The Effects of Irradiation on Structure and Leaching of Pure and Doped Thin-Film Ceria SIMFUEL Models Prepared via Polymer-Templated Deposition
Abstract
1. Introduction
- The non-ionising (thermal or fast) neutron flux which sustains the chain reaction.
- Ionising radiation:
- ○
- The recoil effects of highly energetic FP ions following fission events;
- ○
- α flux from MA (minor actinide) decay and (n, α) reactions;
- ○
- β− flux from FP (fission product) and MA β− decay;
- ○
- The intense γ emissions that accompany both of these.
2. Experimental
2.1. Materials
2.2. Substrate Preparation
2.3. Synthesis of Model ε-Particles
2.4. Preparation of CeO2 Films
2.5. Characterisation
2.6. Irradiation
2.7. Leaching Experiments
3. Results and Discussion
3.1. Sample Preparation of CeO2 SIMFUEL Films
3.2. Physicochemical Studies of CeO2 SIMFUEL Films—Variations with Dopants and Irradiation
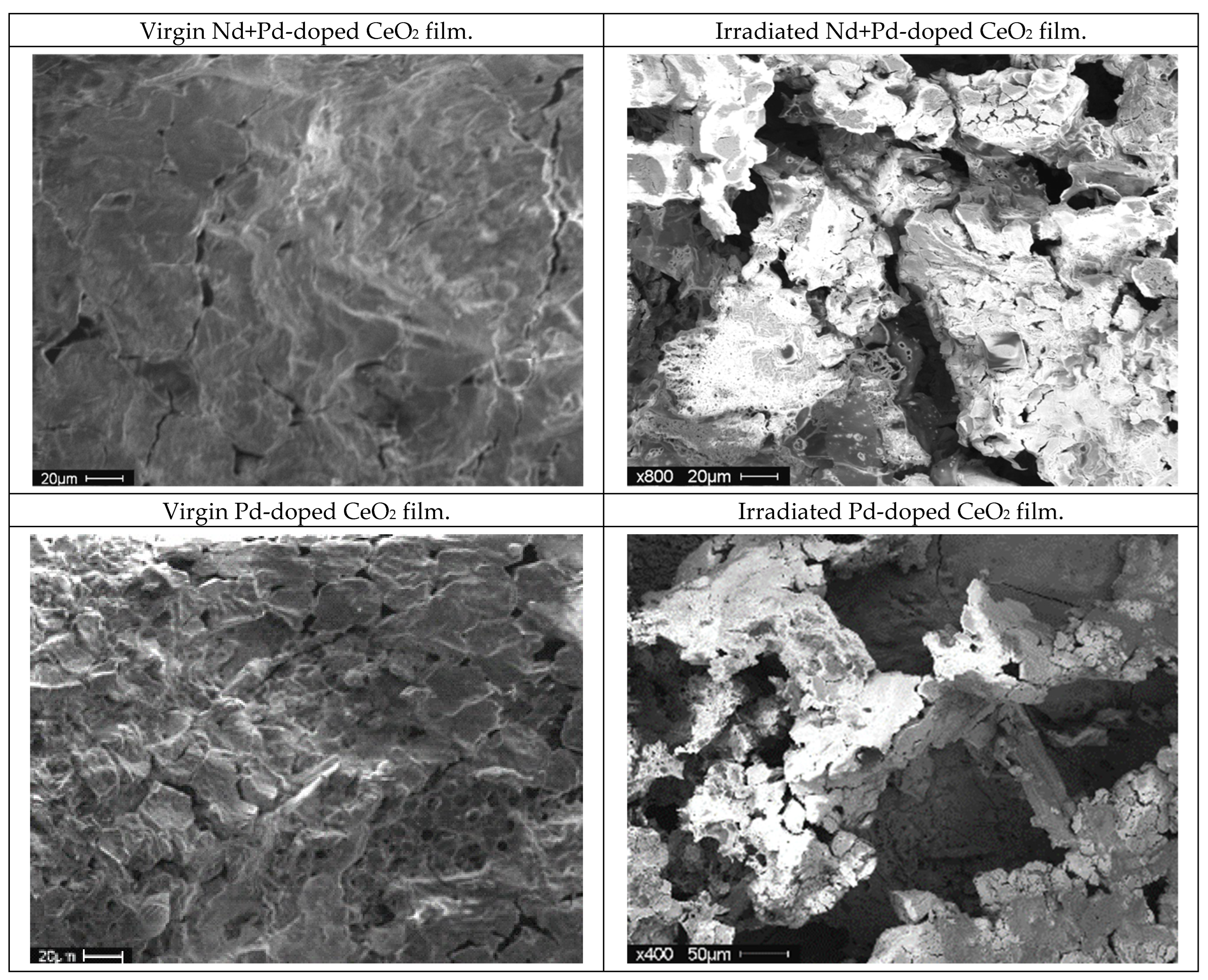
3.2.1. Effects of Dopants and Irradiation on Film Surface Morphology
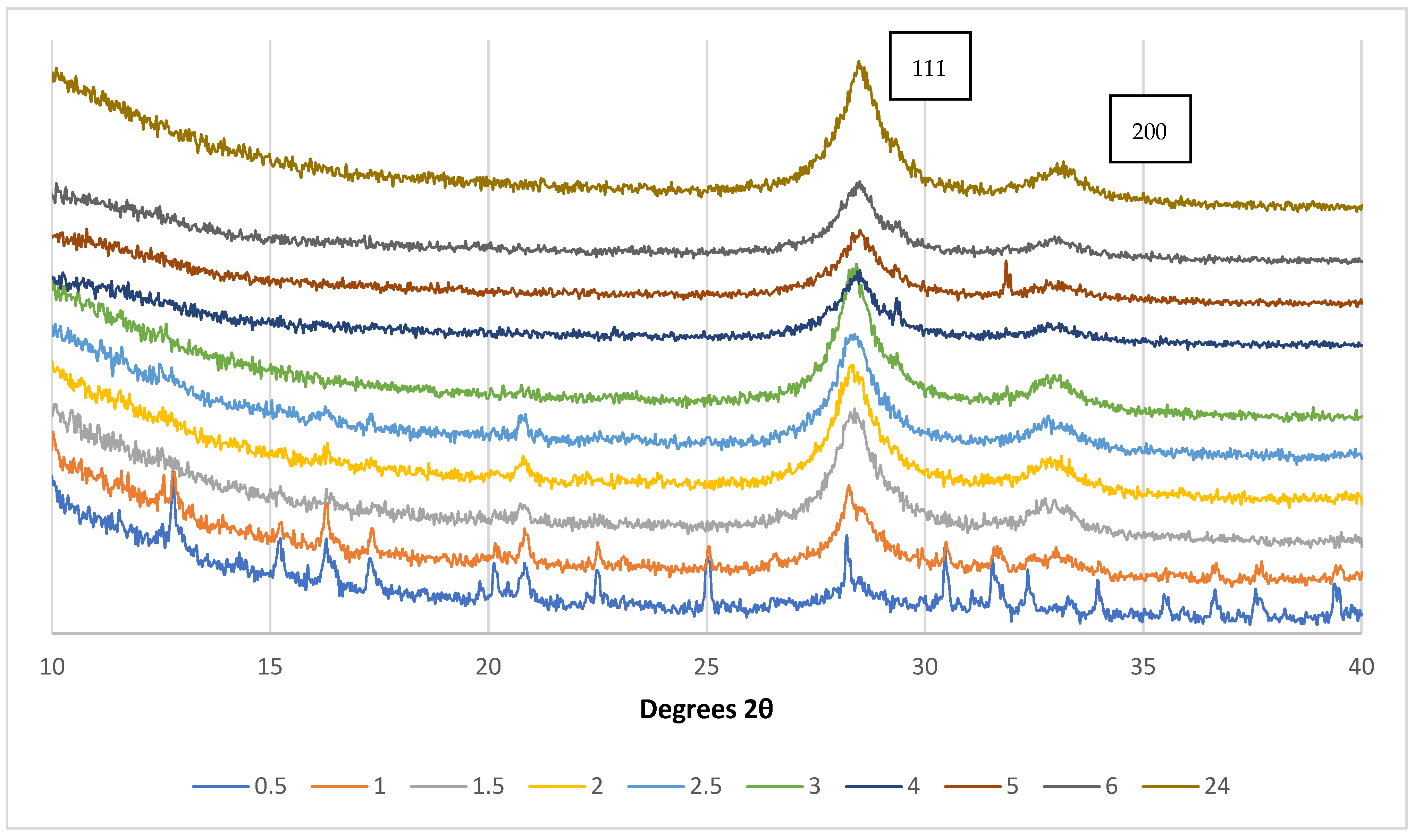
3.2.2. Effects of Dopants and Irradiation on Film Crystal Structures
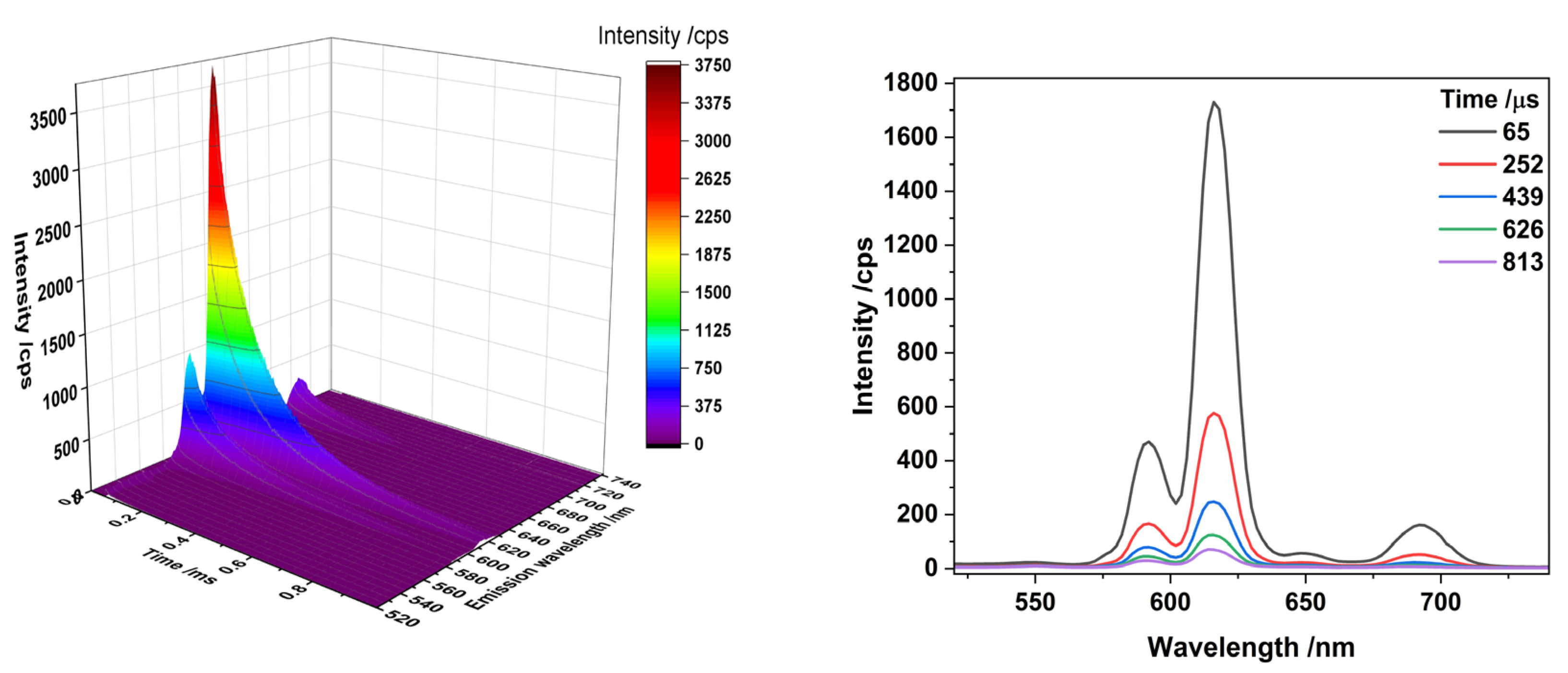
3.2.3. Effect of Dopants and Irradiation on Chemical Environments
3.3. Effects of Dopants and/or Irradiation on the Leaching of CeO2 SIMFUEL Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holdsworth, A.F.; George, K.; Adams, S.J.S.; Sharrad, C.A. An accessible statistical regression approach for the estimation of spent nuclear fuel compositions and decay heats to support the development of nuclear fuel management strategies. Prog. Nucl. Energy 2021, 141, 103935. [Google Scholar] [CrossRef]
- Mayhew, C.; He, Z.; Corcoran, E.C. Application of Auger Electron Spectroscopy to the Study of Molybdenum Oxidation in Oxide Nuclear Fuel; Report: AECL-CW--124540-CONF-003; Chalk River Laboratories: Chalk River, ON, Canada, 2015; Available online: https://inis.iaea.org/collection/NCLCollectionStore/_Public/49/103/49103669.pdf (accessed on 21 April 2024).
- Willman, C.; Håkansson, A.; Osifo, O.; Bäcklin, A.; Svärd, S.J. Nondestructive assay of spent nuclear fuel with gamma-ray spectroscopy. Ann. Nucl. Energy 2006, 33, 427–438. [Google Scholar] [CrossRef]
- Makarova, T.P.; Bibichev, B.A.; Domkin, V.D. Destructive analysis of the nuclide composition of spent fuel of WWER-440, WWER-1000, and RBMK-1000 reactors. Radiochemistry 2008, 50, 414–426. [Google Scholar] [CrossRef]
- Wang, T.; Xie, T.; Xu, C. Microextractors applied in nuclear-spent fuel reprocessing: Micro/mini plants and radiochemical analysis. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1–31. [Google Scholar] [CrossRef]
- Bruchet, A.; Taniga, V.; Descroix, S.; Malaquin, L.; Goutelard, F.; Mariet, C. Centrifugal microfluidic platform for radiochemistry: Potentialities for the chemical analysis of nuclear spent fuels. Talanta 2013, 116, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Parrish, R.J.; Cappia, F.; Aitkaliyeva, A. Comparison of the radial effects of burnup on fast reactor MOX fuel microstructure and solid fission products. J. Nucl. Mater. 2020, 531, 152003. [Google Scholar] [CrossRef]
- Oettingen, M. The Application of Radiochemical Measurements of PWR Spent Fuel for the Validation of Burnup Codes. Energies 2022, 15, 3041. [Google Scholar] [CrossRef]
- Hill, C. Worldwide Approaches towards Minimizing the Burden of High Level Waste. On-going IAEA Activities on Innovative Systems. In Proceedings of the Third GENIORS Workshop on Processes and Process Simulation, Milan, Italy, 4–6 November 2019. [Google Scholar]
- Bauhn, L.; Hansson, N.; Ekberg, C.; Fors, P.; Spahiu, K. The fate of hydroxyl radicals produced during H2O2 decomposition on a SIMFUEL surface in the presence of dissolved hydrogen. J. Nucl. Mater. 2018, 507, 38–43. [Google Scholar] [CrossRef]
- Corcoran, E.C.; Lewis, B.J.; Thompson, W.T.; Mouris, J.; He, Z. Controlled oxidation experiments of simulated irradiated UO2 fuel in relation to thermochemical modelling. J. Nucl. Mater. 2011, 414, 73–82. [Google Scholar] [CrossRef]
- Oversby, V.M.; Konsult, V.M.O. Uranium Dioxide, SIMFUEL, and Spent Fuel Dissolution Rates a Review of Published Data Report: TR-99-22; Swedish Nuclear Fuel and Waste Management Co.: Stockholm, Sweden, 1999. Available online: https://www.osti.gov/etdeweb/servlets/purl/20045929 (accessed on 21 April 2024).
- Nilsson, S.; Jonsson, M. H2O2 and radiation induced dissolution of UO2 and SIMFUEL pellets. J. Nucl. Mater. 2011, 410, 89–93. [Google Scholar] [CrossRef]
- Santos, B.G.; Noël, J.J.; Shoesmith, D.W. The effect of pH on the anodic dissolution of SIMFUEL (UO2). J. Electroanal. Chem. 2006, 586, 1–11. [Google Scholar] [CrossRef]
- Santos, B.G.; Nesbitt, H.W.; Noël, J.J.; Shoesmith, D.W. X-ray photoelectron spectroscopy study of anodically oxidized SIMFUEL surfaces. Electrochim. Acta 2004, 49, 1863–1873. [Google Scholar] [CrossRef]
- Ollila, K. SIMFUEL dissolution studies in granitic groundwater. J. Nucl. Mater. 1992, 190, 70–77. [Google Scholar] [CrossRef]
- Bruno, J.; Casas, I.; Sandino, A. Static and dynamic SIMFUEL dissolution studies under oxic conditions. J. Nucl. Mater. 1992, 190, 61–69. [Google Scholar] [CrossRef]
- Lucuta, P.G.; Matzke, H.; Verrall, R.A.; Tasman, H.A. Thermal conductivity of SIMFUEL. J. Nucl. Mater. 1992, 188, 198–204. [Google Scholar] [CrossRef]
- Lucuta, P.G.; Matzke, H.; Verrall, R.A. Modelling of UO2-based SIMFUEL thermal conductivity—The effect of the burnup. J. Nucl. Mater. 1994, 217, 279–286. [Google Scholar] [CrossRef]
- Lucuta, P.G.; Matzke, H.; Verrall, R.A. Thermal conductivity of hyperstoichiometric SIMFUEL. J. Nucl. Mater. 1995, 223, 51–60. [Google Scholar] [CrossRef]
- Verrall, R.A.; Lucuta, P.G. Specific heat measurements of UO2 and SIMFUEL. J. Nucl. Mater. 1996, 228, 251–253. [Google Scholar] [CrossRef]
- Stennett, M.C.; Corkhill, C.L.; Marshall, L.A.; Hyatt, N.C. Preparation, characterisation and dissolution of a CeO2 analogue for UO2 nuclear fuel. J. Nucl. Mater. 2013, 432, 182–188. [Google Scholar] [CrossRef]
- Popel, A.J.; Le Solliec, S.; Lampronti, G.I.; Day, J.; Petrov, P.K.; Farnan, I. The effect of fission-energy Xe ion irradiation on the structural integrity and dissolution of the CeO2 matrix. J. Nucl. Mater. 2017, 484, 332–338. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Bailey, D.J.; Tocino, F.Y.; Stennett, M.C.; Miller, J.A.; Provis, J.L.; Travis, K.P.; Hyatt, N.C. Role of Microstructure and Surface Defects on the Dissolution Kinetics of CeO2, a UO2 Fuel Analogue. ACS Appl. Mater. Interfaces 2016, 8, 10562–10571. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Conroy, M.A.; Kruska, K.; Olszta, M.J.; Droubay, T.C.; Schwantes, J.M.; Taylor, C.A.; Price, P.M.; Hattar, K.; Devanathan, R. In Situ Study of Particle Precipitation in Metal-Doped CeO2 during Thermal Treatment and Ion Irradiation for Emulation of Irradiating Fuels. J. Phys. Chem. C 2019, 123, 2591–2601. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Myllykylä, E.; Bailey, D.J.; Thornber, S.M.; Qi, J.; Maldonado, P.; Stennett, M.C.; Hamilton, A.; Hyatt, N.C. Contribution of Energetically Reactive Surface Features to the Dissolution of CeO2 and ThO2 Analogues for Spent Nuclear Fuel Microstructures. ACS Appl. Mater. Interfaces 2014, 6, 12279–12289. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.; Ye, B.; Oaks, A.J.; Chen, W.; Kirk, M.A.; Rest, J.; Yacout, A.M.; Stubbins, J.F. Fission gas transport and its interactions with irradiation-induced defects in lanthanum doped ceria. Nucl. Instrum. Methods Phys. Res. B 2012, 272, 239–243. [Google Scholar] [CrossRef]
- Melchers, R.E. On the ALARP approach to risk management. Reliab. Eng. Syst. Saf. 2001, 71, 201–208. [Google Scholar] [CrossRef]
- Cheng, F.; George, K.; Hector, A.L.; Jura, M.; Kroner, A.; Levason, W.; Nesbitt, J.; Reid, G.; Smith, D.C.; Wilson, J.W. Chemical Vapor Deposition of GaP and GaAs Thin Films From [nBu2Ga(μ-EtBu2)2GanBu2] (E = P or As) and Ga(PtBu2)3. Chem. Mater. 2011, 23, 5217–5222. [Google Scholar] [CrossRef]
- George, K.; de Groot, C.H.; Gurnani, C.; Hector, A.L.; Huang, R.; Jura, M.; Levason, W.; Reid, G. Telluroether and Selenoether Complexes as Single Source Reagents for Low Pressure Chemical Vapor Deposition of Crystalline Ga2Te3 and Ga2Se3 Thin Films. Chem. Mater. 2013, 25, 1829–1836. [Google Scholar] [CrossRef]
- Klemens, P.G. Theory of Thermal Conduction in Thin Ceramic Films. Int. J. Thermophys. 2001, 22, 265–275. [Google Scholar] [CrossRef]
- Trummer, M.; Roth, O.; Jonsson, M. H2 inhibition of radiation induced dissolution of spent nuclear fuel. J. Nucl. Mater. 2009, 383, 226–230. [Google Scholar] [CrossRef]
- Eriksen, T.E.; Jonsson, M.; Merino, J. Modelling of time resolved and long contact time dissolution studies of spent nuclear fuel in 10 mM carbonate solution—A comparison between two different models and experimental data. J. Nucl. Mater. 2008, 375, 331–339. [Google Scholar] [CrossRef]
- Holdsworth, A.F. Manufacture, Applications, and Opportunities of Thin Film Palladium-Silver (PdAg) Alloys: A Review. Preprints 2019, 2019080261. [Google Scholar] [CrossRef]
- Rossnagel, S.M. Thin film deposition with physical vapor deposition and related technologies. J. Vac. Sci. Technol. A 2003, 21, S74–S87. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Jia, Q.X.; McCleskey, T.M.; Burrell, A.K.; Lin, Y.; Collis, G.E.; Wang, H.; Li, A.D.Q.; Foltyn, S.R. Polymer-assisted deposition of metal-oxide films. Nat. Mater. 2004, 3, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Gao, Z.; Sun, J.; Shen, J. Fabrication of Lanthanide Phosphate Nanocrystals with Well-Controlled Morphologies by Layer-by-Layer Adsorption and Reaction Method at Room Temperature. Cryst. Growth Des. 2009, 9, 3707–3713. [Google Scholar] [CrossRef]
- Baranov, V.G.; Lunev, A.V.; Reutov, V.F.; Tenishev, A.V.; Isaenkova, M.G.; Khlunov, A.V. An attempt to reproduce high burn-up structure by ion irradiation of SIMFUEL. J. Nucl. Mater. 2014, 452, 147–157. [Google Scholar] [CrossRef]
- Nuclear Power. Available online: https://www.nuclear-power.com/nuclear-power/reactor-physics/atomic-nuclear-physics/radiation/ (accessed on 17 February 2024).
- Mincher, B.J.; Modolo, G.; Mezyk, S.P. Review Article: The Effects of Radiation Chemistry on Solvent Extraction: 1. Conditions in Acidic Solution and a Review of TBP Radiolysis. Solvent Extr. Ion Exch. 2009, 27, 1–25. [Google Scholar] [CrossRef]
- Narasimharao, K.L.; Sarma, K.S.; Mathew, C.; Jadhav, A.V.; Shukla, J.P.; Natarajan, V.; Seshagiri, T.K.; Sali, S.K.; Dhiwar, V.I.; Pande, B.; et al. Physico-chemical and ion exchange characteristics of ammonium molybdophosphate irradiated with electrons. J. Chem. Soc. Faraday Trans. 1998, 94, 1641–1647. [Google Scholar] [CrossRef]
- Malmbeck, R.; Magnusson, D.; Bourg, S.; Carrott, M.; Geist, A.; Hérès, X.; Miguirditchian, M.; Modolo, G.; Müllich, U.; Sorel, C.; et al. Homogenous recycling of transuranium elements from irradiated fast reactor fuel by the EURO-GANEX solvent extraction process. Radiochim. Acta 2019, 107, 917–929. [Google Scholar] [CrossRef]
- Audebrand, N.; Guillou, N.; Auffrédic, J.P.; Louër, D. The thermal behaviour of ceric ammonium nitrate studied by temperature-dependent X-ray powder diffraction. Thermochim. Acta 1996, 286, 83–87. [Google Scholar] [CrossRef]
- Semagina, N.; Renken, A.; Laub, D.; Kiwi-Minsker, L. Synthesis of monodispersed palladium nanoparticles to study structure sensitivity of solvent-free selective hydrogenation of 2-methyl-3-butyn-2-ol. J. Catal. 2007, 246, 308–314. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K.; Price, D. The potential of metal oxalates as novel flame retardants and synergists for engineering polymers. Polym. Degrad. Stab. 2014, 110, 290–297. [Google Scholar] [CrossRef]
- Holdsworth, A.F.; Horrocks, A.R.; Kandola, B.K. Synthesis and thermal analytical screening of metal complexes as potential novel fire retardants in polyamide 6.6. Polym. Degrad. Stab. 2017, 144, 420–433. [Google Scholar] [CrossRef]
- Holdsworth, A.F. Novel Metal Complex Fire Retardants for Engineering Polymers. Ph.D. Thesis, University of Bolton, Bolton, UK, 2015. [Google Scholar]
- IAEA-TECDOC-1537. Strategy and Methodology for Radioactive Waste Characterization; International Atomic Energy Agency: Vienna, Austria, March 2007; Available online: https://www-pub.iaea.org/MTCD/Publications/PDF/te_1537_web.pdf (accessed on 21 April 2024).
- Thorpe, C.L.; Neeway, J.J.; Pearce, C.I.; Hand, R.J.; Fisher, A.J.; Walling, S.A.; Hyatt, N.C.; Kruger, A.A.; Schweiger, M.; Kosson, D.S.; et al. Forty years of durability assessment of nuclear waste glass by standard methods. npj Mater. Degrad. 2021, 5, 61. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E.; Kalmykov, S.N. Characterisation of Radioactive Waste. In An Introduction to Nuclear Waste Immobilisation, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 14. [Google Scholar]
- Xu, J.; Wang, Z.; Wen, L.; Zhou, X.; Xu, J.; Yang, S. Dynamics of the layer-by-layer assembly of a poly(acrylic acid)–lanthanide complex colloid and poly(diallyldimethyl ammonium). Soft Matter 2016, 12, 867–875. [Google Scholar] [CrossRef]
- Springer Materials Crystallographic Data. Available online: https://materials.springer.com/isp/crystallographic/docs/sd_1101276 (accessed on 17 February 2024).
- Beineke, T.A.; Delgaudio, J. Crystal structure of ceric ammonium nitrate. Inorg. Chem. 1968, 7, 715–721. [Google Scholar] [CrossRef]
- Guillou, N.; Auffrédic, J.P.; Louër, D. Cerous Potassium Nitrate, K3Ce2(NO3)9. Acta Crystallogr. C 1995, 51, 1032–1034. [Google Scholar] [CrossRef]
- Evans, A.G.; Cannon, R.M. Overview no. 48: Toughening of brittle solids by martensitic transformations. Acta Metall. 1986, 34, 761–800. [Google Scholar] [CrossRef]
- Melnikov, P.; Arkhangelsky, I.V.; Nascimento, V.A.; de Oliveira, L.C.S.; Silva, A.F.; Zanoni, L.Z. Thermal properties of europium nitrate hexahydrate Eu(NO3)3·6H2O. J. Therm. Anal. Calorim. 2017, 128, 1353–1358. [Google Scholar] [CrossRef]
- Karppinen, M.; Kyläkoski, P.; Niinistö, L.; Rodellas, C. Thermal decomposition as a preparative route to anhydrous rare earth nitrates. J. Therm. Anal. 1989, 35, 347–353. [Google Scholar] [CrossRef]
- Bharathi, R.N.; Sankar, S. Structural, Optical, and Magnetic Properties of Nd-Doped CeO2 Nanoparticles Codoped with Transition Metal Elements (Cu, Zn, Cr). J. Supercond. Nov. Magn. 2018, 31, 2603–2615. [Google Scholar] [CrossRef]
- Raj, A.K.V.; Rao, P.P.; Sreena, T.S.; Thara, T.R.A. Influence of local structure on photoluminescence properties of Eu3+ doped CeO2 red phosphors through induced oxygen vacancies by contrasting rare earth substitutions. Phys. Chem. Chem. Phys. 2017, 19, 20110–20120. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.A.; Catto, A.C.; Longo, E.; Nossol, E.; Lima, R.C. Characterization and electrochemical performance of CeO2 and Eu-doped CeO2 films as a manganese redox flow battery component. J. Rare Earths 2018, 36, 1074–1083. [Google Scholar] [CrossRef]
- Vujčić, I.; Gavrilović, T.; Sekulić, M.; Mašić, S.; Putić, S.; Papan, J.; Dramićanin, M.D. Gamma-radiation effects on luminescence properties of Eu3+ activated LaPO4 phosphor. Nucl. Instrum. Methods Phys. Res. B 2018, 422, 85–90. [Google Scholar] [CrossRef]
- IAEA Technical Report #218. Storage of Water Reactor Spent Fuel in Water Pools; International Atomic Energy Agency: Vienna, Austria, 1982; Available online: https://www.iaea.org/publications/1319/storage-of-water-reactor-spent-fuel-in-water-pools (accessed on 21 April 2024).
- Cornelis, G.; Ryan, B.; McLaughlin, M.J.; Kirby, J.K.; Beak, D.; Chittleborough, D. Solubility and Batch Retention of CeO2 Nanoparticles in Soils. Environ. Sci. Technol. 2011, 45, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Tok, A.I.Y.; Boey, F.Y.C.; Dong, Z.; Sun, X.L. Hydrothermal synthesis of CeO2 nano-particles. J. Mater. Process. Technol. 2007, 190, 217–222. [Google Scholar] [CrossRef]
- Spahiu, K.; Evins, L.Z. Metal Alloy Particles in Spent Nuclear Fuel; SKB Public Memo 1415408; SKB: Stockholm, Sweden, 2013; Available online: https://skb.se/wp-content/uploads/2015/05/Bilaga-t-1418271_1415408-Metal-alloy-particles-in-spent-nuclear-fuel.pdf (accessed on 21 April 2024).
- Imperial College Database of Ionic Radii. Available online: http://abulafia.mt.ic.ac.uk/shannon/ptable.php (accessed on 16 January 2024).
- Le Caër, S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Petrik, N.G.; Alexandrov, A.B.; Vall, A.I. Interfacial Energy Transfer during Gamma Radiolysis of Water on the Surface of ZrO2 and Some Other Oxides. J. Phys. Chem. B 2001, 105, 5935–5944. [Google Scholar] [CrossRef]
- Rhodes, C.; Harris, R.; Graham, D. Concept Design of a Euro-GANEX Reprocessing Plant; GENIORS Report D8.1; National Nuclear Laboratory: Sellafield, UK, 2018. [Google Scholar]
- Jonsson, M.; Nielsen, F.; Roth, O.; Ekeroth, E.; Nilsson, S.; Hossain, M.M. Radiation Induced Spent Nuclear Fuel Dissolution under Deep Repository Conditions. Environ. Sci. Technol. 2007, 41, 7087–7093. [Google Scholar] [CrossRef]
- Pont, A.-L.; Marcilla, R.; De Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Schild, H.G. Thermal degradation of poly(methacrylic acid): Further studies applying TGA/FTIR. J. Polym. Sci. A 1993, 31, 2403–2405. [Google Scholar] [CrossRef]
- Purohit, R.D.; Saha, S.; Tyagi, A.K. Nanocrystalline Ceria Powders Through Citrate–Nitrate Combustion. J. Nanosci. Nanotechnol. 2006, 6, 209–214. [Google Scholar] [CrossRef] [PubMed]


| Sample | Ce (%) | Nd (%) | Pd (%) | Sample | Ce (%) | Eu (%) |
|---|---|---|---|---|---|---|
| Control | 100 | - | - | Control | 100 | - |
| Nd | 98 | 2 | - | 1% | 99 | 1 |
| Pd | 99 | - | 1 | 2% | 98 | 2 |
| Nd-Pd | 97 | 2 | 1 | 5% | 95 | 5 |
| Sample | Ce (%) | Nd (%) | Pd (CPS) a | Sample | Ce (%) | Eu (%) |
|---|---|---|---|---|---|---|
| CeO2 | 5 | - | - | - | - | - |
| [Irrad] | 31 | - | - | - | - | - |
| Nd | 5 | 33 | - | 1% Eu | 55 | 100 |
| [Irrad] | 9 | 33 | - | [Irrad] | 34 | 60 |
| Pd | 81 | - | 5000 | 2% Eu | 45 | 63 |
| [Irrad] | 53 | - | 4000 | [Irrad] | 23 | 36 |
| Nd+Pd | 40 | 62 | 4000 | 5% Eu | 39 | 68 |
| [Irrad] | 40 | 62 | 3600 | [Irrad] | 34 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holdsworth, A.F.; Feng, Z.; Edge, R.; Waters, J.P.; Halman, A.M.; Collison, D.; George, K.; Natrajan, L.S.; Denecke, M.A. The Effects of Irradiation on Structure and Leaching of Pure and Doped Thin-Film Ceria SIMFUEL Models Prepared via Polymer-Templated Deposition. J. Nucl. Eng. 2024, 5, 150-167. https://doi.org/10.3390/jne5020011
Holdsworth AF, Feng Z, Edge R, Waters JP, Halman AM, Collison D, George K, Natrajan LS, Denecke MA. The Effects of Irradiation on Structure and Leaching of Pure and Doped Thin-Film Ceria SIMFUEL Models Prepared via Polymer-Templated Deposition. Journal of Nuclear Engineering. 2024; 5(2):150-167. https://doi.org/10.3390/jne5020011
Chicago/Turabian StyleHoldsworth, Alistair F., Zizhen Feng, Ruth Edge, John P. Waters, Alice M. Halman, David Collison, Kathryn George, Louise S. Natrajan, and Melissa A. Denecke. 2024. "The Effects of Irradiation on Structure and Leaching of Pure and Doped Thin-Film Ceria SIMFUEL Models Prepared via Polymer-Templated Deposition" Journal of Nuclear Engineering 5, no. 2: 150-167. https://doi.org/10.3390/jne5020011
APA StyleHoldsworth, A. F., Feng, Z., Edge, R., Waters, J. P., Halman, A. M., Collison, D., George, K., Natrajan, L. S., & Denecke, M. A. (2024). The Effects of Irradiation on Structure and Leaching of Pure and Doped Thin-Film Ceria SIMFUEL Models Prepared via Polymer-Templated Deposition. Journal of Nuclear Engineering, 5(2), 150-167. https://doi.org/10.3390/jne5020011







