Synergistic Effect of Pd Co-Catalyst and rGO–TiO2 Hybrid Support for Enhanced Photoreforming of Oxygenates
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of GO/TiO2 Support
2.2. Metal Co-catalyst Loading
2.3. Hydrogen Production Experiments
2.4. Photocatalysts’ Characterization
3. Results and Discussion
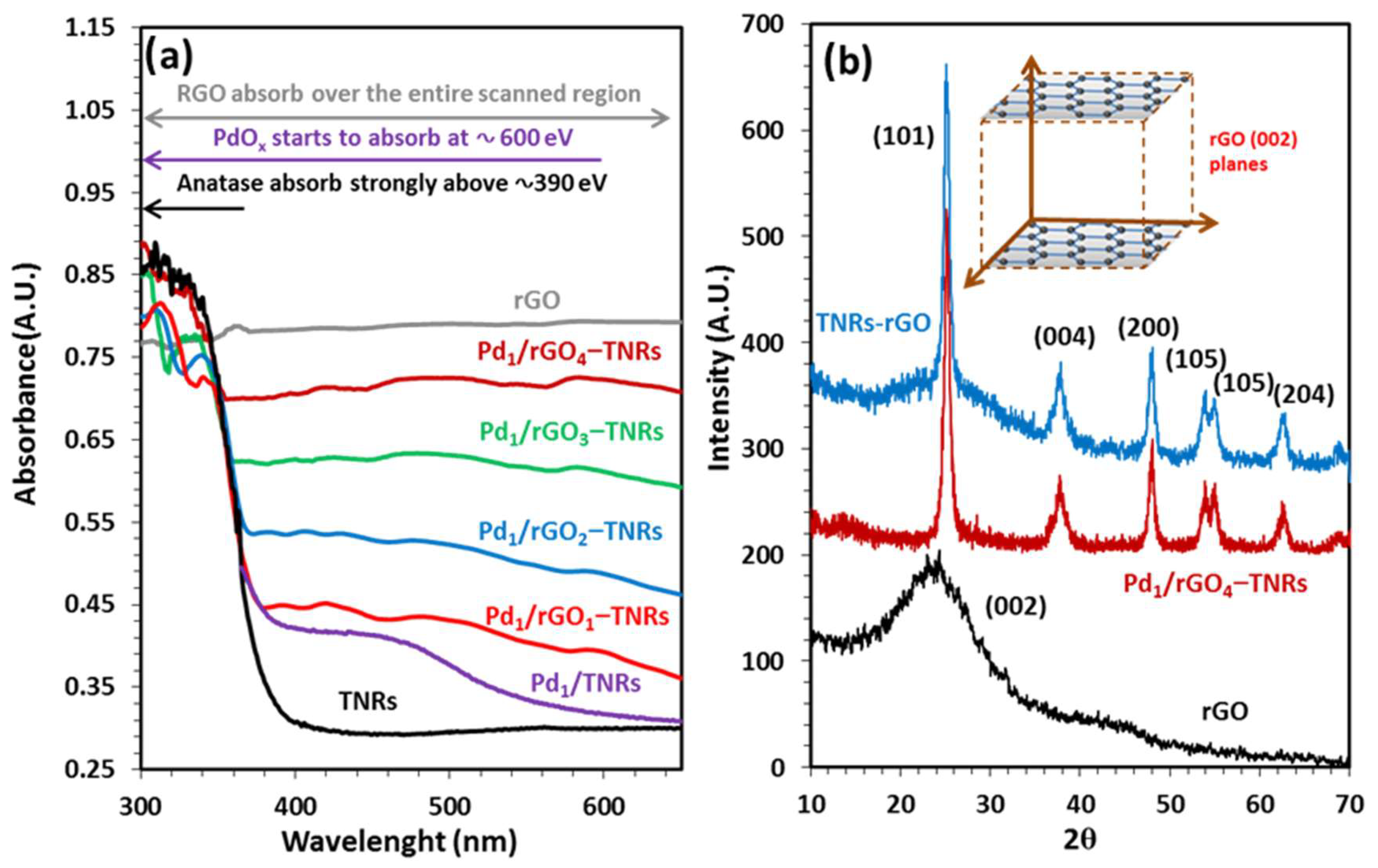
3.1. Characterization
3.2. Photoreforming Activity
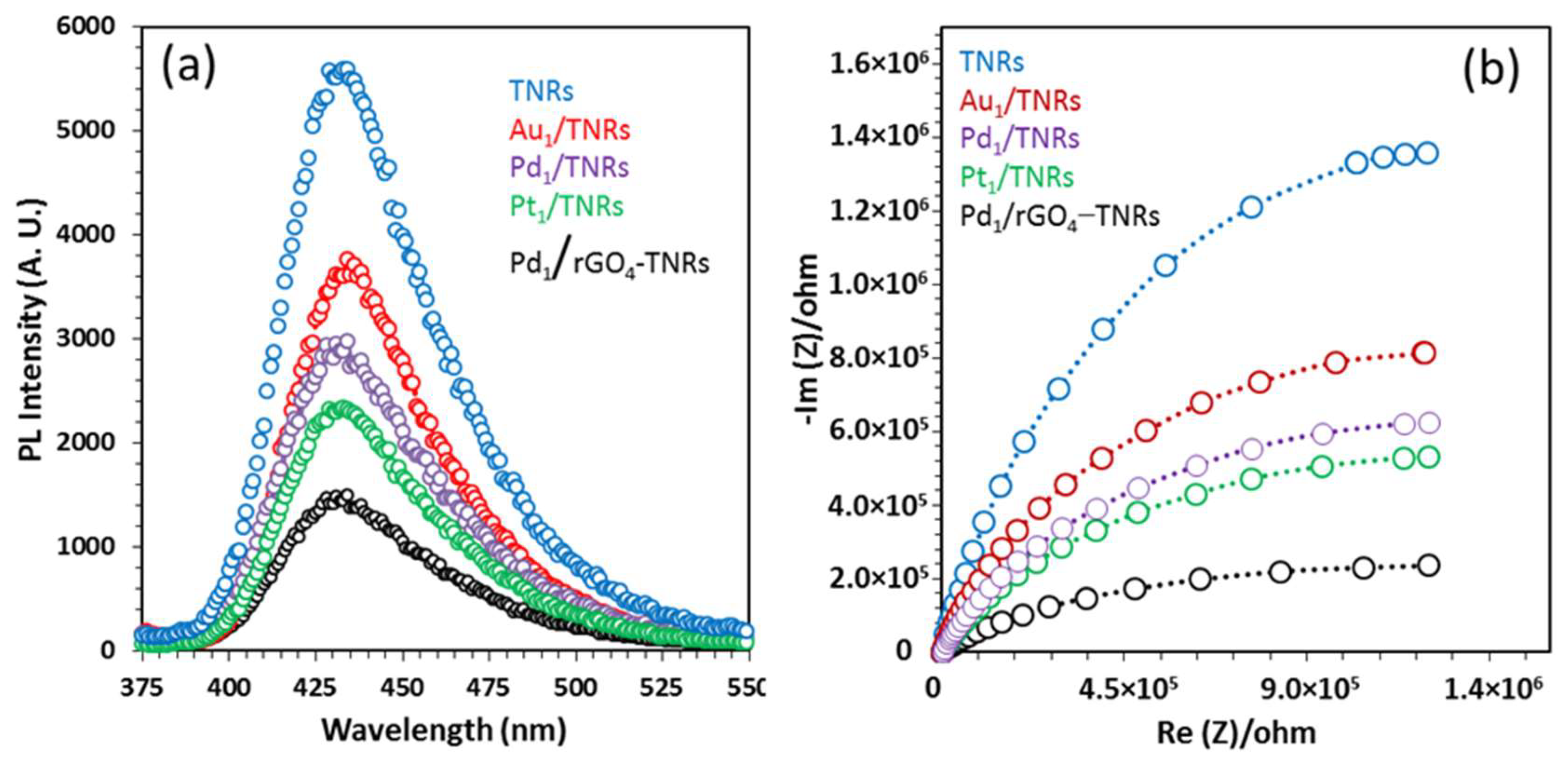
3.3. Hydrogen Production Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nadeem, M.A.; Connelly, K.A.; Idriss, H. The photoreaction of TiO2 and Au/TiO2 single crystal and powder with organic adsorbates. Int. J. Nanotechnol. 2012, 9, 121–162. [Google Scholar] [CrossRef]
- Li, L.; Hasan, I.M.U.; Farwa; He, R.; Peng, L.; Xu, N.; Niazi, N.K.; Zhang, J.-N.; Qiao, J. Copper as a single metal atom based photo-, electro-, and photoelectrochemical catalyst decorated on carbon nitride surface for efficient CO2 reduction: A review. Nano Res. Energy 2022, 1, e9120015. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Tsuji, I. Strategies for the Development of Visible-light-driven Photocatalysts for Water Splitting. Chem. Lett. 2004, 33, 1534–1539. [Google Scholar] [CrossRef]
- Maeda, K.; Kentaro, T.; Daling, L.; Tsuyoshi, T.; Nobuo, S.; Yasunobu, I.; Kazunari, D. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, A.; Nukumizu, K.; Takata, T.; Kondo, J.N.; Hara, M.; Kobayashi, H.; Domen, K. LaTiO2N as a Visible-Light (≤600 nm)-Driven Photocatalyst. J. Phys. Chem. B 2002, 107, 791–797. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Deluga, G.A.; Salge, J.R.; Schmidt, L.D.; Verykios, X.E. Renewable Hydrogen from Ethanol by Autothermal Reforming. Science 2004, 303, 993–997. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef]
- James, B.D.; Baum, G.N.; Perez, J.; Baum, K.N. Technoeconomic analysis of photoelectrochemical (PEC) hydrogen production. DOE Rep. 2009. [Google Scholar]
- Majeed, I.; Nadeem, M.A.; Hussain, E.; Waterhouse, G.I.; Badshah, A.; Iqbal, A.; Nadeem, M.A.; Idriss, H. On the synergism between Cu and Ni for photocatalytic hydrogen production and their potential as substitutes of noble metals. ChemCatChem 2016, 8, 3146–3155. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.-T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol–water mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Zhurenok, A.V.; Vasilchenko, D.B.; Kozlova, E.A. Comprehensive Review on g-C3N4-Based Photocatalysts for the Photocatalytic Hydrogen Production under Visible Light. Int. J. Mol. Sci. 2023, 24, 346. [Google Scholar] [CrossRef]
- Rothenberger, G.; Moser, J.; Graetzel, M.; Serpone, N.; Sharma, D.K. Charge carrier trapping and recombination dynamics in small semiconductor particles. J. Am. Chem. Soc. 1985, 107, 8054–8059. [Google Scholar] [CrossRef]
- Wad, A. Photocatalytic properties of TiO2. Chem. Mater. 1993, 5, 280–283. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Kanodarwala, F.K.; Hussain, E.; Badshah, A.; Hussain, I.; Stride, J.A.; Nadeem, M.A. Controlled Synthesis of TiO2 Nanostructures: Exceptional Hydrogen Production in Alcohol-Water Mixtures over Cu(OH)2-Ni (OH)2/TiO2 Nanorods. ChemistrySelect 2017, 2, 7497–7507. [Google Scholar] [CrossRef]
- Hong, Y.; Shi, P.; Wang, P.; Yao, W. Improved photocatalytic activity of CdS/reduced graphene oxide (RGO) for H2 evolution by strengthening the connection between CdS and RGO sheets. Int. J. Hydrog. Energy 2015, 40, 7045–7051. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.E.; Kamat, P.V. Influence of Metal/Metal Ion Concentration on the Photocatalytic Activity of TiO2-Au Composite Nanoparticles. Langmuir 2002, 19, 469–474. [Google Scholar] [CrossRef]
- Muscetta, M.; Clarizia, L.; Garlisi, C.; Palmisano, G.; Marotta, R.; Andreozzi, R.; Di Somma, I. Hydrogen production upon UV-light irradiation of Cu/TiO2 photocatalyst in the presence of alkanol-amines. Int. J. Hydrog. Energy 2020, 45, 26701–26715. [Google Scholar] [CrossRef]
- Hoang, A.T.; Pandey, A.; Chen, W.-H.; Ahmed, S.F.; Nižetić, S.; Ng, K.H.; Said, Z.; Duong, X.Q.; Ağbulut, Ü.; Hadiyanto, H.; et al. Hydrogen Production by Water Splitting with Support of Metal and Carbon-Based Photocatalysts. ACS Sustain. Chem. Eng. 2023, 11, 1221–1252. [Google Scholar] [CrossRef]
- Wu, C.; Guo, J.; Zhang, J.; Zhao, Y.; Tian, J.; Isimjan, T.T.; Yang, X. Palladium nanoclusters decorated partially decomposed porous ZIF-67 polyhedron with ultrahigh catalytic activity and stability on hydrogen generation. Renew. Energy 2019, 136, 1064–1070. [Google Scholar] [CrossRef]
- Dai, Y.; Xiong, Y. Control of selectivity in organic synthesis via heterogeneous photocatalysis under visible light. Nano Res. Energy 2022, 1, 9120006. [Google Scholar] [CrossRef]
- Fisher, A.; Peter, L.; Ponomarev, E.; Walker, A.; Wijayantha, K. Intensity dependence of the back reaction and transport of electrons in dye-sensitized nanocrystalline TiO2 solar cells. J. Phys. Chem. B 2000, 104, 949–958. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Zheng, Z.; Yuan, Y.; Zhao, J.-C.; Waclawik, E.R.; Ke, X.; Zhu, H. An efficient photocatalyst structure: TiO2(B) nanofibers with a shell of anatase nanocrystals. J. Am. Chem. Soc. 2009, 131, 17885–17893. [Google Scholar] [CrossRef]
- Sood, S.; Gouma, P. Polymorphism in nanocrystalline binary metal oxides. Nanomater. Energy 2013, 2, 82–96. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Reddy, N.L.; Srinivas, B.; Durgakumari, V.; Roddatis, V.; Bondarchuk, O.; Karthik, M.; Ikuma, Y.; Shankar, M. Stable and active CuxO/TiO2 nanostructured catalyst for proficient hydrogen production under solar light irradiation. Sol. Energy Mater. Sol. Cells 2016, 146, 63–71. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; Grimes, C.A. Long vertically aligned titania nanotubes on transparent conducting oxide for highly efficient solar cells. Nat. Nanotechnol. 2009, 4, 592–597. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Sasaki, T.; Koshizaki, N. Dispersion of nanosized noble metals in TiO2 matrix and their photoelectrode properties. Thin Solid Film. 2005, 483, 276–282. [Google Scholar] [CrossRef]
- Ohtani, B.; Iwai, K.; Nishimoto, S.-I.; Sato, S. Role of Platinum Deposits on Titanium (IV) Oxide Particles: Structural and Kinetic Analyses of Photocatalytic Reaction in Aqueous Alcohol and Amino Acid Solutions. J. Phys. Chem. B 1997, 101, 3349–3359. [Google Scholar] [CrossRef]
- Kamat, P.V. Photophysical, Photochemical and Photocatalytic Aspects of Metal Nanoparticles. J. Phys. Chem. B 2002, 106, 7729–7744. [Google Scholar] [CrossRef]
- Lee, S.; Scott, J.; Chiang, K.; Amal, R. Nanosized metal deposits on titanium dioxide for augmenting gas-phase toluene photooxidation. J. Nanopart. Res. 2009, 11, 209–219. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Tabata, S.; Nishida, H.; Masaki, Y.; Tabata, K. Stoichiometric photocatalytic decomposition of pure water in Pt/TiO2 aqueous suspension system. Catal. Lett. 1995, 34, 245–249. [Google Scholar] [CrossRef]
- Sato, S.; White, J. Photodecomposition of water over Pt/TiO2 catalysts. Chem. Phys. Lett. 1980, 72, 83–86. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Tian, H.; Kang, S.-Z.; Li, X.; Qin, L.; Ji, M.; Mu, J. Fabrication of an efficient noble metal-free TiO2-based photocatalytic system using Cu–Ni bimetallic deposit as an active center of H2 evolution from water. Sol. Energy Mater. Sol. Cells 2015, 134, 309–317. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Badshah, A.; Kanodarwala, F.K.; Ali, H.; Khan, M.A.; Stride, J.A.; Nadeem, M.A. Titania supported MOF-199 derived Cu-Cu2O nanoparticles: Highly efficient non-noble metal photocatalysts for hydrogen production from alcohol–water mixtures. Catal. Sci. Technol. 2017, 7, 677–686. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Moriga, T.; Idriss, H.; Waterhouse, G.I. Ni/TiO2: A promising low-cost photocatalytic system for solar H2 production from ethanol–water mixtures. J. Catal. 2015, 326, 43–53. [Google Scholar] [CrossRef]
- Majeed, I.; Arif, A.; Faizan, M.; Khan, M.A.; Imran, M.; Ali, H.; Nadeem, M.A.; Nadeem, M.A. CdS nanorods supported copper-nickel hydroxide for hydrogen production under direct sunlight irradiation. J. Environ. Chem. Eng. 2021, 9, 105670. [Google Scholar] [CrossRef]
- Yu, J.; Ran, J. Facile preparation and enhanced photocatalytic H2-production activity of Cu(OH)2 cluster modified TiO2. Energy Environ. Sci. 2011, 4, 1364–1371. [Google Scholar] [CrossRef]
- Ismail, A.A.; Al-Sayari, S.A.; Bahnemann, D. Photodeposition of precious metals onto mesoporous TiO2 nanocrystals with enhanced their photocatalytic activity for methanol oxidation. Catal. Today 2013, 209, 2–7. [Google Scholar] [CrossRef]
- Bowker, M.; Morton, C.; Kennedy, J.; Bahruji, H.; Greves, J.; Jones, W.; Davies, P.R.; Brookes, C.; Wells, P.; Dimitratos, N. Hydrogen production by photoreforming of biofuels using Au, Pd and Au-Pd/TiO2 photocatalysts. J. Catal. 2014, 310, 10–15. [Google Scholar] [CrossRef]
- Majeed, I.; Manzoor, U.; Kanodarwala, F.K.; Nadeem, M.A.; Hussain, E.; Ali, H.; Badshah, A.; Stride, J.A.; Nadeem, M.A. Pd–Ag decorated gC3N4 as an efficient photocatalyst for hydrogen production from water under direct solar light irradiation. Catal. Sci. Technol. 2018, 8, 1183–1193. [Google Scholar] [CrossRef]
- Hussain, E.; Majeed, I.; Nadeem, M.A.; Badshah, A.; Chen, Y.; Nadeem, M.A.; Jin, R. Titania-supported palladium/strontium nanoparticles (Pd/Sr-NPs@ P25) for photocatalytic H2 production from water splitting. J. Phys. Chem. C 2016, 120, 17205–17213. [Google Scholar] [CrossRef]
- Majeed, I.; Ali, H.; Idrees, A.; Arif, A.; Ashraf, W.; Rasul, S.; Khan, M.A.; Nadeem, M.A.; Nadeem, M.A. Understanding the role of metal supported on TiO2 in photoreforming of oxygenates. Energy Adv. 2022, 1, 842–867. [Google Scholar] [CrossRef]
- Ali, H.; Kanodarwala, F.K.; Majeed, I.; Stride, J.A.; Nadeem, M.A. La2O3 Promoted Pd/rGO electro-catalysts for formic acid oxidation. ACS Appl. Mater. Interfaces 2016, 8, 32581–32590. [Google Scholar] [CrossRef]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Hussain, E.; Majeed, I.; Nadeem, M.A.; Iqbal, A.; Chen, Y.; Choucair, M.; Jin, R.; Nadeem, M.A. Remarkable effect of BaO on photocatalytic H2 evolution from water splitting via TiO2 (P25) supported palladium nanoparticles. J. Environ. Chem. Eng. 2019, 7, 102729. [Google Scholar] [CrossRef]
- Dosado, A.G.; Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Waterhouse, G.I.N. Novel Au/TiO2 photocatalysts for hydrogen production in alcohol-water mixtures based on hydrogen titanate nanotube precursors. J. Catal. 2015, 330, 238–254. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Hussain, E.; Badshah, A.; Gilani, R.; Nadeem, M.A. Effect of deposition method on metal loading and photocatalytic activity of Au/CdS for hydrogen production in water electrolyte mixture. Int. J. Hydrog. Energy 2017, 42, 3006–3018. [Google Scholar] [CrossRef]
- Nadeem, A.; Muir, J.; Connelly, K.; Adamson, B.; Metson, B.; Idriss, H. Ethanol photo-oxidation on a rutile TiO2 (110) single crystal surface. Phys. Chem. Chem. Phys. 2011, 13, 7637–7643. [Google Scholar] [CrossRef]
- Majeed, I.; Nadeem, M.A.; Al-Oufi, M.; Nadeem, M.A.; Waterhouse, G.I.N.; Badshah, A.; Metson, J.B.; Idriss, H. On the role of metal particle size and surface coverage for photo-catalytic hydrogen production: A case study of the Au/CdS system. Appl. Catal. B Environ. 2016, 182, 266–276. [Google Scholar] [CrossRef]
- Dou, S.; Zhou, S.; Huang, H.; Yan, P.; Shoko, E.; Isimjan, T.T.; Yang, X. Metal—Organic Framework (MOF)-Derived Electron-Transfer Enhanced Homogeneous PdO-Rich Co3O4 as a Highly Efficient Bifunctional Catalyst for Sodium Borohydride Hydrolysis and 4-Nitrophenol Reduction. Chem.-A Eur. J. 2020, 26, 16923–16931. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Sheha, E. Studies on TiO2/reduced graphene oxide composites as cathode materials for magnesium-ion battery. Graphene 2014, 2014, 48098. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. Graphite oxide and graphene nanoribbons reduction with hydrogen iodide. Fuller. Nanotub. Carbon Nanostructures 2011, 19, 461–468. [Google Scholar] [CrossRef]
- Hodkiewicz, J.; Scientific, T. Characterizing carbon materials with Raman spectroscopy. Sci. Appl. Note 2010, 51946. [Google Scholar]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Ali, H.; Zaman, S.; Majeed, I.; Kanodarwala, F.K.; Nadeem, M.A.; Stride, J.A.; Nadeem, M.A. Porous Carbon/rGO Composite: An Ideal Support Material of Highly Efficient Palladium Electrocatalysts for the Formic Acid Oxidation Reaction. ChemElectroChem 2017, 4, 3126–3133. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Al-Oufi, M.; Wahab, A.K.; Anjum, D.; Idriss, H. Hydrogen Production on Ag-Pd/TiO2 Bimetallic Catalysts: Is there a Combined Effect of Surface Plasmon Resonance with Schottky Mechanism on the Photo-Catalytic Activity? ChemistrySelect 2017, 2, 2754–2762. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Waterhouse, G.I.N.; Idriss, H. A study of ethanol reactions on O2-treated Au/TiO2. Effect of support and metal loading on reaction selectivity. Surf. Sci. 2016, 650, 40–50. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; D’Arienzo, M.; Altomare, M.; Marelli, M.; Scotti, R.; Morazzoni, F.; Selli, E.; Dal Santo, V. Pt and Au/TiO2 photocatalysts for methanol reforming: Role of metal nanoparticles in tuning charge trapping properties and photoefficiency. Appl. Catal. B Environ. 2013, 130, 239–248. [Google Scholar] [CrossRef]
- Zhu, G.; Su, F.; Lv, T.; Pan, L.; Sun, Z. Au nanoparticles as interfacial layer for CdS quantum dot-sensitized solar cells. Nanoscale Res. Lett. 2010, 5, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Tiruvalam, R.; Logsdail, A.J.; He, Q.; Downing, C.A.; Jensen, M.T.; Dimitratos, N.; Kesavan, L.; Wells, P.P.; Bechstein, R. Designer titania-supported Au-Pd nanoparticles for efficient photocatalytic hydrogen production. ACS Nano 2014, 8, 3490–3497. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Z.; Wang, H.; Cheng, W.; Chen, M.; Shangguan, W.; Ding, G. TiO2—Graphene nanocomposites for photocatalytic hydrogen production from splitting water. Int. J. Hydrog. Energy 2012, 37, 2224–2230. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Enhanced photocatalytic H2-production activity of graphene-modified titania nanosheets. Nanoscale 2011, 3, 3670–3678. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Cui, X.; Jiang, Z. A green and facile synthesis of TiO2/graphene nanocomposites and their photocatalytic activity for hydrogen evolution. Int. J. Hydrog. Energy 2012, 37, 811–815. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Li, H.-P.; Cui, X.-L.; Lin, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Kim, H.-I.; Moon, G.-H.; Monllor-Satoca, D.; Park, Y.; Choi, W. Solar photoconversion using graphene/TiO2 composites: Nanographene shell on TiO2 core versus TiO2 nanoparticles on graphene sheet. J. Phys. Chem. C 2012, 116, 1535–1543. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Q.; Liu, S.; Li, Y. Synergetic catalysis of CuO and graphene additives on TiO2 for photocatalytic water splitting. Int. J. Hydrog. Energy 2013, 38, 7232–7240. [Google Scholar] [CrossRef]
- Chen, W.-T.; Jovic, V.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. The role of CuO in promoting photocatalytic hydrogen production over TiO2. Int. J. Hydrog. Energy 2013, 38, 15036–15048. [Google Scholar] [CrossRef]
- Yu, J.; Hai, Y.; Cheng, B. Enhanced photocatalytic H2-production activity of TiO2 by Ni(OH)2 cluster modification. J. Phys. Chem. C 2011, 115, 4953–4958. [Google Scholar] [CrossRef]
- Rayalu, S.S.; Jose, D.; Joshi, M.V.; Mangrulkar, P.A.; Shrestha, K.; Klabunde, K. Photocatalytic water splitting on Au/TiO2 nanocomposites synthesized through various routes: Enhancement in photocatalytic activity due to SPR effect. Appl. Catal. B Environ. 2013, 142–143, 684–693. [Google Scholar] [CrossRef]
- Ortega Méndez, J.A.; López, C.R.; Pulido Melián, E.; González Díaz, O.; Doña Rodríguez, J.M.; Fernández Hevia, D.; Macías, M. Production of hydrogen by water photo-splitting over commercial and synthesised Au/TiO2 catalysts. Appl. Catal. B Environ. 2014, 147, 439–452. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Selli, E.; Forni, L. Photocatalytic hydrogen production over flame spray pyrolysis-synthesised TiO2 and Au/TiO2. Appl. Catal. B Environ. 2008, 84, 332–339. [Google Scholar] [CrossRef]
- Gärtner, F.; Losse, S.; Boddien, A.; Pohl, M.M.; Denurra, S.; Junge, H.; Beller, M. Hydrogen evolution from water/alcohol mixtures: Effective in situ generation of an active Au/TiO2 catalyst. ChemSusChem 2012, 5, 530–533. [Google Scholar] [CrossRef]
- Gallo, A.; Marelli, M.; Psaro, R.; Gombac, V.; Montini, T.; Fornasiero, P.; Pievo, R.; Dal Santo, V. Bimetallic Au-Pt/TiO2 photocatalysts active under UV-A and simulated sunlight for H2 production from ethanol. Green Chem. 2012, 14, 330–333. [Google Scholar] [CrossRef]
- Wu, M.-C.; Lee, P.-H.; Lee, D.-L. Enhanced photocatalytic activity of palladium decorated TiO2 nanofibers containing anatase-rutile mixed phase. Int. J. Hydrog. Energy 2015, 40, 4558–4566. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Kawamori, H.; Honda, D.; Yoshida, H.; Arai, M. Photocatalytic hydrogen production from aqueous glycerol solution using NiO/TiO2 catalysts: Effects of preparation and reaction conditions. Appl. Catal. B Environ. 2016, 181, 818–824. [Google Scholar] [CrossRef]
- Agegnehu, A.K.; Pan, C.-J.; Rick, J.; Lee, J.-F.; Su, W.-N.; Hwang, B.-J. Enhanced hydrogen generation by cocatalytic Ni and NiO nanoparticles loaded on graphene oxide sheets. J. Mater. Chem. 2012, 22, 13849–13854. [Google Scholar] [CrossRef]







| Photocatalyst Structure | Sacrificial Reagents | Irradiation | H2 Production (mmol g−1h−1) | Ref. |
|---|---|---|---|---|
| Pd1−rGO4/TNRs | 5% glycerol–water | 100 W (UV) | 41.0 | Present Study |
| Cu0.8-Ni0.2/TNR130-400 | 5% glycerol–water | 100 W (UV) | 35.1 | 2017 [18] |
| Pt0.5−rGO0.5/P25 | 30% methanol–water | 300 W(UV) | 0.750 | 2012 [73] |
| Pt0.5−rGO1/TiO2-A (NS) | 25% methanol–water | 350 W (UV) | 0.736 | 2011 [74] |
| rGO(sheets)2/P25 (NR) | Na2S and M Na2SO3 | 500 W (UV) | 0.054 | 2012 [75] |
| rGO5/TiO2-A | Na2S and Na2SO3 | (UV) | 0.085 | 2010 [76] |
| rGO20/P25 | 20% ethanol–water | 200 W (UV) | 0.74 | 2011 [60] |
| Pt0.05−Graphen0.7/P25 | 10% methanol–water | 300 W(UV) | 0.1 | 2012 [77] |
| MoS2(0.5)/GO0.5−TiO2-A | 25% ethanol–water | 300 W (UV) | 2.06 | 2012 [69] |
| GO/CuO2−P25 | 10% methanol–water | 500 W (UV) | 2.9 | 2013 [78] |
| CuO1.25/TiO2 | 80% ethanol–water | 100 W (UV) | 20.3 | 2013 [79] |
| Ni(OH)(0.5)/P25 | 25% methanol–water | 3 W (UV) | 3.056 | 2011 [80] |
| Ni1.25/P25 | 95% ethanol–water | 100 W (UV) | 20.7 | 2015 [19] |
| Ni(OH)2(0.5)/P25 | 25% methanol–water | 3 W (UV) | 3.056 | 2011 [80] |
| Au4/P25 | 5% ethanol–water | 450 W (UV-Vis) | 6.12 | 2013 [81] |
| Au0.8/TiO2-A | 25% methanol–water | 400 W (UV) | 1.54 | 2014 [82] |
| Au1/TiO2-A | 6% methanol–water | 250 W (UV-Vis) | 8 | 2008 [83] |
| Au1/TiO2-A | 50% methanol–water | 2.4 W (UV) | 8.4 | 2012 [84] |
| Au0.5-Pt0.5/TiO2-A | 50% ethanol–water | 125 W (UV) | 8 | 2013 [85] |
| Pd1/TiO2-A+R (NS) | 50% ethanol–water | 8 W UV-B | 16.2 | 2015 [86] |
| Au1.5/P25 | 80% ethanol–water | 100 W (UV) | 32.2 | 2015 [54] |
| Au0.25-Pd0.75/P25 | 25% glycerol–water | 100 W (UV) | 19.6 | 2014 [72] |
| Pt1/P25 | 10% glycerol–water | 200 W (UV) | 27.1 | 2015 [13] |
| NiO(2)/TiO2-A+ R | 16% glycerol–water | 500 W (UV) | 1.23 | 2016 [87] |
| Photocatalyst | BET Surface Area (m2 g−1) | TNR Length (nm) | rGO Nominal (wt.%) | Pd wt.% by XPS | H2 Production (mmol h−1g−1) |
|---|---|---|---|---|---|
| TNR | 60.45 | 50–70 | − | − | 2.00 |
| Pd1−rGO1/TNRs(TiO2) | 60.25 | 50–70 | 1 | − | 30.60 |
| Pd1−rGO2/TNRs(TiO2) | 61.05 | 50–70 | 2 | − | 34.16 |
| Pd1−rGO3/TNRs(TiO2) | 61.75 | 50–70 | 3 | − | 37.66 |
| Pd1−rGO4/TNRs(TiO2) | 62 | 50–70 | 4 | 0.85 | 41.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, I.; Arif, A.; Idrees, A.; Ullah, H.; Ali, H.; Mehmood, A.; Rashid, A.; Nadeem, M.A.; Nadeem, M.A. Synergistic Effect of Pd Co-Catalyst and rGO–TiO2 Hybrid Support for Enhanced Photoreforming of Oxygenates. Hydrogen 2023, 4, 192-209. https://doi.org/10.3390/hydrogen4010014
Majeed I, Arif A, Idrees A, Ullah H, Ali H, Mehmood A, Rashid A, Nadeem MA, Nadeem MA. Synergistic Effect of Pd Co-Catalyst and rGO–TiO2 Hybrid Support for Enhanced Photoreforming of Oxygenates. Hydrogen. 2023; 4(1):192-209. https://doi.org/10.3390/hydrogen4010014
Chicago/Turabian StyleMajeed, Imran, Ayesha Arif, Afifa Idrees, Hafeez Ullah, Hassan Ali, Arshad Mehmood, Ashi Rashid, Muhammad Arif Nadeem, and Muhammad Amtiaz Nadeem. 2023. "Synergistic Effect of Pd Co-Catalyst and rGO–TiO2 Hybrid Support for Enhanced Photoreforming of Oxygenates" Hydrogen 4, no. 1: 192-209. https://doi.org/10.3390/hydrogen4010014
APA StyleMajeed, I., Arif, A., Idrees, A., Ullah, H., Ali, H., Mehmood, A., Rashid, A., Nadeem, M. A., & Nadeem, M. A. (2023). Synergistic Effect of Pd Co-Catalyst and rGO–TiO2 Hybrid Support for Enhanced Photoreforming of Oxygenates. Hydrogen, 4(1), 192-209. https://doi.org/10.3390/hydrogen4010014






