, 3, 43–49. [Google Scholar] [CrossRef]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Pastors, J.G.; Warshaw, H.; Daly, A.; Franz, M.; Kulkarni, K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 2002, 25, 608–613. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Kulshresht, A.; Qureshi, T.N. Antidiabetic activity of some herbal plants in streptozotocin induced diabetic albino rats. Pak. J. Nutr. 2009, 8, 551–557. [Google Scholar] [CrossRef]
- Bnouham, M.; Ziyyat, A.; Mekhfi, H.; Tahri, A.; Legssyer, A. Medicinal plants with potential antidiabetic activity—A review of ten years of herbal medicine research (1990–2000). Int. J. Diabetes Metab. 2006, 14, 1–25. [Google Scholar] [CrossRef]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.-H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzin, P.; Eisensmith, R.C.; Copeland, K.C.; Woo, S.L.C. Hepatic insulin gene expression as treatment for type 1 diabetes mellitus in rats. Mol. Endocrinol. 1997, 11, 833–837. [Google Scholar] [CrossRef]
- Auricchio, A.; Gao, G.-P.; Yu, Q.; Raper, S.; Rivera, V.; Clackson, T.; Wilson, J. Constitutive and regulated expression of processed insulin following in vivo hepatic gene transfer. Gene Ther. 2002, 9, 963–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Short, D.K.; Okada, S.; Yamauchi, K.; Pessin, J.E. Adenovirus-mediated transfer of a modified human proinsulin gene reverses hyperglycemia in diabetic mice. Am. J. Physiol. Metab. 1998, 275, E748–E756. [Google Scholar] [CrossRef]
- O’Doherty, R.M.; Lehman, D.L.; Telemaque-Potts, S.; Newgard, C.B. Metabolic impact of glucokinase overexpression in liver: Lowering of blood glucose in fed rats is accompanied by hyperlipidemia. Diabetes 1999, 48, 2022–2027. [Google Scholar] [CrossRef]
- Morral, N.; McEvoy, R.; Dong, H.; Meseck, M.; Altomonte, J.; Thung, S.; Woo, S.L. Adenovirus-mediated expression of glucokinase in the liver as an adjuvant treatment for type 1 diabetes. Hum. Gene Ther. 2002, 13, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, R.M.; Jensen, P.B.; Anderson, P.; Jones, J.G.; Berman, H.K.; Kearney, D.; Newgard, C.B. Activation of direct and indirect pathways of glycogen synthesis by hepatic overexpression of protein targeting to glycogen. J. Clin. Investig. 2000, 105, 479–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzucchi, S.E.; Lipska, K.J.; Mayo, H.; Bailey, C.J.; McGuire, D.K. Metformin in patients with type 2 diabetes and kidney disease. JAMA 2014, 312, 2668–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Features of Diabetes | Gene Involved | Clinical Outcome |
|---|---|---|
| MODY | GCK [26] | GCK-MODY; stable, nonprogressive, normally does not require treatment. Microvascular complications developed in rare cases |
| HNF1A [27] | HNF1A-MODY; progressive defect in insulin secretion, requires a low dose of sulfonylurea therapy | |
| HNF4A [28] | HNF4A-MODY; progressive insulin secretory defect, sensitive to sulfonylurea | |
| HNF1B [29] | HNF1B-MODY; atrophy of the pancreas, renal disease, hyperuricemia, and gout may develop | |
| Kir6.2 [30] | Requires high dose of sulfonylurea therapy | |
| Neonatal diabetes | KCNJ11 [30] | Maybe permanent or transient intrauterine growth restriction, delay development, sensitive to sulfonylurea |
| INS [31] | Require insulin therapy | |
| ABCC8 [32] | Sensitive to sulfonylurea therapy, in some cases delay development | |
| 6q24 (PLAG1, HYMA1) [33] | Transient in nature, intrauterine growth restriction, macroglossia; umbilical hernia, treatment possible other than insulin | |
| GATA6 [34] | Pancreatic hypoplasia and exocrine insufficiency require insulin therapy | |
| EIF2AK3 [35] | Develop Wolcott–Rallison syndrome, requires insulin therapy | |
| FOXP3 [36] | Immunodysregulation, polyendocrinopathy, enteropathy X-linked (IPEX) syndrome; autoimmune diabetes; autoimmune thyroid disease; exfoliative dermatitis; insulin-requiring |
| Drug Class | Example | Mode of Action | Common Side Effects | References |
|---|---|---|---|---|
| Insulin secretagogues | Sulfonylureas | Inhibit β-cell K+ ATP channel and facilitate insulin secretion | Hypoglycemia | [111] |
| Meglitinides | Weight gain | |||
| GLP-1 agonists | Liraglutide | Increase glucose-dependent insulin secretion, reduces glucagon secretion, and delays gastric emptying | [112,113] | |
| Exenatide | Pancreatitis | |||
| Lixisenatide | ||||
| α–glucosidase inhibitors | Acarbose | Reduces the rate of digestion of carbohydrate in the intestine hence less glucose absorption | Abdominal pain | [114] |
| Miglitol | Diarrhea | |||
| Voglibose | Flatulence | |||
| Canagliflozin | Increases glucose excretion in urine | Hypotension | [115] | |
| SGLT-2 inhibitors | Dapagliflozin | Urinary tract infection | ||
| Empagliflozin | ||||
| Vildagliptin | Increases endogenous GLP-1 and GIP levels | Respiratory tract infection | [116,117] | |
| DPP4 inhibitors | Linagliptin | Nasopharyngitis | ||
| Saxagliptin | Headache | |||
| Sitagliptin | ||||
| Activate AMPK, decreasing glucose production and insulin resistance | Lactic acidosis | [118] | ||
| Biguanides | Metformin | Gastrointestinal irritation | ||
| Pioglitazone | Activate PPAR-γ, decreasing insulin resistance | Fluid retention | [119] | |
| Thiazolidinediones | Rosiglitazone | Weight gain |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36-50. https://doi.org/10.3390/diabetology2020004
Alam S, Hasan MK, Neaz S, Hussain N, Hossain MF, Rahman T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology. 2021; 2(2):36-50. https://doi.org/10.3390/diabetology2020004
Chicago/Turabian StyleAlam, Saruar, Md. Kamrul Hasan, Sharif Neaz, Nazmul Hussain, Md. Faruk Hossain, and Tania Rahman. 2021. "Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management" Diabetology 2, no. 2: 36-50. https://doi.org/10.3390/diabetology2020004
APA StyleAlam, S., Hasan, M. K., Neaz, S., Hussain, N., Hossain, M. F., & Rahman, T. (2021). Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology, 2(2), 36-50. https://doi.org/10.3390/diabetology2020004