Influence of Surface Treatments on the Electrochemical Performance of Lab-Made 3D-Printed Electrodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
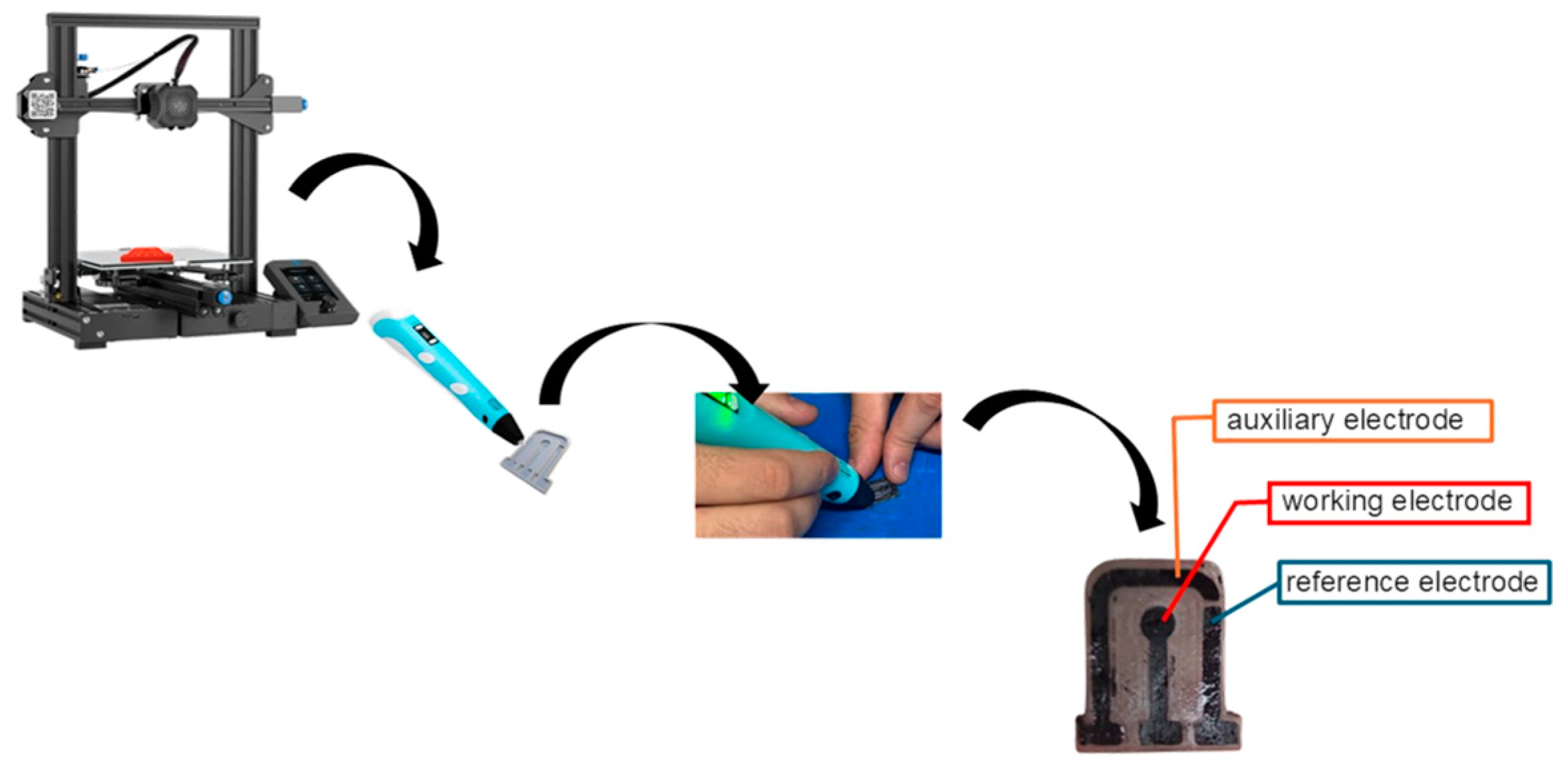
2.2. Manufacture of Composite Filament and CB-PLA Electrodes
2.3. CB-PLA Electrochemical Sensor Treatment
2.4. Electrochemical Characterization of Sensors
3. Results
3.1. Manufacture of Conductive Filaments and CB-PLA Electrodes
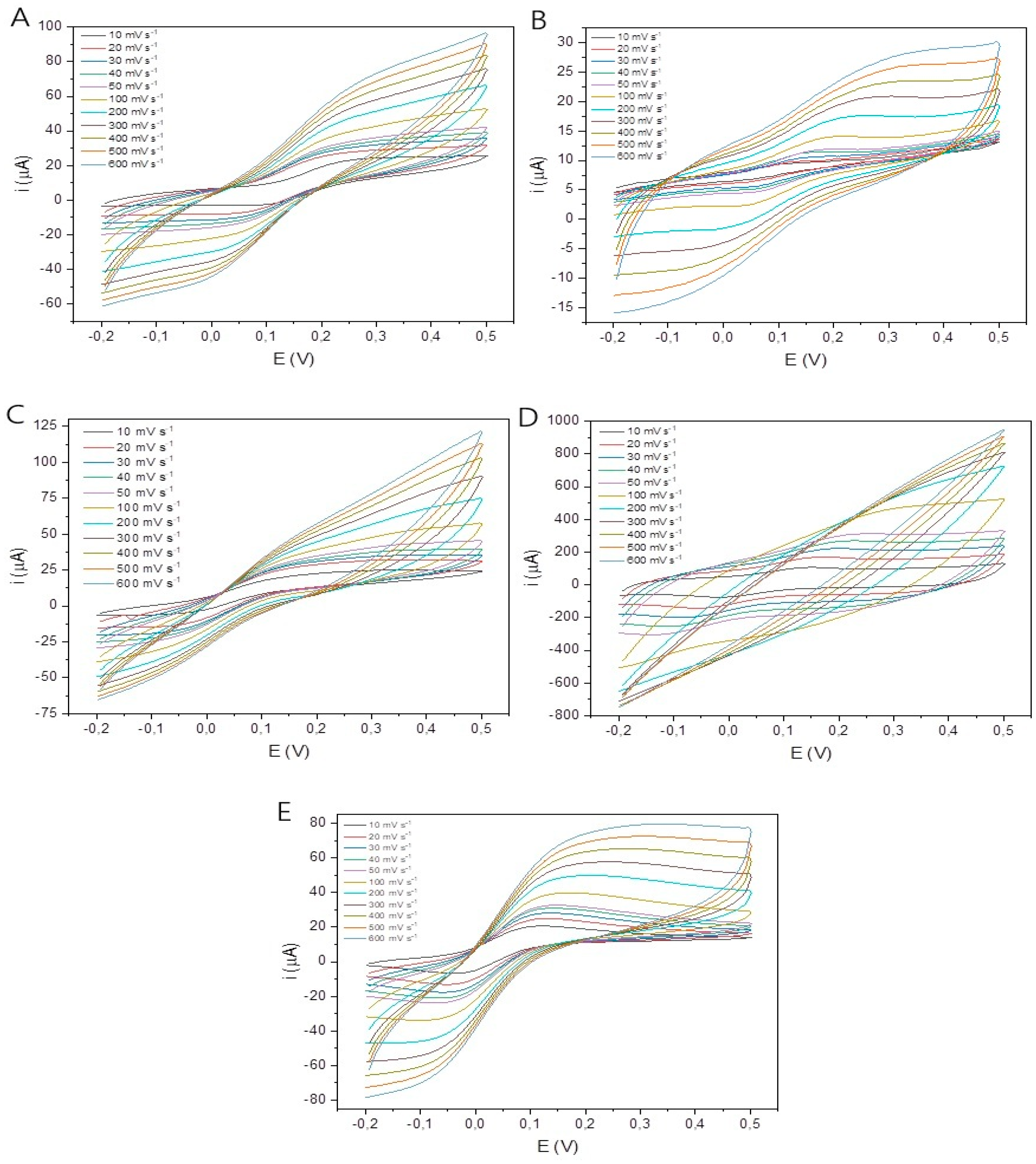
3.2. Electrochemical Characterization of Sensor
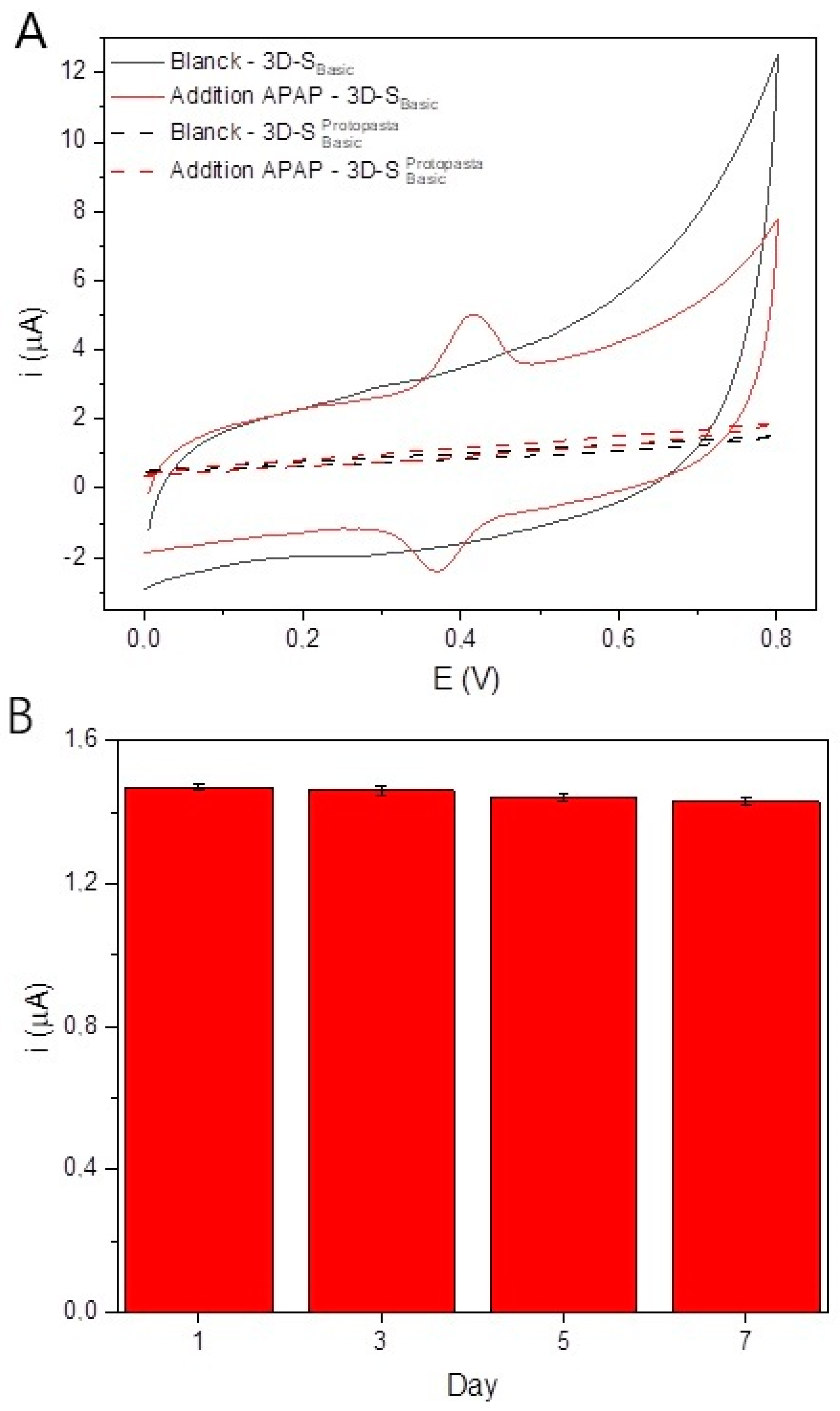
3.3. Evaluation of the Potential of 3D-SBasic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Jiang, S.; Zhang, S. Mapping technological trajectories and exploring knowledge sources: A case study of 3D printing technologies. Technol. Forecast. Soc. Change 2020, 161, 120251. [Google Scholar] [CrossRef]
- Al Salaheen, M.; Alaloul, W.S.; Ali Musarat, M.; Johari, M.A.B.; Alzubi, K.M.; Alawag, A.M. Women career in construction industry after industrial revolution 4.0 norm. J. Open Innov. Technol. Mark. Complex. 2024, 10, 100277. [Google Scholar] [CrossRef]
- Praveena, B.A.; Lokesh, N.; Buradi, A.; Santhosh, N.; Praveena, B.L.; Vignesh, R. A comprehensive review of emerging additive manufacturing (3D printing technology): Methods, materials, applications, challenges, trends and future potential. Mater. Today Proc. 2022, 52, 1309–1313. [Google Scholar] [CrossRef]
- Mohanavel, V.; Ashraff Ali, K.S.; Ranganathan, K.; Allen Jeffrey, J.; Ravikumar, M.M.; Rajkumar, S. The roles and applications of additive manufacturing in the aerospace and automobile sector. Mater. Today Proc. 2021, 47, 405–409. [Google Scholar] [CrossRef]
- Shakor, P.; Nejadi, S.; Paul, G.; Malek, S. Review of Emerging Additive Manufacturing Technologies in 3D Printing of Cementitious Materials in the Construction Industry. Front. Built Environ. 2019, 4, 85. [Google Scholar] [CrossRef]
- Hojati, M.; Li, Z.; Memari, A.M.; Park, K.; Zahabi, M.; Nazarian, S.; Duarte, J.P.; Radlińska, A. 3D-printable quaternary cementitious materials towards sustainable development: Mixture design and mechanical properties. Results Eng. 2022, 13, 100341. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Bhandari, B.; Li, J. Future perspective of additive manufacturing of food for children. Trends Food Sci. Technol. 2023, 136, 120–134. [Google Scholar] [CrossRef]
- Millan, M.G.D.; de la Torre, M.G.M.V. 3D food printing: Technological advances, personalization and future challenges in the food industry. Int. J. Gastron. Food Sci. 2024, 37, 100963. [Google Scholar] [CrossRef]
- Alami, A.H.; Olabi, A.G.; Alashkar, A.; Alasad, S.; Aljaghoub, H.; Rezk, H.; Abdelkareem, M.A. Additive manufacturing in the aerospace and automotive industries: Recent trends and role in achieving sustainable development goals. Ain Shams Eng. J. 2023, 14, 102516. [Google Scholar] [CrossRef]
- Careri, F.; Khan, R.H.U.; Todd, C.; Attallah, M.M. Additive manufacturing of heat exchangers in aerospace applications: A review. Appl. Therm. Eng. 2023, 235, 121387. [Google Scholar] [CrossRef]
- Bozkurt, Y.; Karayel, E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 2021, 14, 1430–1450. [Google Scholar] [CrossRef]
- Abdalla, A.; Patel, B.A. 3D Printed Electrochemical Sensors. Annu. Rev. Anal. Chem. 2021, 14, 47–63. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; dos Santos, P.L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 118, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Cândido, T.C.O.; Silva, D.N.d.; Borges, M.M.C.; Barbosa, T.G.; Trindade, S.O.D.d.; Pereira, A.C. 3D-Printed Electrochemical Sensors: A Comprehensive Review of Clinical Analysis Applications. Analytica 2024, 5, 552–575. [Google Scholar] [CrossRef]
- Abdalla, A.; Patel, B.A. 3D-printed electrochemical sensors: A new horizon for measurement of biomolecules. Curr. Opin. Electrochem. 2020, 20, 78–81. [Google Scholar] [CrossRef]
- Zhou, X.; Deng, J.; Fang, C.; Lei, W.; Song, Y.; Zhang, Z.; Huang, Z.; Li, Y. Additive manufacturing of CNTs/PLA composites and the correlation between microstructure and functional properties. J. Mater. Sci. Technol. 2021, 60, 27–34. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, Z.; He, X.L.; Tang, X.P.; Wu, N.Y.; Huang, D.; Dong, M.; Huang, J. Electrical gas sensors based on metal–organic frameworks for breath diagnosis. Microchem. J. 2024, 199, 109992. [Google Scholar] [CrossRef]
- Li, W.K.; Shi, Y.-P. Recent advances of carbon materials on pesticides removal and extraction based determination from polluted water. Trends Anal. Chem. 2024, 171, 117534. [Google Scholar] [CrossRef]
- Wan, X.; Du, H.; Tuo, D.; Qi, X.; Wang, T.; Wu, J.; Li, G. UiO-66/Carboxylated Multiwalled Carbon Nanotube Composites for Highly Efficient and Stable Voltammetric Sensors for Gatifloxacin. ACS Appl. Nano Mater. 2023, 6, 19403–19413. [Google Scholar] [CrossRef]
- Wang, T.; Guo, W.; Xia, Y.; Zhu, Y.; Chen, L.; Zhang, Y.; Li, G. Bimetallic UiO-66(Zr/Ce) MOF encapsulated reduced graphene oxide nanocomposite for voltametric detection of trace Lomefloxacin residues in milk. J. Food Compos. Anal. 2025, 139, 107086. [Google Scholar] [CrossRef]
- Zouaoui, F.; Menassol, G.; Ducros, C.; Mailley, P.; Thomas, Y. Electrochemical sensors based on amorphous carbon electrode: A review. Microchem. J. 2025, 209, 112650. [Google Scholar] [CrossRef]
- Raoof, J.B.; Darvishnejad, F.; Ghani, M. A sensitive electrochemical sensor based on polyoxometalates@carbon spheres-multi-walled carbon nanotubes-β-cyclodextrin composite modified carbon paste electrode for simultaneous determination of some phenolic compounds in environmental samples. Microchem. J. 2025, 208, 112548. [Google Scholar] [CrossRef]
- Joshi, A.; Slaughter, G. Electrochemical carbon-based sensors for non-enzymatic uric acid sensing. Microchem. J. 2025, 208, 112331. [Google Scholar] [CrossRef]
- Stefano, J.S.; Silva, L.R.G.; Rocha, R.G.; Brazaca, L.C.; Richter, E.M.; Muñoz, R.A.A.; Janegitz, B.C. New conductive filament ready-to-use for 3D-printing electrochemical (bio)sensors: Towards the detection of SARS-CoV-2. Anal. Chim. Acta 2022, 1191, 339372. [Google Scholar] [CrossRef]
- Dul, S.; Fambri, L.; Pegoretti, A. Fused Deposition Modelling with ABS–Graphene Nanocomposites. Compos. Part. A Appl. Sci. Manuf. 2016, 85, 181–191. [Google Scholar] [CrossRef]
- Lopes, C.E.C.; De Faria, L.V.; Araújo, D.A.G.; Richter, E.M.; Paixão, T.R.L.C.; Dantas, L.M.F.; Muñoz, R.A.A.; Da Silva, I.S. Lab-Made 3D-Printed Electrochemical Sensors for Tetracycline Determination. Talanta 2023, 259, 124536. [Google Scholar] [CrossRef] [PubMed]
- Musenich, L.; Berardengo, M.; Avalle, M.; Haj-Ali, R.; Sharabi, M.; Libonati, F. Anisotropic Mechanical and Sensing Properties of Carbon Black-Polylactic Acid Nanocomposites Produced by Fused Filament Fabrication. Smart Mater. Struct. 2024, 33, 095010. [Google Scholar] [CrossRef]
- Rocha, D.P.; Rocha, R.G.; Castro, S.V.F.; Trindade, M.A.G.; Munoz, R.A.A.; Richter, E.M.; Angnes, L. Posttreatment of 3D-printed Surfaces for Electrochemical Applications: A Critical Review on Proposed Protocols. Electrochem. Sci. Adv. 2022, 2, e2100136. [Google Scholar] [CrossRef]
- Novotný, F.; Urbanová, V.; Plutnar, J.; Pumera, M. Preserving Fine Structure Details and Dramatically Enhancing Electron Transfer Rates in Graphene 3D-Printed Electrodes via Thermal Annealing: Toward Nitroaromatic Explosives Sensing. ACS Appl. Mater. Interfaces 2019, 11, 35371–35375. [Google Scholar] [CrossRef]
- João, A.F.; Rocha, R.G.; Matias, T.A.; Richter, E.M.; Flávio, S.; Petruci, J.; Muñoz, R.A.A. 3D-Printing in Forensic Electrochemistry: Atropine Determination in Beverages Using an Additively Manufactured Graphene-Polylactic Acid Electrode. Microchem. J. 2021, 167, 106324. [Google Scholar] [CrossRef]
- Singh Shergill, R.; Perez, F.; Abdalla, A.; Anil Patel, B. Comparing Electrochemical Pre-Treated 3D Printed Native and Mechanically Polished Electrode Surfaces for Analytical Sensing. J. Electroanal. Chem. 2022, 905, 115994. [Google Scholar] [CrossRef]
- Redondo, E.; Muñoz, J.; Pumera, M. Green Activation Using Reducing Agents of Carbon-Based 3D Printed Electrodes: Turning Good Electrodes to Great. Carbon 2021, 175, 413–419. [Google Scholar] [CrossRef]
- Negahdary, M.; do Lago, C.L.; Gutz, I.G.R.; Buoro, R.M.; Durazzo, M.; Angnes, L. Developing a nanomaterial-based 3D-printed platform: Application as a cancer aptasensor via detection of heat shock protein 90 (HSP90). Sens. Actuators B Chem. 2024, 409, 135592. [Google Scholar] [CrossRef]
- Veloso, W.B.; Ataide, V.N.; Rocha, D.P.; Nogueira, H.P.; De Siervo, A.; Angnes, L.; Muñoz, R.A.A.; Paixão, T.R.L.C. 3D-Printed Sensor Decorated with Nanomaterials by CO2 Laser Ablation and Electrochemical Treatment for Non-Enzymatic Tyrosine Detection. Microchim. Acta 2023, 190, 63. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, G.P.; Rocha, R.G.; Nascimento, A.B.; Richter, E.M.; Muñoz, R.A.A. Portable Atmospheric Air Plasma Jet Pen for the Surface Treatment of Three-Dimensionally (3D)-Printed Electrodes. Anal. Chem. 2024, 96, 15852–15858. [Google Scholar] [CrossRef]
- Zhong, L.; Liao, M.; Ou, J.; Yang, Y.; Wen, J.; Jiang, Y.; Yang, H.; Dai, X.; Wang, L. Gold particles modified 3D printed carbon black nanonetwork electrode for improving the detection sensitivity of dopamine. Microchem. J. 2024, 201, 110630. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, I.Y.; Song, W.S. Biodegradation of Polylactic Acid (PLA) Fibers Using Different Enzymes. Macromol. Res. 2014, 22, 657–663. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and Biodegradation of Poly(Lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Burke, J. Solubility Parameters: Theory and Application. AIC Book Pap. Group Annu. 1984, 3, 13–58. [Google Scholar]
- Abbott, S. Chemical compatibility of poly(lactic acid): A practical framework using Hansen solubility parameters. In Poly(lactic acid): Synthesis, Structures, Properties, Processing, and Applications; Auras, R., Lim, L.T., Selke, S.E.M., Tsuji, H., Eds.; John Wiley & Son, Inc.: Hoboken, NJ, USA, 2010; pp. 83–95. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Katic, V.; Loureiro, H.C.; dos Santos, M.F.; dos Santos, D.P.; Formiga, A.L.B.; Bonacin, J.A. Enhanced performance of 3D printed graphene electrodes after electrochemical pre-treatment: Role of exposed graphene sheets. Sens. Actuators B Chem. 2019, 281, 837–848. [Google Scholar] [CrossRef]
- Kalinke, C.; Neumsteir, N.V.; Aparecido, G.O.; Ferraz, T.V.B.; dos Santos, P.L.; Janegitz, B.C.; Bonacin, J.A. Comparison of activation processes for 3D printed PLA-graphene electrodes: Electrochemical properties and application for sensing of dopamine. Analyst 2020, 145, 1207. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, R.; Browne, M.P.; Sofer, Z.; Pumera, M. The capacitance and electron transfer of 3D-printed graphene electrodes are dramatically influenced by the type of solvent used for pre-treatment. Electrochem. Commun. 2019, 102, 83–88. [Google Scholar] [CrossRef]
- Sharma, A.; Faber, H.; Khosla, A.; Anthopoulos, T.D. 3D printed electrochemical devices for bio-chemical sensing: A review. Mater. Sci. Eng. R Rep. 2023, 156, 100754. [Google Scholar] [CrossRef]
- Silva, V.A.O.P.; Fernandes-Junior, W.S.; Rocha, D.P.; Stefano, J.S.; Munoz, R.A.A.; Bonacin, J.A.; Janegitz, B.C. 3D-printed reduced graphene oxide/polylactic acid electrodes: A new prototyped platform for sensing and biosensing applications. Biosens. Bioelectron. 2020, 170, 112684. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Skoog, D.A.; West, D.M.; Holler, J. Fundamentals of Analytical Chemistry, 9th ed.; Cengage Learnin: Singapore, 2014. [Google Scholar]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry: Principles, Methods and Applications; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Trachioti, M.G.; Lazanas, A.C.; Prodromidis, M.I. Shedding light on the calculation of electrode electroactive area and heterogeneous electron transfer rate constants at graphite screen-printed electrodes. Microchim. Acta 2023, 190, 251. [Google Scholar] [CrossRef]
- Washe, A.P.; Lozano-Sánchez, P.; Bejarano-Nosas, D.; Katakis, I. Facile and versatile approaches to enhancing electrochemical performance of screen printed electrodes. Electrochim. Acta. 2013, 91, 166–172. [Google Scholar] [CrossRef]
- Tanimoto, S.; Ichimura, A. Discrimination of Inner- and Outer-Sphere Electrode Reactions by Cyclic Voltammetry Experiments. J. Chem. Educ. 2013, 90, 778–781. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Yuan, H.; Chang, G.; Zhu, J.; Dai, H.; Wei, X. Review of electrochemical impedance spectroscopy in fault diagnosis for proton exchange membrane fuel cells. Renew. Sustain. Energy Rev. 2025, 211, 115226. [Google Scholar] [CrossRef]
- Chang, C.; Wang, S.; Tao, C.; Jiang, J.; Jiang, Y.; Wang, L. An improvement of equivalent circuit model for state of health estimation of lithium-ion batteries based on mid-frequency and low-frequency electrochemical impedance spectroscopy. Measurement 2022, 202, 111795. [Google Scholar] [CrossRef]
- Finšgar, M.; Xhanari, K.; Petovar, B. Copper-film electrodes for Pb(II) trace analysis and a detailed electrochemical impedance spectroscopy study. Microchem. J. 2019, 147, 863–871. [Google Scholar] [CrossRef]
- Sadeghi, M.; Shabani-Nooshabadi, M. High sensitive titanium/chitosan-coated nanoporous gold film electrode for electrochemical determination of acetaminophen in the presence of piroxicam. Prog. Org. Coat. 2021, 151, 106100. [Google Scholar] [CrossRef]
- Gu, H.; Shui, X.; Zhang, Y.; Zeng, T.; Yang, J.; Wu, Z.; Zhang, X.; Yang, N. Porous carbon scaffolded Fe-based alloy nanoparticles for electrochemical quantification of acetaminophen and rutin. Carbon 2024, 221, 118954. [Google Scholar] [CrossRef]
- Ghadirinataj, M.; Hassaninejad-Darzi, S.K.; Emadi, H. An electrochemical nanosensor for simultaneous quantification of acetaminophen and acyclovir by ND@Dy2O3-IL/CPE. Electrochim. Acta 2023, 450, 142274. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Alsareii, S.A.; Jalalah, M.; Alsaiari, M.; Harraz, F.A. Mn2O3 nanoparticle-porous silicon nanocomposite based amperometric sensor for sensitive detection and quantification of Acetaminophen in real samples. Ceram. Int. 2023, 49, 933–943. [Google Scholar] [CrossRef]
- Pereira, A.C.S.; Silva, D.N.; Porto, L.S.; Pereira, A.C. Development of electrochemical biosensor based on nanostructured carbon materials for paracetamol determination. Electroanalysis 2020, 32, 1905–1913. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Yan, X.; Li, K.; Deng, D.; He, H.; Lei, Y.; Luo, L. Electrochemical sensor based on Ni3S2-MoS2 hollow nanospheres for sensitive detection of acetaminophen. Microchem. J. 2025, 208, 112487. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, D.; Wang, J.; Lin, X.; Diao, G. Three-dimensional nitrogen-doped graphene-based metal-free electrochemical sensors for simultaneous determination of ascorbic acid, dopamine, uric acid, and acetaminophen. Analyst 2021, 146, 964. [Google Scholar] [CrossRef]
- Yang, K.; Yang, H.; Zheng, Y.; Chen, H.; Liu, W.; Yang, X. Electrochemical sensor based on cobalt single-atom anchored porous carbon composite for sensitive detection of acetaminophen. Microchem. J. 2024, 203, 110874. [Google Scholar] [CrossRef]
- Sriprasertsuk, S.; Mathias, S.C.; Varcoe, J.R.; Crean, C. Polypyrrole-coated carbon fibre electrodes for paracetamol and clozapine drug sensing. J. Electroanal. Chem. 2021, 897, 115608. [Google Scholar] [CrossRef]
- Kozak, J.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. Electrochemically Activated Screen-Printed Carbon Sensor Modified with Anionic Surfactant (aSPCE/SDS) for Simultaneous Determination of Paracetamol, Diclofenac and Tramadol. Materials 2021, 14, 3581. [Google Scholar] [CrossRef]
- Yang, H.; Dai, X.; Liao, M.; Ou, J.; Yang, Y.; Wan, M.; Zhou, J.; Wang, L. Spherical covalent organic framework and gold nanoparticles modified 3D-printed nanocarbon electrode for the sensor of acetaminophen. Microchem. J. 2023, 189, 108547. [Google Scholar] [CrossRef]
- Rumack, B.H.; Matthew, H. Acetaminophen poisoning and toxicity. Pediatrics 1975, 55, 871–876. [Google Scholar] [CrossRef] [PubMed]



| Sensor | α | Electrochemical Area (mm2) | K° (cm s−1) | RΩ (Ω) | Rp (Ω) | Q |
|---|---|---|---|---|---|---|
| 3D sensor | 0.40 | 1.02 ± 0.03 | 0.001534 | 151.00 | 2000.00 | 23.9 μS s0.279 |
| 3D-SE | 0.50 | 0.20 ± 0.01 | 0.000403 | 462.00 | 3990.00 | 8.75 μS s0.657 |
| 3D-SAcid | 0.30 | 7.22 ± 0.27 | 0.000139 | 642.00 | 3670.00 | 41.30 μS s0.537 |
| 3D-SBasic | 0.50 | 10.03 ± 0.42 | 0.000529 | 276.00 | 118.00 | 20.60 μS s0.500 |
| 3D-SSolvent | 0.40 | 5.62 ± 0.13 | 0.000442 | 500.00 | 1600.00 | 45.03 μS s0.763 |
| Sensor | Sensitivity (μA/μM) |
|---|---|
| 3D-SBasic | 0.20809 |
| Glass Carbon | 0.03633 |
| Carbon Paste | 0.02444 |
| Platinum Disc | 0.00282 |
| Sensor | Sensitivity (μA/μM) | LOD (μM) | [Ref.] |
|---|---|---|---|
| SG-CS-TiNPGF a | 0.486 | 0.10 | [55] |
| FeCo@C/GCE b | 38.22 | 5.9 × 10−4 | [56] |
| ND@Dy2O3-IL/CPE c | - | 0.031 | [57] |
| Mn2O3@PSi/GCE d | 0.058 | 0.033 | [58] |
| MWCNT-LAC/GCE e | 0.339 | 7.00 | [59] |
| Ni3S2-MoS2/GCE f | - | 0.093 | [60] |
| 3D-NG/DMF/ GCE g | - | 0.020 | [61] |
| Co-N-C@PC/GCE h | 5.12 | 0.0341 | [62] |
| PPy.SDS i | 14.7 | 34.00 | [63] |
| SPCE/SDS j | 0.44 | 0.0148 | [64] |
| 3DE/Au/COF k | 0.170 | 0.076 | [65] |
| 3D-SBasic | 0.519 | 0.198 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, T.G.; Silva, D.N.d.; Borges, M.M.C.; Trindade, S.O.D.d.; de Oliveira Cândido, T.C.; Pereira, A.C. Influence of Surface Treatments on the Electrochemical Performance of Lab-Made 3D-Printed Electrodes. Analytica 2025, 6, 9. https://doi.org/10.3390/analytica6010009
Barbosa TG, Silva DNd, Borges MMC, Trindade SODd, de Oliveira Cândido TC, Pereira AC. Influence of Surface Treatments on the Electrochemical Performance of Lab-Made 3D-Printed Electrodes. Analytica. 2025; 6(1):9. https://doi.org/10.3390/analytica6010009
Chicago/Turabian StyleBarbosa, Thiago Gabry, Daniela Nunes da Silva, Marcella Matos Cordeiro Borges, Scarlat Ohanna Dávila da Trindade, Thaís Cristina de Oliveira Cândido, and Arnaldo César Pereira. 2025. "Influence of Surface Treatments on the Electrochemical Performance of Lab-Made 3D-Printed Electrodes" Analytica 6, no. 1: 9. https://doi.org/10.3390/analytica6010009
APA StyleBarbosa, T. G., Silva, D. N. d., Borges, M. M. C., Trindade, S. O. D. d., de Oliveira Cândido, T. C., & Pereira, A. C. (2025). Influence of Surface Treatments on the Electrochemical Performance of Lab-Made 3D-Printed Electrodes. Analytica, 6(1), 9. https://doi.org/10.3390/analytica6010009









