1. Introduction
Lead-cooled Fast Reactors (LFR) are emerging as a promising technology within the framework of Generation IV nuclear power systems, attracting global interest due to their distinctive features and potential advantages in terms of sustainability, safety and proliferation resistance [
1,
2]. These reactors use liquid lead or lead bismuth eutectic (LBE) as the primary coolant, benefiting from its high boiling point, excellent thermal conductivity, and strong shielding capabilities against gamma radiation [
3]. Moreover, the fast neutron spectrum improves fuel utilisation efficiency and supports a closed fuel cycle, thereby reducing long-term radiotoxic waste [
4]. The simplified design of LFRs contributes to enhanced safety and reliability by allowing the primary coolant loop to operate at atmospheric pressure and relatively low temperatures [
5]. The use of Mixed Oxide (MOX) fuels diminishes proliferation risks, while lead’s chemical inertness mitigates hazards associated with severe nuclear accidents [
6]. Current research efforts in the European Union focuses on the development of lead and LBE technologies, particularly through two significant projects: MYRRHA [
7], a subcritical research reactor, and ALFRED, the Advanced Lead Fast Reactor European Demonstrator, aimed at advancing essential knowledge in reactor design, operation, maintenance, and materials technology for LFR [
8].
A key aspect of LFR development involves characterisation of the radionuclide source term, which encompasses the types, inventories, and potential release rates of radioactive species under both normal and accidental conditions. Accurate assessment of the source term is essential for conducting robust safety analyses, supporting regulatory licensing processes, and planning emergency response strategies. While source term behaviour has been widely studied for other reactor types [
9], LFR-specific challenges remain underexplored, particularly those involving the interaction between radionuclides and the lead coolant.
Among the radionuclides of concern, volatile species that migrate into the reactor cover gas system demand particular attention. Polonium-210 (
210Po), a highly radiotoxic alpha emitter produced through neutron activation of lead, poses a unique safety consideration due to its potential for escaping the coolant and accumulation in the cover gas [
10]. Understanding the behaviour of
210Po and other volatile radionuclides is therefore essential for ensuring both environmental and occupational safety in reactors such as ALFRED.
The derivation of a reactor source term depends on multiple factors, including operating power, inventories of fission and activation products, designed and implemented safety features, and the nature of the postulated accident scenarios. Where realistic data is not available, conservative assumptions are often applied to simplify analyses, typically leading to overestimations of potential consequences. Conversely, realistic assumptions offer more accurate and typically less severe assessments of the source term. Still, they may require substantial modelling effort, particularly for complex phenomena such as fission product release and transport within the reactor system and containment building [
9].
This paper presents a focused review of radionuclide production and transport in LFRs, with an emphasis on volatile impurities that may escape the lead coolant and accumulate in the cover gas system. Using the ALFRED design as a reference case [
11], the study evaluates the maximum expected inventories of key radionuclides, examines their behaviour under both normal and accidental conditions, and assesses their radiological implications. The analysis is based on a conservative bounding scenario, assuming the instantaneous release of fission products from a single failed fuel assembly (FA) into the primary circuit, followed by equilibrium transfer into the cover gas, based on thermodynamic relations. This scenario does not correspond to a specific deterministic accident but serves as a simplified upper-bound estimate of the source term in this case. The purpose of this approach is to provide an initial evaluation for a single failed FA that could be further used as starting point in the conceptual design of gas purification and monitoring systems. The findings offer practical insights for optimising next-generation LFR safety systems, coolant chemistry control, cover gas purification strategies, radioprotection protocols, and radioactive waste management.
2. Radionuclide Generation in LFRs
For reactor safety analysis, postulated initiating events require both qualitative and quantitative information regarding source term derivation and associated radiological consequences. The source term is defined as the magnitude, composition, form (physical and chemical) and mode of release (puff, intermittent or continuous) of radioactive elements (fission and/or activation products) released during a reactor accident. The mechanism, time and location of the release must also be identified [
9].
In LFR systems, radionuclide inventories arise from various nuclear and physicochemical processes, including fission reactions, neutron activation of coolant and structural materials, and interactions with trace impurities or corrosion products. Characterising the nature, quantity, and behaviour of these radionuclides is essential for accurately assessing their contribution to the source term under both normal and accidental conditions.
2.1. Fission Products
The primary source of radioactivity in any nuclear reactor is the fission process. During operation, the fission of actinides such as uranium-235 and plutonium-239 in Mixed Oxide (MOX) fuel generates a wide spectrum of fission products. For reactor safety assessments, particular attention is given to radionuclides across several chemical groups, including noble gases (Xe, Kr), halogens (I, Br), alkali metals (Cs, Rb), chalcogens (Po, Te, Se,), alkaline earths (Sr, Ba), transition metals (Ru, Mo, Pd, Rh, Tc), as well as lanthanides and actinides (La, Eu, Y, Ce, Pr, Pm, Sm, Np, Pu, Zr, Nb) [
12]. Many of these radionuclides are either volatile or exhibit a non-negligible volatility at typical reactor operating temperatures, posing a potential release risk.
The chemical form of radionuclides is a critical factor influencing their volatility, transport behaviour, retention within reactor systems, and associated radiological impact, particularly for species like iodine and caesium. Regulatory guidance for water-cooled reactors recommends that, under postulated accident scenarios, 95% of the released iodine be assumed to occur as caesium iodide (CsI), 4.85% as elemental iodine (I
2), and 0.15% as organic iodide compounds [
13]. These fractions apply to iodine released both from the fuel-cladding gap and the fuel matrix.
In lead-cooled reactors, however, iodine released into the primary system is expected to react with lead, forming lead-iodine compounds that can significantly alter the distribution and speciation of iodine compared to water-cooled systems. Furthermore, caesium and iodine may chemically interact in both the liquid and gas phases, enhancing the formation of volatile compounds such as CsI. These interactions may increase the volatility of both elements under certain conditions. The chemical behaviour of iodine and caesium in lead-cooled systems is not yet fully understood, and ongoing research is investigating these mechanisms [
14]. These aspects will be experimental investigated in a dedicated facility currently under development at RATEN [
15].
For the ALFRED reactor, a detailed inventory of the fission products (FPs) present in the irradiated MOX fuel at End Of Cycle (EOC)—average residence time of the whole core is 3 years (at equilibrium)—was calculated using the nuclear analysis codes FISPACT and MCNPX [
16]. This data serves as a fundamental input for evaluating the source term. The key parameters of the ALFRED design applied in this assessment are as follows [
11]:
Thermal power: 300 MW;
Fuel type—MOX-(Pu,U)O1.97 with a maximum Pu enrichment of 30%;
Core configuration: 171 fuel assemblies (FA) enclosing 127 fuel pins;
Peak burn-up: 100 MWd/kg;
Clad material: stainless steel 15–15 Ti;
Lead Coolant: inlet temperature 400 °C, outlet temperature 480 °C;
Maximum clad temperature during Unprotected loss of flow (ULOF) 750 °C;
Lead mass: 3400 tonnes.
Cover gas: inert gas (argon) in a static configuration, volume 80 m3; no gas flow dynamics and purification systems are considered in this analysis.
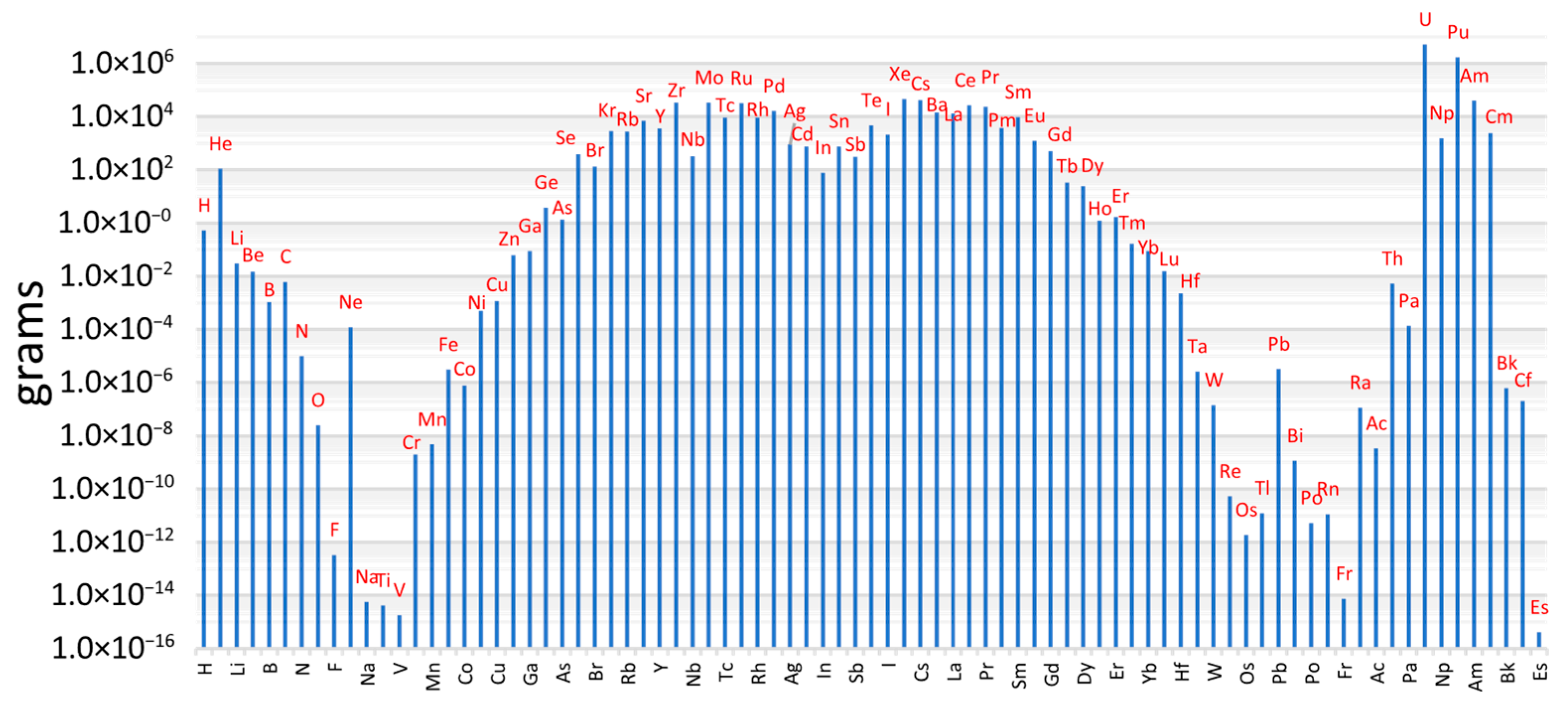
Figure 1 presents the total mass inventory of fission products averaged for all fuel assemblies at EOC [
16].
While most of the fission products are expected to remain confined within the fuel matrix or be captured by cladding materials, some may escape through microcracks or fuel-cladding gaps. Volatile radionuclides, such as iodine, caesium, tellurium, and noble gases, pose a particular safety concern due to their potential for release and environmental dispersion.
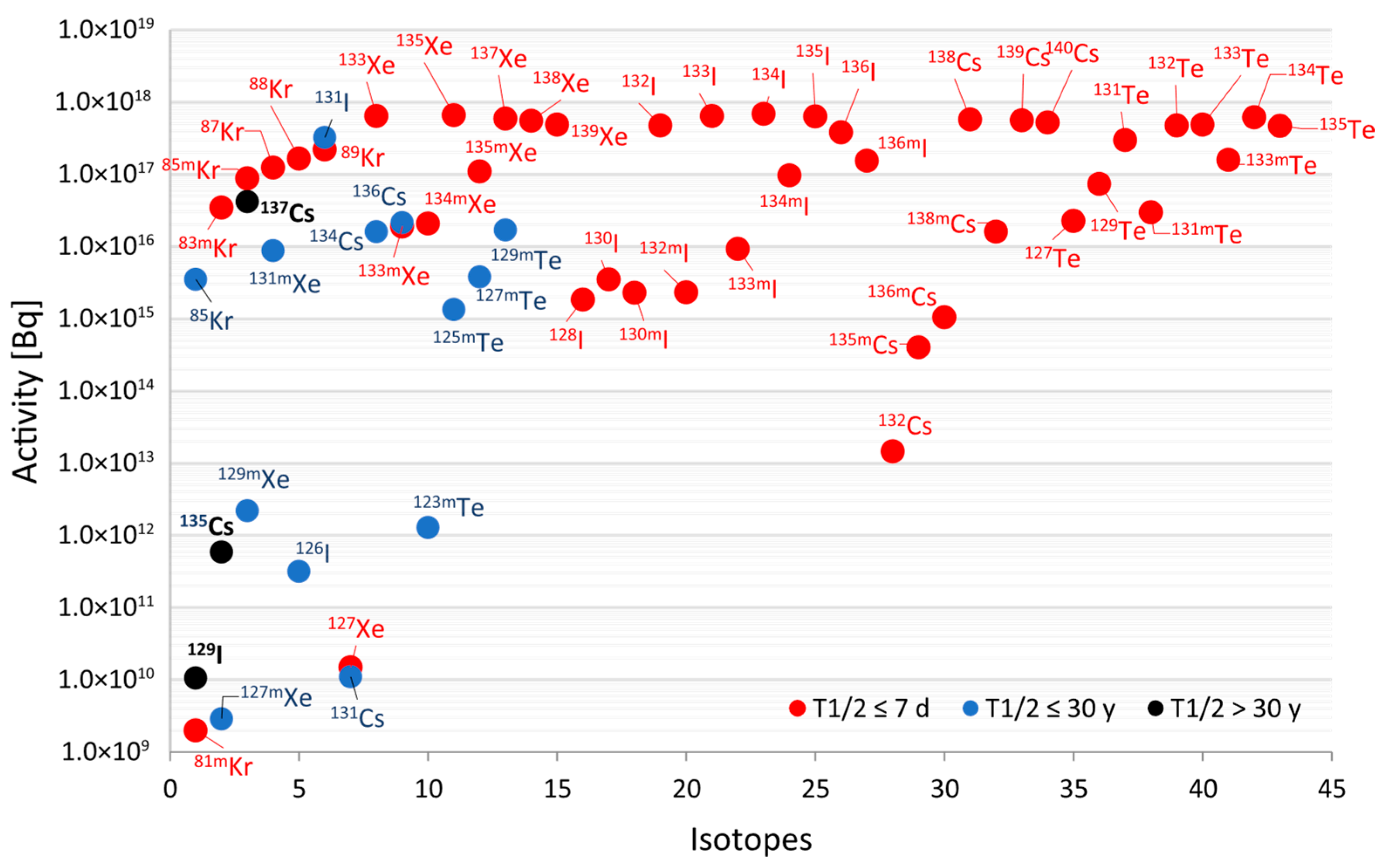
The calculated activities of these volatile fission products (FPs) for the ALFRED core at EOC are represented in
Figure 2. To emphasise radiologically significant contributors, only those radionuclides with an activity exceeding 1GBq are included. Isotopes depicted with red dots are very short-lived isotopes (T½ ≤ 7 days) and account for the majority of the total activity among the most volatile FPs. Short-lived isotopes (T½ ≤ 30 years), shown in blue, and long-lived isotopes (T½ >30 years), shown in black, contribute less than 4% to the total volatile activity.
2.2. Activation Products
Activation products (APs) are continuously generated during the operation of an LFR reactor through neutron activation of materials present within the reactor system, including the lead coolant, structural components, and trace impurities. These radionuclides are significant both in terms of total activity and their potential to contribute to radiological dose.
An inventory of activation impurities was calculated within the framework of the LEADER project, using the nuclear analysis codes FISPACT and MCNPX [
17]. The analysis assumed continuous irradiation of the entire lead volume (approximately 3400 t) over the reactor’s lifetime, applying a flux spectrum averaged across the primary circuit and using the parameters for the core outlined previously. The resulting radionuclide inventory provides the critical input for evaluating source terms and radiological impact studies.
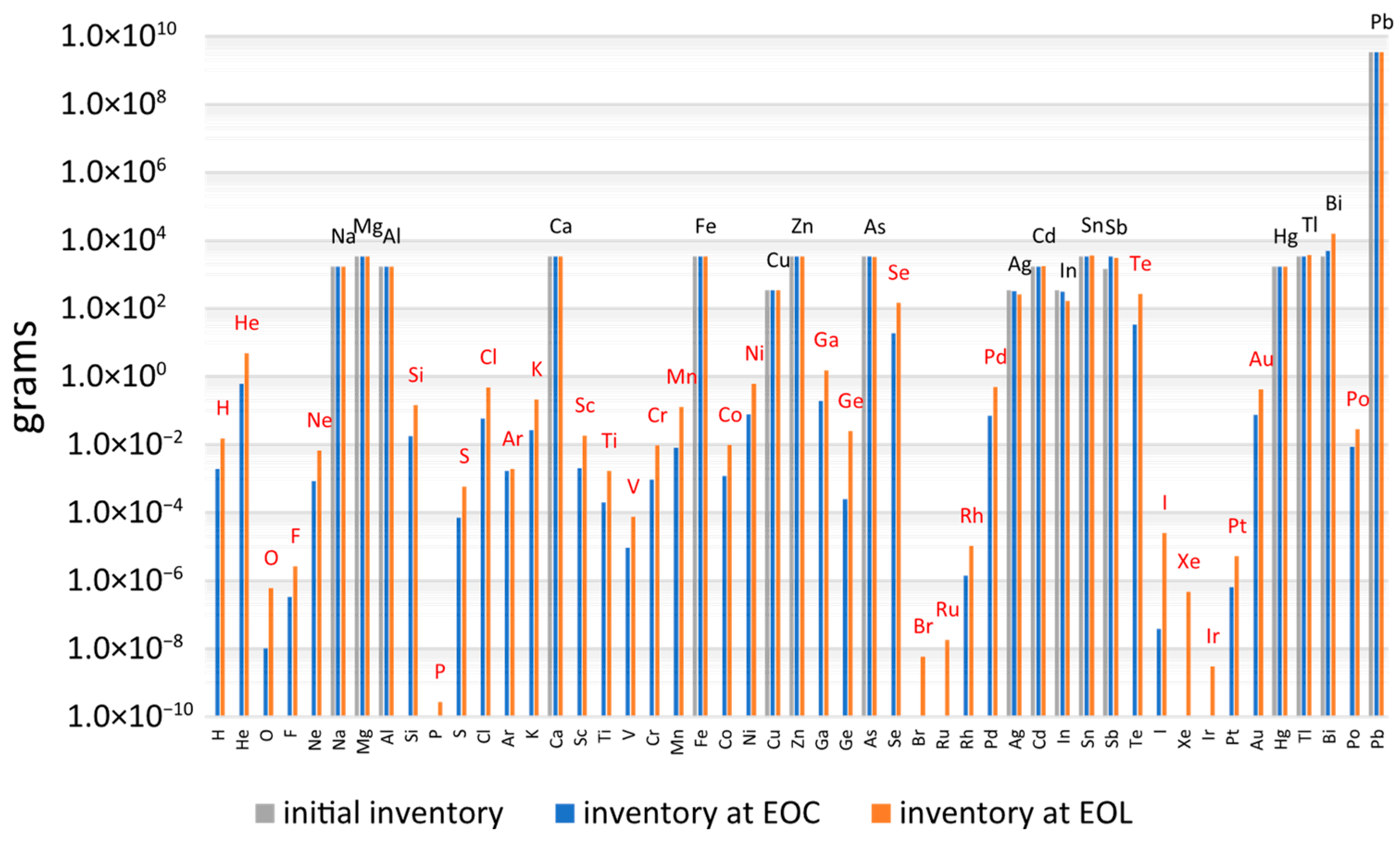
Figure 3 illustrates the mass inventory of the APs within the ALFRED lead coolant. Elements depicted in black represent intrinsic impurities, based on the composition of the C00 commercial-grade lead [
17], while the elements shown in red correspond to the elements produced by neutron activation. Results are presented for two operational stages: after 5 years of irradiation, corresponding to EOC, and after 40 years, representing the End of Life (EOL). The intrinsic impurities inventory remains nearly unchanged over 40 years due to the limited activation pathways of most of these stable isotopes in the fast neutron spectrum of an LFR system, and the transmutation rate for most of these elements is low.
Among these products,
210Po is of particular concern. Produced through neutron activation of
209Bi, an intrinsic impurity in lead, and
208Pb [
18] (which accounts for 52.4% of natural lead [
19]),
210Po poses a unique radiological hazard due to its high radiotoxicity [
20], volatility, and mobility within the primary system, especially under accident conditions and during maintenance [
21,
22,
23].
In addition to
210Po, other volatile activation products such as mercury, thallium, cadmium, and tellurium can contribute significantly to potential radiological risks [
24]. These elements may volatilise at elevated temperatures, increasing the impurity inventory in the reactor’s cover gas system and, if not properly filtered, posing potential release risks to the reactor hall or environment [
25,
26,
27].
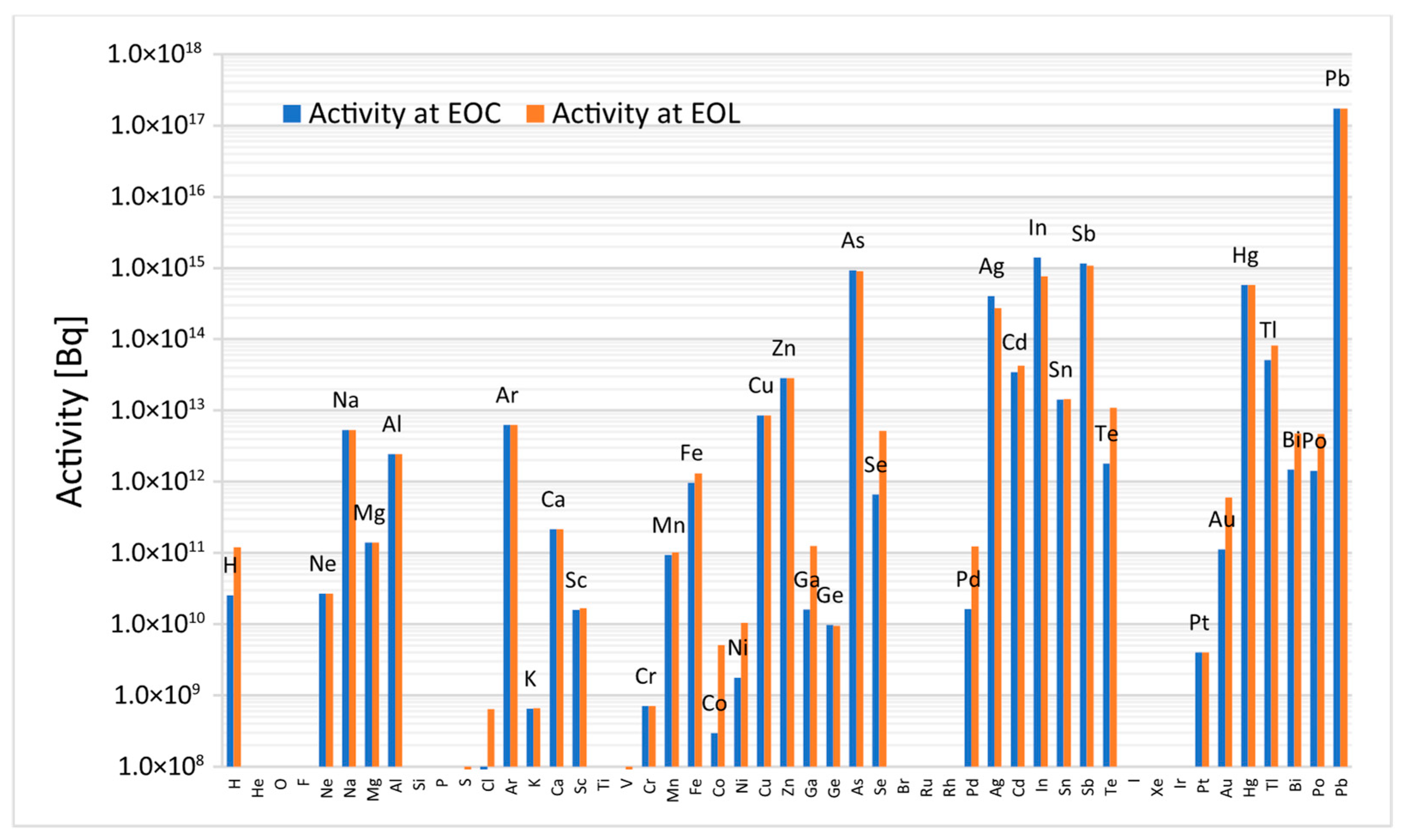
Figure 4 presents the total activity of lead activation products in the ALFRED reactor at both EOC and EOL [
17]. After 40 years of irradiation, the total specific activity of the coolant reaches 5.214 × 10
10 Bq/kg, with over 97% attributed to lead radionuclides. Notably, a significant part of this activity (81%) is attributed to the short-lived isotope
207mPb (T½-0.8 s), which decays almost entirely within seconds after shutdown.
As a result, after just one day of cooling, the total activity drops significantly to 5.48 × 108 Bq/kg (1% of the EOL value) with nearly 90% of this activity arising from short-lived radioisotopes such as 122Sb (27.55%, T½-2.7 d), 76As (25.65%, T½-26.3 h), 124Sb (19.1%, T½-60.2 d), 209Pb (9.33%, T½-3.2 h), and 203Pb (6.62%, T½-51.9 h). After seven days, the activity decreases further to 1.85 × 108 Bq/kg, representing less than 0.4% of the initial EOL activity. At this stage, approximately 70% of the residual activity is due to short-lived isotopes—124Sb (52.5%) and 122Sb (17.3%)—while 204Tl (T½-3.78 y) contributes 11.5%.
This reduction continues over time and, after one year of cooling, 99.95% of the initial activity has decayed, leaving a residual activity of 2.56 × 107 Bq/kg. The dominant contributors at this stage are: 204Tl (69.52%), 65Zn (6.94%, T½-244.26 d), 124Sb (6.17%) and 110mAg (4.25%, T½-249.8 d).
After 100 years, the remaining activity constitutes only 0.0003% of the EOL value, equivalent to 1.34 × 105 Bq/kg, primarily due to: 204Tl (87.65%), followed by 113mCd (4.74%, T½-14.1 y) and 205Pb (3.07%, T½-1.53 × 107 y).
Although lead dominates the activation inventory in terms of mass and activity, the potential radiological impact is heavily influenced by the volatility and toxicity of individual species.
Figure 5 shows the estimated inhalation dose contributions of each activation product in a conservative, unmitigated release scenario after 5 years of irradiation, assuming no retention by lead, filters or containment.
210Po alone accounts for 45.3% of the total dose impact, underscoring its dominant role in radioprotection assessments, particularly during maintenance and decontamination operations.
2.3. Other Impurities
In addition to fission and activation products, various other impurities can affect coolant chemistry, system performance, and safety in ALFRED operation. These include intrinsic impurities introduced during manufacturing (Ag, Cu, Sn), corrosion products from structural materials (Fe, Cr, Ni), and dissolved gases (O2, H2O) introduced during maintenance, air ingress, or from minor leaks in the steam generator.
While some of these impurities are relatively benign under nominal operating conditions, they can lead to plugging, deposition, or localised corrosion, particularly in cooler regions of the primary system. Effective impurity management, through cover gas purification, oxygen control, and rigorous operational protocols, is essential for maintaining coolant quality and ensuring long-term safe operation of ALFRED.
3. Behaviour of the Volatile Impurities in Liquid Lead
Under normal reactor operation, various chemical processes—oxidation-reduction, dissolution, and precipitation—occur within the lead coolant, influencing the distribution and retention of radioactive impurities. These impurities, introduced as fission or activation products, interact with the liquid lead, structural materials, or other contaminants. Some remain dissolved, while others volatilise, accumulate in the cover gas, and may later condense back into the coolant.
The extent to which a radionuclide evaporates from the lead is governed by system conditions, its chemical properties and thermodynamic equilibrium, which tends toward a state of minimum free energy [
21]. Due to the high temperature of liquid lead (above 400 °C) and inert cover gas, equilibrium in AFLRED is reached quickly. Given the extreme dilution of impurities, the system can be approximated as an ideal solution, where the coolant behaviour remains constant regardless of the degree of contamination [
28].
In ideal solutions, the vapour pressure of component A (
in a solvent B is proportional to its molar fraction
, following Raoult’s Law:
However, real systems deviate from this behaviour, requiring the activity coefficient (γ) to account for solute-solvent interactions, leading to Henry’s Law for very dilute solutions:
where
KH is the Henry constant (
and it is determined experimentally.
The number of moles of a volatile impurity in the cover gas can be estimated using the ideal gas law, and its total radioactivity can be calculated from its molar quantity, molar mass, and specific activity. This equilibrium-based assessment was previously applied to ALFRED for postulated accidents involving the release of key volatile elements such as Po, Cs, Sr and I [
29]. The present study revisits that analysis with updated data on Henry constants and extends it to additional volatile impurities—including Hg, Cd and Te, enabling a more comprehensive characterisation of the potential source term.
3.1. Noble Gases
The noble gases Xe and Kr are among the most important fission products for source term monitoring, especially under accident conditions. Due to their high volatility and chemical inertness, they do not form stable compounds and rapidly migrate into the reactor cover gas once released from the fuel in case of cladding breach. Radioactive isotopes such as
133Xe,
135Xe,
85Kr and
88Kr are significant β-γ emitters, contributing to external dose rates and environmental contamination, and are routinely monitored as indicators of fuel cladding integrity and radiological risk [
30]. Moreover, their release characteristics can be used for early detection of cladding failure and even to pin-point the failed fuel assembly using a tagging method [
31,
32].
Given their extremely low solubility in liquid heavy metals [
33] and the absence of retention mechanisms, any release of these gases into the coolant will almost immediately and completely transfer to the reactor cover gas. Thus, the amount of noble gases present in the cover gas will directly correspond to the amount released from the failed fuel pins.
3.2. Polonium
Polonium is one of the most radiotoxic elements produced by activation in heavy liquid metal-cooled nuclear systems. The median lethal dose (LD
50/60) for acute radiation exposure is 4.8 Sv [
34]. With an effective dose coefficient for
210Po of 0.51 μSv/Bq via ingestion and 2.54 μSv/Bq via inhalation [
35], and a specific activity of 1.66 × 10
14 Bq/g, the estimated lethal dose is approximately 56 ng by ingestion and 11 ng by inhalation, placing Po among the most critical radionuclides for safety assessments in LFR systems.
The vapour pressure of Po dissolved in heavy liquid metals has been determined experimentally using various techniques, including vapour transport, transpiration and collection in trapping solutions [
36], as well as the transpiration method, which involves condensation of vapour in a cold zone downstream of the gas flow [
23,
37,
38]. Aerts combined experimental data with ab initio calculations and thermochemical modelling, concluding that the predominant gas species are Po
(g), Po
2(g), PbPo
(g), and BiPo
(g) [
10]. The activity coefficient of Po dissolved in LBE was derived by fitting a thermodynamic model to experimental Henry’s law constants, confirming that polonium is fully soluble in the molten metal, with PbPo
(s) identified as the most stable compound formed in this system. These results support the applicability of Henry’s law for Po evaporation at molar fractions below 10
−3—significantly higher than those typically expected in heavy metal-cooled reactors.
Recent investigations by Zivadinovic et al. examined Po behaviour in proton-irradiated LBE material containing corrosion and activation impurities, with concentrations representative of those found in an actual LFR. The research focused on the influence of such impurities on the Po evaporation behaviour [
39]. Using the transpiration method, Po release was examined under high-temperature conditions (700–950 °C). The results indicate that the presence of impurities does not substantially enhance Po evaporation, suggesting that existing data from pure Po-LBE systems are applicable for heavy metals cooled reactor safety assessments.
Based on the available experimental data, this study adopts the correlation proposed by Gonzalez Prieto et al. [
38], which is valid for temperatures ranging from 600 to 1000 °C. This correlation aligns well with other experimental studies performed at lower temperatures [
36]:
3.3. Mercury
Experimental studies on mercury volatilisation from LBE have shown that significant evaporation begins at temperatures as low as 200 °C, with over 80% of Hg released at 352 °C in less than one hour [
25]. Research conducted by Neuhausen using gamma spectrometry with
203Hg tracers and the transpiration method confirmed rapid Hg release under inert atmospheres, while oxidative conditions notably reduced evaporation rates [
40]. Henry constants derived from these data indicated near-ideal behaviour at high concentrations, but considerably lower volatility at trace levels.
Additional experiments by Aerts et al. evaluated both liquid and solid LBE samples, finding that Hg evaporation from liquid LBE closely resembles that of pure Hg [
41] at concentrations ranging from ppm to ppb levels [
42]. Notably, solid samples displayed unexpectedly high evaporation rates, which may pose significant operational safety concerns for heavy liquid metal reactors. The experimental data confirmed Henry’s law applicability over molar fractions from 10
−6 to 10
−12, with an activity coefficient effectively equal to unity. The recommended empirical relation is:
3.4. Cadmium
Cadmium is generated both through neutron activation of lead and as a fission product. Among its activation isotopes,
113mCd and
109Cd are particularly relevant due to their relatively long half-lives and associated gamma emissions, contributing to the radiological inventory of heavy liquid metal reactor systems. Like mercury, cadmium is a volatile metal, though it has a higher boiling point of 767 °C compared to Hg, resulting in lower vapour pressures under the same conditions. The vapour pressure of pure cadmium as a function of temperature can be expressed by the empirical relation [
43]:
The Pb-Cd binary system exhibits eutectic behaviour, with Cd being completely miscible in liquid Pb above its melting point [
44]. Experimental studies indicate a significant positive deviation from ideal behaviour in this system, suggesting a weak affinity between Cd and Pb in the liquid state, hence the tendency of cadmium to volatilise from the melt. This trend is consistent even in ternary systems like Cd-Tl-Pb [
45]. Based on thermodynamic assessments, the recommended activity coefficient for cadmium in lead is 3.376 [
28], leading to a Henry constant relation:
3.5. Tellurium
Equilibrium studies conducted by Ohno demonstrated that within the 450–750 °C range, Te’s vapour pressure in LBE is directly proportional to its concentration in the liquid alloy, confirming Henry’s law behaviour for molecular fractions below 0.002 [
37]. Complementary experiments by Neuhausen using the transpiration method on samples doped with radioactive
121Te at lower molar fractions (10
−12 and 10
−13) showed detectable evaporation only above 700 °C, with 50% of the volatile content released around 1000 °C [
23]. At lower temperatures (200–450 °C), volatilisation remained negligible, and water vapour in the atmosphere had no measurable effect on evaporation rates.
Follow-up experiments at higher Te concentrations (up to 8 × 10
−3) in a He atmosphere over 220–600 °C, confirmed the ideal behaviour at temperatures above 400 °C [
46]. Evaporation rates between 400 and 600 °C correlated well with Neuhausen’s data, while at lower temperatures, deviations appeared, indicating higher Henry constants, similar to the behaviour seen for Po [
47]. Across all studies, Te demonstrated significantly lower vapour pressures compared to pure Te [
48], indicating strong retention in LBE.
3.6. Iodine
Iodine is a key radionuclide in nuclear safety assessments due to its high radiological impact and volatility. Among its isotopes, notably
131I (T½ = 8.02 days) is a significant fission product with the potential for environmental release in accident scenarios [
49]. In ALFRED, iodine is primarily generated within fuel assemblies, accumulating in the gas plenum before potential release into the coolant and subsequently into the cover gas following cladding failure.
In Pb-I systems, iodine interacts strongly with lead, forming compounds such as PbI
2, PbI, PbI
4, with no free molecular iodine expected in the gas phase under diluted conditions [
50]. Experimental studies by Neuhausen and Eichler on LBE doped with uranium (subsequently generating iodine by neutron activation) showed detectable iodine volatilisation only above 530 °C, with ~50% released at 630 °C in short-term tests, and maximum release near 85% at higher temperatures [
51]. Long-term experiments confirmed negligible volatilisation below 500 °C, with rapid release occurring above 600 °C. Notably, measured vapour pressures for PbI
2 in LBE were substantially higher than theoretical estimates based on pure PbI
2 behaviour, highlighting notable deviations from ideal behaviour [
21]. These findings emphasise the need for refined experimental data and models under operational and post-accident conditions to accurately predict iodine transport in heavy metal-cooled reactor systems.
Until more conclusive data becomes available, the estimation of iodine release from lead in source term assessments will rely on the best-fit expression for the vapour pressure of pure PbI
2 in its liquid phase, as proposed by Knake [
52]:
3.7. Caesium
In lead-cooled nuclear systems, Cs is produced exclusively as a fission product within the nuclear fuel, gradually accumulating in the plenum of fuel pins during irradiation. In the event of cladding failure, Cs can be released into the coolant and subsequently into the reactor cover gas. Experimental studies indicate that Cs does not form stable compounds with liquid lead [
53] but can interact with other elements, notably iodine, both in the liquid phase and the gas phase to form volatile species such as caesium iodide (CsI), which exhibits significant vapour pressure at elevated temperatures [
14].
For conservative safety assessments of Cs release from lead melts under operational or accidental conditions, a fixed activity coefficient of 0.032 is recommended [
28]. Applying this value to the vapour pressure relation for pure Cs [
54] results in the following Henry constant correlation:
Transpiration method experiments performed between 200 and 1000 °C with LBE samples at various Cs concentrations have shown good agreement with Henry’s law predictions at high concentrations and temperatures, while significant deviations were observed at lower concentrations and temperatures, underscoring the need for additional studies [
55,
56]. In the absence of a validated model for the Cs behaviour in liquid metals, this study adopts the Henry constant of pure Cs, corrected with the recommended activity coefficient.
4. Results on ALFRED Source Term Estimation
The release potential of volatile impurities from the coolant was evaluated based on empirical Henry constant relations and their molar fractions in the molten lead. This assessment allowed the calculation of the equilibrium partial pressures for each element, representing their maximum potential concentrations in the gas phase above the coolant.
The radionuclide inventory considered includes both activation products (APs), assessed after five years of irradiation, and fission products (FPs) produced in a single fuel assembly, representing approximately 0.6% of the total core inventory at end-of-cycle (EOC). The release of the FPs from the failed assembly into the primary circuit is assumed to occur instantaneously.
Table 1 summarises the relevant volatile elements, their inventory, corresponding activities, and molar fractions in lead at full dissolution.
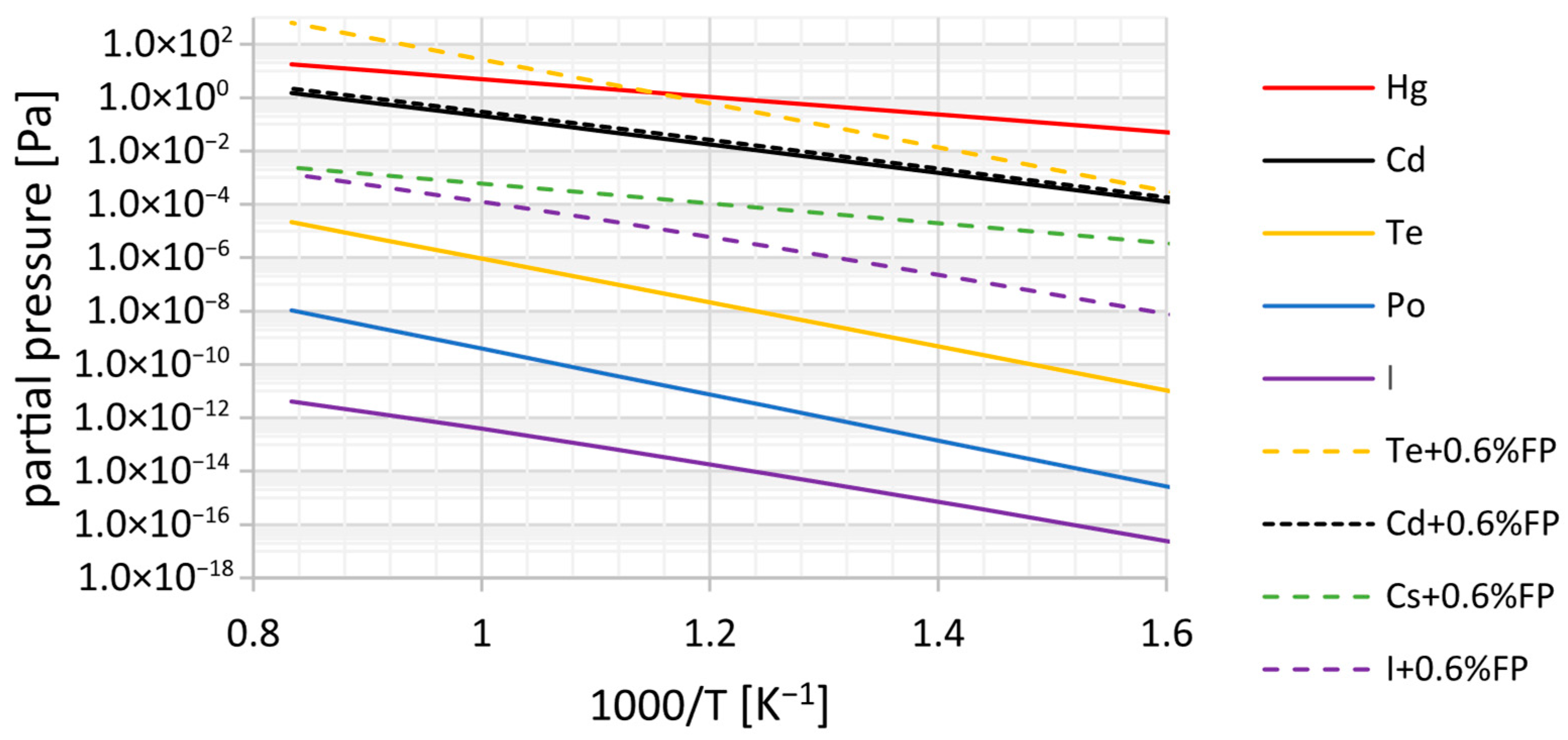
The variation in these impurities’ equilibrium partial pressures with temperature is presented in
Figure 6, illustrating both normal operation (APs after five years—solid lines) and a postulated accident scenario involving the release of FPs from a defective fuel assembly (dashed lines). The results show that Hg, a lead neutron activation product, has the highest volatility, followed by Cd and Te. Po displays much lower partial pressures, indicating strong retention in the liquid lead. However, due to its high radiotoxicity, its monitoring and filtration remain essential for safe operation.
In accident scenarios, the partial pressures of Te and I (as PbI2) increase notably, with Cs isotopes also migrating into the gas phase. These trends confirm the high release potential of certain species under abnormal conditions, supporting the need for effective cover gas purification systems and source term control strategies.
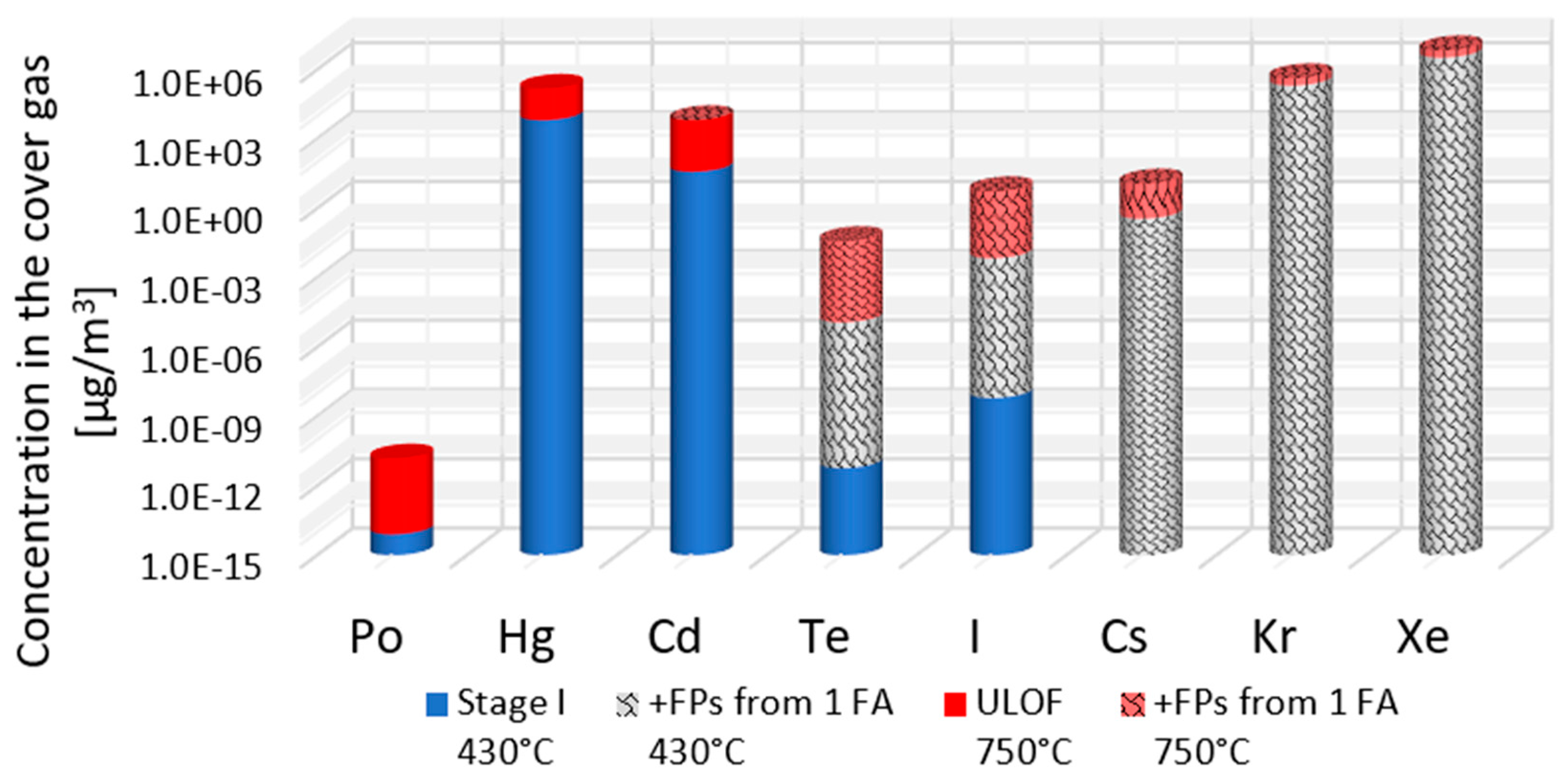
Using the ideal gas law and the previously estimated equilibrium partial pressures, the maximum concentrations of each volatile impurity in the ALFRED cover gas volume (80 m
3) were calculated for different scenarios.
Figure 7 presents these concentrations for APs at normal operating temperatures (430 °C, blue columns), an elevated temperature corresponding to the ULOF scenario (750 °C, red columns), and an accidental release case (striped columns) including FPs from a single fuel assembly. As expected, higher temperatures and the release of FPs significantly raise the gas-phase concentrations, underlining the importance of efficient online monitoring of the cover gas chemical composition as an indicator of abnormal conditions in the reactor.
Furthermore, based on the molar amounts of the dominant radionuclides (T½ higher than 6.5 h) and their specific activity, the maximum potential activity in the ALFRED cover gas was estimated as a sum of the individual radionuclide contributions assuming no decay or removal mechanisms. This was calculated using the following equation:
where
Ai is the activity of the volatilised impurity,
ni is the molar amount of the impurity in the cover gas,
Mi is the molar mass (g/mol), and
Asp is the specific activity of each radionuclide (Bq/g).
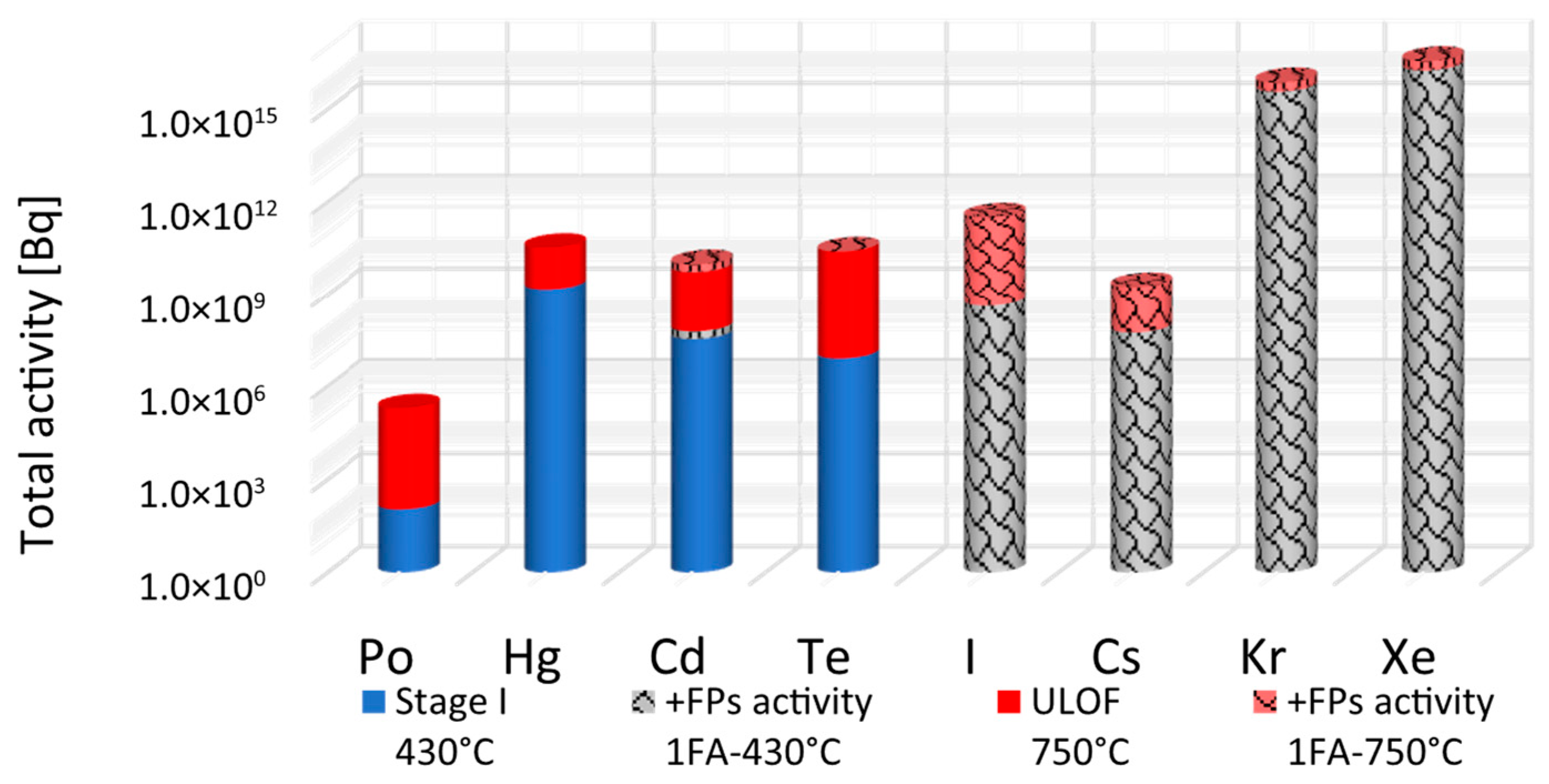
This represents the potential radiological source term in the case of unmitigated release. The results are shown in
Figure 8.
Under normal operating conditions at 430 °C, the total activity released to the cover gas is estimated at 2.110 × 109 Bq, with over 99.995% of the initial inventory retained by the coolant. The gas phase activity is heavily dominated by Hg radionuclides, contributing 98.87% of the total released activity, primarily from 203Hg (67.01%) and 197Hg (29.98%).
Although present in much smaller amounts, Po remains a significant safety hazard because of its high radiotoxicity. As an alpha emitter, it poses no external hazard; however, it becomes extremely dangerous if inhaled or ingested. As outlined in
Section 3.2, a fatal dose of
210Po can result from ingesting 9.4 MBq (254 µCi) or inhaling 1.9 MBq (51 µCi). Regulatory guidelines set the maximum permissible airborne concentration for
210Po in workplace environments at 11.1 Bq/m3 (3.0 × 10
−10 µCi/mL) [
20]. Under equilibrium conditions, the maximum activity of
210Po in the cover gas is estimated at 109 Bq, a relatively low activity but still significant due to its biological impact in case of accidental release.
In the ULOF scenario (750 °C), the total activity in the cover gas increases significantly, driven by the enhanced volatility of Hg, Cd, and Te. Additionally, in the accidental release scenario where fission products from one fuel assembly are added to the coolant inventory, the contributions from I, Cs, and the noble gases (Kr, Xe) become highly significant, with noble gases dominating the gas-phase radioactivity.
An experimental programme is planned to support the safety analysis of the ALFRED reactor, accompanied by the development of dedicated infrastructure to address the challenges associated with severe accident scenarios in Lead-cooled Fast Reactor (LFR) systems. To this end, a specialised research facility, named Meltin’Pot, will be built in Romania on the RATEN-ICN platform [
57]. This facility is designed to investigate the behaviour of impurities in liquid lead and to study key phenomena relevant to severe accident conditions.
The Meltin’Pot facility will consist in four experimental modules, each dedicated to a specific research area: fuel–coolant interaction; fuel dispersion and relocation following severe accident conditions; fission product retention in liquid lead and migration into the cover gas; and the behaviour of polonium isotopes, including their retention in lead and stripping into the cover gas in the presence of gases or steam. This infrastructure aims to generate key experimental data that are essential for the licensing process and a deeper understanding of source term behaviour in LFR systems.
5. Conclusions
This study assessed the release potential and cover gas concentrations of volatile radionuclides in the ALFRED reactor under both normal operating and postulated accident conditions. Using updated data on Henry constants, the temperature-dependent equilibrium partial pressures were evaluated, indicating that Hg, Cd, and Te exhibit the highest volatility among the studied radionuclides. While the partial pressures of Po remain significantly lower, its high radiotoxicity continues to warrant special monitoring and handling measures. The analysis confirmed that, under normal operating conditions (430 °C), the molten lead coolant effectively retains volatile radionuclides, with more than 99.995% of the total inventory remaining in the liquid phase. The cover gas activity is largely dominated by Hg radionuclides, accounting for over 98% of the total released activity.
Under the ULOF scenario (750 °C), a significant increase in the cover gas activity is anticipated due to the enhanced volatility of Hg, Cd, and Te. Additionally, in the event of fuel cladding breach leading to the release of fission products into the coolant, the contributions from iodine, caesium, krypton, and xenon become significant, with noble gases dominating the gas-phase radiological source term.
Understanding the behaviour of volatile species is essential for the safe design and operation of LFR systems. Accurate prediction of source terms, both under normal and accident conditions, relies on robust thermodynamic data for these impurities, as their release into the reactor cover gas directly impacts external radiation dose rates and environmental risk assessments. Effective cover gas purification systems are mandatory to manage migrating hazardous radionuclides like polonium and mercury, while strict operational temperature control can help mitigate their increased volatility at higher temperatures.
Despite progress in characterising impurity behaviour in lead-bismuth systems, notable research gaps remain. These include the need for extended experimental data on vapour pressures and solubility at reactor-relevant conditions, investigation of the combined effects of multiple impurities, development of real-time in situ monitoring techniques, and refinement of thermodynamic models to address the non-ideal behaviour of the impurities at the trace concentration level and dynamic thermal conditions.
To support ALFRED’s safety analysis and to address severe accident challenges in LFR systems, the dedicated Meltin’Pot experimental facility, currently under development at RATEN, will serve as a key infrastructure for investigating impurity behaviour in liquid lead and exploring critical phenomena related to severe accidents.
Building on the current study, future work will incorporate time-dependent evaluations based on specific deterministic postulated accident scenarios—such as unprotected loss-of-flow (ULOF) and cladding failure—and integrate probabilistic safety assessments to quantify the likelihood and consequences of such events. This combined approach will support a more comprehensive and regulatory-aligned characterisation of the source term, enhancing the safety basis for next-generation LFRs like ALFRED.