Optimizing Hydrogen Storage and Fuel Cell Performance Using Carbon-Based Materials: Insights into Pressure and Surface Area Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide and Reduced Graphene Oxide
2.2. Hydrogen Adsorption–Desorption Processes
2.3. Characterization of Graphite, Graphene Oxide, and rGO
2.4. PEM Fuel Cell Application
3. Results
3.1. Hydrogen Storage Properties of Graphite
3.2. Hydrogen Storage Properties of GO
3.3. Hydrogen Storage Properties of rGO
3.4. Hydrogen Storage Potential of Graphite, GO, and rGO
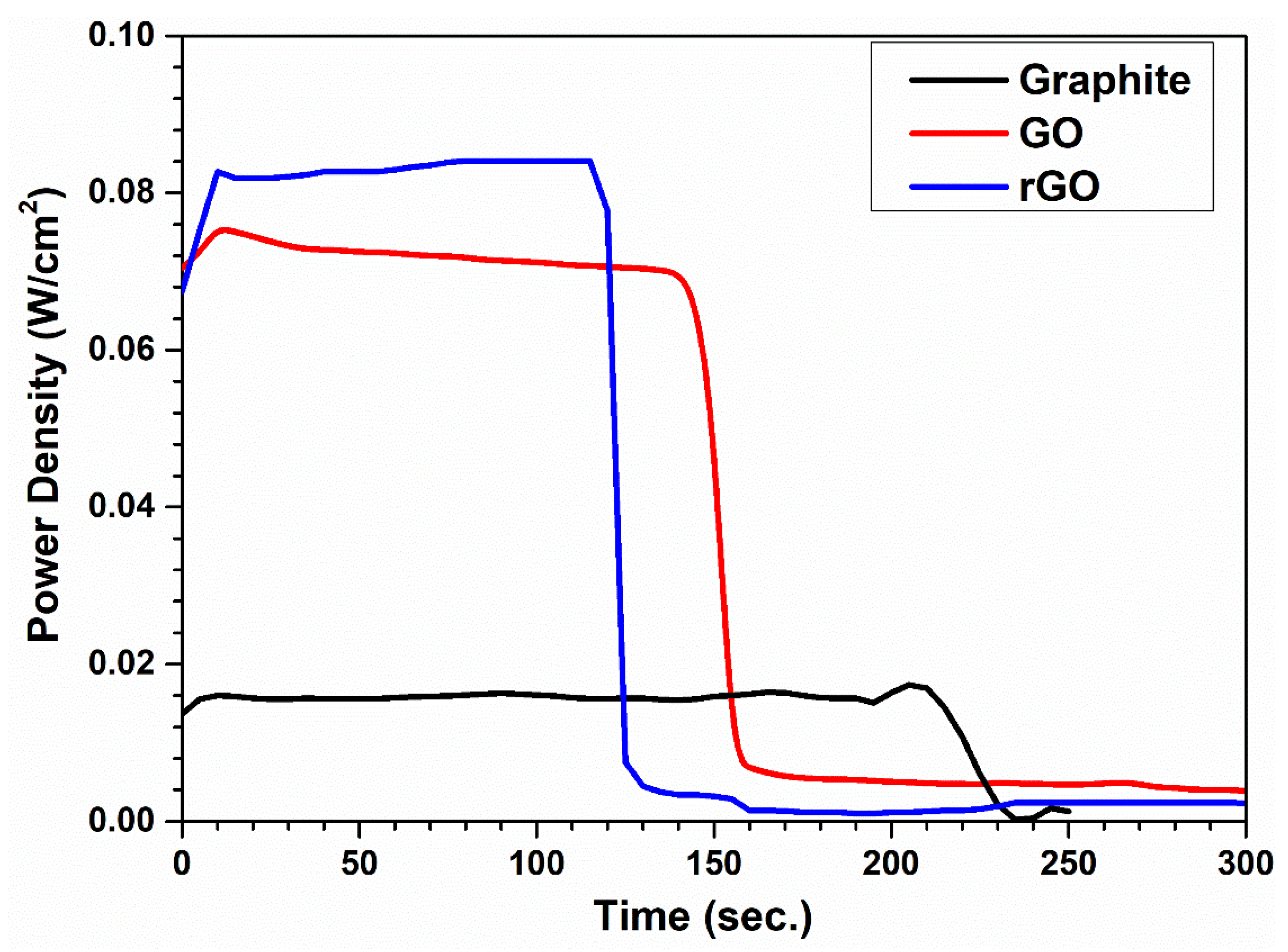
3.5. Utilization of Hydrogen-Adsorbed Graphite, GO, and rGO in PEM Fuel Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Ye, T.; Meng, X.; He, D.; Li, L.; Song, K.; Jiang, J.; Sun, C. Advances in the Application of Sulfonated Poly (Ether Ether Ketone)(SPEEK) and Its Organic Composite Membranes for Proton Exchange Membrane Fuel Cells (PEMFCs). Polymers 2024, 16, 2840. [Google Scholar] [CrossRef] [PubMed]
- Abusnina, M.M. Synthesis and Characterization of Kesterite Cu2ZnSnS4 (Czts) Thin Films for Solar Cell Application; University of Denver: Denver, CO, USA, 2016. [Google Scholar]
- Lin, H.-J.; Li, H.-W.; Shao, H.; Lu, Y.; Asano, K. In situ measurement technologies on solid-state hydrogen storage materials: A review. Mater. Today Energy 2020, 17, 100463. [Google Scholar]
- Niaz, S.; Manzoor, T.; Pandith, A.H.; Reviews, S.E. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar]
- Brinkman, L.; Bulfin, B.; Steinfeld, A. Thermochemical hydrogen storage via the reversible reduction and oxidation of metal oxides. Energy Fuels 2021, 35, 18756–18767. [Google Scholar] [CrossRef]
- So, S.H.; Ha, S.; Min, C.G.; Lee, Y.-S.; Park, C.R. Effects of nitrogen plasma treatments on hydrogen storage capacity of microporous carbon at room temperature and its feasibility as a hydrogen storage material. Carbon Lett. 2023, 33, 1027–1034. [Google Scholar]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Abbasi, G.R.; Lashari, N.; Patil, S.; Abdurrahman, M. A mini-review on underground hydrogen storage: Production to field studies. Energy Fuels 2023, 37, 8128–8141. [Google Scholar]
- Seayad, A.M.; Antonelli, D.M. Recent advances in hydrogen storage in metal-containing inorganic nanostructures and related materials. Adv. Mater. 2004, 16, 765–777. [Google Scholar]
- Zhao, P.; Zeng, X.; Wang, Y. Hydrogen absorption/desorption performance analysis and optimization on thin double-layered annular hydrogen storage bed: Heat and mass transfer. J. Energy Storage 2024, 100, 113647. [Google Scholar]
- Zubizarreta, L.; Arenillas, A.; Pis, J.J. Carbon materials for H2 storage. Int. J. Hydrog. Energy 2009, 34, 4575–4581. [Google Scholar]
- Attia, N.F.; Elashery, S.E.; Nour, M.A.; Policicchio, A.; Agostino, R.G.; Abd-Ellah, M.; Jiang, S.; Oh, H. Recent advances in sustainable and efficient hydrogen storage nanomaterials. J. Energy Storage 2024, 100, 113519. [Google Scholar] [CrossRef]
- Ströbel, R.; Garche, J.; Moseley, P.; Jörissen, L.; Wolf, G. Hydrogen storage by carbon materials. J. Power Sources 2006, 159, 781–801. [Google Scholar]
- Ao, Z.; Tan, T.; Li, S.; Jiang, Q. Molecular hydrogen storage in Al-doped bulk graphite with wider layer distances. Solid State Commun. 2009, 149, 1363–1367. [Google Scholar]
- Lueking, A.D.; Pan, L.; Narayanan, D.L.; Clifford, C.E.B. Effect of expanded graphite lattice in exfoliated graphite nanofibers on hydrogen storage. J. Phys. Chem. B 2005, 109, 12710–12717. [Google Scholar]
- Kothandam, G.; Singh, G.; Guan, X.; Lee, J.M.; Ramadass, K.; Joseph, S.; Benzigar, M.; Karakoti, A.; Yi, J.; Kumar, P.; et al. Recent advances in carbon-based electrodes for energy storage and conversion. Adv. Sci. 2023, 10, 2301045. [Google Scholar]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar]
- Pei, P.; Whitwick, M.B.; Kureshi, S.; Cannon, M.; Quan, G.; Kjeang, E. Hydrogen storage mechanism in transition metal decorated graphene oxide: The symbiotic effect of oxygen groups and high layer spacing. Int. J. Hydrog. Energy 2020, 45, 6713–6726. [Google Scholar]
- Klechikov, A.; Sun, J.; Hu, G.; Zheng, M.; Wågberg, T.; Talyzin, A.V. Graphene decorated with metal nanoparticles: Hydrogen sorption and related artefacts. Microporous Mesoporous Mater. 2017, 250, 27–34. [Google Scholar] [CrossRef]
- Bonanno, M.; Müller, K.; Bensmann, B.; Hanke-Rauschenbach, R.; Aili, D.; Franken, T.; Chromik, A.; Peach, R.; Freiberg, A.T.; Thiele, S. Review and prospects of PEM water electrolysis at elevated temperature operation. Adv. Mater. Technol. 2024, 9, 2300281. [Google Scholar] [CrossRef]
- Zan, R.; Altuntepe, A. Nitrogen doping of graphene by CVD. J. Mol. Struct. 2020, 1199, 127026. [Google Scholar]
- Srinivas, G.; Zhu, Y.; Piner, R.; Skipper, N.; Ellerby, M.; Ruoff, R. Synthesis of graphene-like nanosheets and their hydrogen adsorption capacity. Carbon 2010, 48, 630–635. [Google Scholar] [CrossRef]
- Kim, B.H.; Hong, W.G.; Yu, H.Y.; Han, Y.-K.; Lee, S.M.; Chang, S.J.; Moon, H.R.; Jun, Y.; Kim, H.J. Thermally modulated multilayered graphene oxide for hydrogen storage. Phys. Chem. Chem. Phys. 2012, 14, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Rajaura, R.S.; Srivastava, S.; Sharma, V.; Sharma, P.; Lal, C.; Singh, M.; Palsania, H.; Vijay, Y. Role of interlayer spacing and functional group on the hydrogen storage properties of graphene oxide and reduced graphene oxide. Int. J. Hydrog. Energy 2016, 41, 9454–9461. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.; Li, J.; Mei, J.; Jiang, J.; Fan, F.; Yang, W.; Zhuo, R.; Song, K. Self-Tuning Oxygen Excess Ratio Control for Proton Exchange Membrane Fuel Cells Under Dynamic Conditions. Processes 2024, 12, 2807. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Xu, C.; Khatami, Y.; Banerjee, K. Synthesis of high-quality monolayer and bilayer graphene on copper using chemical vapor deposition. Carbon 2011, 49, 4122–4130. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T.L. Probing layer number and stacking order of few-layer graphene by Raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.; Morozov, S.; Blake, P.; Halsall, M.; Ferrari, A.C.; Boukhvalov, D.; Katsnelson, M.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef]
- Albetran, H.M. Structural Characterization of Graphite Nanoplatelets Synthesized from Graphite Flakes. Preprints 2020, 100, 520 2020080325. [Google Scholar] [CrossRef]
- Monteserín, C.; Blanco, M.; Aranzabe, E.; Aranzabe, A.; Laza, J.M.; Larrañaga-Varga, A.; Vilas, J.L. Effects of graphene oxide and chemically-reduced graphene oxide on the dynamic mechanical properties of epoxy amine composites. Polymers 2017, 9, 449. [Google Scholar] [CrossRef] [PubMed]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. B 1939, 56, 978. [Google Scholar]
- Fouda, A.N.; El Shazly, M.D.; Almaqwashi, A.A. Facile and scalable green synthesis of N-doped graphene/CNTs nanocomposites via ball milling. Ain Shams Eng. J. 2021, 12, 1017–1024. [Google Scholar]
- Hoang, N.B.; Nguyen, T.T.; Nguyen, T.S.; Bui, T.P.Q.; Bach, L.G. The application of expanded graphite fabricated by microwave method to eliminate organic dyes in aqueous solution. Cogent Eng. 2019, 6, 1584939. [Google Scholar]
- Krishna, R.; Titus, E.; Okhay, O.; Gil, J.C.; Ventura, J.; Ramana, E.V.; Gracio, J.J. Rapid electrochemical synthesis of hydrogenated graphene oxide using Ni nanoparticles. Int. J. Electrochem. Sci. 2014, 9, 4054–4069. [Google Scholar]
- Rout, D.R.; Senapati, P.; Sutar, H.; Sau, D.C.; Murmu, R. Graphene oxide (GO) supported palladium (Pd) nanocomposites for enhanced hydrogenation. Graphene 2020, 8, 33–51. [Google Scholar]
- Gupta, R.; Alahmed, Z.; Yakuphanoglu, F. Graphene oxide based low cost battery. Mater. Lett. 2013, 112, 75–77. [Google Scholar]
- Ariharan, A.; Viswanathan, B.; Nandhakumar, V. Nitrogen doped graphene as potential material for hydrogen storage. Graphene 2017, 6, 41–60. [Google Scholar]
- Esmaeili, A.; Entezari, M. Facile and fast synthesis of graphene oxide nanosheets via bath ultrasonic irradiation. J. Colloid Interface Sci. 2014, 432, 19–25. [Google Scholar]
- Fatima, S.; Ali, S.I.; Younas, D.; Islam, A.; Akinwande, D.; Rizwan, S. Graphene nanohybrids for enhanced catalytic activity and large surface area. MRS Commun. 2019, 9, 27–36. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Kim, J.; Eum, J.-H.; Kang, J.; Kwon, O.; Kim, H.; Kim, D.W. Tuning the hierarchical pore structure of graphene oxide through dual thermal activation for high-performance supercapacitor. Sci. Rep. 2021, 11, 2063. [Google Scholar]
- Shen, J.; Yan, B.; Shi, M.; Ma, H.; Li, N.; Ye, M. One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 2011, 21, 3415–3421. [Google Scholar]
- Jindal, H.; Oberoi, A.S.; Sandhu, I.S.; Chitkara, M.; Singh, B. Graphene for hydrogen energy storage-A comparative study on GO and rGO employed in a modified reversible PEM fuel cell. Int. J. Energy Res. 2021, 45, 5815–5826. [Google Scholar]
- Mehta, S.S.; Nadargi, D.Y.; Tamboli, M.S.; Alshahrani, T.; Minnam Reddy, V.R.; Kim, E.S.; Mulla, I.S.; Park, C.; Suryavanshi, S.S. RGO/WO3 hierarchical architectures for improved H2S sensing and highly efficient solar-driving photo-degradation of RhB dye. Sci. Rep. 2021, 11, 5023. [Google Scholar] [CrossRef]
- Wei, L.; Mao, Y. Enhanced hydrogen storage performance of reduced graphene oxide hybrids with nickel or its metallic mixtures based on spillover mechanism. Int. J. Hydrog. Energy 2016, 41, 11692–11699. [Google Scholar] [CrossRef]
- Ganesha, H.; Veeresh, S.; Nagaraju, Y.; Vandana, M.; Basappa, M.; Vijeth, H.; Devendrappa, H. 2-Dimensional layered molybdenum disulfide nanosheets and CTAB-assisted molybdenum disulfide nanoflower for high performance supercapacitor application. Nanoscale Adv. 2022, 4, 521–531. [Google Scholar]
- Li, G.; Jing, M.; Chen, Z.; He, B.; Zhou, M.; Hou, Z. Self-assembly of porous CuO nanospheres decorated on reduced graphene oxide with enhanced lithium storage performance. RSC Adv. 2017, 7, 10376–10384. [Google Scholar]
- Georgiev, P.; Ross, D.; Albers, P.; Ramirez-Cuesta, A. The rotational and translational dynamics of molecular hydrogen physisorbed in activated carbon: A direct probe of microporosity and hydrogen storage performance. Carbon 2006, 44, 2724–2738. [Google Scholar]
- Aboutalebi, S.H.; Aminorroaya-Yamini, S.; Nevirkovets, I.; Konstantinov, K.; Liu, H.K. Enhanced hydrogen storage in graphene oxide-MWCNTs composite at room temperature. Adv. Energy Mater. 2012, 2, 1439–1446. [Google Scholar]
- Hynek, S.; Fuller, W.; Bentley, J. Hydrogen storage by carbon sorption. Int. J. Hydrog. Energy 1997, 22, 601–610. [Google Scholar] [CrossRef]
- Yadav, S.; Zhu, Z.; Singh, C.V. Defect engineering of graphene for effective hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 4981–4995. [Google Scholar] [CrossRef]
- Darkrim, F.L.; Malbrunot, P.; Tartaglia, G. Review of hydrogen storage by adsorption in carbon nanotubes. Int. J. Hydrog. Energy 2002, 27, 193–202. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Hydrogen adsorption of acid-treated multi-walled carbon nanotubes at low temperature. Bull. Korean Chem. Soc. 2010, 31, 1596–1600. [Google Scholar] [CrossRef]
- Xia, Y.; Walker, G.S.; Grant, D.M.; Mokaya, R. Hydrogen storage in high surface area carbons: Experimental demonstration of the effects of nitrogen doping. J. Am. Chem. Soc. 2009, 131, 16493–16499. [Google Scholar] [CrossRef]
- Ozturk, Z. The effect of surface area and dopant percentage on hydrogen storage of Pt@ ac loaded activated carbon and Cu-BTC composites. Int. J. Renew. Energy Res. 2016, 6, 1007–1014. [Google Scholar]
- Blackman, J.M.; Patrick, J.W.; Arenillas, A.; Shi, W.; Snape, C.E. Activation of carbon nanofibres for hydrogen storage. Carbon 2006, 44, 1376–1385. [Google Scholar] [CrossRef]
- Chan, Y.; Hill, J.M. Hydrogen storage inside graphene-oxide frameworks. Nanotechnology 2011, 22, 305403. [Google Scholar] [CrossRef]
- Kim, T.H.; Bae, J.; Lee, T.H.; Hwang, J.; Jung, J.H.; Kim, D.K.; Lee, J.S.; Kim, D.O.; Lee, Y.H.; Ihm, J. Room-temperature hydrogen storage via two-dimensional potential well in mesoporous graphene oxide. Nano Energy 2016, 27, 402–411. [Google Scholar]
- Ghosh, A.; Subrahmanyam, K.; Krishna, K.S.; Datta, S.; Govindaraj, A.; Pati, S.K.; Rao, C. Uptake of H2 and CO2 by graphene. J. Phys. Chem. C 2008, 112, 15704–15707. [Google Scholar] [CrossRef]
- Saeed, E.W.; Warkozek, E.G. Modeling and analysis of renewable PEM fuel cell system. Energy Procedia 2015, 74, 87–101. [Google Scholar]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development–a review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Chen, Z.; Shu, C.; Gan, Z.; Cao, J.; Qiu, P.; Sun, X.; Deng, C.; Wu, Y.; Tang, W. Research Progress and Perspectives on Anti-Poisoning Hydrogen Oxidation Reaction Electrocatalysts for Hydrogen Fuel Cells. Small 2025, 21, 2411049. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Huang, H.; Lawson, T.; Xia, Z.; Giusto, P.; Antonietti, M.; Jaroniec, M.; Chhowalla, M.; Baek, J.B.; Liu, Y.; et al. Recent advances on carbon-based metal-free electrocatalysts for energy and chemical conversions. Adv. Mater. 2024, 36, 2405664. [Google Scholar]


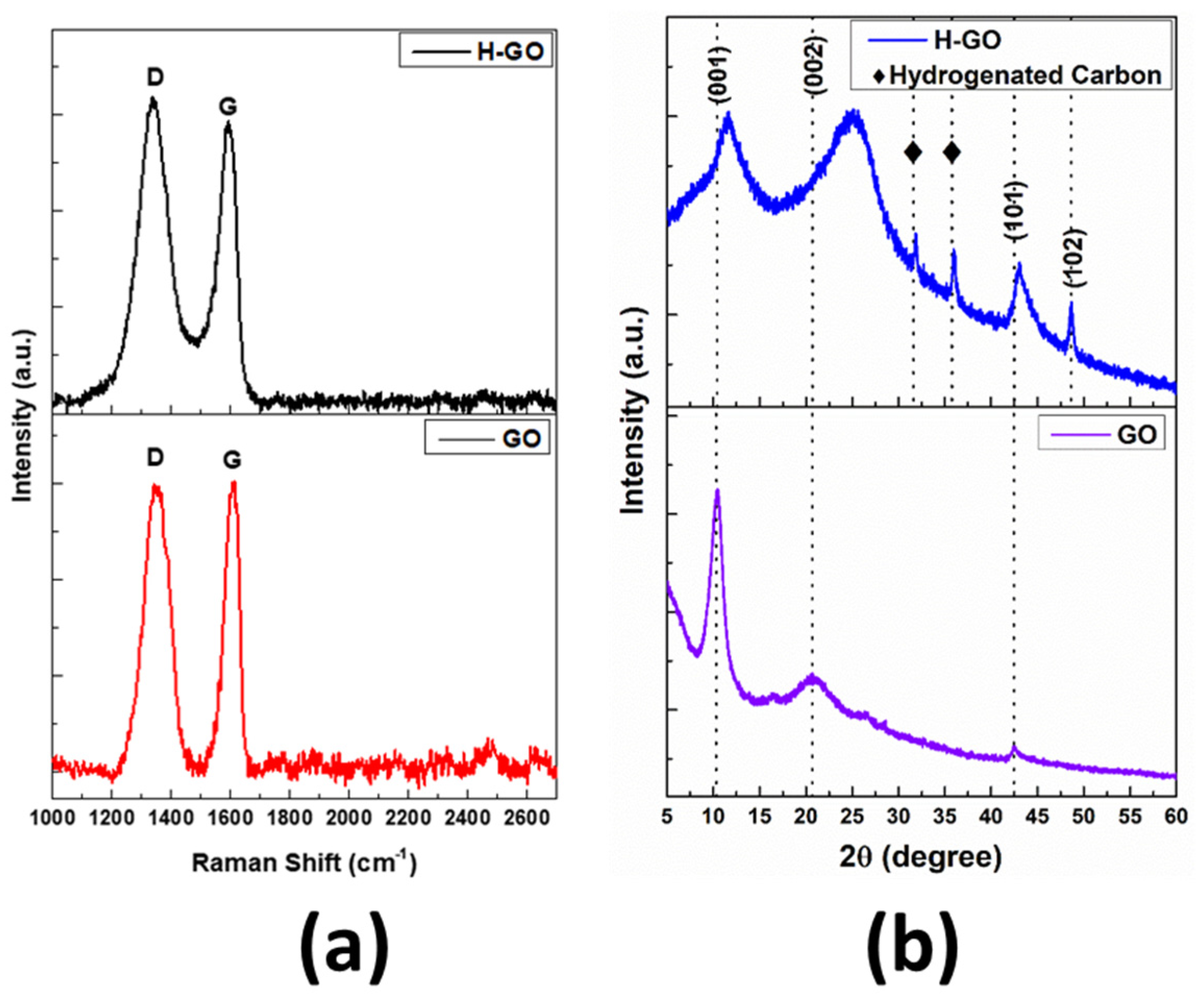
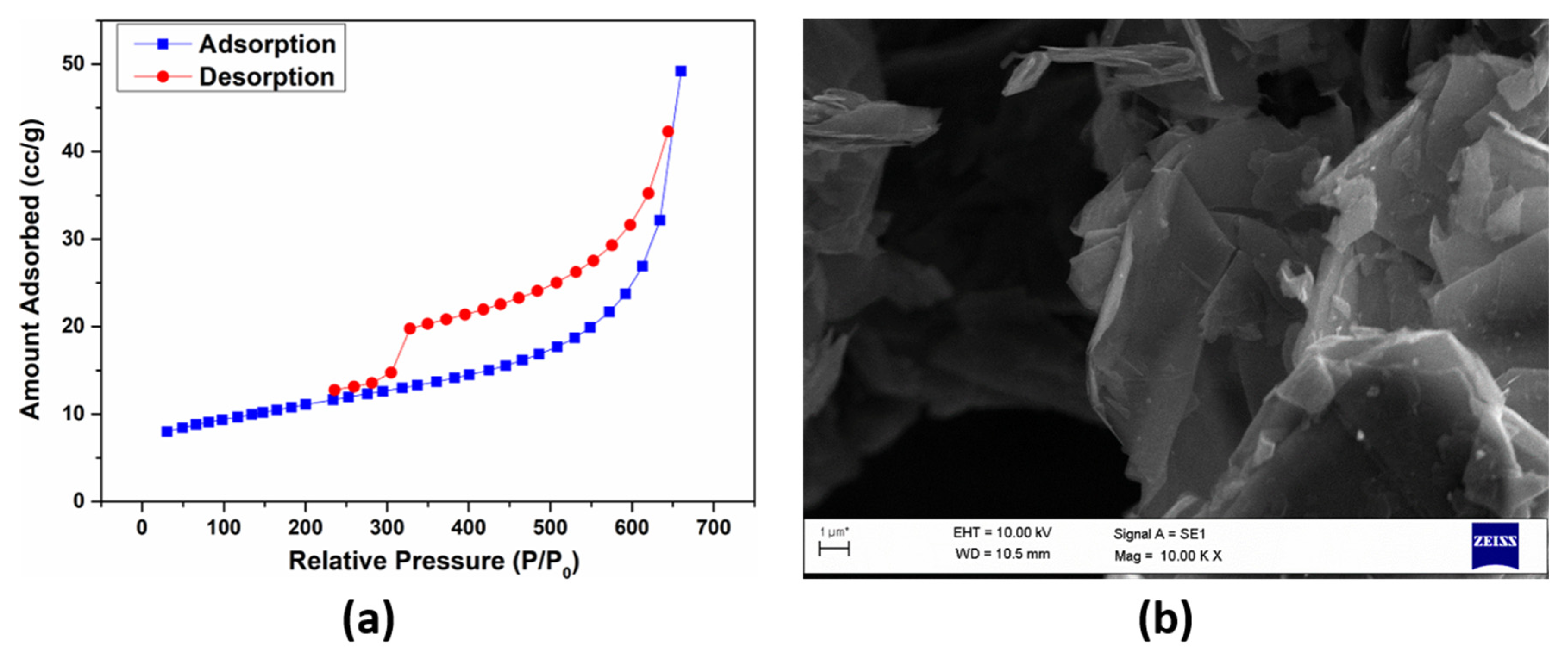

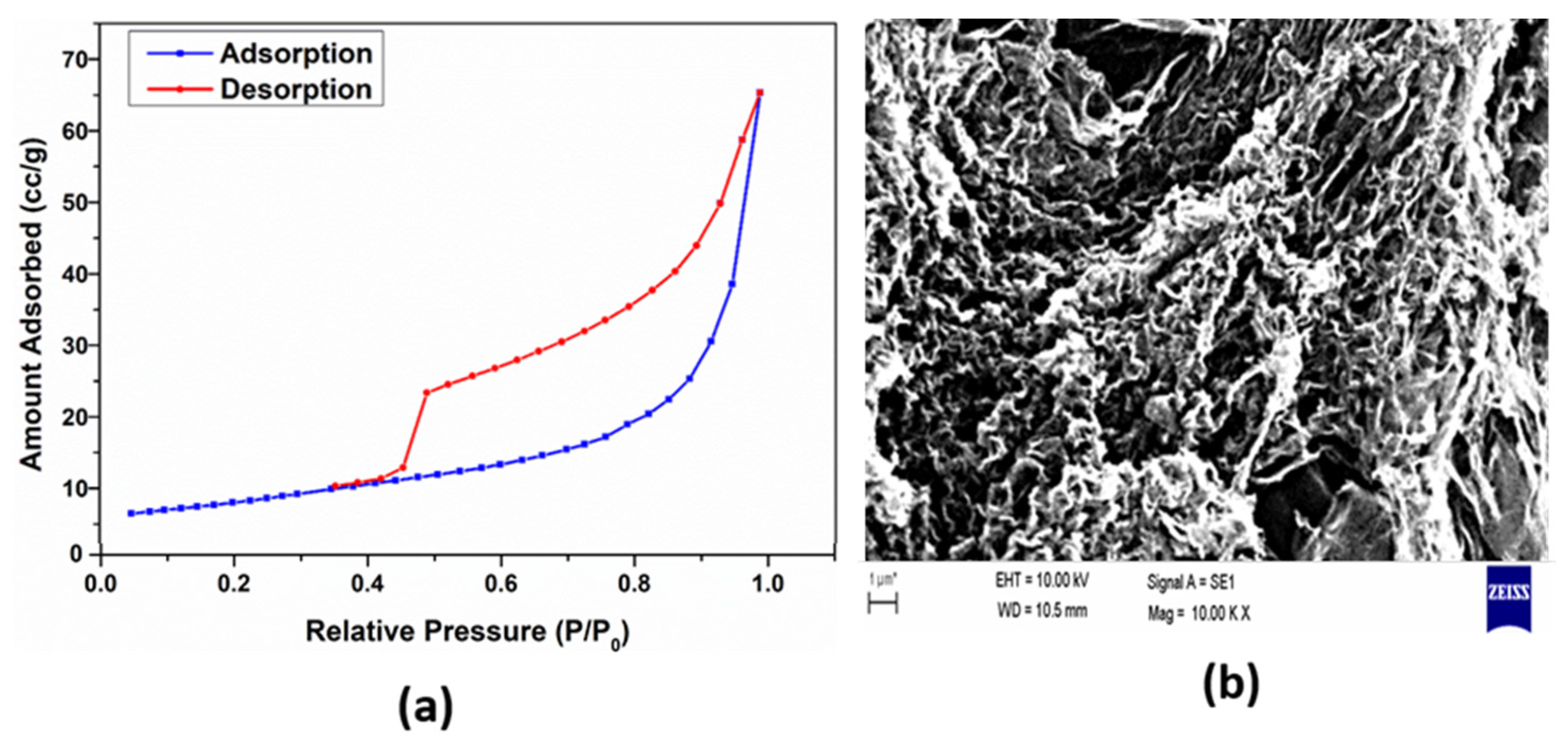

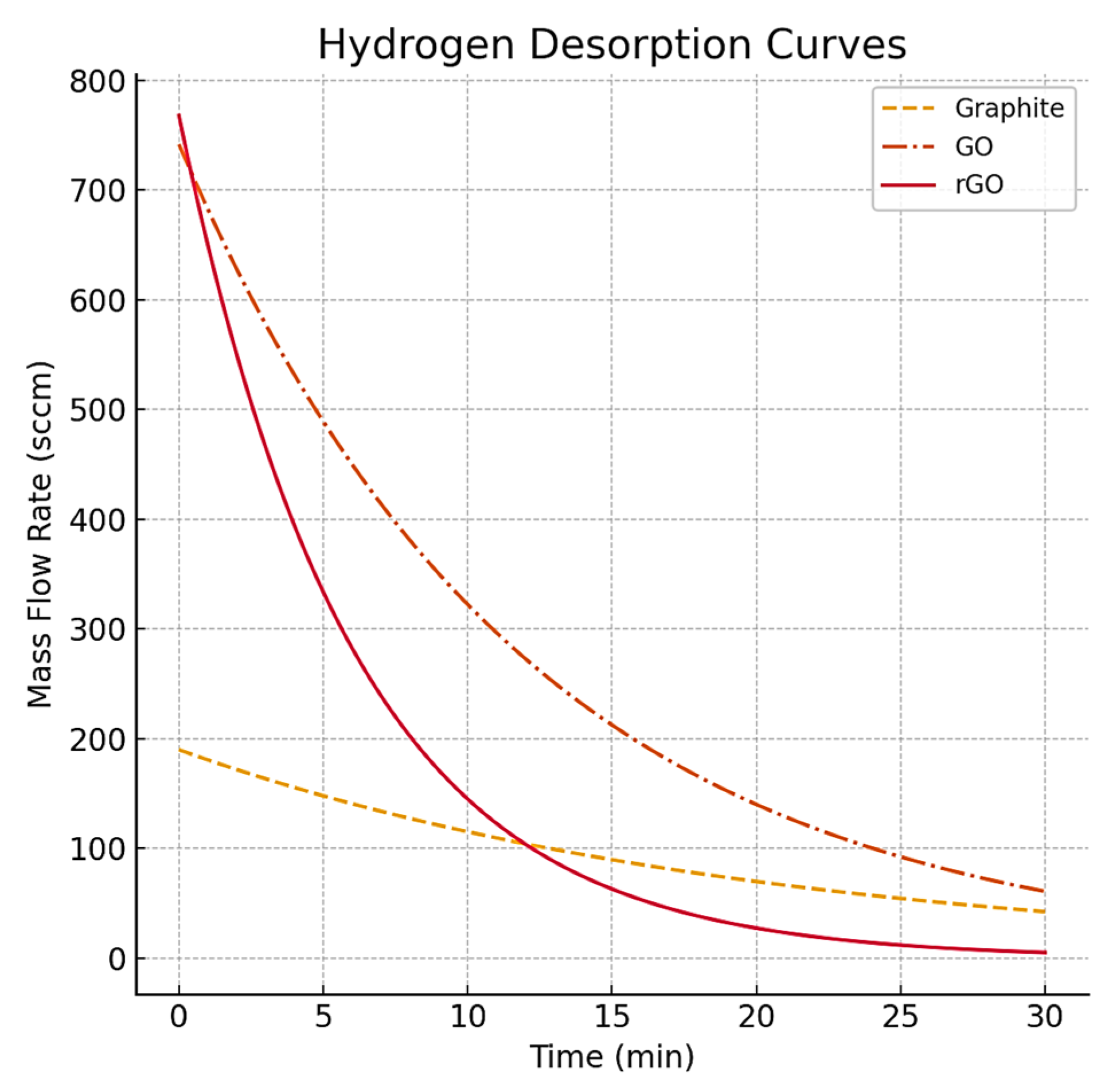
| Sample | G Band Position | 2D Band Position | D Band Position | D’ Band Position | I2D/G |
|---|---|---|---|---|---|
| Gr | 1574 cm−1 | 2660 cm−1 | 1332 cm−1 | - | 0.32 |
| H-Gr | 1571 cm−1 | 2653 cm−1 | 1329 cm−1 | 1620 cm−1 | 0.25 |
| Sample | 2θ (°) | FWHM (°) | Crystallite Size (nm) |
|---|---|---|---|
| Gr | 26.65 | 0.23 | 37.09 |
| H-Gr | 26.69 | 0.32 | 26.66 |
| Sample | 2θ (°) | FWHM (°) | Crystallite Size (nm) |
|---|---|---|---|
| GO | 10.43 | 1.32 | 6.31 |
| H-GO | 11.58 | 2.83 | 2.95 |
| Sample | 2θ (°) | FWHM (°) | Crystallite Size (nm) |
|---|---|---|---|
| rGO | 24.42 | 7.59 | 1.59 |
| H-rGO | 24.42 | 7.61 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altuntepe, A.; Çelik, S.; Zan, R. Optimizing Hydrogen Storage and Fuel Cell Performance Using Carbon-Based Materials: Insights into Pressure and Surface Area Effects. Hydrogen 2025, 6, 22. https://doi.org/10.3390/hydrogen6020022
Altuntepe A, Çelik S, Zan R. Optimizing Hydrogen Storage and Fuel Cell Performance Using Carbon-Based Materials: Insights into Pressure and Surface Area Effects. Hydrogen. 2025; 6(2):22. https://doi.org/10.3390/hydrogen6020022
Chicago/Turabian StyleAltuntepe, Ali, Selahattin Çelik, and Recep Zan. 2025. "Optimizing Hydrogen Storage and Fuel Cell Performance Using Carbon-Based Materials: Insights into Pressure and Surface Area Effects" Hydrogen 6, no. 2: 22. https://doi.org/10.3390/hydrogen6020022
APA StyleAltuntepe, A., Çelik, S., & Zan, R. (2025). Optimizing Hydrogen Storage and Fuel Cell Performance Using Carbon-Based Materials: Insights into Pressure and Surface Area Effects. Hydrogen, 6(2), 22. https://doi.org/10.3390/hydrogen6020022






