Simultaneous Detection of Drug-Induced Liver Injury Protein and microRNA Biomarkers Using Dynamic Chemical Labelling on a Luminex MAGPIX System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clinical Samples
2.3. Calibration Curves for ARG1 and miR-122 Assays
2.3.1. Calibration Curve for ARG1 Assay
2.3.2. Calibration Curve for miR-122 Assay
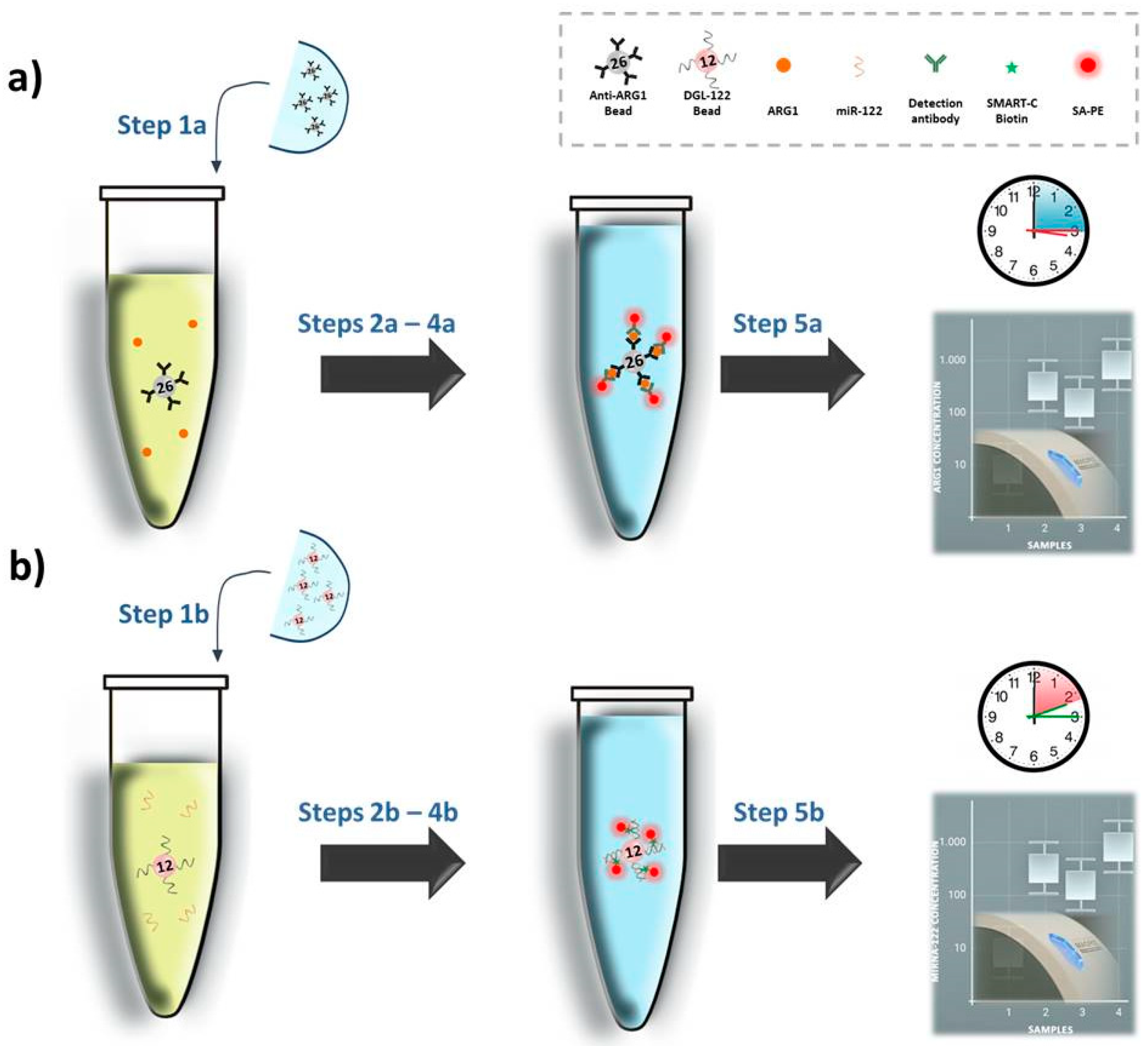
2.4. ARG1 Singleplex Assay
2.5. miR-122 Singleplex Assay
2.6. SeqCOMBO Assay
3. Results and Discussion
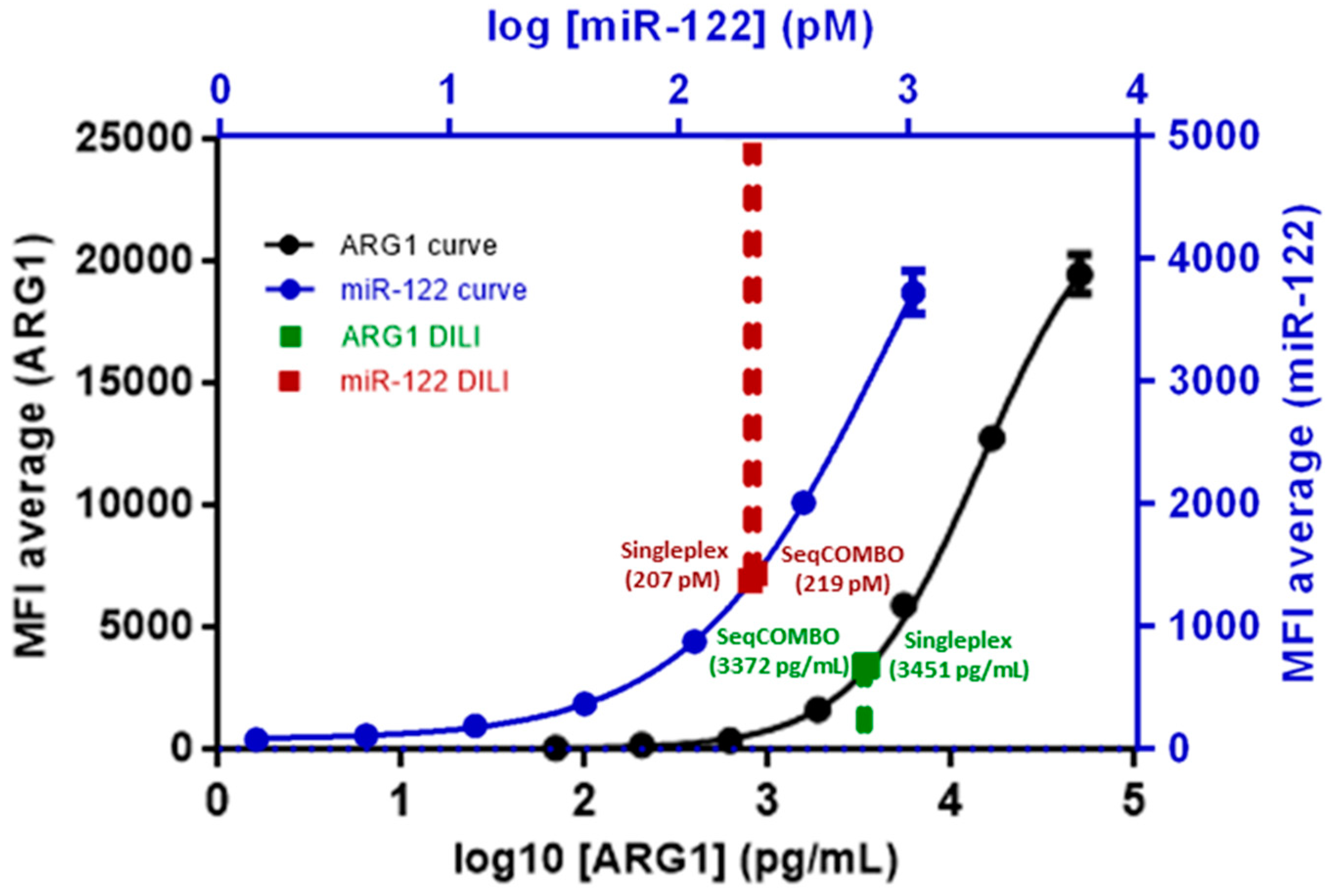
3.1. Singleplex Assay—Analysis of ARG1 and miR-122
3.2. SeqCOMBO Assay—Analysis of ARG1 and miR-122 Simultaneously
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Comission. Strengthening Pharmacovigilance to Reduce Adverse Effects of Medicines. 2008. Available online: https://ec.europa.eu/commission/presscorner/detail/en/MEMO_08_782 (accessed on 14 July 2021).
- Juntti-Patinen, L.; Neuvonen, P.J. Drug-related deaths in a university central hospital. Eur. J. Clin. Pharmacol. 2002, 58, 479–482. [Google Scholar]
- Devarbhavi, H. An Update on Drug-induced Liver Injury. J. Clin. Exp. Hepatol. 2012, 2, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Suzuki, A.; Borlak, J.; Andrade, R.J.; Lucena, M.I. Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 2015, 63, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björnsson, E.S. Drug-induced liver injury: An overview over the most critical compounds. Arch. Toxicol. 2015, 89, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Howell, L.S.; Ireland, L.; Park, B.K.; Goldring, C.E. MiR-122 and other microRNAs as potential circulating biomarkers of drug-induced liver injury. Expert Rev. Mol. Diagn. 2018, 18, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Correction to: Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2019, 17, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babai, S.; Auclert, L.; Le-Louët, H. Safety data and withdrawal of hepatotoxic drugs. Therapie 2018. [Google Scholar] [CrossRef] [PubMed]
- Robles-Díaz, M.; Medina-Caliz, I.; Stephens, C.; Andrade, R.J.; Lucena, M.I. Biomarkers in DILI: One More Step Forward. Front. Pharmacol. 2016, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular Biomarkers in Drug-Induced Liver Injury: Challenges and Future Perspectives. Front. Pharmacol. 2019, 10, 1667. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, N.; Iguchi, H.; Ochiya, T. Circulating microRNA in body fluid: A new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010, 101, 2087–2092. [Google Scholar] [CrossRef]
- Ortiz-Quintero, B. Cell-free microRNAs in blood and other body fluids, as cancer biomarkers. Cell Prolif. 2016, 49, 281–303. [Google Scholar] [CrossRef]
- Gloor, Y.; Schvartz, D.F.; Samer, C. Old problem, new solutions: Biomarker discovery for acetaminophen liver toxicity. Expert Opin. Drug Metab. Toxicol. 2019, 15, 659–669. [Google Scholar] [CrossRef]
- Lin, H.; Ewing, L.E.; Koturbash, I.; Gurley, B.J.; Miousse, I.R. MicroRNAs as biomarkers for liver injury: Current knowledge, challenges and future prospects. Food Chem. Toxicol. 2017, 110, 229–239. [Google Scholar] [CrossRef]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C. Liver-specific microRNA-122: Biogenesis and function. RNA Biol. 2012, 9, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Longarela, B.; Morrison, E.E.; Tranter, J.D.; Chahman-Vos, L.; Léonard, J.F.; Gautier, J.C.; Laurent, S.; Lartigau, A.; Boitier, E.; Sautier, L.; et al. Direct Detection of miR-122 in Hepatotoxicity Using Dynamic Chemical Labeling Overcomes Stability and isomiR Challenges. Anal. Chem. 2020, 92, 3388–3395. [Google Scholar] [CrossRef] [PubMed]
- Detassis, S.; Grasso, M.; Tabraue-Chávez, M.; Marín-Romero, A.; López-Longarela, B.; Ilyine, H.; Ress, C.; Ceriani, S.; Erspan, M.; Maglione, A.; et al. New Platform for the Direct Profiling of microRNAs in Biofluids. Anal. Chem. 2019, 91, 5874–5880. [Google Scholar] [CrossRef] [PubMed]
- Marín-Romero, A.; Robles-Remacho, A.; Tabraue-Chávez, M.; López-Longarela, B.; Sánchez-Martín, R.M.; Guardia-Monteagudo, J.J.; Fara, M.A.; López-Delgado, F.J.; Pernagallo, S.; Díaz-Mochón, J.J. A PCR-free technology to detect and quantify microRNAs directly from human plasma. Analyst 2018, 143, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; López-Longarela, B.; Pernagallo, S.; Ilyine, H.; Vliegenthart, A.D.B.; Dear, J.W.; Díaz-Mochón, J.J.; Duffy, D.C. Polymerase-free measurement of microRNA-122 with single base specificity using single molecule arrays: Detection of drug-induced liver injury. PLoS ONE 2017, 12, e0179669. [Google Scholar] [CrossRef] [PubMed]
- McCreight, J.C.; Schneider, S.E.; Wilburn, D.B.; Swanson, W.J. Evolution of microRNA in primates. PLoS ONE 2017, 12, e0176596. [Google Scholar] [CrossRef]
- Dear, J.W.; Clarke, J.I.; Francis, B.; Allen, L.; Wraight, J.; Shen, J.; Dargan, P.I.; Wood, D.; Cooper, J.; Thomas, S.H.; et al. Risk stratification after paracetamol overdose using mechanistic biomarkers: Results from two prospective cohort studies. Lancet Gastroenterol. Hepatol. 2018, 3, 104–113. [Google Scholar] [CrossRef] [Green Version]
- SAFE-T Consortium. Novel Clinical Biomarkers of Drug-Induced Liver Injury. 2016. Available online: http://www.imi-safe-t.eu/files/files-inline/DILI%20BM%20Summary%20Data%20Package%20-%2020170105_final_updated.pdf (accessed on 14 April 2021).
- Comission, E. Translational Safety Biomarker Pipeline (TransBioLine): Enabling Development and Implementation of Novel Safety Biomarkers in Clinical Trials and Diagnosis of Disease. 2019. Available online: https://cordis.europa.eu/project/id/821283 (accessed on 14 April 2021).
- (FDA) FaDA. Letter of Support for Drug-Induced Liver Injury (DJU) Biomarker(s). 2016. Available online: https://www.fda.gov/media/99532/download (accessed on 14 July 2021).
- Wang, X.; Walt, D.R. Simultaneous detection of small molecules, proteins and microRNAs using single molecule arrays. Chem. Sci. 2020, 11, 7896–7903. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Luque-González, M.A.; Tabraue-Chávez, M.; Fara, M.A.; López-Longarela, B.; Cano-Cortes, V.; López-Delgado, F.J.; Sánchez-Martín, R.M.; Ilyine, H.; Bradley, M.; et al. Novel bead-based platform for direct detection of unlabelled nucleic acids through Single Nucleobase Labelling. Talanta 2016, 161, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Gonzalez, A.; Robles-Remacho, A.; Marin-Romero, A.; Detassis, S.; Lopez-Longarela, B.; Lopez-Delgado, F.J.; de Miguel-Perez, D.; Guardia-Monteagudo, J.J.; Fara, M.A.; Tabraue-Chavez, M.; et al. PCR-free and chemistry-based technology for miR-21 rapid detection directly from tumour cells. Talanta 2019, 200, 51–56. [Google Scholar] [CrossRef]
- Garcia-Fernandez, E.; Gonzalez-Garcia, M.C.; Pernagallo, S.; Ruedas-Rama, M.J.; Fara, M.A.; López-Delgado, F.J.; Dear, J.W.; Ilyine, H.; Ress, C.; Díaz-Mochón, J.J.; et al. miR-122 direct detection in human serum by time-gated fluorescence imaging. Chem. Commun. (Camb.) 2019, 55, 14958–14961. [Google Scholar] [CrossRef]
- Marín-Romero, A.; Tabraue-Chávez, M.; Dear, J.W.; Sánchez-Martín, R.M.; Ilyine, H.; Guardia-Monteagudo, J.J.; Fara, M.A.; López-Delgado, F.J.; Díaz-Mochón, J.J.; Pernagallo, S. Amplification-free profiling of microRNA-122 biomarker in DILI patient serums, using the luminex MAGPIX system. Talanta 2020, 219, 121265. [Google Scholar] [CrossRef] [PubMed]
- Luque-González, M.A.; Tabraue-Chávez, M.; López-Longarela, B.; Sánchez-Martín, R.M.; Ortiz-González, M.; Soriano-Rodríguez, M.; García-Salcedo, J.A.; Pernagallo, S.; Díaz-Mochón, J.J. Identification of Trypanosomatids by detecting Single Nucleotide Fingerprints using DNA analysis by dynamic chemistry with MALDI-ToF. Talanta 2018, 176, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Pernagallo, S.; Ventimiglia, G.; Cavalluzzo, C.; Alessi, E.; Ilyine, H.; Bradley, M.; Diaz-Mochon, J.J. Novel biochip platform for nucleic acid analysis. Sensors 2012, 12, 8100–8111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, F.R.; Diaz-Mochon, J.J.; Swift, M.D.; Bradley, M. DNA analysis by dynamic chemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 1809–1812. [Google Scholar] [CrossRef] [PubMed]
- Bailey, W.J.; Holder, D.; Patel, H.; Devlin, P.; Gonzalez, R.J.; Hamilton, V.; Muniappa, N.; Hamlin, D.M.; Thomas, C.E.; Sistare, F.D.; et al. A performance evaluation of three drug-induced liver injury biomarkers in the rat: Alpha-glutathione S-transferase, arginase 1, and 4-hydroxyphenyl-pyruvate dioxygenase. Toxicol. Sci. 2012, 130, 229–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ahwany, E.G.; Mourad, L.; Zoheiry, M.M.; Abu-Taleb, H.; Hassan, M.; Atta, R.; Hassanien, M.; Zada, S. MicroRNA-122a as a non-invasive biomarker for HCV genotype 4-related hepatocellular carcinoma in Egyptian patients. Arch. Med. Sci. 2019, 15, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- McCrae, J.C.; Sharkey, N.; Webb, D.J.; Vliegenthart, A.D.; Dear, J.W. Ethanol consumption produces a small increase in circulating miR-122 in healthy individuals. Clin. Toxicol. (Phila.) 2016, 54, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Perraut, R.; Richard, V.; Varela, M.-L.; Trape, J.-F.; Guillotte, M.; Tall, A.; Toure, A.; Sokhna, C.; Vigan-Womas, I.; Mercereau-Puijalon, O. Comparative analysis of IgG responses to Plasmodium falciparum MSP1p19 and PF13-DBL1α1 using ELISA and a magnetic bead-based duplex assay (MAGPIX®-Luminex) in a Senegalese meso-endemic community. Malar. J. 2014, 13, 410. [Google Scholar] [CrossRef] [Green Version]
- Patino, C.M.; Ferreira, J.C. Inclusion and exclusion criteria in research studies: Definitions and why they matter. J. Bras. Pneumol. 2018, 44, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaso, H.; Murata, T.; Day, N.; Yokoyama, K.K. Simultaneous detection of RNA and protein by in situ hybridization and immunological staining. J. Histochem. Cytochem. 2001, 49, 1177–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Singleplex ARG1 MFI Average | Singleplex miR-122 MFI Average | seqCOMBO ARG1 MFI Average | seqCOMBO miR-122 MFI Average | * MFI Average | SD | CV | |
|---|---|---|---|---|---|---|---|
| ARG1 | 3340.5 | ---- | 3256.0 | ---- | 3298.0 | 59.8 | 1.81% |
| miR-122 | ---- | 1376.8 | ---- | 1436.5 | 1406.3 | 42.2 | 3.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-Romero, A.; Tabraue-Chávez, M.; López-Longarela, B.; Fara, M.A.; Sánchez-Martín, R.M.; Dear, J.W.; Ilyine, H.; Díaz-Mochón, J.J.; Pernagallo, S. Simultaneous Detection of Drug-Induced Liver Injury Protein and microRNA Biomarkers Using Dynamic Chemical Labelling on a Luminex MAGPIX System. Analytica 2021, 2, 130-139. https://doi.org/10.3390/analytica2040013
Marín-Romero A, Tabraue-Chávez M, López-Longarela B, Fara MA, Sánchez-Martín RM, Dear JW, Ilyine H, Díaz-Mochón JJ, Pernagallo S. Simultaneous Detection of Drug-Induced Liver Injury Protein and microRNA Biomarkers Using Dynamic Chemical Labelling on a Luminex MAGPIX System. Analytica. 2021; 2(4):130-139. https://doi.org/10.3390/analytica2040013
Chicago/Turabian StyleMarín-Romero, Antonio, Mavys Tabraue-Chávez, Bárbara López-Longarela, Mario A. Fara, Rosario M. Sánchez-Martín, James W. Dear, Hugh Ilyine, Juan J. Díaz-Mochón, and Salvatore Pernagallo. 2021. "Simultaneous Detection of Drug-Induced Liver Injury Protein and microRNA Biomarkers Using Dynamic Chemical Labelling on a Luminex MAGPIX System" Analytica 2, no. 4: 130-139. https://doi.org/10.3390/analytica2040013
APA StyleMarín-Romero, A., Tabraue-Chávez, M., López-Longarela, B., Fara, M. A., Sánchez-Martín, R. M., Dear, J. W., Ilyine, H., Díaz-Mochón, J. J., & Pernagallo, S. (2021). Simultaneous Detection of Drug-Induced Liver Injury Protein and microRNA Biomarkers Using Dynamic Chemical Labelling on a Luminex MAGPIX System. Analytica, 2(4), 130-139. https://doi.org/10.3390/analytica2040013






