Adsorption of Pharmaceutical Compounds from Water on Chitosan/Glutaraldehyde Hydrogels: Theoretical and Experimental Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Section
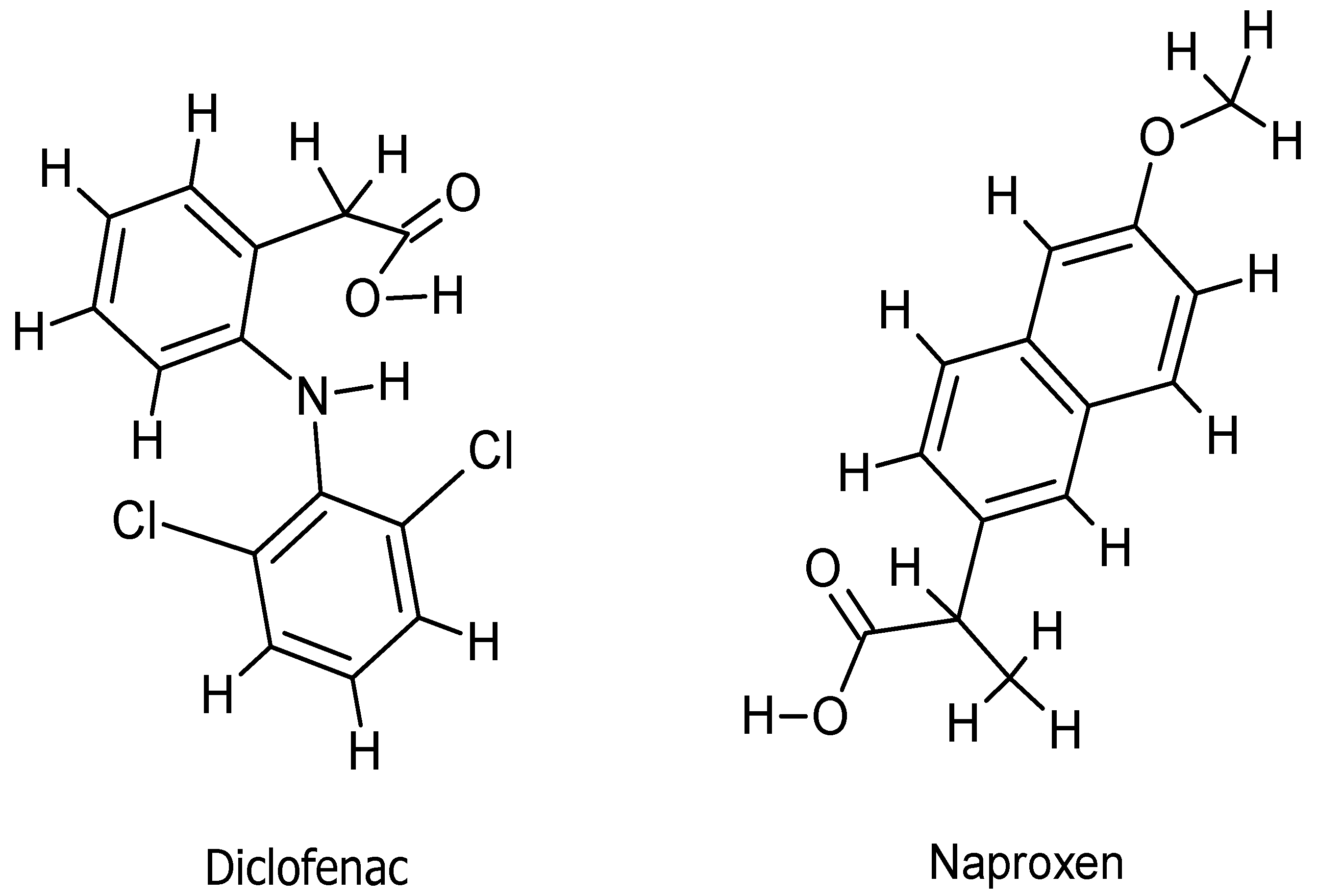
2.1.1. Geometry Optimization
2.1.2. Determination of QSAR and Thermodynamic Properties
2.1.3. Electrostatic Potential Map (EPM)
2.1.4. Adsorption Process
2.2. Experimental Studies
2.2.1. Reagents
2.2.2. Hydrogel Synthesis
2.3. Characterization of Hydrogel
2.3.1. FTIR
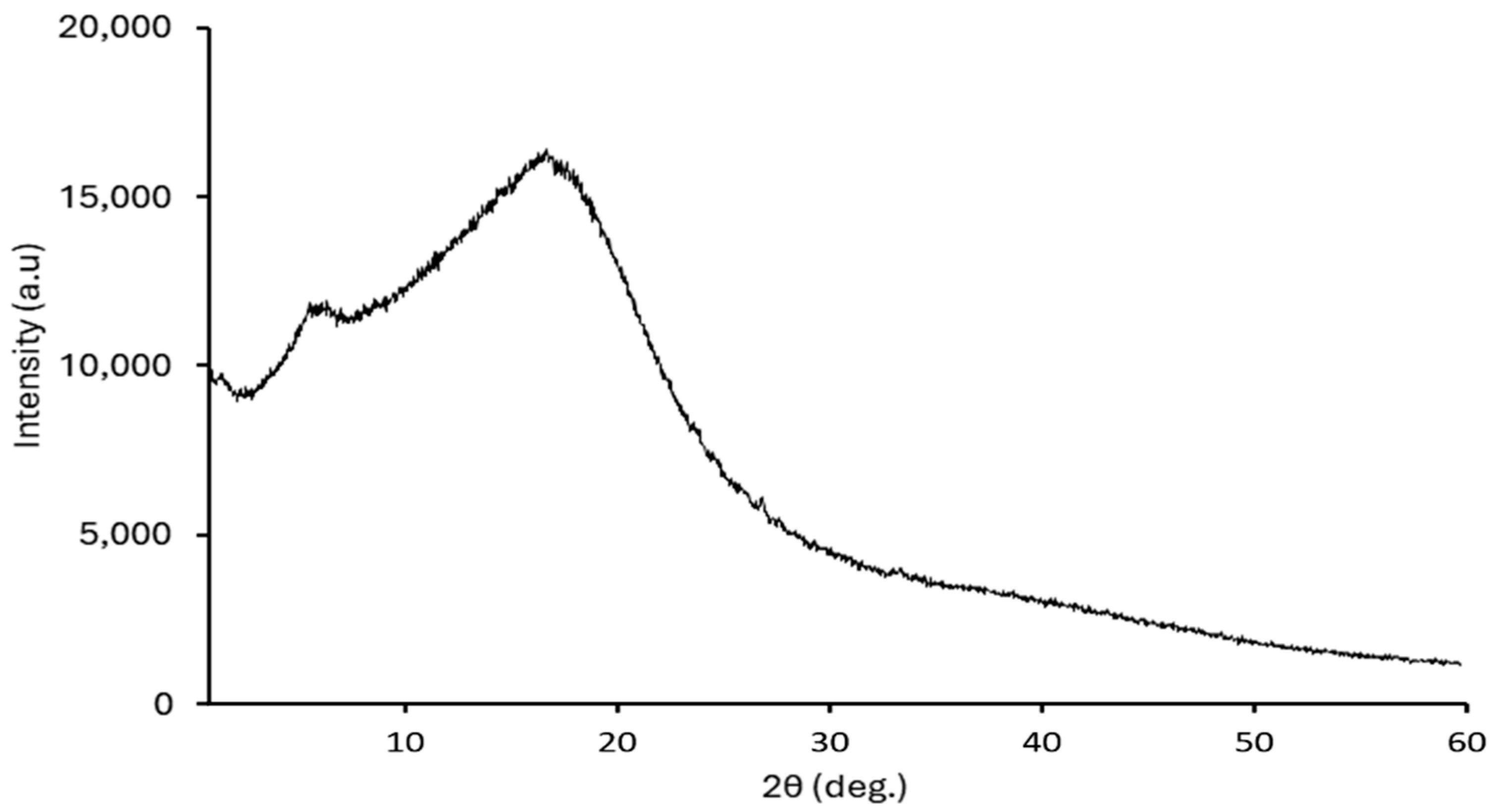
2.3.2. XRD
2.4. Adsorption Studies of Pharmaceuticals
3. Results
3.1. Theoretical Studies
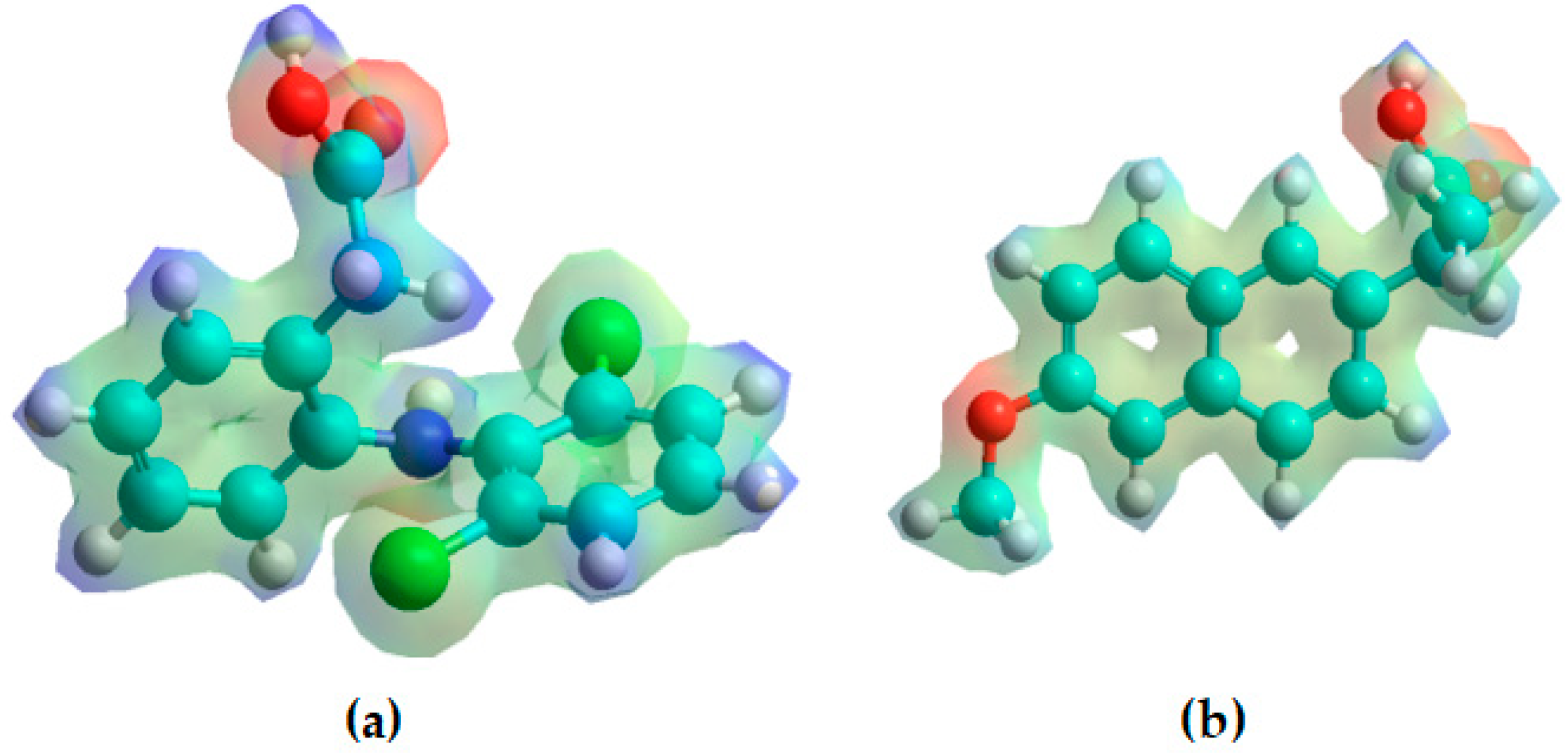
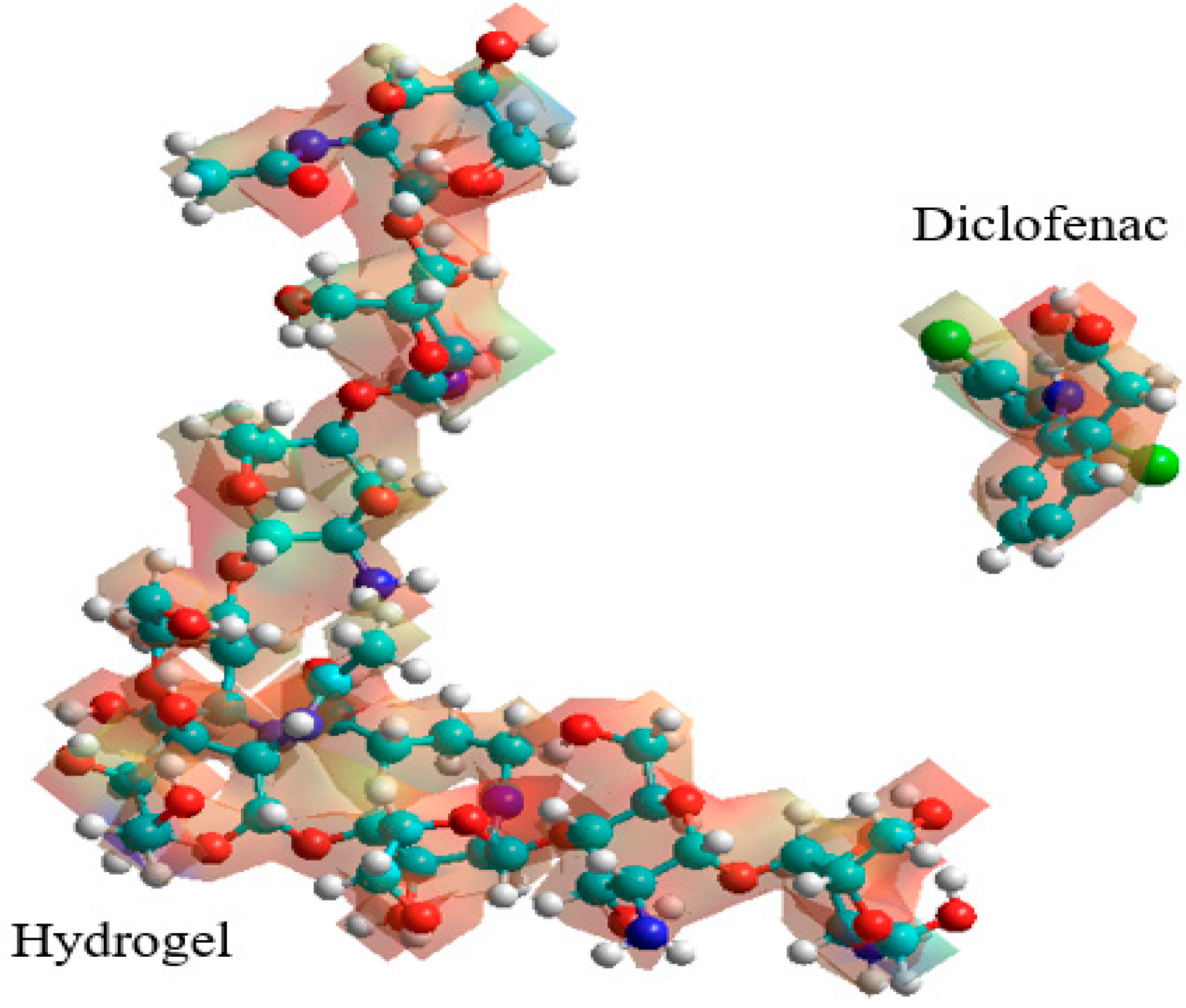
3.2. Monte Carlo Simulation at 308.15 K
3.2.1. Hydrogel–Diclofenac
3.2.2. Hydrogel–Naproxen
3.3. Experimental Analysis
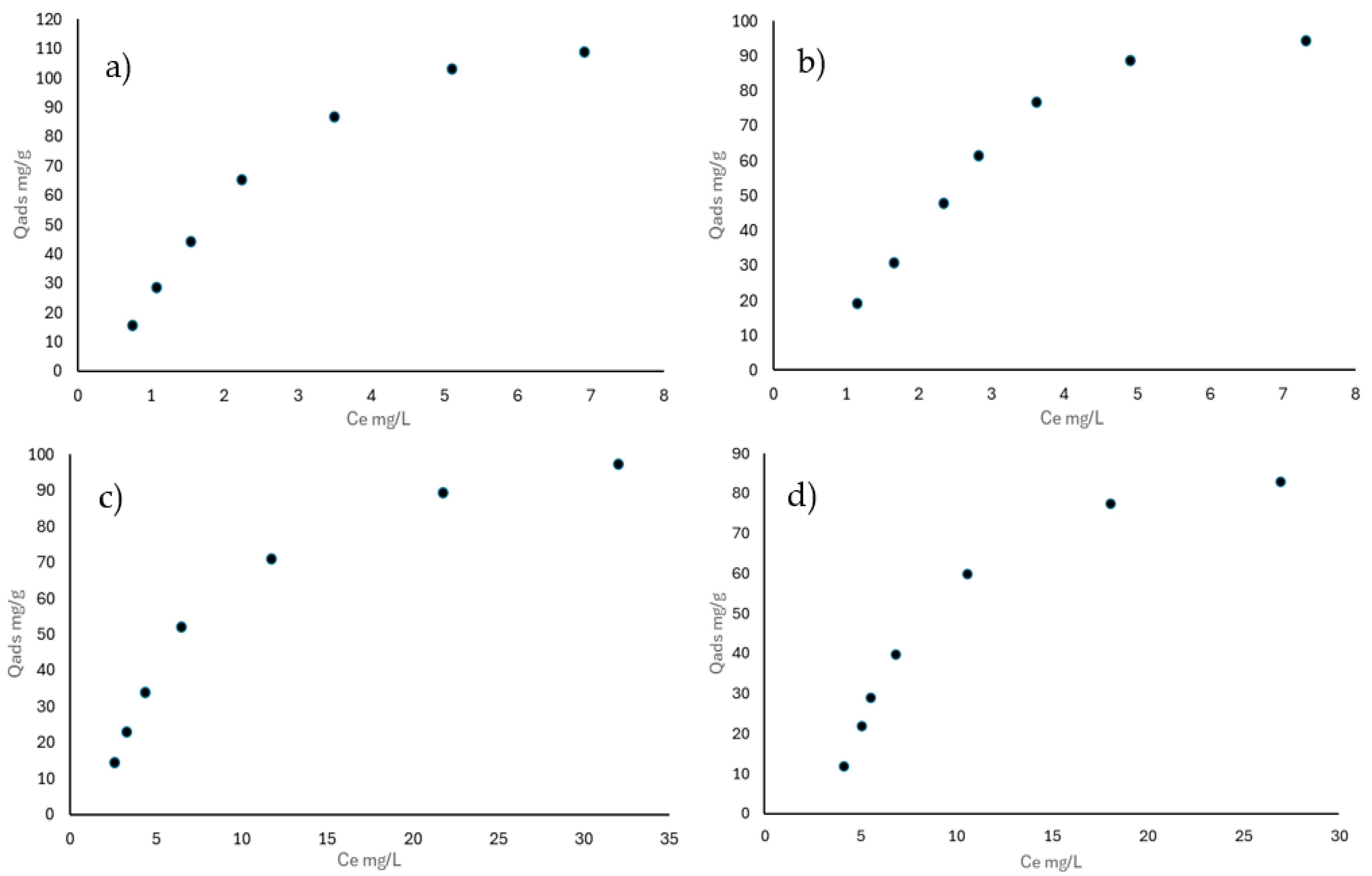
3.3.1. Adsorption Isotherms in a Single System
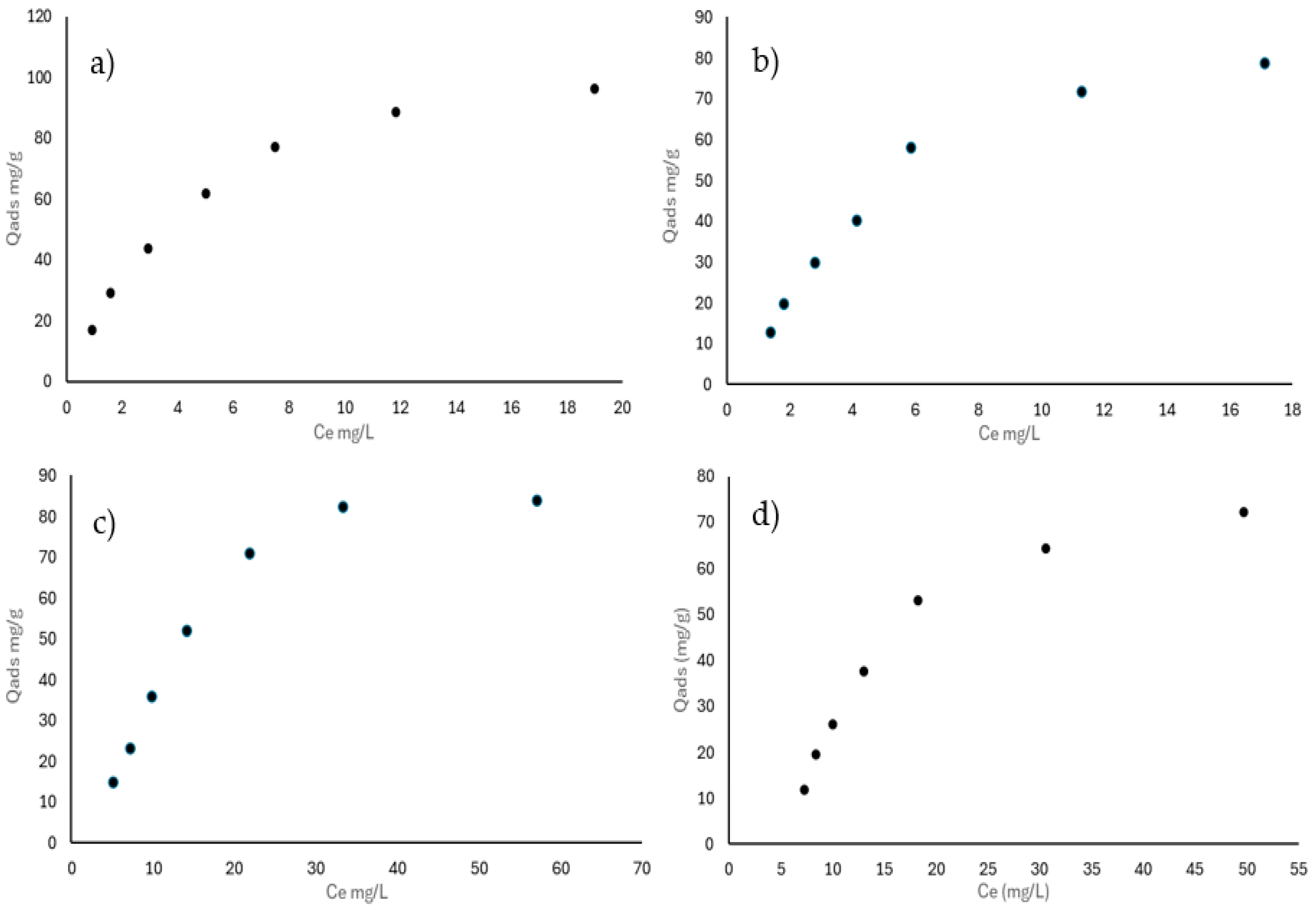
3.3.2. Adsorption Isotherms of Naproxen and Diclofenac in a Binary System
3.3.3. FTIR After Adsorption Experiments
3.3.4. XRD After Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, L.; Yang, H.; Xu, X. Effects of water pollution on human health and disease heterogeneity: A Review. Front. Environ. Sci. 2022, 10, 880246. [Google Scholar] [CrossRef]
- De Souza, S.A.; de Souza, A. Invisible pollutants: Environmental, economic and social impacts as threats to water quality. Rev. Juríd. 2019, 16, 95–107. [Google Scholar] [CrossRef]
- Ariho, A.; Aja, L.; Muhammad, T.; Mohammad, L. Water pollution: Causes, impacts and current efforts to address the issues of water pollution along river Meizimera-kihihi, Kanugu District, Uganda. F1000Reserch 2024, 13, 1298. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Rachna, S.A. Water purification by using Adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of adsorption process for effective removal of emerging contaminants from water and wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Ghosh, S.; Falyouna, O.; Malloum, A.; Othmani, A.; Bedair, H.; Onyeaka, H.; Al-sharify, Z.T.; Oluwaseun, A.; Miri, T.; Osagie, C.; et al. A general review on the use of advance oxidation and adsorption processes for the removal of furfural from industrial effluents. Micropor. Mesopor. Mater. 2022, 331, 2021. [Google Scholar] [CrossRef]
- Far, F.; Omrani, M.; Jamal, M.N.; Javanshir, S. Multi-responsive chitosan-based hydrogels for controlled release of vincristine. Commun. Chem. 2023, 6, 28. [Google Scholar]
- Hidaka, M.; Kojima, M.; Sakai, S.; Delattre, C. Characterization of chitosan hydrogels obtained through phenol and tripolyphosphate anionic crosslinking. Polymers 2024, 16, 1274. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, L.; Zhai, X.; Ji, S.; Ye, J.; Zhu, Z.; Teng, C.; Dong, W.; Wei, W. A multi-functional double cross-linked chitosan hydrogel with tunable mechanical and antibacterial properties for skin wound dressing. Carbohyd. Polym. 2023, 322, 121344. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and interfacial properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharmaceut. Sci. 2015, 10, 1–16. [Google Scholar]
- Ávila-Camacho, B.A.; Rangel-Vázquez, N.A. Analysis of the adsorption of Hg2+, Ni2+ and Cu2+ on chitosan hydrogels. Polimeros 2024, 34, e20240035. [Google Scholar] [CrossRef]
- Oliveira-Gonçalves, J.; Strieder, M.M.; Oliveira-Silva, L.F.; dos Reis, G.S.; Dotto, G.L. Advanced technologies in water treatment: Chitosan and its modifications as effective agents in the adsorption of contaminants. Int. J. Biol. Macromol. 2024, 270, 132307. [Google Scholar] [CrossRef]
- Grisales-Cifuentes, C.M.; Galvis, E.A.S.; Porras, J.; Flórez, E.; Torres-Palma, R.A.; Acelas, N. Kinetics, isotherms, effect of structure, and computational analysis during the removal of three representative pharmaceuticals from water by adsorption using a biochar obtained from oil palm fiber. Bioresour. Technol. 2021, 326, 124753. [Google Scholar] [CrossRef] [PubMed]
- Can, C.; Jianlong, W. Correlating metal ionic characteristics with biosorption capacity using QSAR model. Chemosphere 2007, 69, 1610–1616. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Arianita, A.; Amalia, B.; Pudjiastuti, W.; Melanie, S.; Fauzia, V.; Imawan, C. Effect of glutaraldehyde to the mechanical properties of chitosan/nanocellulose. J. Phys. Conf. Ser. 2019, 1317, 012045. [Google Scholar] [CrossRef]
- Kümmerer, K. The presence of pharmaceuticals in the environment due to human use–present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Boxall, A.B. Pharmaceuticals in the environment and human health. In Health Care Environ Contamination, 1st ed.; Boxall, A.B.A., Kookana, R.S., Eds.; Elsevier: London, UK, 2018; Volume 1, pp. 123–136. [Google Scholar]
- Phillips, P.; Chalmers, A. Wastewater effluent, combined sewer overflows, and other sources of organic compounds to Lake Champlain 1. J. Am. Water Resour. Assoc. 2009, 45, 45–57. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; Von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Bavumiragira, J.P.; Yin, H. Fate and transport of pharmaceuticals in water systems: A processes review. Sci. Total Environ. 2022, 823, 153635. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Yu, T.H.; Lin, C.F. Pharmaceutical contamination in residential, industrial, and agricultural waste streams: Risk to aqueous environments in Taiwan. Chemosphere 2008, 74, 131–141. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Spongberg, A.L.; Witter, J.D. Pharmaceutical compounds in the wastewater process stream in northwest Ohio. Sci. Total Environ. 2008, 397, 148–157. [Google Scholar] [CrossRef]
- Yoon, Y.; Ryu, J.; Oh, J.; Choi, B.G.; Snyder, S.A. Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han River (Seoul, South Korea). Sci. Total Environ. 2010, 408, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Tyumina, E.; Subbotina, M.; Polygalov, M.; Tyan, S.; Ivshina, I. Ketoprofen as an emerging contaminant: Occurrence, ecotoxicity and (bio) removal. Front. Microbiol. 2023, 14, 1200108. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.J.S.; Barbosa, S.C.; Primel, E.G. Emerging contaminants in Brazilian aquatic environment: Identifying targets of potential concern based on occurrence and ecological risk. Environ. Sci. Pollut. Res. Int. 2021, 28, 67528–67543. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- Hofman-Caris, C.H.M.; Siegers, W.G.; van de Merlen, K.; De Man, A.W.A.; Hofman, J.A.M.H. Removal of pharmaceuticals from WWTP effluent: Removal of EfOM followed by advanced oxidation. Chem. Eng. J. 2017, 327, 514–521. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Bastami, T.R.; Ahmadpour, A.; Hekmatikar, F.A. Synthesis of Fe3O4/Bi2WO6 nanohybrid for the photocatalytic degradation of pharmaceutical ibuprofen under solar light. J. Ind. Eng. Chem. 2017, 51, 244–254. [Google Scholar] [CrossRef]
- Beni, F.A.; Gholami, A.; Ayati, A.; Shahrak, M.N.; Sillanpää, M. UV-switchable phosphotungstic acid sandwiched between ZIF-8 and Au nanoparticles to improve simultaneous adsorption and UV light photocatalysis toward tetracycline degradation. Micropor. Mesopor. Mater. 2020, 303, 110275. [Google Scholar] [CrossRef]
- Gomes, J.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Application of ozonation for pharmaceuticals and personal care products removal from water. Sci. Total Environ. 2017, 586, 265–283. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Fu, J.; Lazaridis, N.K.; Bikiaris, D.N.; Matis, K.A. New approaches on the removal of pharmaceuticals from wastewaters with adsorbent materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Ayati, A.; Ranjbari, S.; Tanhaei, B.; Sillanpää, M. Ionic liquid-modified composites for the adsorptive removal of emerging water contaminants: A review. J. Mol. Liq. 2019, 275, 71–83. [Google Scholar] [CrossRef]
- Ranjbari, S.; Tanhaei, B.; Ayati, A.; Sillanpää, M. Novel Aliquat-336 impregnated chitosan beads for the adsorptive removal of anionic azo dyes. Int. J. Biol. Macromol. 2019, 125, 989–998. [Google Scholar] [CrossRef]
- Arya, V.; Philip, L. Adsorption of pharmaceuticals in water using Fe3O4 coated polymer clay composite. Micropor. Mesopor. Mater. 2016, 232, 273–280. [Google Scholar]
- Ravi, S.; Choi, Y.; Choe, J.K. Novel phenyl-phosphate-based porous organic polymers for removal of pharmaceutical contaminants in water. Chem. Eng. J. 2020, 379, 122290. [Google Scholar]
- Abdolmaleki, A.Y.; Zilouei, H.; Khorasani, S.N.; Zargoosh, K. Adsorption of tetracycline from water using glutaraldehyde-crosslinked electrospun nanofibers of chitosan/poly (vinyl alcohol). Water Sci. Technol. 2018, 77, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, R.; Li, M.; Yu, F.; He, C. Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohyd. Polym. 2019, 220, 141–148. [Google Scholar]
- Mojiri, A.; Andasht Kazeroon, R.; Gholami, A. Cross-linked magnetic chitosan/activated biochar for removal of emerging micropollutants from water: Optimization by the artificial neural network. Water 2019, 11, 551. [Google Scholar]
- Pereira, A.K.D.S.; Reis, D.T.; Barbosa, K.M.; Scheidt, G.N.; da Costa, L.S.; Santos, L.S.S. Antibacterial effects and ibuprofen release potential using chitosan microspheres loaded with silver nanoparticles. Carbohyd. Res. 2020, 488, 107891. [Google Scholar]
- Li, J.; Zhao, T.; Yang, Q.; Du, S.; Xu, L. A review of quantitative structure-activity relationship: The development and current status of data sets, molecular descriptors and mathematical models. Chemometr. Intell. Lab. 2025, 256, 105278. [Google Scholar]
- Uzzaman, M.; Hasan, M.K.; Mahmud, S.; Yousuf, A.; Islam, S.; Uddin, M.N.; Barua, A. Physicochemical, spectral, molecular docking and ADMET studies of bisphenol analogues: A computational approach. Inform. Med. Unlocked 2021, 25, 100706. [Google Scholar]
- Alberti, G.; Amendola, V.; Pesavento, M.; Biesuz, R. Beyond the synthesis of novel solid phases: Review on modelling of sorption phenomena. Coordin. Chem. Rev. 2012, 256, 28–45. [Google Scholar]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Quintero-Álvarez, F.G.; Rojas-Mayorga, C.K.; Mendoza-Castillo, D.I.; Aguayo-Villarreal, I.A.; Bonilla-Petriciolet, A. Physicochemical modeling of the adsorption of pharmaceuticals on mil-100-Fe and mil-101-Fe MOFs. Adsorpt. Sci. Technol. 2022, 2022, 4482263. [Google Scholar]
- Varma, R.; Vasudevan, S.; Chelladurai, S.; Narayanasamy, A. Synthesis and physicochemical characteristics of chitosan extracted from Pinna deltoides. Lett. Appl. NanoBioSci. 2021, 11, 4061–4070. [Google Scholar]
- Medeiros, R.S.; Ferreira, A.P.; Venâncio, T.; Cavalheiro, É.T. Preparation, characterization and study of the dissociation of naproxen from its chitosan salt. Molecules 2022, 27, 5801. [Google Scholar] [CrossRef] [PubMed]






| Pharmaceutical | Water Source | Concentration (ng/L) | Reference |
|---|---|---|---|
| Ampicillin | Industrial effluents | 5.8 × 103 | [25] |
| Caffeine | Urban effluents | 23–776 | [26] |
| Surface water | 2.9–194 | [26] | |
| WWTP influents | 2448–4865 | [27] | |
| Diclofenac | Urban effluents | 8.8–127 | [26] |
| Surface water | 1.1–6.8 | [26] | |
| WWTP influents | 9–13 | [27] | |
| River | 21–98 | [28] | |
| Ibuprofen | Urban effluents | 10–137 | [26] |
| Surface water | 11–38 | [26] | |
| River | 35–270 | [28] | |
| Ketoprofen | Hospital effluents | 1034.5 | [29] |
| WWTP influents | 24–177 | [29] | |
| River | 17–620 | [30] | |
| Naproxen | Urban effluents | 20–483 | [26] |
| Surface water | 1.8–18 | [26] | |
| River | 81–360 | [28] | |
| Salicylic acid | WWTP influents | 433–8036 | [27] |
| Tetracycline | Industrial effluents | 1.5 × 103 | [25] |
| Energetic Properties | Diclofenac | Naproxen | Hydrogel–Diclofenac | Hydrogel–Naproxen |
| Gibbs free energy (kcal/mol) | −81,614 | −67,954 | −586,105 | −572,472 |
| Enthalpy of formation (kcal/mol) | −48 | −100 | 1683 | 1721 |
| Dipolar moment (Debyes) | 1.79 | 2.21 | 9.72 | 16.38 |
| QSAR Properties | Diclofenac | Naproxen | Hydrogel–Diclofenac | Hydrogel–Naproxen |
| Volume (Å3) | 768 | 710 | 4018 | 3969 |
| Surface area (Å2) | 460 | 438 | 2027 | 2006 |
| Log P | −0.21 | −0.56 | −14.34 | −12.86 |
| Polarizability (Å3) | 29.69 | 25.32 | 160.68 | 156.31 |
| Energetic Properties | 298.15 K | 308.15 K |
| Gibbs free energy (kcal/mol) | −585,943 | −585,950 |
| Dipolar moment (Debyes) | 11.89 | 12.3 |
| QSAR Properties | 298.15 K | 308.15 K |
| 3989 | 3987 | |
| 1922 | 1928 | |
| Log P | −14.34 | −14.34 |
| Functional Group | 298.15 K | 308.15 K | Vibrational Mode |
|---|---|---|---|
| OH * | 3637, 3577, 3375 | 3634, 3535, 3382 | Stretching |
| C=O * | 2057 | 2042 | Stretching |
| C=O Δ | 1979 | 1976 | Stretching |
| C=N * | 1961 | 2042 | Stretching |
| NH2 * | 1709 | 1727 | Scissoring |
| Energetic Properties | 298.15 K | 308.15 K |
| Gibbs free energy (kcal/mol) | −572,290 | −572,251 |
| Dipolar moment (Debyes) | 16.35 | 16.02 |
| QSAR Properties | 298.15 K | 308.15 K |
| 3970 | 3978 | |
| 2002 | 2015 | |
| Log P | −12.86 | −12.86 |
| Functional Group | 298.15 K | 308.15 K | Vibrational Mode |
|---|---|---|---|
| OH * | 3489, 3418, 3292 | 3470, 3407, 3288 | Stretching |
| C=O Δ | 2077 | 2164 | Stretching |
| C=O * | 1996, 1978 | 1964 | Stretching |
| C=N * | 1971 | 2036, 1876 | Stretching |
| NH2 * | 1713 | 1731 | Scissoring |
| C-O * | 1583 | 1593 | Stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila Camacho, B.A.; Rojas Pabón, M.A.; Rangel Vázquez, N.A.; Márquez Brazón, E.A.; Reynel Ávila, H.E.; Mendoza Castillo, D.I.; Huerta, Y.A. Adsorption of Pharmaceutical Compounds from Water on Chitosan/Glutaraldehyde Hydrogels: Theoretical and Experimental Analysis. Polysaccharides 2025, 6, 90. https://doi.org/10.3390/polysaccharides6040090
Ávila Camacho BA, Rojas Pabón MA, Rangel Vázquez NA, Márquez Brazón EA, Reynel Ávila HE, Mendoza Castillo DI, Huerta YA. Adsorption of Pharmaceutical Compounds from Water on Chitosan/Glutaraldehyde Hydrogels: Theoretical and Experimental Analysis. Polysaccharides. 2025; 6(4):90. https://doi.org/10.3390/polysaccharides6040090
Chicago/Turabian StyleÁvila Camacho, Billy Alberto, Miguel Andrés Rojas Pabón, Norma Aurea Rangel Vázquez, Edgar A. Márquez Brazón, Hilda Elizabeth Reynel Ávila, Didilia Ileana Mendoza Castillo, and Yectli A. Huerta. 2025. "Adsorption of Pharmaceutical Compounds from Water on Chitosan/Glutaraldehyde Hydrogels: Theoretical and Experimental Analysis" Polysaccharides 6, no. 4: 90. https://doi.org/10.3390/polysaccharides6040090
APA StyleÁvila Camacho, B. A., Rojas Pabón, M. A., Rangel Vázquez, N. A., Márquez Brazón, E. A., Reynel Ávila, H. E., Mendoza Castillo, D. I., & Huerta, Y. A. (2025). Adsorption of Pharmaceutical Compounds from Water on Chitosan/Glutaraldehyde Hydrogels: Theoretical and Experimental Analysis. Polysaccharides, 6(4), 90. https://doi.org/10.3390/polysaccharides6040090








