Distribution Patterns and Habitat Preferences of Five Globally Threatened and Endemic Montane Orthoptera (Parnassiana and Oropodisma)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Target Species
| Genus | Species | Mountains | ||||||
|---|---|---|---|---|---|---|---|---|
| Τ | H | Κ | Ox | Oi | V | G | ||
| Parnassiana | P. coracis | ○ | ● | |||||
| P. tymphrestos | ● | ○ | ○ | ● | ||||
| P. gionica | ● | |||||||
| Oropodisma | O. willemsei | ○ | ○ | ○ | ○ | ○ | ● | |
| O. tymphrestosi | ● | x | x | |||||
2.2. Orthoptera Sampling
2.3. Microhabitat Parameters
2.4. Environmental Data
2.5. Data Analysis
2.5.1. Current Distribution Range
2.5.2. Species Distribution Models
2.5.3. Modeling Microhabitat Effects on Population Densities
3. Results
3.1. Current Distribution Pattern and Population Densities
3.2. Habitat Suitability
3.3. Microhabitat
3.3.1. Microhabitat Description
| Parnassiana | Oropodisma | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P. coracis | P. tymphrestos | P. gionica | O. willemsei | O. tymphrestosi | ||||||
| Variable | Mean | Min–Max | Mean | Min–Max | Mean | Min–Max | Mean | Min–Max | Mean | Min–Max |
| Elevation (m) | 1782 | 1555–2403 | 1890 | 1542–2241 | 2037 | 1758–2135 | 1948 | 1567–2403 | 1970 | 1648–2241 |
| Slope (o) | 23.5 | 0–45 | 19.2 | 0–43 | 21 | 4–41 | 22.18 | 0–45 | 22.09 | 0–42 |
| Soil cover (%) | 7.1 | 0–35 | 6.4 | 0–25 | 3.4 | 0–10 | 22.18 | 0–45 | 4.57 | 0–12 |
| Rock cover (%) | 8.8 | 0–60 | 9.3 | 0–38 | 7.6 | 0–20 | 10.44 | 0–38 | 13.48 | 0–38 |
| Stone cover (%) | 11.8 | 0–60 | 15.48 | 0–65 | 29.8 | 5–55 | 20.63 | 0–60 | 23.09 | 0–45 |
| Herb/grass cover (%) | 66.4 | 30–97 | 66.5 | 20–100 | 56.8 | 35–90 | 63.36 | 30–98 | 67.52 | 30–98 |
| Shrub/robust plant cover (%) | 33.1 | 0–70 | 33.3 | 0–80 | 43 | 10–65 | 35.25 | 3–70 | 32.48 | 2–70 |
| Grass/herb height (cm) | 28.1 | 10–120 | 25.1 | 2.75–125 | 25.1 | 2.75–125 | 23.41 | 7–107 | 25.2 | 12.5–80 |
| Shrub/robust plant height (cm) | 33.1 | 10–140 | 16.4 | 0–90 | 16.4 | 0–90 | 14.66 | 6–120 | 11.33 | 5–65 |
| Plant Species | Percentage of Plots | |||||||||
| Festuca jeanpertii | 74.4% | 69.6% | 35.6% | 68.7% | 52.63% | |||||
| Eryngium amethystinum | 51.8% | 31.2% | 54.6% | 48.2% | 7.37% | |||||
| Trisetum flavescens | 50.0% | 40.9% | 18.3% | 39.9% | 31.58% | |||||
| Astragalus creticus subsp. rumelicus | 52.8% | 60.5% | 67.8% | 60.8% | 28.42% | |||||
| Thymus longicaulis | 65.6% | 57.6% | 28.3% | 49.0% | 53.68% | |||||
3.3.2. Predictors of Population Density
4. Discussion
4.1. Species Distribution and Population Densities
4.2. Habitat Suitability
4.3. Microhabitat Preferences
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Appendix A
| Mountain | E (m) | N2000 | R (mm) | T (°C) |
|---|---|---|---|---|
| Giona | 2508 | GR2450002 | 897 | 7.5 |
| Helidona | 1974 | - | 1024 | 9.5 |
| Kaliakouda | 2099 | - | 1027 | 7 |
| Oiti | 2151 | GR2440007 | 835 | 9 |
| Oxia | 1923 | - | 1028 | 10 |
| Tymphrestos | 2313 | GR2430001 | 1045 | 7 |
| Vardousia | 2495 | GR2450001 | 833 | 9.5 |
| Total (7) | - | 4 | 833–1045 | 7–9.5 |
Appendix B
| Genus | Taxa | Rank | Model | k | AICc | ΔAICc | wi | R2m | R2c |
|---|---|---|---|---|---|---|---|---|---|
| Parnassiana | Parnassiana Complex (GLMM) | 1 | Alt + St | 2 | 731.6 | 0 | 0.294 | 0.1 | 0.9 |
| 2 | Alt + St + Rpmean | 3 | 732.1 | 0.51 | 0.228 | 0.1 | 0.9 | ||
| 3 | Alt + St + Ghmean | 3 | 732.9 | 1.30 | 0.154 | 0.11 | 0.9 | ||
| 4 | Alt + St + Rpmean + Ghmean | 4 | 733.4 | 1.79 | 0.120 | 0.11 | 0.9 | ||
| P. coracis (GLM) | 1 | Alt + Slope | 2 | 272.2 | 0 | 0.243 | 0.29 | - | |
| 2 | Alt + Slope + St | 3 | 272.3 | 0.07 | 0.235 | 0.33 | - | ||
| 3 | Alt + St | 2 | 273 | 0.8 | 0.163 | 0.38 | - | ||
| 4 | Alt | 1 | 273.1 | 0.86 | 0.158 | 0.31 | - | ||
| 5 | Alt + Rpmean + St | 3 | 273.8 | 1.56 | 0.112 | 0.34 | - | ||
| P. tymphrestos (GLM) | 1 | St | 1 | 374 | 0 | 0.257 | 0.15 | - | |
| 2 | St + Ghmean | 2 | 374.6 | 0.64 | 0.187 | 0.17 | - | ||
| 3 | St + R | 2 | 374.6 | 0.65 | 0.185 | 0.17 | - | ||
| 4 | St + Ghmean + R | 3 | 374.9 | 0.92 | 0.162 | 0.21 | - | ||
| 5 | St + Sl | 2 | 375.4 | 1.44 | 0.125 | 0.16 | - | ||
| P. gionica (GAM) | 1 | St + Sl + R | 3 | 596.7 | 0 | 1 | 0.990 | - | |
| Oropodisma | Oropodisma Complex (GLMM) | 1 | Sl + R + Ghmean | 1 | 536 | 0 | 0.246 | 0.11 | 0.91 |
| 2 | Sl + Ghmean | 2 | 536.5 | 0.45 | 0.197 | 0.09 | 0.91 | ||
| 3 | Sl + Ghmean + Hcover + R | 2 | 537 | 1.01 | 0.149 | 0.13 | 0.92 | ||
| 4 | Sl + So | 2 | 537 | 1.01 | 0.149 | 0.08 | 0.91 | ||
| 5 | Sl + R | 2 | 537.2 | 1.14 | 0.139 | 0.07 | 0.91 | ||
| 6 | Sl + Ghmean + So | 3 | 537.5 | 1.44 | 0.12 | 0.10 | 0.91 | ||
| 7 | Sl + Ghmean + Hcover | 3 | 537.6 | 1.57 | 0.98 | 0.10 | 0.92 | ||
| 8 | Sl + Ghmean + R + Rpmean | 4 | 537.7 | 1.64 | 0.043 | 0.12 | 0.91 | ||
| 9 | Sl | 1 | 537.8 | 1.77 | 0.035 | 0.04 | 0.91 | ||
| 10 | Sl + R + So | 3 | 537.9 | 1.91 | 0.026 | 0.09 | 0.91 | ||
| 11 | Sl + R + Rpmean | 3 | 538 | 1.97 | 0.026 | 0.09 | 0.91 | ||
| O. willemsei (GLMM) | 1 | R + Sl + Rpmean + Ghmean | 4 | 399.6 | 0 | 0.402 | 0.24 | 0.55 | |
| 2 | R + Sl + Ghmean | 3 | 401 | 1.43 | 0.197 | 0.21 | 0.52 | ||
| O. tymphrestosi (GLM) | 1 | So | 1 | 137.3 | 0 | 0.367 | 0.19 | - | |
| 2 | Hcover + So | 2 | 138.3 | 0.94 | 0.229 | 0.28 | - |
Appendix C
| Parnassiana | Oropodisma | ||||
|---|---|---|---|---|---|
| Taxon | Variable | Importance | Taxon | Variable | Importance |
| Parnassiana complex | HLI | 1.15 | Oropodisma complex | bio3 | 1.20 |
| bio17 | 1.09 | PETWQ | 1.18 | ||
| PETDQ | 1.08 | TPI | 1.06 | ||
| NDVI | 1.04 | NDVI | 1.02 | ||
| aspect | 1.03 | HLI | 1.00 | ||
| slope | 1.02 | slope | 0.97 | ||
| bio3 | 0.95 | bio4 | 0.94 | ||
| TPI | 0.88 | O. tymphrestosi | AIT | 1.33 | |
| bio4 | 0.80 | NDVI | 1.02 | ||
| P. coracis | bio17 | 1.72 | TPI | 0.86 | |
| TPI | 1.27 | slope | 0.79 | ||
| NDVI | 1.23 | HLI | 0.69 | ||
| aspect | 1.06 | O. willemsei | PETDQ | 1.16 | |
| PETDQ | 1.05 | NDVI | 1.11 | ||
| HLI | 0.81 | PETWQ | 1.11 | ||
| slope | 0.69 | bio2 | 1.04 | ||
| bio3 | 1.43 | bio17 | 0.97 | ||
| P. tymphrestosi | HLI | 1.26 | TPI | 0.97 | |
| PETDQ | 1.22 | bio3 | 0.94 | ||
| PETWQ | 1.20 | HLI | 0.93 | ||
| slope | 1.12 | aspect | 0.86 | ||
| NDVI | 1.01 | ||||
| bio2 | 0.90 | ||||
| bio3 | 0.80 | ||||
| aspect | 0.80 | ||||
| TPI | 0.76 | ||||
| P. gionica | PETDQ | 1.47 | |||
| bio3 | 1.43 | ||||
| continentality | 1.07 | ||||
| NDVI | 0.965 | ||||
| TPI | 0.912 | ||||
| HLI | 0.741 | ||||
| aspect | 0.629 | ||||
References
- Mizsei, E.; Boros, Z.; Lovas-Kiss, Á.; Szepesváry, C.; Szabolcs, M.; Rák, G.; Ujszegi, J.; Gál, Z.; Lengyel, S.; Puskás, G. A trait-based framework for understanding predator–prey relationships: Trait matching between a specialist snake and its insect prey. Funct. Ecol. 2019, 33, 2354–2368. [Google Scholar] [CrossRef]
- Sow, A.; Haran, J.; Benoit, L.; Galan, M.; Brévault, T. DNA Metabarcoding as a Tool for Disentangling Food Webs in Agroecosystems. Insects 2020, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Li, F.-F.; Bai, J.; Liang, K.; Li, K.; Qin, G.-Q.; Zhang, Y.-L.; Li, X.-J. Isolation and characterization of intestinal bacteria associated with cellulose degradation in grasshoppers (Orthoptera). J. Insect Sci. 2023, 23, 7. [Google Scholar] [CrossRef]
- Stebaev, I.V.; Molodtsov, V.V. Typology of Landscape-Regional Distribution of the Acridoidea Species in Grass Biotopes of South-West Siberia Based on Isopleth Portraits of Their Quantitative Distribution. Biol. Bull. Russ. Acad. Sci. 2001, 28, 408–416. [Google Scholar] [CrossRef]
- Weking, S.; Kämpf, I.; Mathar, W.; Hölzel, N. Effects of land use and landscape patterns on Orthoptera communities in the Western Siberian forest steppe. Biodivers. Conserv. 2016, 25, 2341–2359. [Google Scholar] [CrossRef]
- Zografou, K.; Adamidis, G.C.; Komnenov, M.; Kati, V.; Sotirakopoulos, P.; Pitta, E.; Chatzaki, M. Diversity of spiders and orthopterans respond to intra-seasonal and spatial environmental changes. J. Insect Conserv. 2017, 21, 531–543. [Google Scholar] [CrossRef]
- Helbing, F.; Blaeser, T.P.; Löffler, F.; Fartmann, T. Response of Orthoptera communities to succession in alluvial pine woodlands. J. Insect Conserv. 2014, 18, 215–224. [Google Scholar] [CrossRef]
- Löffler, F.; Poniatowski, D.; Fartmann, T. Orthoptera community shifts in response to land-use and climate change—Lessons from a long-term study across different grassland habitats. Biol. Conserv. 2019, 236, 315–323. [Google Scholar] [CrossRef]
- Tiede, Y.; Hemp, C.; Schmidt, A.; Nauss, T.; Farwig, N.; Brandl, R. Beyond body size: Consistent decrease of traits within orthopteran assemblages with elevation. Ecology 2018, 99, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, T.; Casey, D. Topographic heterogeneity influences diversity and abundance of Orthoptera in a rewilding scheme. J. Orthoptera Res. 2024, 33, 255–266. [Google Scholar] [CrossRef]
- Kenyeres, Z.; Takács, G.; Király, G. Challenges of Orthoptera conservation in grasslands with land use-determined sizes and structural heterogeneity. Landsc. Ecol. Eng. 2024, 20, 441–453. [Google Scholar] [CrossRef]
- Hochkirch, A.; Nieto, A.; Criado, M. European Red List of Grasshoppers, Crickets and Bush-Crickets; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Stefanidis, A.; Zografou, K.; Tzortzakaki, O.; Kati, V. Orthoptera Community Dynamics and Conservation in a Natura 2000 Site (Greece): The Role of Beta Diversity. Diversity 2024, 16, 11. [Google Scholar] [CrossRef]
- Spehn, E.; Rudmann-Maurer, K.; Korner, C.; Maselli, D. Mountain Biodiversity and Global Change; Global Mountain Biodiversity Assessment: Basel, Switzerland, 2010. [Google Scholar]
- Barve, S.; Dhondt, A.A. Elevational replacement of two Himalayan titmice: Interspecific competition or habitat preference? J. Avian Biol. 2017, 48, 1189–1194. [Google Scholar] [CrossRef]
- Graham, C.H.; Carnaval, A.C.; Cadena, C.D.; Zamudio, K.R.; Roberts, T.E.; Parra, J.L.; McCain, C.M.; Bowie, R.C.K.; Moritz, C.; Baines, S.B.; et al. The origin and maintenance of montane diversity: Integrating evolutionary and ecological processes. Ecography 2014, 37, 711–719. [Google Scholar] [CrossRef]
- Willemse, L.; Kleukers, R.; Odé, B. The Grasshoppers of Greece; EIS Kenniscentrum Insecten en Andere Ongewervelden: Leiden, The Netherlands, 2018. [Google Scholar]
- Stefanidis, A.; Kougioumoutzis, K.; Zografou, K.; Fotiadis, G.; Tzortzakaki, O.; Willemse, L.; Kati, V. Mitigating the extinction risk of globally threatened and endemic mountainous Orthoptera species: Parnassiana parnassica and Oropodisma parnassica. Insect Conserv. Divers. 2025, 18, 54–68. [Google Scholar] [CrossRef]
- Kati, V.; Dufrêne, M.; Legakis, A.; Grill, A.; Lebrun, P. Conservation management for Orthoptera in the Dadia reserve, Greece. Biol. Conserv. 2004, 115, 33–44. [Google Scholar] [CrossRef]
- Zografou, K.; Sfenthourakis, S.; Pullin, A.; Kati, V. On the surrogate value of red-listed butterflies for butterflies and grasshoppers: A case study in Grammos site of Natura 2000, Greece. J. Insect Conserv. 2009, 13, 505–514. [Google Scholar] [CrossRef]
- Kati, V.; Mani, P.; von Helversen, O.; Willemse, F.; Elsner, N.; Dimopoulos, P. Human land use threatens endemic wetland species: The case of Chorthippus lacustris (La Greca and Messina 1975) (Orthoptera: Acrididae) in Epirus, Greece. J. Insect Conserv. 2006, 10, 65–74. [Google Scholar] [CrossRef]
- Jackson, C.R.; Robertson, M.P. Predicting the potential distribution of an endangered cryptic subterranean mammal from few occurrence records. J. Nat. Conserv. 2011, 19, 87–94. [Google Scholar] [CrossRef]
- Thomaes, A.; Kervyn, T.; Maes, D. Applying species distribution modelling for the conservation of the threatened saproxylic Stag Beetle (Lucanus cervus). Biol. Conserv. 2008, 141, 1400–1410. [Google Scholar] [CrossRef]
- Grzywacz, B.; Heller, K.-G.; Chobanov, D.P.; Warchałowska-Śliwa, E. Conventional and molecular chromosome study in the European genus Parnassiana Zeuner, 1941 (Orthoptera, Tettigoniinae, Platycleidini). Folia Biol. 2017, 65, 1–8. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Peterson, A.T. Predicting Species’ Geographic Distributions Based on Ecological Niche Modeling. Condor 2001, 103, 599–605. [Google Scholar] [CrossRef]
- Poniatowski, D.; Fartmann, T. The classification of insect communities: Lessons from orthopteran assemblages of semi-dry calcareous grasslands in central Germany. Eur. J. Entomol. 2008, 105, 659–671. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. GLMM and GAMM. In Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 323–341. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. GLM and GAM for Count Data. In Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 209–243. [Google Scholar]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Kati, V.; Petridou, M.; Tzortzakaki, O.; Papantoniou, E.; Galani, A.; Psaralexi, M.; Gotsis, D.; Papaioannou, H.; Kassara, C. How much wilderness is left? A roadless approach under the Global and the European Biodiversity Strategy focusing on Greece. Biol. Conserv. 2023, 281, 110015. [Google Scholar] [CrossRef]
- Kosztra, B.; Büttner, G. Updated CLC Illustrated Nomenclature Guidelines; Environment Agency Austria: Wien, Austria, 2019. [Google Scholar]
- Economou-Eliopoulos, M.; Kanellopoulos, C. Abundance and Genetic Significance of Lithium in Karst-Type Bauxite Deposits: A Comparative Review. Minerals 2023, 13, 962. [Google Scholar] [CrossRef]
- RAE. Geospatial Map for Energy Units and Requests. Available online: https://geo.rae.gr/?lang=EN (accessed on 24 October 2024).
- Willemse, L.; Hochkirch, A.; Heller, K.-G.; Kati, V.; Papapavlou, K.; Tzirkalli, E. Parnassiana coracis. The IUCN Red List of Threatened Species. 2016: e.T68450152A70624651. Available online: https://www.iucnredlist.org/species/68450152/70624651 (accessed on 22 October 2024). [CrossRef]
- Willemse, L.; Hochkirch, A.; Heller, K.-G.; Kati, V.; Papapavlou, K.; Tzirkalli, E. Parnassiana tymphrestos. The IUCN Red List of Threatened Species. 2016: e.T68450372A70624904. Available online: https://www.iucnredlist.org/species/68450372/70624904 (accessed on 22 October 2024). [CrossRef]
- Willemse, L.; Hochkirch, A.; Heller, K.-G.; Kati, V.; Papapavlou, K.; Tzirkalli, E. Parnassiana gionica. The IUCN Red List of Threatened Species. 2016: e.T68450237A70624696. Available online: https://www.iucnredlist.org/species/68450237/70624696 (accessed on 22 October 2024). [CrossRef]
- Willemse, L.; Hochkirch, A.; Kati, V.; Tzirkalli, E.; Papapavlou, K.; Heller, K.-G. Oropodisma willemsei. The IUCN Red List of Threatened Species. 2016: e.T16084523A18053580. Available online: https://www.iucnredlist.org/species/16084523/18053580 (accessed on 23 October 2024). [CrossRef]
- Willemse, L.; Hochkirch, A.; Kati, V.; Papapavlou, K.; Tzirkalli, E.; Heller, K.-G. Oropodisma tymphrestosi. The IUCN Red List of Threatened Species. 2016: E.T16084584A70566762. Available online: https://www.iucnredlist.org/species/16084584/70566762 (accessed on 22 October 2024). [CrossRef]
- Reinhardt, K.; Köhler, G.; Maas, S.; Detzel, P. Low dispersal ability and habitat specificity promote extinctions in rare but not in widespread species: The Orthoptera of Germany. Ecography 2005, 28, 593–602. [Google Scholar] [CrossRef]
- Strid, A. Mountain Flora of Greece; CUP Archive: Cambridge, UK, 1986; Volume 1. [Google Scholar]
- Marchi, M.; Castellanos-Acuña, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A. ClimateEU, scale-free climate normals, historical time series, and future projections for Europe. Sci. Data 2020, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.; Philipps, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling, R Package Version 1.1-4; R Core Team: Cary, CA, USA, 2017.
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography 2018, 41, 291–307. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T.; Spittlehouse, D.L.; Murdock, T.Q. A Comprehensive, High-Resolution Database of Historical and Projected Climate Surfaces for Western North America. Bull. Am. Meteorol. Soc. 2013, 94, 1307–1309. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Murdock, T.Q. ClimateWNA—High-Resolution Spatial Climate Data for Western North America. J. Appl. Meteorol. Climatol. 2012, 51, 16–29. [Google Scholar] [CrossRef]
- Hijmans, R. terra: Spatial Data Analysis, R Package Version 1.7-46; R Core Team: Cary, CA, USA, 2023.
- Evans, J.S. spatialEco, R Package Version 1.2-0; R Core Team: Cary, CA, USA, 2019.
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Benito, B. collinear: R Package for Seamless Multicollinearity Management; Zonodo: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Dauby, G.; Lima, R.A.F.d. ConR: Computation of Parameters Used in Preliminary Assessment of Species Conservation Status, R package version 2.1; R Core Team: Cary, CA, USA, 2024. Available online: https://gdauby.github.io/ConR/ (accessed on 16 October 2024).
- Burgman, M.A.; Fox, J.C. Bias in species range estimates from minimum convex polygons: Implications for conservation and options for improved planning. Anim. Conserv. 2003, 6, 19–28. [Google Scholar] [CrossRef]
- Jetz, W.; Sekercioglu, C.H.; Watson, J.E.M. Ecological Correlates and Conservation Implications of Overestimating Species Geographic Ranges. Conserv. Biol. 2008, 22, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Duarte Ritter, C.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R. CoordinateCleaner: Standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Smith, A.B. enmSdm: Tools for Modeling Species Niches and Distributions, R Package Version 0.5.1.5; R Core Team: Cary, CA, USA, 2020.
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R package for assessing and improving data quality of occurrence record datasets. Ecography 2016, 39, 394–401. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. blockCV: An r package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Methods Ecol. Evol. 2019, 10, 225–232. [Google Scholar] [CrossRef]
- Dubos, N.; Préau, C.; Lenormand, M.; Papuga, G.; Monsarrat, S.; Denelle, P.; Louarn, M.L.; Heremans, S.; May, R.; Roche, P.; et al. Assessing the effect of sample bias correction in species distribution models. Ecol. Indic. 2022, 145, 109487. [Google Scholar] [CrossRef]
- Inman, R.; Franklin, J.; Esque, T.; Nussear, K. Comparing sample bias correction methods for species distribution modeling using virtual species. Ecosphere 2021, 12, e03422. [Google Scholar] [CrossRef]
- Velazco, S.J.E.; Rose, M.B.; de Andrade, A.F.A.; Minoli, I.; Franklin, J. flexsdm: An r package for supporting a comprehensive and flexible species distribution modelling workflow. Methods Ecol. Evol. 2022, 13, 1661–1669. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Guisan, A. ecospat: Spatial Ecology Miscellaneous Methods, R package version 3.2; R Core Team: Cary, CA, USA, 2021.
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Modelling species presence-only data with random forests. Ecography 2021, 44, 1731–1742. [Google Scholar] [CrossRef]
- Collart, F.; Hedenäs, L.; Broennimann, O.; Guisan, A.; Vanderpoorten, A. Intraspecific differentiation: Implications for niche and distribution modelling. J. Biogeogr. 2021, 48, 415–426. [Google Scholar] [CrossRef]
- Konowalik, K.; Nosol, A. Evaluation metrics and validation of presence-only species distribution models based on distributional maps with varying coverage. Sci. Rep. 2021, 11, 1482. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Velazco, S.J.E.; Rose, M.B.; De Marco, P., Jr.; Regan, H.M.; Franklin, J. How far can I extrapolate my species distribution model? Exploring shape, a novel method. Ecography 2024, 2024, e06992. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Cook, R.D. Cook’s Distance. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 301–302. [Google Scholar]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.H.; White, J.-S.S. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference, R package version 1.47.5; R Core Team: Cary, CA, USA, 2022. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 20 October 2024).
- Richards, S.A. Testing ecological theory using the information-theoretic approach: Examples and cautionary results. Ecology 2005, 86, 2805–2814. [Google Scholar] [CrossRef]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 October 2024).
- Jones, D.G.; Kobelt, J.; Ross, J.M.; Powell, T.H.Q.; Prior, K.M. Latitudinal gradient in species diversity provides high niche opportunities for a range-expanding phytophagous insect. J. Anim. Ecol. 2022, 91, 2037–2049. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, M.; Dudaniec, R.Y.; Neu, A.; Lessard, J.-P.; Bridle, J.; Carbonell, J.A.; Diamond, S.E.; Marshall, K.E.; Parmesan, C.; Singer, M.C.; et al. The importance of eco-evolutionary dynamics for predicting and managing insect range shifts. Curr. Opin. Insect Sci. 2022, 52, 100939. [Google Scholar] [CrossRef]
- Illich, I.; Zuna-Kratky, T. Population dynamics of an alpine grasshopper (Orthoptera) community over 30 years and the effects of climate warming and grazing. J. Insect Conserv. 2022, 26, 435–451. [Google Scholar] [CrossRef]
- Samways, M.J.; Pryke, J.S. Large-scale ecological networks do work in an ecologically complex biodiversity hotspot. Ambio 2016, 45, 161–172. [Google Scholar] [CrossRef]
- Nolte, D.; Boutaud, E.; Kotze, D.J.; Schuldt, A.; Assmann, T. Habitat specialization, distribution range size and body size drive extinction risk in carabid beetles. Biodivers. Conserv. 2019, 28, 1267–1283. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide decline of the entomofauna: A review of its drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Tucker, C.M. Difficult decisions: Strategies for conservation prioritization when taxonomic, phylogenetic and functional diversity are not spatially congruent. Biol. Conserv. 2018, 225, 128–133. [Google Scholar] [CrossRef]
- Kenyeres, Z.; Bauer, N. Conservation possibilities of Isophya costata (Orthoptera: Tettigoniidae: Phaneropterinae) based on frequency, population size, and habitats. J. Orthoptera Res. 2021, 30, 35–41. [Google Scholar] [CrossRef]
- Deveson, E. Satellite normalized difference vegetation index data used in managing Australian plague locusts. J. Appl. Remote Sens. 2013, 7, 075096. [Google Scholar] [CrossRef]
- Levanoni, O.; Levin, N.; Pe’er, G.; Turbé, A.; Kark, S. Can we predict butterfly diversity along an elevation gradient from space? Ecography 2011, 34, 372–383. [Google Scholar] [CrossRef]
- Njovu, H.K.; Steffan-Dewenter, I.; Gebert, F.; Schellenberger Costa, D.; Kleyer, M.; Wagner, T.; Peters, M.K. Plant traits mediate the effects of climate on phytophagous beetle diversity on Mt. Kilimanjaro. Ecology 2021, 102, e03521. [Google Scholar] [CrossRef] [PubMed]
- Dezsi, Ş.; Mîndrescu, M.; Petrea, D.; Rai, P.K.; Hamann, A.; Nistor, M.-M. High-resolution projections of evapotranspiration and water availability for Europe under climate change. Int. J. Climatol. 2018, 38, 3832–3841. [Google Scholar] [CrossRef]
- Unnisa, Z.; Govind, A.; Lasserre, B.; Marchetti, M. Water Balance Trends along Climatic Variations in the Mediterranean Basin over the Past Decades. Water 2023, 15, 1889. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Petrlic, K.; Verachtert, E.; Bochet, E.; Poesen, J. Effects of slope angle and aspect on plant cover and species richness in a humid Mediterranean badland. Earth Surf. Process. Landf. 2014, 39, 1705–1716. [Google Scholar] [CrossRef]
- Scherrer, D.; Schmid, S.; Körner, C. Elevational species shifts in a warmer climate are overestimated when based on weather station data. Int. J. Biometeorol. 2011, 55, 645–654. [Google Scholar] [CrossRef]
- Chappell, M.A. Metabolism and thermoregulation in desert and montane grasshoppers. Oecologia 1983, 56, 126–131. [Google Scholar] [CrossRef]
- O’Neill, K.M.; Rolston, M.G. Short-term dynamics of behavioral thermoregulation by adults of the grasshopper Melanoplus sanguinipes. J. Insect Sci. 2007, 7, 27. [Google Scholar] [CrossRef]
- Samietz, J.; Salser, M.A.; Dingle, H. Altitudinal variation in behavioural thermoregulation: Local adaptation vs. plasticity in California grasshoppers. J. Evol. Biol. 2005, 18, 1087–1096. [Google Scholar] [CrossRef]
- König, S.; Krauss, J.; Classen, A.; Hof, C.; Prietzel, M.; Wagner, C.; Steffan-Dewenter, I. Micro- and macroclimate interactively shape diversity, niches and traits of Orthoptera communities along elevational gradients. Divers. Distrib. 2024, 30, e13810. [Google Scholar] [CrossRef]
- Pitteloud, C.; Descombes, P.; Sànchez-Moreno, S.; Kergunteuil, A.; Ibanez, S.; Rasmann, S.; Pellissier, L. Contrasting responses of above- and below-ground herbivore communities along elevation. Oecologia 2020, 194, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Berner, D.; Körner, C.; Blanckenhorn, W.U. Grasshopper populations across 2000 m of altitude: Is there life history adaptation? Ecography 2004, 27, 733–740. [Google Scholar] [CrossRef]
- Wilson, R.J.; Bennie, J.; Lawson, C.R.; Pearson, D.; Ortúzar-Ugarte, G.; Gutiérrez, D. Population turnover, habitat use and microclimate at the contracting range margin of a butterfly. J. Insect Conserv. 2015, 19, 205–216. [Google Scholar] [CrossRef]
- Schaffers, A.P.; Raemakers, I.P.; Sýkora, K.V.; ter Braak, C.J.F. Arthropod assemblages are best predicted by plant species composition. Ecology 2008, 89, 782–794. [Google Scholar] [CrossRef]
- Willemse, L.; Tilmans, J.; Kotitsa, N.; Trichas, A.; Heller, K.G.; Chobanov, D.; Odé, B. A review of Eupholidoptera (Orthoptera, Tettigoniidae) from Crete, Gavdos, Gavdopoula, and Andikithira. ZooKeys 2023, 1151, 67–158. [Google Scholar] [CrossRef]
- Gardiner, T. Hillside lagomorph grazing and its influence on Orthoptera. J. Orthoptera Res. 2022, 31, 157–162. [Google Scholar] [CrossRef]
- Neilly, H.; Jones, H.; Schwarzkopf, L. Ants drive invertebrate community response to cattle grazing. Agric. Ecosyst. Environ. 2020, 290, 106742. [Google Scholar] [CrossRef]
- Spalinger, L.C.; Haynes, A.G.; Schütz, M.; Risch, A.C. Impact of wild ungulate grazing on Orthoptera abundance and diversity in subalpine grasslands. Insect Conserv. Divers. 2012, 5, 444–452. [Google Scholar] [CrossRef]
- NOA. National Observatory of Athens. Available online: https://www.noa.gr (accessed on 23 October 2024).
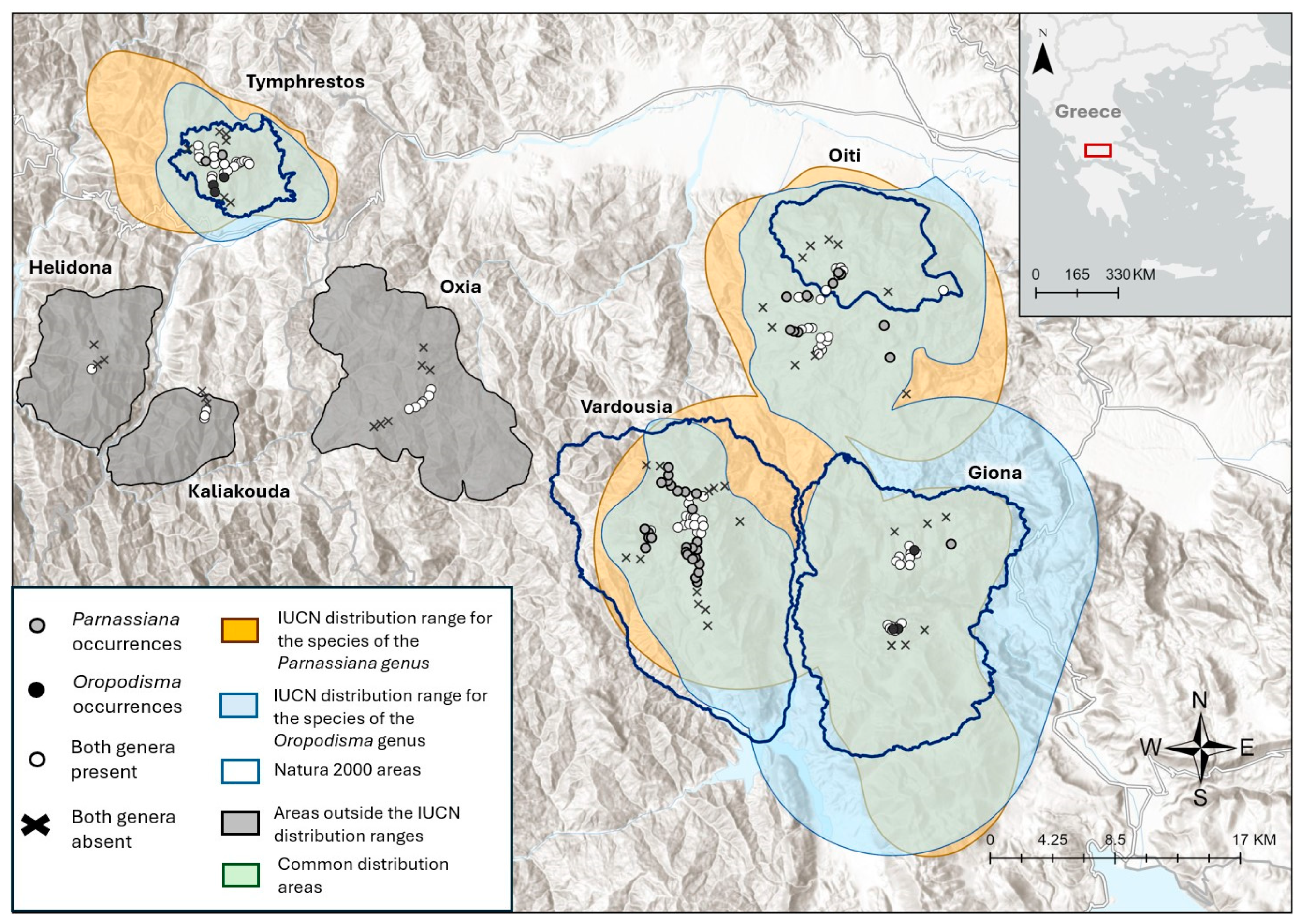
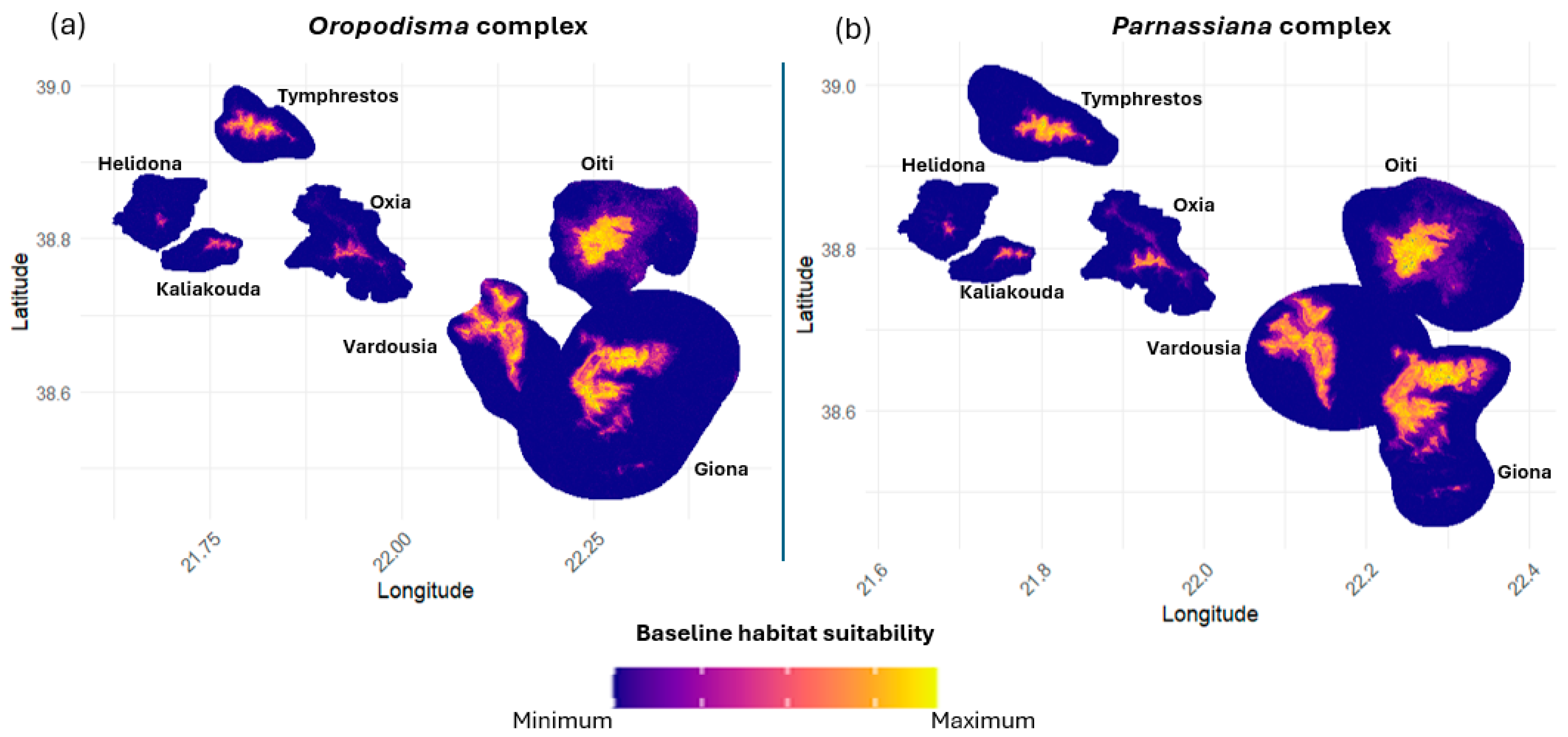
| Genus | Species | N (n) | Presence in Sites Sampled (%) | Distribution Range (km2) | Population Density (ind/m2) |
|---|---|---|---|---|---|
| Parnassiana | P. coracis | 49 (47) | 28 | 896 | 7.2 (±6.9) |
| P. tymphrestos | 57 (54) | 33 | 2138 | 12.2 (±13.4) | |
| P. gionica | 16 (16) | 9 | 474 | 3.8 (±2.2) | |
| Subtotal | 122 (117) | 70 | 2802 | 8.9 (±10.6) | |
| Oropodisma | O. willemsei | 66 (63) | 38 | 2114 | 8.5 (±9.6) |
| O. tymphrestosi | 23 (23) | 13 | 410 | 2.5 (±8.1) | |
| Subtotal | 89 (86) | 51 | 2438 | 8.5 (±9.6) |
| Species | P. coracis | P. tymphrestos | P. gionica | O. willemsei | O. tymphrestosi |
|---|---|---|---|---|---|
| P. coracis | - | ||||
| P. tymphrestos | 0 (25.4) | - | |||
| P. gionica | 0 (13.8) | 0 (23.8) | - | ||
| O. willemsei | 34.8 (41.3) | 50.8 (68.8) | 27.7 (21.1) | - | |
| O. tymphrestosi | 0 (1.1) | 36.8 (19.9) | 0 (0) | 0 (4.7) | - |
| Genus | Taxa | NDVI | PETDQ (mm/month) | PDQ (mm) | PETWQ (mm/month) |
|---|---|---|---|---|---|
| Parnassiana | Parnassiana complex | 0.2–0.4 | 100–200 | 133–147 | - |
| P. coracis | 0.2–0.4 | 100–200 | 133–136, 139–140 | - | |
| P. tymphrestos | 0.3–0.4 | 150–200 | 143–147 | - | |
| P. gionica | 0.3–0.4 | 150–200 | 143–147 | - | |
| Oropodisma | Oropodisma complex | 0.2–0.4 | 100–220 | - | 100–120, 190–240 |
| O. willemsei | 0.2–0.4 | 150–210 | - | 100–120, 190–240 | |
| O. tymphrestosi | 0.3–0.4 | 100–200 | - | 100–120, 190–240 |
| Genus | Taxa | Variable | Coefficient | Pr (>|z|) | Cumulative Weight |
|---|---|---|---|---|---|
| Parnassiana | Parnassiana complex | Alt | 0.0018 | 0.0028 * | 0.796 |
| St | −0.0173 | 0.0037 * | 0.228 | ||
| Rpmean | −0.0070 | 0.1684 | 0.154 | ||
| Ghmean | −0.0035 | 0.3143 | 0.120 | ||
| P. coracis | Alt | 0.0035 | 0.00003 *** | 0.911 | |
| Sl | 0.0131 | 0.0849 | 0.235 | ||
| St | −0.0144 | 0.1192 | 0.163 | ||
| Rpmean | −0.0074 | 0.2059 | 0.112 | ||
| P. tymphrestos | St | −0.0278 | 0.0012 ** | 0.916 | |
| Ghmean | −0.0053 | 0.1407 | 0.187 | ||
| R | −0.0186 | 0.1960 | 0.185 | ||
| Slope | −0.0125 | 0.3440 | 0.125 | ||
| P. gionica | St | −0.1003 | 0.0275 * | 1 | |
| R | 0.1808 | 0.0584 | 0.654 | ||
| Sl | 0.0675 | 0.0822 | 0.086 | ||
| Oropodisma | Oropodisma complex | Slope | 0.0174 | 0.0208 * | 0.969 |
| Ghmean | −0.0215 | 0.0550 | 0.197 | ||
| R | −0.0214 | 0.0892 | 0.149 | ||
| Hcover | −0.0085 | 0.2298 | 0.139 | ||
| So | 0.0398 | 0.2448 | 0.120 | ||
| Rpmean | 0.0398 | 0.3620 | 0.118 | ||
| O. willemsei | R | −0.3394 | 0.0059 ** | 0.625 | |
| Sl | 0.0169 | 0.0266 * | 0.316 | ||
| Ghmean | −0.0246 | 0.0519 | 0.192 | ||
| Rpmean | −0.0179 | 0.0540 | 0.117 | ||
| O. tymphrestosi | So | −0.1502 | 0.0058 ** | 0.5960 | |
| Hcover | 0.0165 | 0.1391 | 0.2290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanidis, A.; Kougioumoutzis, K.; Zografou, K.; Fotiadis, G.; Willemse, L.; Tzortzakaki, O.; Kati, V. Distribution Patterns and Habitat Preferences of Five Globally Threatened and Endemic Montane Orthoptera (Parnassiana and Oropodisma). Ecologies 2025, 6, 5. https://doi.org/10.3390/ecologies6010005
Stefanidis A, Kougioumoutzis K, Zografou K, Fotiadis G, Willemse L, Tzortzakaki O, Kati V. Distribution Patterns and Habitat Preferences of Five Globally Threatened and Endemic Montane Orthoptera (Parnassiana and Oropodisma). Ecologies. 2025; 6(1):5. https://doi.org/10.3390/ecologies6010005
Chicago/Turabian StyleStefanidis, Apostolis, Konstantinos Kougioumoutzis, Konstantina Zografou, Georgios Fotiadis, Luc Willemse, Olga Tzortzakaki, and Vassiliki Kati. 2025. "Distribution Patterns and Habitat Preferences of Five Globally Threatened and Endemic Montane Orthoptera (Parnassiana and Oropodisma)" Ecologies 6, no. 1: 5. https://doi.org/10.3390/ecologies6010005
APA StyleStefanidis, A., Kougioumoutzis, K., Zografou, K., Fotiadis, G., Willemse, L., Tzortzakaki, O., & Kati, V. (2025). Distribution Patterns and Habitat Preferences of Five Globally Threatened and Endemic Montane Orthoptera (Parnassiana and Oropodisma). Ecologies, 6(1), 5. https://doi.org/10.3390/ecologies6010005









