Methylcellulose Bionanocomposite Films Incorporated with Zein Nanoparticles Containing Propolis and Curcumin for Functional Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Zein Nanoparticles Containing Curcumin and Propolis
2.3. Preparation of Nanocomposite Films by Casting
2.4. Characterization
2.4.1. Encapsulation Efficiency
2.4.2. Antioxidant Activity (AA) and Total Phenolic Content (TPC)
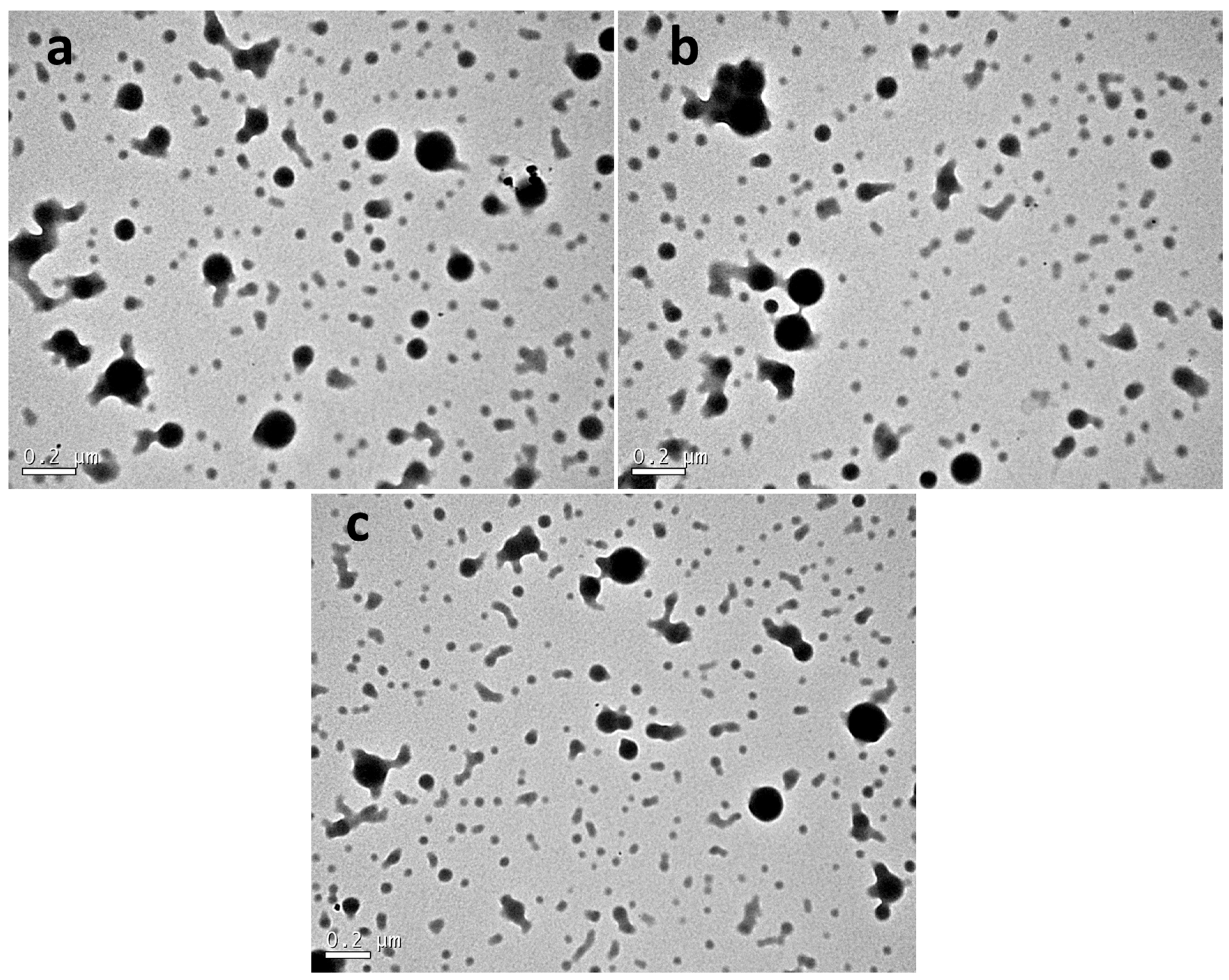
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. Zeta Potential, Polydispersity Index, and Average Size
2.4.5. Release Studies
2.4.6. Evaluation of the Mechanical Properties
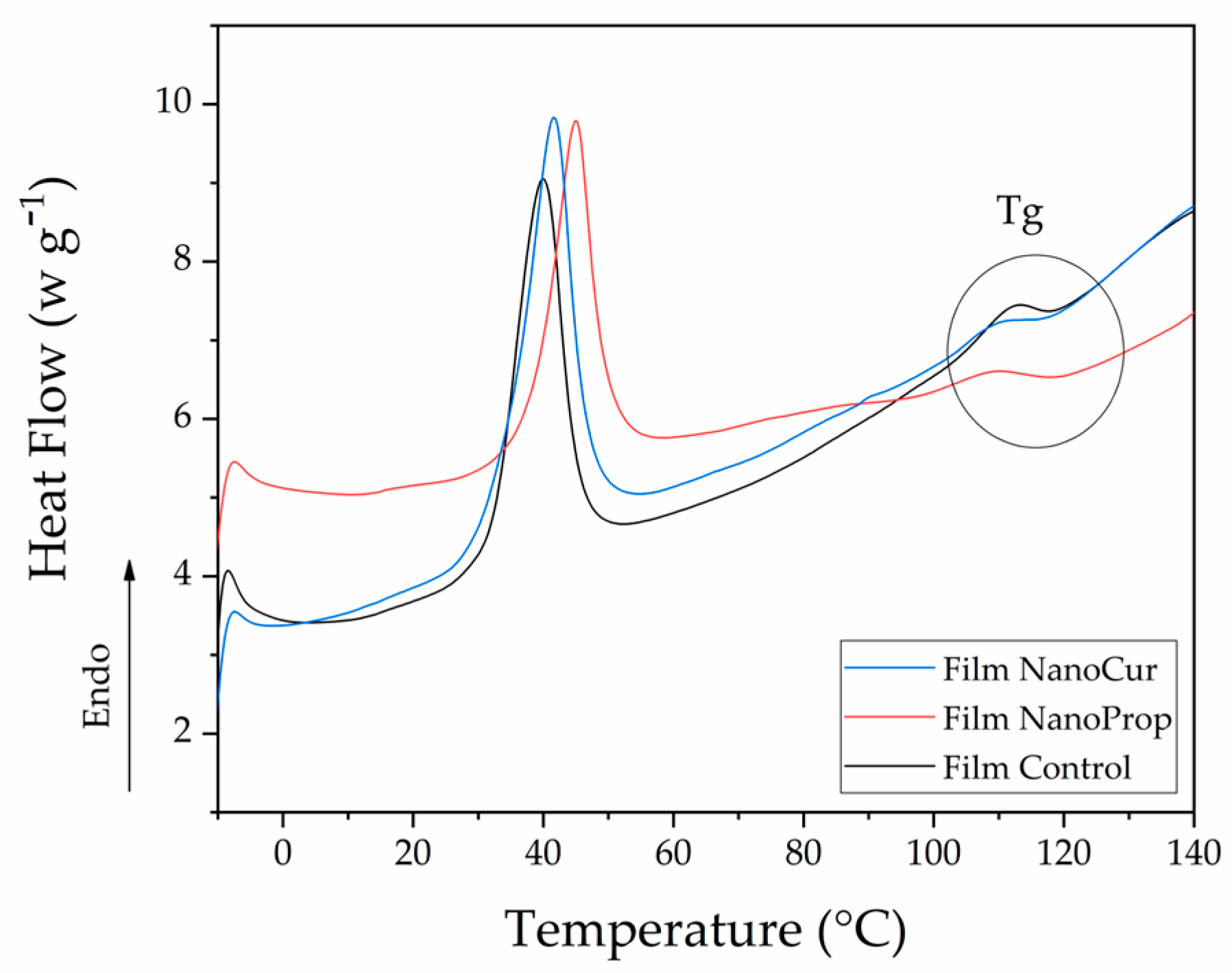
2.4.7. Thermal Analysis
2.4.8. Color Analysis, Transmittance, and Opacity of the Films
2.4.9. Contact Angle Measurement
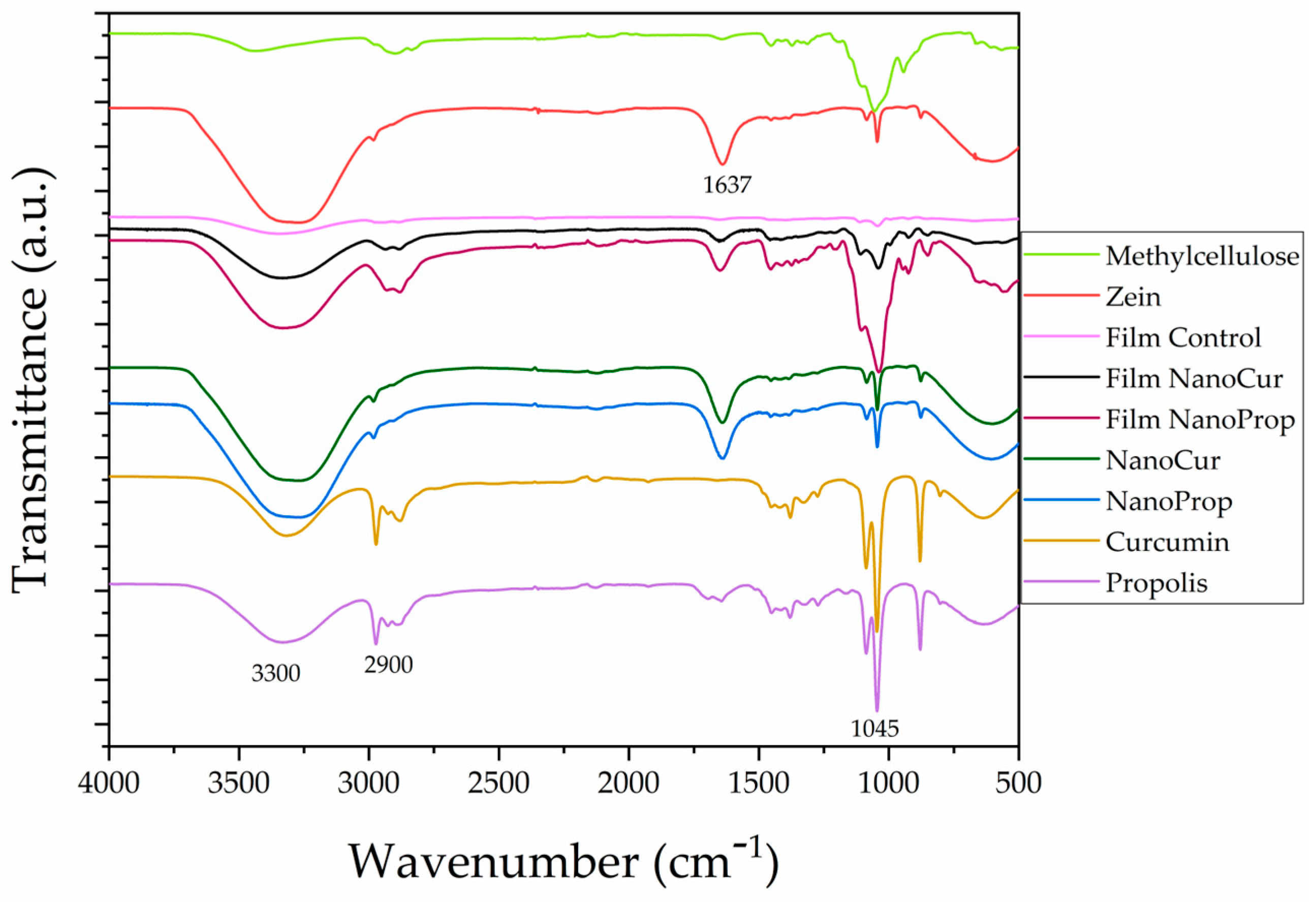
2.4.10. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.11. Water Vapor Permeability
2.4.12. Scanning Electron Microscopy (SEM) of Films
2.4.13. Antibacterial Activity
2.4.14. Application of Films as Packaging
2.4.15. Statistical Analysis
3. Results and Discussion
3.1. Stability and Size Distribution of Synthesized Nanoparticles
3.2. Encapsulation Efficiency and Release Studies
3.2.1. Encapsulation Efficiency
3.2.2. Release Studies of the Nanoparticles
3.2.3. Release Studies of the Films
3.3. Microstructural Analysis of Nanoparticles
3.4. Structural Analysis of Nanocomposite Films
3.5. Mechanical Characterization of Nanocomposite Films
3.6. Surface Energy and Color Analysis of Nanocomposite Films
3.7. Thermal Behavior of Methylcellulose Films
3.8. Antioxidant Activity
3.9. Antibacterial Activity
3.10. Scanning Electron Microscopy (SEM)
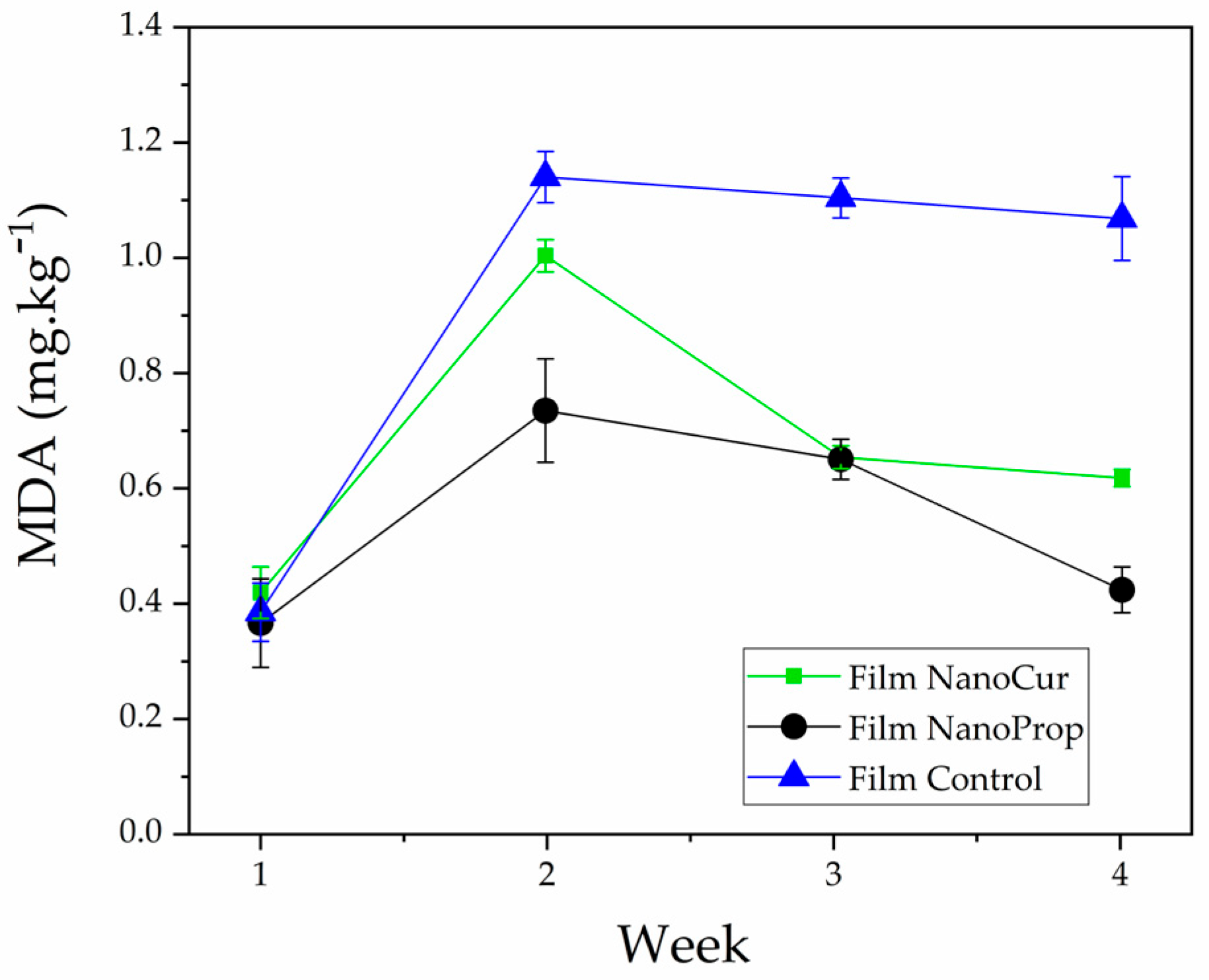
3.11. Application of Films in Packaging
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nunes, M.R.; De Souza Maguerroski Castilho, M.; De Lima Veeck, A.P.; Da Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and Antimicrobial Methylcellulose Films Containing Lippia Alba Extract and Silver Nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Noronha, C.M.; De Carvalho, S.M.; Lino, R.C.; Barreto, P.L.M. Characterization of Antioxidant Methylcellulose Film Incorporated with α-Tocopherol Nanocapsules. Food Chem. 2014, 159, 529–535. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Rosa, G.B.; Ferreira, A.L.A.; Da Rosa, C.G.; Beling, P.C.; Xavier, L.O.; Hansen, C.M.; Ferrareze, J.P.; Nunes, M.R.; Barreto, P.L.M.; et al. Bioactive Food Packaging Based on Starch, Citric Pectin and Functionalized with Acca Sellowiana Waste by-Product: Characterization and Application in the Postharvest Conservation of Apple. Int. J. Biol. Macromol. 2020, 147, 295–303. [Google Scholar] [CrossRef]
- Dag, D.; Jung, J.; Zhao, Y. Development and Characterization of Cellulose Nanofiber Reinforced Hydroxypropyl Methylcellulose Films Functionalized with Propolis-Loaded Zein Nanoparticles and Its Application for Cheddar Cheese Storage. Int. J. Biol. Macromol. 2024, 261, 129790. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, H.; Zhu, J.; Liu, C.; Sun, X.; Wang, D.; Xu, Y. Fabrication and Characterization of Zein Nanoparticles by Dextran Sulfate Coating as Vehicles for Delivery of Curcumin. Int. J. Biol. Macromol. 2020, 151, 1074–1083. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Polysaccharides/Propolis Composite as Promising Materials with Biomedical and Packaging Applications: A Review. Biomass Convers. Biorefinery 2024, 14, 4555–4565. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; Mohamed, S.A.A. Propolis Applications in Food Industries and Packaging. Biomass Convers. Biorefinery 2024, 14, 13731–13746. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. ISBN 978-0-12-800173-8. [Google Scholar]
- Taghinejad, F.; Masoumi, B.; Tabibiazar, M.; Bagheri, V.; Pezeshki, A.; Mahmoudzadeh, M. Curcumin-Thymol Loaded Hydrolyzed Zein-Ethyl Cellulose as Active Packaging Film for Extended Minced Mutton Shelf-Life. J. Food Meas. Charact. 2024, 18, 6342–6355. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Guo, F.; Yu, Y.; Lu, J.; Li, Y.; Cheng, Q.; Peng, J.; Yu, G. Biodegradable Cellulose/Curcumin Films with Janus Structure for Food Packaging and Freshness Monitoring. Carbohydr. Polym. 2024, 324, 121516. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Yang, M.; Abdalkarim, S.Y.H.; Yu, H.-Y. In Situ Growth of Curcumin-Loaded Cellulose Composite Film for Real-Time Monitoring of Food Freshness in Smart Packaging. Int. J. Biol. Macromol. 2024, 279, 135090. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; Zapelini De Melo, A.P.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; Vinicius De Oliveira Brisola Maciel, M.; Bertoldi, F.C.; Manique Barreto, P.L. Application in Situ of Zein Nanocapsules Loaded with Origanum Vulgare Linneus and Thymus Vulgaris as a Preservative in Bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Nunes, M.R.; Agostinetto, L.; Da Rosa, C.G.; Sganzerla, W.G.; Pires, M.F.; Munaretto, G.A.; Rosar, C.R.; Bertoldi, F.C.; Barreto, P.L.M.; Veeck, A.P.D.L.; et al. Application of Nanoparticles Entrapped Orange Essential Oil to Inhibit the Incidence of Phytopathogenic Fungi during Storage of Agroecological Maize Seeds. Food Res. Int. 2024, 175, 113738. [Google Scholar] [CrossRef]
- Cheng, C.J.; Jones, O.G. Effect of Drying Temperature and Extent of Particle Dispersion on Composite Films of Methylcellulose and Zein Nanoparticles. J. Food Eng. 2019, 250, 26–32. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Da Rosa, C.G.; Da Silva, A.P.G.; Ferrareze, J.P.; Azevedo, M.S.; Forster-Carneiro, T.; Nunes, M.R.; De Lima Veeck, A.P. Application in Situ of Biodegradable Films Produced with Starch, Citric Pectin and Functionalized with Feijoa (Acca Sellowiana (Berg) Burret) Extracts: An Effective Proposal for Food Conservation. Int. J. Biol. Macromol. 2021, 189, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; Da Rosa, C.G.; Agostinetto, L.; Veeck, A.P.D.L.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertoldi, F.C.; et al. Chitosan Packaging Functionalized with Cinnamodendron Dinisii Essential Oil Loaded Zein: A Proposal for Meat Conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Ikegaki, M. Preparation of Water and Ethanolic Extracts of Propolis and Evaluation of the Preparations. Biosci. Biotechnol. Biochem. 1998, 62, 2230–2232. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The Phenolic Constituents of Prunus Domestica. I.—The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; De Oliveira Brisola Maciel, M.V.; De Carvalho, S.M.; De Melo, A.P.Z.; Jummes, B.; Da Silva, T.; Martelli, S.M.; Villetti, M.A.; Bertoldi, F.C.; Barreto, P.L.M. Characterization and Evaluation of Physicochemical and Antimicrobial Properties of Zein Nanoparticles Loaded with Phenolics Monoterpenes. Colloids Surf. Physicochem. Eng. Asp. 2015, 481, 337–344. [Google Scholar] [CrossRef]
- USFDA. Guidance for Industry: Preparation of Premarket Notifications for Food Contact Substances: Chemistry Recommendations; USFDA: Silver Spring, MD, USA, 2007.
- ASTM D882-95a; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: Philadelphia, PA, USA, 1995.
- ASTM E96-22; Test Methods for Gravimetric Determination of Water Vapor Transmission Rate of Materials. ASTM International: Philadelphia, PA, USA, 2022. [CrossRef]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental Methods in Chemical Engineering: Zeta Potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- De Melo, A.P.Z.; Da Rosa, C.G.; Noronha, C.M.; Machado, M.H.; Sganzerla, W.G.; Bellinati, N.V.D.C.; Nunes, M.R.; Verruck, S.; Prudêncio, E.S.; Barreto, P.L.M. Nanoencapsulation of Vitamin D3 and Fortification in an Experimental Jelly Model of Acca Sellowiana: Bioaccessibility in a Simulated Gastrointestinal System. LWT 2021, 145, 111287. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar] [CrossRef]
- Khan, R.A.; Salmieri, S.; Dussault, D.; Uribe-Calderon, J.; Kamal, M.R.; Safrany, A.; Lacroix, M. Production and Properties of Nanocellulose-Reinforced Methylcellulose-Based Biodegradable Films. J. Agric. Food Chem. 2010, 58, 7878–7885. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, T.G.; Da Silva, P.F.; Azevedo, L.F.; Da Rocha, L.G.; De Moraes Porto, I.C.C.; Lima E Moura, T.F.A.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; Fonseca, E.J.D.S.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanoscale Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-N.; Lu, K.-Y.; Wang, P.; Ho, Y.-C.; Tsai, M.-L.; Mi, F.-L. Development of Bacterial Cellulose/Chitin Multi-Nanofibers Based Smart Films Containing Natural Active Microspheres and Nanoparticles Formed in Situ. Carbohydr. Polym. 2020, 228, 115370. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; Sganzerla, W.G.; De Oliveira Brisola Maciel, M.V.; De Melo, A.P.Z.; Da Rosa Almeida, A.; Ramos Nunes, M.; Bertoldi, F.C.; Manique Barreto, P.L. Development of Poly (Ethylene Oxide) Bioactive Nanocomposite Films Functionalized with Zein Nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2020, 586, 124268. [Google Scholar] [CrossRef]
- Francisco, A.P.; Sganzerla, W.G.; Nochi Castro, L.E.; Cruz Tabosa Barroso, T.L.; Da Silva, A.P.G.; Da Rosa, C.G.; Nunes, M.R.; Forster-Carneiro, T.; Rostagno, M.A. Pressurized Liquid Extraction of Bioactive Compounds from Grape Peel and Application in pH-Sensing Carboxymethyl Cellulose Films: A Promising Material to Monitor the Freshness of Pork and Milk. Food Res. Int. 2024, 179, 114017. [Google Scholar] [CrossRef]
- Castillo, M.L.R.D.; López-Tobar, E.; Sanchez-Cortes, S.; Flores, G.; Blanch, G.P. Stabilization of Curcumin against Photodegradation by Encapsulation in Gamma-Cyclodextrin: A Study Based on Chromatographic and Spectroscopic (Raman and UV–Visible) Data. Vib. Spectrosc. 2015, 81, 106–111. [Google Scholar] [CrossRef]
- De Carvalho, S.M.; Noronha, C.M.; Da Rosa, C.G.; Sganzerla, W.G.; Bellettini, I.C.; Nunes, M.R.; Bertoldi, F.C.; Manique Barreto, P.L. PVA Antioxidant Nanocomposite Films Functionalized with Alpha-Tocopherol Loaded Solid Lipid Nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2019, 581, 123793. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Sajkiewicz, P.; Gradys, A. Toward a Better Understanding of the Gelation Mechanism of Methylcellulose via Systematic DSC Studies. Polymers 2022, 14, 1810. [Google Scholar] [CrossRef]
- Li, L.; Liu, E.; Lim, C.H. Micro-DSC and Rheological Studies of Interactions between Methylcellulose and Surfactants. J. Phys. Chem. B 2007, 111, 6410–6416. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, İ. Antioxidant and Radical Scavenging Properties of Curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; De Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of Bioactive Compounds Potential and Antioxidant Activity of Brown, Green and Red Propolis from Brazilian Northeast Region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Suphrom, N.; Pipatsawasdikul, K.; Kongbangkerd, A.; Chikun, K.; Ngobkhonburi, S.; Muaklek, B.; Pitsamai, W.; Nisaipham, B.; Chuaimueang, W.; Sasiri, P.; et al. Bioactivity Assay of Arundina Graminifolia (D.Don) Hochr. Extracts from Diverse Plant Parts in Thailand: An Assay-Based Investigation. Sci. Hortic. 2024, 327, 112876. [Google Scholar] [CrossRef]
- Ibeogu, I.H.; Bako, H.K.; Yar, M.S.; Zhao, Q.; Zhu, J.; Zhao, D.; Zhang, M.; Ke, W.; Shan, K.; Zhou, G.; et al. Gelatin-Serum Plasma Film Incorporated with Curcumin for Improvement of Antioxidant and Antibacterial Properties for Fresh Pork Packaging Application. Food Hydrocoll. 2024, 149, 109617. [Google Scholar] [CrossRef]
- Dos Santos Alves, M.J.; De Sousa, M.H.O.; De Moura, N.F.; Cesca, K.; Verruck, S.; Monteiro, A.R.; Valencia, G.A. Starch Nanoparticles Containing Phenolic Compounds from Green Propolis: Characterization and Evaluation of Antioxidant, Antimicrobial and Digestibility Properties. Int. J. Biol. Macromol. 2024, 255, 128079. [Google Scholar] [CrossRef] [PubMed]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus Aureus and Staphylococcal Food-Borne Disease: An Ongoing Challenge in Public Health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [PubMed]
- Beuchat, L.R.; Ryu, J.-H. Produce Handling and Processing Practices. Emerg. Infect. Dis. 1997, 3, 459–465. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and Antibiofilm Studies of Curcumin Loaded Chitosan Nanoparticles against Polymicrobial Biofilms of Candida albicans and Staphylococcus aureus. Carbohydr. Polym. 2020, 241, 116254. [Google Scholar] [CrossRef] [PubMed]
- Bouchelaghem, S. Propolis Characterization and Antimicrobial Activities against Staphylococcus aureus and Candida albicans: A Review. Saudi J. Biol. Sci. 2022, 29, 1936–1946. [Google Scholar] [CrossRef]
- Lino, R.C.; De Carvalho, S.M.; Noronha, C.M.; Sganzerla, W.G.; Da Rosa, C.G.; Nunes, M.R.; D’Avila, R.F.; Zambiazi, R.C.; Barreto, P.L.M. Production of Methylcellulose Films Functionalized with Poly-ε-Caprolactone Nanocapsules Entrapped β-Carotene for Food Packaging Application. Food Res. Int. 2022, 160, 111750. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wang, B.; Dutta, P. Nanoparticle Processing: Understanding and Controlling Aggregation. Adv. Colloid Interface Sci. 2020, 279, 102162. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; Longo, M.; De Oliveira, J.L.; Da Rosa, C.G.; De Lima Veeck, A.P.; De Aquino, R.S.; Masiero, A.V.; Bertoldi, F.C.; Barreto, P.L.M.; Nunes, M.R. Nanocomposite Poly (Ethylene Oxide) Films Functionalized with Silver Nanoparticles Synthesized with Acca Sellowiana Extracts. Colloids Surf. Physicochem. Eng. Asp. 2020, 602, 125125. [Google Scholar] [CrossRef]
- Gerde, J.; Hardy, C.; Fehr, W.; White, P.J. Frying Performance of No-trans, Low-Linolenic Acid Soybean Oils. J. Am. Oil Chem. Soc. 2007, 84, 557–563. [Google Scholar] [CrossRef]




| Aspect | Present Work | [4] | [9] | [14] |
|---|---|---|---|---|
| Polymeric matrix | Methylcellulose (MC) reinforced with zein nanoparticles loaded with propolis or curcumin. | HPMC reinforced with cellulose nanofibers and zein nanoparticles loaded with propolis. | Hydrolyzed zein–ethylcellulose films containing curcumin and thymol. | Methylcellulose (MC) and zein nanoparticles. |
| Bioactive compounds | Propolis or curcumin. | Propolis. | Curcumin and thymol. | Indications of phenolic compounds encapsulated. |
| Mechanical properties | Increase in tensile strength and elongation. | Elongation at break increased and TS unchanged. | Hydrolysis increased tensile strength; thymol/curcumin affected solubility. | Tensile strength, elongation at break improved. |
| Barrier properties | Lower water vapor permeability and improved hydrophobicity compared with control. | WVP and hydrophobic contact improved. | WVP slightly increased after bioactive addition. | Water vapor permeability improved. |
| Antioxidant/antimicrobial activity | High DPPH scavenging; confirmed bacterial inhibition. | Films reduced lipid oxidation in cheese; antioxidant properties and sealing suitable for food packaging. | Significant bactericidal effect. Great antioxidant activity. | Not reported. |
| Food application tested | Primary packaging for fresh ground peanuts. | Active packaging for refrigerated cheddar cheese. | Preservation of minced mutton under refrigeration. | Suggested for active food packaging. |
| Sample | Zeta Potential (mV) | PI | Z-Ave (nm) |
|---|---|---|---|
| NanoControl | 39.8 ± 2.7 a | 0.270 ± 0.0275 ab | 128.5 ± 0.7 c |
| NanoCur | 36.5 ± 1.2 a | 0.236 ± 0.0180 b | 141.2 ± 1.0 a |
| NanoProp | 38.2 ± 1.7 a | 0.280 ± 0.00665 a | 132.6 ± 0.2 b |
| Sample | Zero Order | First Order | Korsmeyer–Peppas | Higuchi | |
|---|---|---|---|---|---|
| NanoCur | R2 | 0.80 | 0.68 | 0.96 | 0.82 |
| K | 0.020 | 0.097 | 0.035 | 18.87 | |
| NanoProp | R2 | 0.75 | 0.50 | 0.91 | 0.65 |
| K | 0.15 | 0.045 | 1.05 | 0.059 |
| Film | Tensile Strength (MPa) | Modulus of Elasticity (MPa) | Elongation (%) | Thickness (mm) | Water Vapor Permeability (g mm/m2 d kPa) |
|---|---|---|---|---|---|
| Film Control | 1041.74 ± 113.02 a | 397.62 ± 174.22 a | 286.00 ± 33.62 a | 0.98 ± 0.22 a | 3.22 ± 0.15 b |
| Film NanoCur | 862.78 ± 152.61 a | 174.66 ± 94.5 ab | 785.33 ± 72.18 b | 1.26 ± 0.21 a | 4.19 ± 0.11 b |
| Film NanoProp | 1156.74 ± 378.37 a | 99.37 ± 19.91 b | 614.00 ± 186.61 b | 1.56 ± 0.67 a | 5.50 ± 0.67 a |
| Films | Contact Angle (°) | Owens-Wendt (mN m−1) | |||||
|---|---|---|---|---|---|---|---|
| Water | Dimethyl Sulfoxide | Ethylene Glycol | Glycerol | γT | γp | γd | |
| Film Control | 16.77 ± 1.07 c | 48.60 ± 0.36 a | 54.57 ± 2.76 a | 65.70 ± 2.34 b | 82.35 | 82.09 | 0.26 |
| Film NanoCur | 34.07 ± 3.56 a | 34.63 ± 2.77 c | 46.60 ± 2.00 b | 70.20 ± 1.35 a | 59.33 | 55.57 | 3.75 |
| Film NanoProp | 24.83 ± 1.59 b | 42.10 ± 2.52 b | 48.33 ± 1.71 b | 62.33 ± 0.83 b | 71.49 | 69.75 | 1.74 |
| Film | Color values | Transmittance (%) | Opacity | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | ∆E | 200 nm | 500 nm | ||
| Film Control | 88.44 ± 0.083 c | 1.31 ± 0.13 a | −4.98 ± 0.015 a | 8.34 ± 0.078 a | 0.1 ± 0.01 a | 15.2 ± 0.1 a | 0.45 ± 0.02 a |
| Film NanoCur | 89.86 ± 0.12 a | −8.79 ± 0.045 b | 56.62 ± 6.01 b | 25.22 ± 1.15 b | 0.1 ± 0.02 a | 6.0 ± 0.01 b | 1.16 ± 0.03 b |
| Film NanoProp | 88.95 ± 0.090 b | −1.28 ± 0.15 c | 13.09 ± 1.53 c | 11.45 ± 0.78 c | 0.1 ± 0.02 a | 7.5 ± 0.02 c | 1.02 ± 0.02 c |
| Sample | FRAP (mg TEAC mL−1) | ABTS (mg TEAC mL−1) | DPPH (% Inhibition) | TPC (mg GAE mL−1) |
|---|---|---|---|---|
| Film NanoCur | 110.86 ± 4.59 c | 15.34 ± 0.49 c | 5.81 ± 1.70 c | 0.078 ± 0.0024 b |
| Film NanoProp | 197.33 ± 8.64 a | 14.70 ± 0.23 c | 8.20 ± 1.71 bc | 0.063 ± 0.0053 b |
| NanoCur | 162.85 ± 5.94 b | 16.77 ± 0.083 b | 11.24 ± 2.52 b | 0.40 ± 0.084 a |
| NanoProp | 194.14 ± 5.61 a | 17.88 ± 0.022 a | 23.93 ± 3.92 a | 0.37 ± 0.11 a |
| Sample | Staphylococcus aureus (106 CFU Ml−1) | Escherichia coli (106 CFU mL−1) |
|---|---|---|
| Film Control | 29.2 ± 9.6 a | 45.3 ± 6.6 a |
| Film NanoCur | 9.01 ± 0.65 b | 9.21 ± 0.53 b |
| Film NanoProp | 9.31 ± 0.3 b | 9.06 ± 0.086 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, M.R.; da Rosa, C.G.; Salvador, G.; Teixeira, S.C.d.O.; da Costa, M.C.M.; Almeida, A.d.R.; dos Santos, V.V.; Siegloch, A.E.; Zinger, F.D.; Suave, J.; et al. Methylcellulose Bionanocomposite Films Incorporated with Zein Nanoparticles Containing Propolis and Curcumin for Functional Packaging. Polysaccharides 2025, 6, 91. https://doi.org/10.3390/polysaccharides6040091
Nunes MR, da Rosa CG, Salvador G, Teixeira SCdO, da Costa MCM, Almeida AdR, dos Santos VV, Siegloch AE, Zinger FD, Suave J, et al. Methylcellulose Bionanocomposite Films Incorporated with Zein Nanoparticles Containing Propolis and Curcumin for Functional Packaging. Polysaccharides. 2025; 6(4):91. https://doi.org/10.3390/polysaccharides6040091
Chicago/Turabian StyleNunes, Michael Ramos, Cleonice Gonçalves da Rosa, Gabriel Salvador, Sarah Cardoso de Oliveira Teixeira, Maria Clara Marinho da Costa, Aline da Rosa Almeida, Vanessa Valgas dos Santos, Ana Emília Siegloch, Fernando Domingo Zinger, Jaqueline Suave, and et al. 2025. "Methylcellulose Bionanocomposite Films Incorporated with Zein Nanoparticles Containing Propolis and Curcumin for Functional Packaging" Polysaccharides 6, no. 4: 91. https://doi.org/10.3390/polysaccharides6040091
APA StyleNunes, M. R., da Rosa, C. G., Salvador, G., Teixeira, S. C. d. O., da Costa, M. C. M., Almeida, A. d. R., dos Santos, V. V., Siegloch, A. E., Zinger, F. D., Suave, J., & Hotza, D. (2025). Methylcellulose Bionanocomposite Films Incorporated with Zein Nanoparticles Containing Propolis and Curcumin for Functional Packaging. Polysaccharides, 6(4), 91. https://doi.org/10.3390/polysaccharides6040091





