Leaf Trait Variability and CSR Strategy Shifts in Mediterranean Woody Species along an Edaphic Gradient
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
2.2. Soil Analyses
2.3. Leaf Trait Measurements and CSR Strategy Evaluation
2.4. Data Analyses
3. Results
3.1. Soil Properties
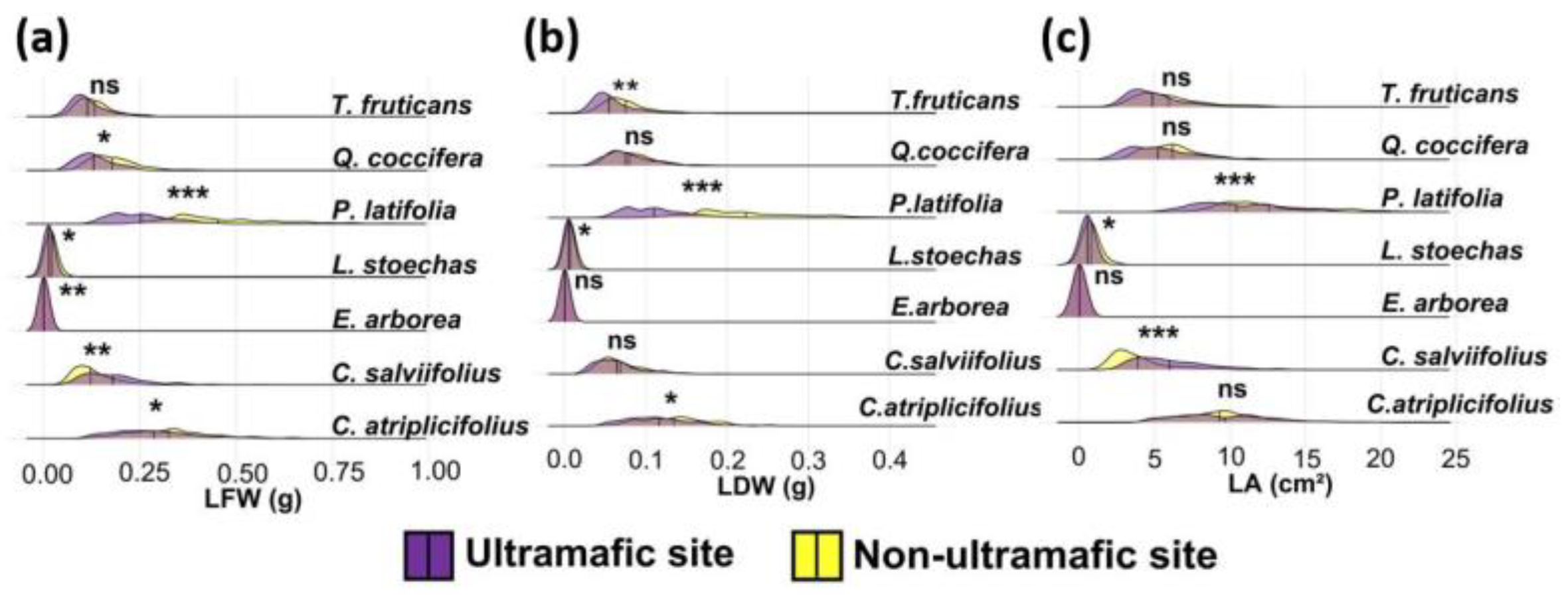
3.2. Intraspecific Variability in Leaf Traits
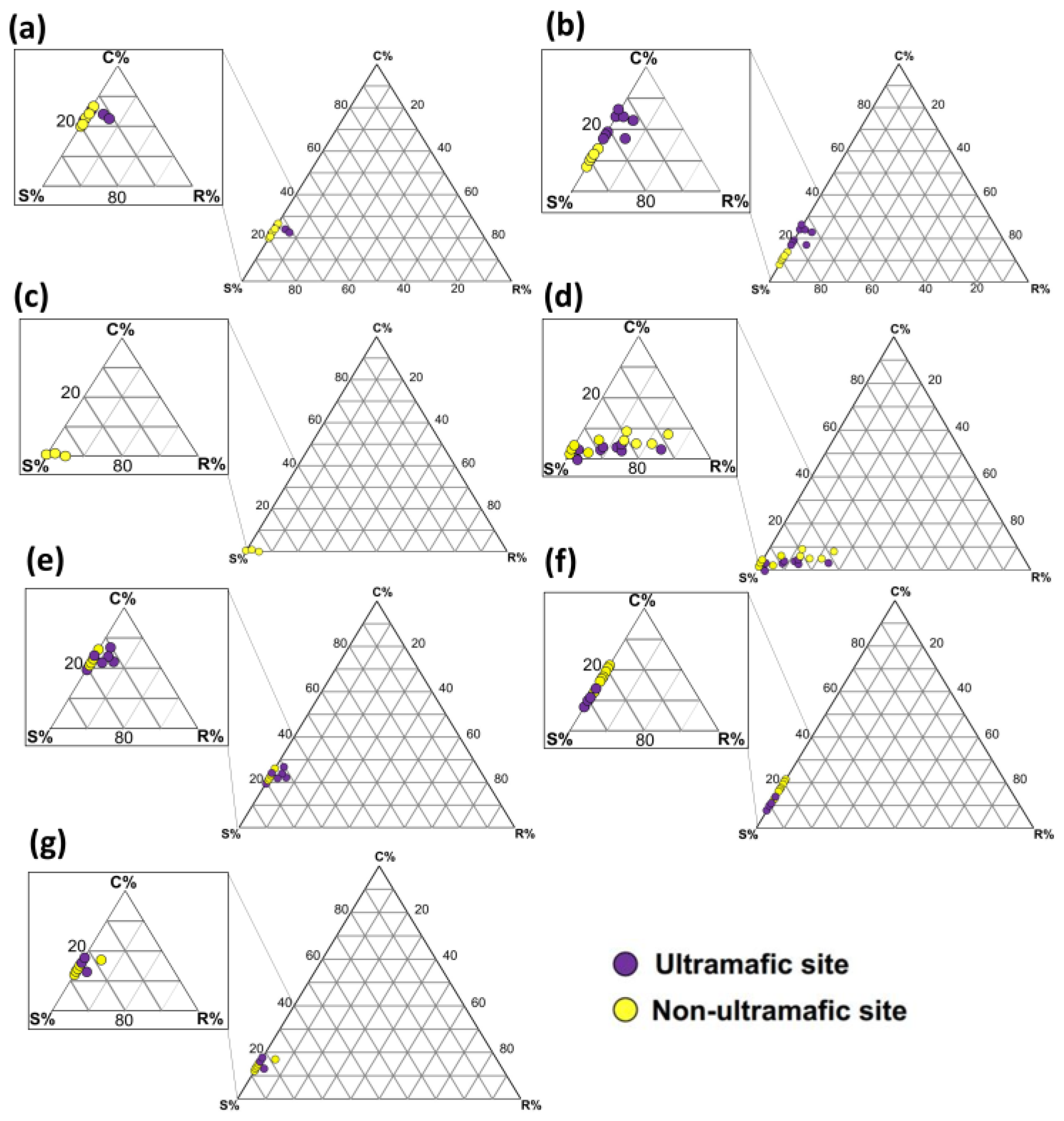
3.3. Intraspecific Variability in CSR Strategies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittermeier, R.A.; Gil, P.R.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A.B. Hotspots Revisited—Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Harrison, S.; Rajakaruna, N. What Have We Learned from Serpentine in Evolution, Ecology, and Other Sciences? In Serpentine: The Evolution and Ecology of a Model System; University of California Press: Berkeley, CA, USA, 2011; ISBN 0520268350. [Google Scholar] [CrossRef]
- Damschen, E.I.; Harrison, S.; Going, B.M.; Anacker, B.L. Climate change and plant communities on unusual soils. In Serpentine: The Evolution and Ecology of a Model System; Harrison, S., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 359–382. ISBN 0520268350. [Google Scholar] [CrossRef]
- Hjort, J.; Gordon, J.E.; Gray, M.; Hunter, M.L., Jr. Why geodiversity matters in valuing nature’s stage. Conserv. Biol. 2015, 29, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.R. Serpentine and Its Vegetation: A Multidisciplinary Approach; Croom Helm: London, UK, 1987; ISBN 0709950632. [Google Scholar]
- Lefèbvre, C.; Vernet, P. Microevolutionary processes on contaminated deposits. In Heavy Metal Tolerance in Plants: Evolutionary Aspects; Shaw, A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1990; pp. 285–299. Available online: https://books.google.co.ma/books?hl=fr&lr=&id=kvsPo4Et5scC&oi=fnd&pg=PA285&dq=lefebvre+and+vernet+1990&ots=wH131FvRS0&sig=pmFrD9mvkM8caUmNZj08EUn7rw&redir_esc=y#v=onepage&q&f=false (accessed on 14 July 2024).
- Garnier, J.; Quantin, C.; Guimarães, E.; Garg, V.K.; Martins, E.S.; Becquer, T. Understanding the Genesis of Ultramafic Soils and Catena Dynamics in Niquelândia, Brazil. Geoderma 2009, 151, 204–214. [Google Scholar] [CrossRef]
- Coleman, R.G.; Jove, C. Geological Origin of Serpentinites. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Ltd.: Andover, UK, 1993; pp. 1–18. [Google Scholar]
- Echevarria, G. Genesis and Behaviour of Ultramafic Soils and Consequences for Nickel Biogeochemistry. In Agromining: Farming for Metals; van der Ent, A., Baker, A.J., Echevarria, G., Simonnot, M.O., Morel, J.L., Eds.; Mineral Resource Reviews; Springer: Cham, Germen, 2021. [Google Scholar] [CrossRef]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D., Jr. Evolutionary Ecology of Plant Adaptation to Serpentine Soils. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Kruckeberg, A.R. An essay: The stimulus of unusual geologies for plant speciation. Syst. Bot. 1986, 11, 455–463. [Google Scholar] [CrossRef]
- Adamidis, C.; Kazakou, E.; Baker, A.J.M.; Reeves, R.D.; Dimitrakopoulos, P.G. The effect of harsh abiotic conditions on the diversity of serpentine plant communities on Lesbos, an eastern Mediterranean island. Plant Ecol. Divers. 2014, 7, 433–444. [Google Scholar] [CrossRef]
- El Ghalabzouri, A.; Ajbilou, R.; Mariotti, M.G.; Targuisti, K.; Ater, M. Vegetation of Beni Bousera (northern Morocco) ultramafic soils and adjacent non-ultramafic soils in relation to edaphic factors. Aust. J. Bot. 2015, 63, 353–366. [Google Scholar] [CrossRef]
- Kazakou, E.; Dimitrakopoulos, P.G.; Baker, A.J.M.; Reeves, R.D.; Troumbis, A.Y. Hypotheses, mechanisms and tradeoffs of tolerance and adaptation to serpentine soils: From species to ecosystem level. Biol. Rev. 2008, 83, 495–508. [Google Scholar] [CrossRef]
- O’Dell, R.E.; Rajakaruna, N. Intraspecific variation, adaptation, and evolution. In Serpentine: Evolution and Ecology in a Model System; Harrison, S., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011. [Google Scholar] [CrossRef]
- Rajakaruna, N.; Bradfield, G.E.; Bohm, B.A.; Whitton, J. Adaptive differentiation in response to water stress by edaphic races of Lasthenia california (Asteraceae). Int. J. Plant Sci. 2003, 164, 371–376. [Google Scholar] [CrossRef][Green Version]
- Stevanović, V.; Tan, K.; Iatrou, G. Distribution of the endemic Balkan flora on serpentine I.—Obligate serpentine endemics. Plant Syst. Evol. 2003, 242, 149–170. [Google Scholar] [CrossRef]
- Chakkour, S.; Kassout, J.; Kadaoui, K.; El Ghalabzouri, A.; Sahli, A.; Kadiri, M.; Ater, M. Arable plant communities of ultramafic and non-ultramafic soils in Beni Bousera (North Morocco). Community Ecol. 2023, 24, 171–187. [Google Scholar] [CrossRef]
- El Ghalabzouri, A.; Kassout, J.; Khamlichi, A.; Ajbilou, R.; Ater, M. Diversity and structure of woody vegetation of the Beni Bousera massif, northern Morocco: Conservation interest. Euro-Mediterr. J. Environ. Integr. 2023, 8, 863–873. [Google Scholar] [CrossRef]
- Selvi, F. Diversity, geographic variation and conservation of the serpentine flora of Tuscany (Italy). Biodivers. Conserv. 2007, 16, 1423–1439. [Google Scholar] [CrossRef]
- Pérez-Latorre, A.V.; Hidalgo-Triana, N.; Cabezudo, B. Composition, ecology and conservation of the south-Iberian serpentine flora in the context of the Mediterranean basin. An. Jard. Bot. Madr. 2013, 70, 62–71. [Google Scholar]
- Moreno-Saiz, J.C.; Martínez García, F.; Gavilán, R.G. Plant Conservation in Spain: Strategies to halt the loss of plant diversity. Mediterr. Bot. 2018, 39, 65–66. [Google Scholar] [CrossRef]
- Lavorel, S.; McIntyre, S.; Landsberg, J.; Forbes, T.D.A. Plant functional classifications: From general groups to specific groups based on response to disturbance. Trends Ecol. Evol. 1997, 12, 474–478. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Udayakumar, M.; Sekar, T. Leaf Traits of Trees in Tropical Dry Evergreen Forests of Peninsular India. Ecologies 2021, 2, 268–284. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies, Vegetation Processes and Ecosystem Properties; Wiley: Winchester, MA, USA, 2001. [Google Scholar]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Díaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Wilson, P.J.; Hunt, R.; Grime, J.P.; Thompson, K. Allocating C-S-R plant functional types: A soft approach to a hard problem. Oikos 1999, 85, 282–294. [Google Scholar] [CrossRef]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L. Allocating CSR plant functional types: The use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef]
- Caccianiga, M.; Luzzaro, A.; Pierce, S.; Ceriani, R.M.; Cerabolini, B. The functional basis of a primary succession resolved by CSR classification. Oikos 2006, 112, 10–20. [Google Scholar] [CrossRef]
- Cerabolini, B.E.; Brusa, G.; Ceriani, R.M.; De Andreis, R.; Luzzaro, A.; Pierce, S. Can CSR classification be generally applied outside Britain? Plant Ecol. 2010, 210, 253–261. [Google Scholar] [CrossRef]
- Yildirmi, C.; Karavin, N.; Cansaran, A. Classification and evaluation of some endemic plants from Turkey using Grime’s CSR strategies. Eurasia J. Biosci. 2012, 6, 97–104. [Google Scholar] [CrossRef]
- Violle, C.; Borgy, B.; Choler, P. Trait databases: Misuses and precautions. J. Veg. Sci. 2015, 26, 826–827. [Google Scholar] [CrossRef]
- Gervais-Bergeron, B.; Paul, A.L.; Chagnon, P.L.; Baker, A.J.; van der Ent, A.; Faucon, M.P.; Quintela-Sabarís, C.; Labrecque, M. Trace element hyperaccumulator plant traits: A call for trait data collection. Plant Soil 2023, 488, 187–196. [Google Scholar] [CrossRef]
- Von Wettberg, E.J.; Ray-Mukherjee, J.; D’Adesky, N.; Nesbeth, D.; Sistla, S. The evolutionary ecology and genetics of stress resistance syndrome (SRS) traits: Revisiting Chapin, Autumn and Pugnaire (1993). In Plant Ecology and Evolution in Harsh Environments; Rajakaruna, N., Boyd, R.S., Harris, T., Eds.; Nova Science Publishers: New York, NY, USA, 2014; pp. 201–226. [Google Scholar]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.; Diemer, M.; et al. The world-wide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Freschet, G.T.; Valverde-Barrantes, O.J.; Tucker, C.M.; Craine, J.M.; McCormack, M.L.; Violle, C.; Fort, F.; Blackwood, C.B.; Urban-Mead, K.R.; Iversen, C.M.; et al. Climate, soil, and plant functional types as drivers of global fine-root trait variation. J. Ecol. 2017, 105, 1182–1196. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Douzet, R.; Aubert, S.; Lavorel, S. A Multi-Trait Approach Reveals the Structure and the Relative Importance of Intra- vs. Interspecific Variability in Plant Traits. Funct. Ecol. 2010, 24, 1192–1201. [Google Scholar] [CrossRef]
- Albert, C.H.; Thuiller, W.; Yoccoz, N.G.; Soudant, A.; Boucher, F.; Saccone, P.; Lavorel, S. Intraspecific Functional Variability: Extent, Structure and Sources of Variation. J. Ecol. 2010, 98, 604–613. [Google Scholar] [CrossRef]
- Kattge, J.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY—A global database of plant traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Lange, B.; Faucon, M.-P.; Delhaye, G.; Hamiti, N.; Meerts, P. Functional traits of a facultative metallophyte from tropical Africa: Population variation and plasticity in response to cobalt. Environ. Exp. Bot. 2017, 136, 1–8. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Lechowicz, M.J. How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett. 2010, 13, 838–848. [Google Scholar] [CrossRef]
- Kassout, J.; Terral, J.-F.; Hodgson, J.G.; Ater, M. Trait-Based Plant Ecology a Flawed Tool in Climate Studies? The Leaf Traits of Wild Olive That Pattern with Climate Are Not Those Routinely Measured. PLoS ONE 2019, 14, e0219908. [Google Scholar] [CrossRef] [PubMed]
- Kassout, J.; Hmimsa, Y.; Fatehi, S.E.; Kadaoui, K.; Houssni, M.; Chakkour, S.; Sahli, A.; El Chami, M.A.; Ariza-Mateos, D.; Palacios-Rodríguez, G.; et al. Aridity Gradients Shape Intraspecific Variability of Morphological Traits in Native Ceratonia siliqua L. of Morocco. Plants 2023, 12, 3447. [Google Scholar] [CrossRef] [PubMed]
- Kassout, J.; Terral, J.F.; Souali, H.; Ater, M. Environment-dependent and intraspecific variations in leaf and size traits of a native wild olive (Olea europaea L.) along an aridity gradient in Morocco: A functional perspective. Plant Ecol. 2024, 225, 1–17. [Google Scholar] [CrossRef]
- May, R.L.; Warner, S.; Wingler, A. Classification of intra-specific variation in plant functional strategies reveals adaptation to climate. Ann. Bot. 2017, 119, 1343–1352. [Google Scholar] [CrossRef]
- Giupponi, L. Intraspecific variation in functional strategy and leaf shape of Campanula elatinoides reveals adaptation to climate. Flora 2020, 268, 151605. [Google Scholar] [CrossRef]
- Baltieri, M.; Fantinato, E.; Del Vecchio, S.; Buffa, G. Intraspecific variability of leaf traits and functional strategy of Himantoglossum adriaticum H. Baumann. Plant Sociol. 2020, 57, 105–112. [Google Scholar] [CrossRef]
- Astuti, G.; Ciccarelli, D.; Roma-Marzio, F.; Trinco, A.; Peruzzi, L. Narrow endemic species Bellevalia webbiana shows significant intraspecific variation in tertiary CSR strategy. Plant Biosyst. 2019, 153, 12–18. [Google Scholar] [CrossRef]
- Lazzaro, L.; Colzi, I.; Ciampi, D.; Gonnelli, C.; Lastrucci, L.; Bazihizina, N.; Viciani, D.; Coppi, A. Intraspecific trait variability and genetic diversity in the adaptive strategies of serpentine and non-serpentine populations of Silene paradoxa L. Plant Soil 2021, 460, 105–121. [Google Scholar] [CrossRef]
- Richardson, S.J.; Allen, R.B.; Doherty, J.E. Shifts in leaf N:P ratio during resorption reflect soil P in temperate rainforest. Funct. Ecol. 2008, 22, 738–745. [Google Scholar] [CrossRef]
- Jung, V.; Violle, C.; Mondy, C.P.; Hoffmann, L.; Muller, S.D. Intraspecific variability and trait-based community assembly. J. Ecol. 2010, 98, 1134–1140. [Google Scholar] [CrossRef]
- Kumordzi, B.B.; Nilsson, M.C.; Gundale, M.J.; Wardle, D.A. Changes in local-scale intraspecific trait variability of dominant species across contrasting island ecosystems. Ecosphere 2014, 5, 1–17. [Google Scholar] [CrossRef]
- Iozia, L.M.; Varone, L. Tackling local ecological homogeneity: Finding intraspecific trait variability in local populations of Mediterranean plants. Ecol. Evol. 2023, 13, e10550. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. World Clim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Manthei, C.D. Geochemical Properties of the Beni Bousera (N. Morocco) Peridotites: A Field and Laboratory Approach to Understanding Melt Infiltration and Extraction in an Orogenic Peridotite Massif. Ph.D. Dissertation, Massachusetts Institute of Technology, Cambridge, MA, USA, 2012. [Google Scholar]
- Reuber, I.; Michard, A.; Chalouan, A.; Juteau, T.; Jermoumi, B. Structure and emplacement of the Alpine-type peridotites from Beni Bousera, Rif, Morocco: A polyphase tectonic interpretation. Tectonophysics 1982, 82, 231–251. [Google Scholar] [CrossRef]
- Saddiqi, O.; Reuber, I.; Michard, A. Sur la tectonique de dénudation du manteau infracontinental dans les Béni Bousera, Rif septentrional, Maroc. C. R. Acad. Sci. 1988, 307, 657–662. [Google Scholar]
- Saddiqi, O.; Feinberg, H.; El Azzab, D.; Michard, A. Paléomagnétisme des péridotites des Beni Bousera (Rif interne, Maroc): Conséquences pour l’évolution miocène de l’Arc de Gibraltar. C. R. Acad. Sci. 1995, 321, 361–368, Série 2. Sciences de la terre et des planètes. [Google Scholar]
- Barbero, M.; Quezel, P.; Rivas-Martinez, S. Contribution à l’étude des groupements forestiers et préforestiers du Maroc. Phytocoenologia 1981, 9, 311–412. [Google Scholar] [CrossRef]
- Benabid, A. Étude Phyto-Écologique Biogéographique et Dynamique des Associations et Series Sylvatiques du Rif Occidental. Ph.D. Dissertation, Université Aix-Marseille-III, Aix-en-Provence, France, 1976. [Google Scholar]
- Quézel, P.; Barbero, M.; Benabid, A.; Loisel, R.; Rivas-Martinez, S. Contribution à l’étude des groupements pré-forestiers et des matorrals rifains. Ecol. Mediterr. 1988, 14, 77–122. [Google Scholar] [CrossRef]
- UNEP. World Atlas of Desertification; UNEP and E. Arnold Ltd.: Kent, UK, 1992; Volume 80. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Steege, H.T.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A handbook of protocols for standardized and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Methods of Soil Analysis, Chemical, and Microbiological Properties; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar]
- Norvell, W.A.; Lindsay, W.L. Reactions of DTPA chelates of iron, zinc, copper, and manganese with soils. Soil Sci. Soc. Am. J. 1972, 36, 778–783. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardized measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R Development Core Team. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 10 July 2024).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistical Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 4. [Google Scholar]
- Hammer, Ø. Reference Manual Paleontological Statistics Version 3.15; Natural History Museum University of Oslo: Oslo, Norway, 2017. [Google Scholar]
- Kazakou, E.; Adamidis, G.C.; Baker, A.J.; Reeves, R.D.; Godino, M.; Dimitrakopoulos, P.G. Species adaptation in serpentine soils in Lesbos Island (Greece): Metal hyperaccumulation and tolerance. Plant Soil 2010, 332, 369–385. [Google Scholar] [CrossRef]
- Navas, M.-L.; Roumet, C.; Bellmann, A.; Laurent, G.; Garnier, E. Suites of plant traits in species from different stages of a Mediterranean secondary succession. Plant Biol. 2010, 12, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulos, P.G.; Galanidis, A.; Siamantziouras, A.-S.D.; Troumbis, A.Y. Short-term invasibility patterns in burnt and unburnt experimental Mediterranean grassland communities of varying diversities. Oecologia 2005, 143, 428–437. [Google Scholar] [CrossRef]
- Spehn, E.M.; Scherer-Lorenzen, M.; Schmid, B.; Hector, A.; Caldeira, M.C.; Dimitrakopoulos, P.G.; Finn, J.A.; Jumpponen, A.; O’Donnovan, G.; Pereira, J.S.; et al. The role of legumes as a component of biodiversity in a cross-European study of grassland biomass nitrogen. Oikos 2002, 98, 205–218. [Google Scholar] [CrossRef]
- Ater, M.; Lefèbvre, C.; Gruber, W.; Meerts, P. A phytogeochemical survey of the flora of ultramafic and adjacent normal soils in North Morocco. Plant Soil 2000, 218, 127–135. [Google Scholar] [CrossRef]
- Hidalgo-Triana, N.; Pérez-Latorre, A.V.; Adomou, A.C.; Rudner, M.; Thorne, J.H. Adaptations to the stressful combination of serpentine soils and Mediterranean climate drive plant functional groups and trait richness. Front. Plant Sci. 2023, 14, 1040839. [Google Scholar] [CrossRef]
- Sambatti, J.B.; Rice, K.J. Functional ecology of ecotypic differentiation in the Californian serpentine sunflower (Helianthus exilis). New Phytol. 2007, 175, 107–119. [Google Scholar] [CrossRef]
- Wright, J.P.; Sutton-Grier, A. Does the leaf economic spectrum hold within local species pools across varying environmental conditions? Funct. Ecol. 2012, 26, 1390–1398. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Kazakou, E.; Fyllas, N.M.; Dimitrakopoulos, P.G. Species Adaptive Strategies and Leaf Economic Relationships across Serpentine and Non-Serpentine Habitats on Lesbos, Eastern Mediterranean. PLoS ONE 2014, 9, e96034. [Google Scholar] [CrossRef]
- Diaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.C.; Jalili, A.; Montserrat-Marti, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2015, 529, 167–171. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Nature 2017, 357, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.; Díaz-Burlinson, N.; Gachet, S. Congruency analysis of species ranking based on leaf traits: Which traits are the more reliable? Plant Ecol. 2004, 174, 37–48. [Google Scholar] [CrossRef]
- Anacker, B.L.; Rajakaruna, N.; Ackerly, D.D.; Harrison, S.; E Keeley, J.; Vasey, M.C. Ecological strategies in California chaparral: Interacting effects of soils, climate, and fire on specific leaf area. Plant Ecol. Divers. 2011, 4, 179–188. [Google Scholar] [CrossRef]
- Nguyen, H.; Maneepong, S.; Suraninpong, P. Effects of potassium, calcium and magnesium ratios in soil on their uptake and fruit quality of pummelo. J. Agric. Sci. 2017, 9, 110–121. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Dìaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [PubMed]
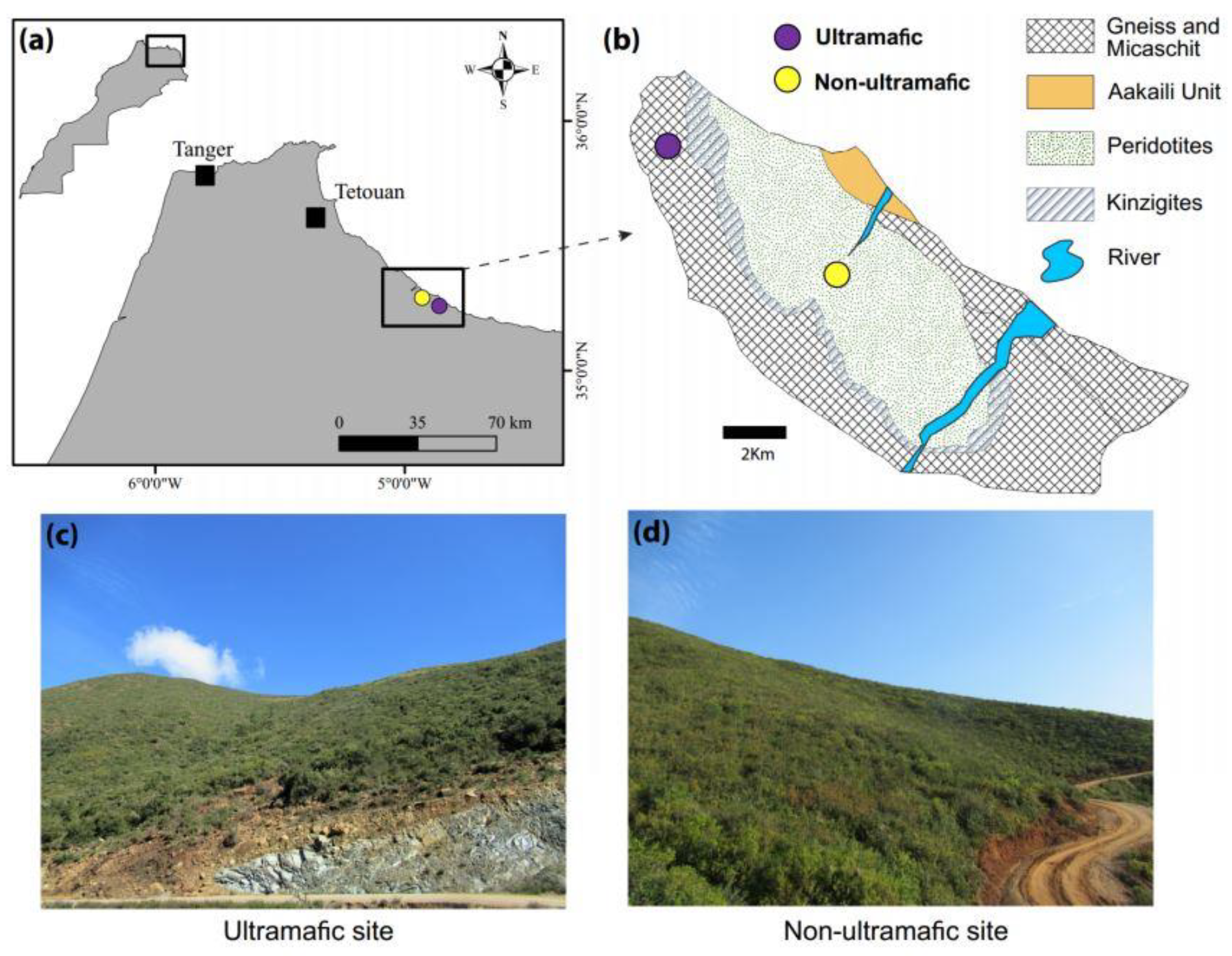
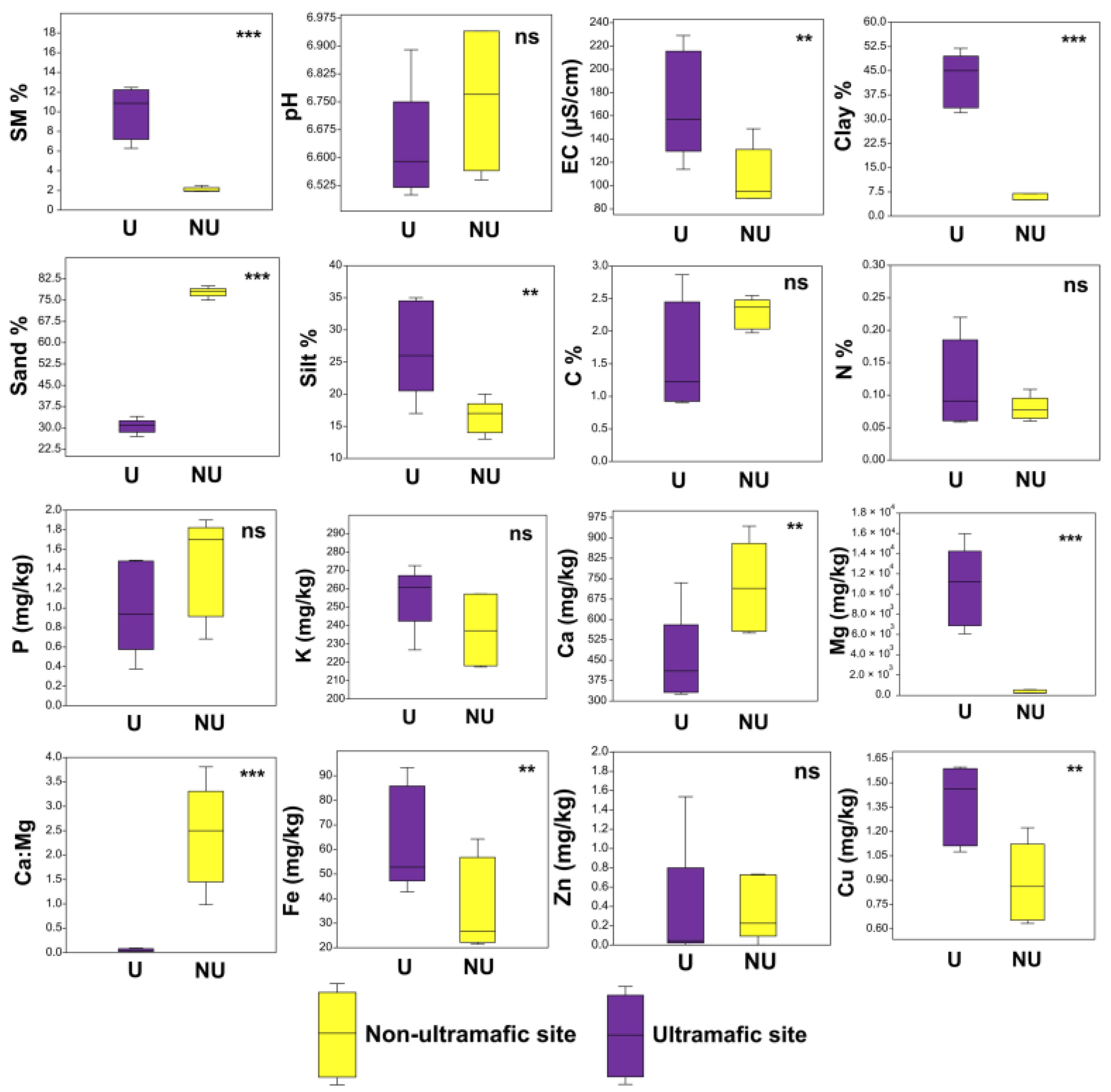
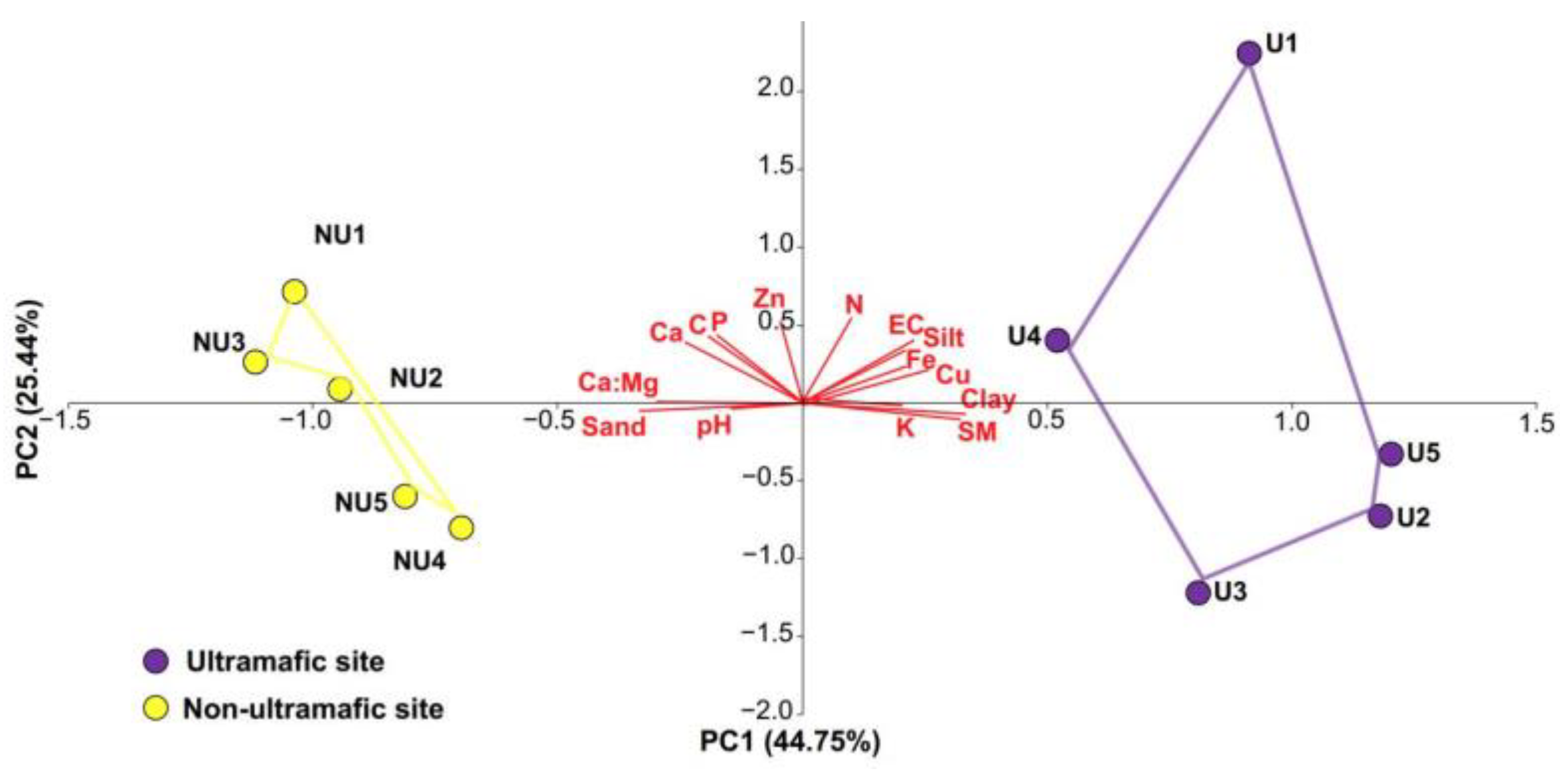
| Study Sites | Ultramafic (U) | Non-Ultramafic (NU) |
|---|---|---|
| Coordinates | 35.245°; −4.882° | 35.301°; −4.928° |
| Altitude (m) | 881 | 490 |
| Slope (°) | 25 | 15 |
| Orientation | Southeast | Southeast |
| Geology | Peridotites | Kinzigte and Micaschist |
| AI ** | 0.65 | 0.43 |
| Aridity class | Sub-humid | Semi-arid |
| Vegetation belt | Mesomediterranean | Thermomediterranean |
| Phytosociological association | Phyllireo latifoliae-Quercetum cocciferae [65] | Calicotomo intermediae-Tetraclinetum articulatae [64] |
| Species | Site | LFW (g) Mean ± SD | LDW (g) Mean ± SD | LA (cm2) Mean ± SD |
|---|---|---|---|---|
| C. atriplicifolius | U | 0.286 ± 0.042 | 0.117 ± 0.017 | 9.75 ± 1.29 |
| NU | 0.331 ± 0.049 | 0.135 ± 0.017 | 9.38 ± 1.28 | |
| F ANOVA | 4.83 * | 6.02 * | 0.40 ns | |
| C. salviifolius | U | 0.179 ± 0.025 | 0.064 ± 0.011 | 6.0 ± 1.0 |
| NU | 0.129 ± 0.038 | 0.071 ± 0.013 | 4.06 ± 0.8 | |
| F ANOVA | 12.07 ** | 1.43 ns | 23.7 *** | |
| E. arborea | U | 6.8 × 10−4 ± 1.6 × 10−5 | 3.8 × 10−4 ± 3.2 × 10−5 | 0.03 ± 0.01 |
| NU | 7.6 × 10−4 ± 6.5 × 10−5 | 3.5 × 10−4 ± 1.6 × 10−5 | 0.03 ± 0.01 | |
| F ANOVA | 15.71 ** | 1.05 ns | 0.06 ns | |
| L. stoechas | U | 0.014 ± 0.004 | 0.005 ± 0.001 | 0.55 ± 0.15 |
| NU | 0.021 ± 0.008 | 0.007 ± 0.002 | 0.80 ± 0.34 | |
| F ANOVA | 5.94 * | 5.49 * | 4.59 * | |
| P. latifolia | U | 0.261 ± 0.044 | 0.112 ± 0.020 | 10.84 ± 1.62 |
| NU | 0.456 ± 0.071 | 0.149 ± 0026 | 12.65 ± 1.64 | |
| F ANOVA | 54.54 *** | 79.10 *** | 6.18 * | |
| Q. coccifera | U | 0.139 ± 0.048 | 0.081 ± 0.026 | 6.34 ± 1.51 |
| NU | 0.179 ± 0.038 | 0.081 ± 0.016 | 5.47 ± 1.99 | |
| F ANOVA | 4.38 * | 0.005 ns | 1.21 ns | |
| U | 0.117 ± 0.030 | 0.056 ± 0.011 | 5.07 ± 1.09 | |
| T. fruticans | NU | 0.134 ± 0.020 | 0.075 ± 0.014 | 5.99 ± 1.08 |
| F ANOVA | 2.05 ns | 10.86 ** | 3.62 ns |
| Species | Site | C% Mean ± SD | S% Mean ± SD | R% Mean ± SD | Strategy Class |
|---|---|---|---|---|---|
| C. atriplicifolius | U | 23.40 ± 1.71 | 75.80 ± 2.57 | 1.00 ± 2.16 | S/CS |
| NU | 22.90 ± 1.79 | 77.10 ± 1.79 | 0.00 | S/CS | |
| Mean | 23.15 ± 1.73 | 76.45 ± 2.26 | 0.50 ± 1.57 | S/CS | |
| F ANOVA | 0.41 ns | 1.72 ns | 2.14 ns | ||
| C. salviifolius | U | 21.10 ± 3.38 | 77.70 ± 3.68 | 1.30 ± 2.31 | S/CS |
| NU | 11.20 ± 1.55 | 88.90 ± 1.37 | 0.00 | S | |
| Mean | 16.15 ± 5.69 | 83.30 ± 6.35 | 0.65 ± 1.73 | S/CS | |
| F ANOVA | 70.85 *** | 81.22 *** | 3.16 ns | ||
| E. arborea | U | 0.00 | 100.00 ± 0.00 | 0.00 | S |
| NU | 0.00 | 99.30 ± 1.64 | 0.70 ± 1.64 | S | |
| Mean | 0.00 | 99.65 ± 1.18 | 0.35 ± 1.18 | S | |
| F ANOVA | - | 1.83 ns | 1.83 ns | ||
| L. stoechas | U | 3.00 ± 1.41 | 88.80 ± 8.30 | 8.30 ± 7.86 | S |
| NU | 5.00 ± 2.36 | 85.40 ± 10.52 | 9.80 ± 8.94 | S | |
| Mean | 4.00 ± 2.15 | 87.10 ± 9.39 | 9.05 ± 8.23 | S | |
| F ANOVA | 5.29 * | 0.64 ns | 0.16 ns | ||
| P. latifolia | U | 23.00 ± 1.83 | 75.70 ± 2.87 | 1.60 ± 2.22 | S/CS |
| NU | 22.80 ± 1.48 | 77.20 ± 1.48 | 0.00 | S/CS | |
| Mean | 22.90 ± 1.62 | 76.45 ± 2.35 | 0.80 ± 1.74 | S/CS | |
| F ANOVA | 0.07 ns | 2.16 ns | 5.19 * | ||
| Q. coccifera | U | 12.60 ± 2.55 | 87.50 ± 2.68 | 0.00 | S |
| NU | 17.40 ± 2.17 | 82.70 ± 2.11 | 0.00 | S/CS | |
| Mean | 15.00 ± 3.37 | 85.10 ± 3.40 | 0.00 | S | |
| F ANOVA | 20.57 *** | 19.82 *** | - | ||
| T. fruticans | U | 14.10 ± 1.91 | 85.60 ± 1.96 | 0.30 ± 0.95 | S |
| NU | 14.00 ± 1.33 | 85.50 ± 2.76 | 0.50 ± 1.58 | S | |
| Mean | 14.05 ± 1.61 | 85.55 ± 2.33 | 0.40 ± 1.27 | S | |
| F ANOVA | 0.02 ns | 0.009 ns | 0.12 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadaoui, K.; Kassout, J.; Boselli, V.A.; Chakkour, S.; Sahli, A.; Houssni, M.; Bouziane, H.; Ater, M. Leaf Trait Variability and CSR Strategy Shifts in Mediterranean Woody Species along an Edaphic Gradient. Ecologies 2024, 5, 455-469. https://doi.org/10.3390/ecologies5030028
Kadaoui K, Kassout J, Boselli VA, Chakkour S, Sahli A, Houssni M, Bouziane H, Ater M. Leaf Trait Variability and CSR Strategy Shifts in Mediterranean Woody Species along an Edaphic Gradient. Ecologies. 2024; 5(3):455-469. https://doi.org/10.3390/ecologies5030028
Chicago/Turabian StyleKadaoui, Khalil, Jalal Kassout, Vladimiro Andrea Boselli, Soufian Chakkour, Abdelouahab Sahli, Mhammad Houssni, Hassan Bouziane, and Mohammed Ater. 2024. "Leaf Trait Variability and CSR Strategy Shifts in Mediterranean Woody Species along an Edaphic Gradient" Ecologies 5, no. 3: 455-469. https://doi.org/10.3390/ecologies5030028
APA StyleKadaoui, K., Kassout, J., Boselli, V. A., Chakkour, S., Sahli, A., Houssni, M., Bouziane, H., & Ater, M. (2024). Leaf Trait Variability and CSR Strategy Shifts in Mediterranean Woody Species along an Edaphic Gradient. Ecologies, 5(3), 455-469. https://doi.org/10.3390/ecologies5030028









