Balancing Livestock Environmental Footprints with Forestry-Based Solutions: A Review
Abstract
:1. Introduction
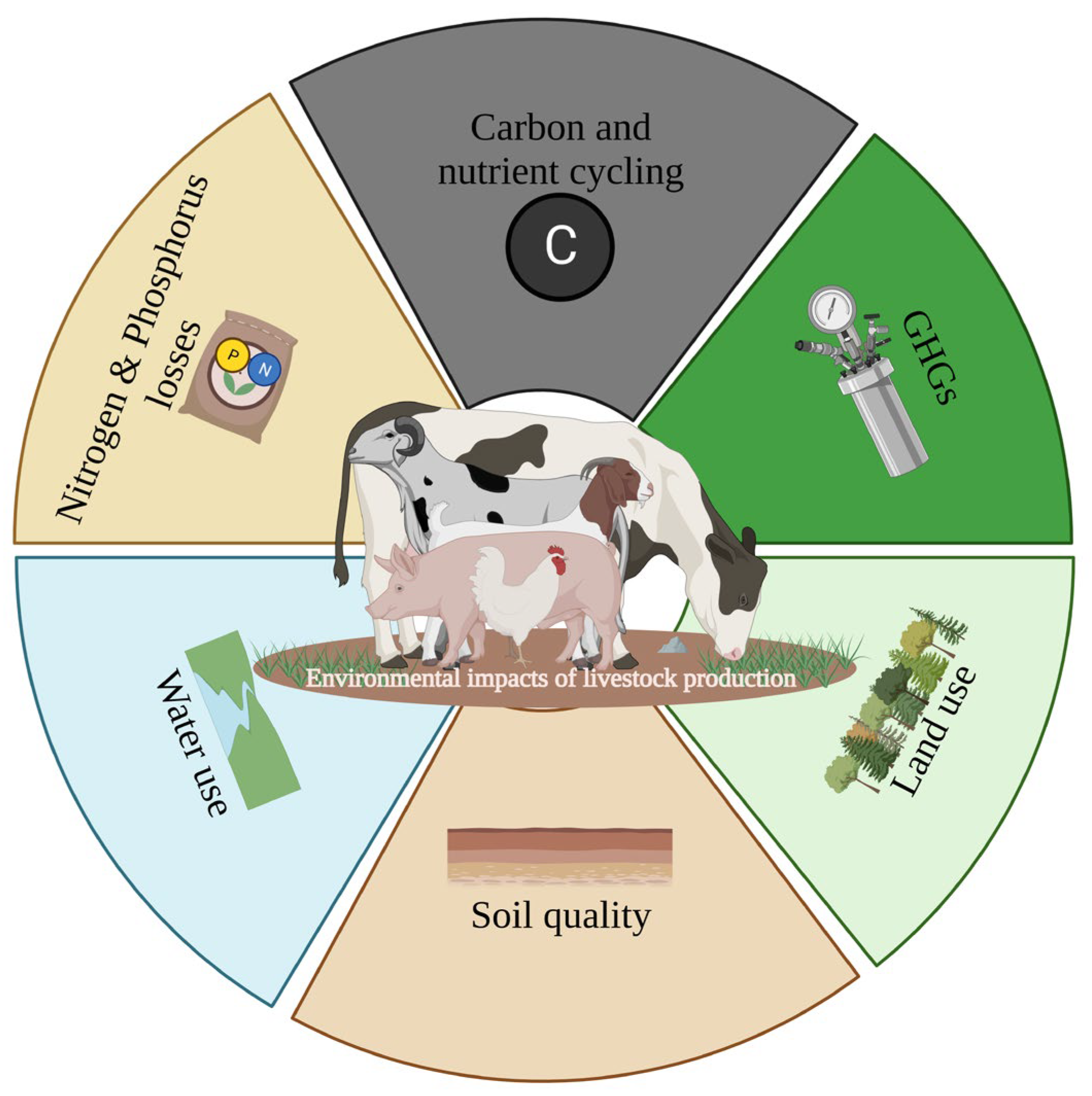
2. Overview of Environmental Impacts of Livestock Production
2.1. Impacts of Livestock Production on Greenhouse Gas (GHG) Emissions
2.1.1. Methane
2.1.2. Nitrous Oxide
2.2. Impacts of Livestock Production on Water and Land Use
2.2.1. Water Use
2.2.2. Land Use
3. Potential Impacts of Forestry on the Environmental Footprint of Livestock Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turnbull, P.F.; Reed, C.A. The fauna from the terminal Pleistocene of Palegawra Cave, a Zarzian occupation site in northeastern Iraq. Fieldiana Anthropol. 1974, 63, 81–146. [Google Scholar]
- Hartung, J. A short history of livestock production. In Livestock Housing: Modern Management to Ensure Optimal Health and Welfare of Farm Animals; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 81–146. [Google Scholar]
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Randolph, T.F.; Schelling, E.; Grace, D.; Nicholson, C.F.; Leroy, J.L.; Cole, D.C.; Demment, M.W.; Omore, A.; Zinsstag, J.; Ruel, M. Invited review: Role of livestock in human nutrition and health for poverty reduction in developing countries. J. Anim. Sci. 2007, 85, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Meadowcroft, J. Minding the Stock: Bringing Public Policy to Bear on Livestock Sector Development; The World Bank: Washington, DC, USA, 2009. [Google Scholar]
- FAO. Meat Market Review: Emerging Trends and Outlook; FAO: Rome, Italy, 2021. [Google Scholar]
- Sakadevan, K.; Nguyen, M.L. Livestock Production and Its Impact on Nutrient Pollution and Greenhouse Gas Emissions. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 141, pp. 147–184. [Google Scholar]
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Simpson, D.; Sutton, M.A.; De Vries, W.; Weiss, F. Impacts of European livestock production: Nitrogen, sulphur, phosphorus and greenhouse gas emissions, land-use, water eutrophication and biodiversity. Environ. Res. Lett. 2015, 10, 115004. [Google Scholar] [CrossRef]
- Bartley, R.; Roth, C.H.; Ludwig, J.; McJannet, D.; Liedloff, A.; Corfield, J.; Hawdon, A.; Abbott, B. Runoff and erosion from Australia’s tropical semi-arid rangelands: Influence of ground cover for differing space and time scales. Hydrol. Process. 2006, 20, 3317–3333. [Google Scholar] [CrossRef]
- Bell, L.W.; Kirkegaard, J.A.; Swan, A.; Hunt, J.R.; Huth, N.I.; Fettell, N.A. Impacts of soil damage by grazing livestock on crop productivity. Soil Tillage Res. 2011, 113, 19–29. [Google Scholar] [CrossRef]
- Whitmore, A. Impact of livestock on soil. In Proceedings of the Workshop 4 on Sustainable Animal Production, Hannover, Germany, 28 September 2000; pp. 39–41. [Google Scholar]
- Moumen, A.; Azizi, G.; Chekroun, K.B.; Baghour, M. The effects of livestock methane emission on the global warming: A review. Int. J. Glob. Warm. 2016, 9, 229–253. [Google Scholar] [CrossRef]
- Sejian, V.; Hyder, I.; Ezeji, T.; Lakritz, J.; Bhatta, R.; Ravindra, J.; Prasad, C.S.; Lal, R. Global warming: Role of livestock. In Climate Change Impact on Livestock: Adaptation and Mitigation; Springer: Berlin/Heidelberg, Germany, 2015; pp. 141–169. [Google Scholar]
- Caro, D.; Davis, S.J.; Bastianoni, S.; Caldeira, K. Global and regional trends in greenhouse gas emissions from livestock. Clim. Chang. 2014, 126, 203–216. [Google Scholar] [CrossRef]
- Casey, K.D.; Bicudo, J.R.; Schmidt, D.R.; Singh, A.; Gay, S.W.; Gates, R.S.; Jacobson, L.D.; Hoff, S.J. Air quality and emissions from livestock and poultry production/waste management systems. In Animal Agriculture and the Environment: National Center for Manure and Animal Waste Management White Papers; ASABE: St. Joseph, MI, USA, 2006. [Google Scholar]
- Banhazi, T.; Aland, A.; Hartung, J. Air Quality and Livestock Farming; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Baroni, L.; Cenci, L.; Tettamanti, M.; Berati, M. Evaluating the environmental impact of various dietary patterns combined with different food production systems. Eur. J. Clin. Nutr. 2007, 61, 279–286. [Google Scholar] [CrossRef]
- Richardson, N.; Shepherd, R.; Elliman, N. Current attitudes and future influence on meat consumption in the UK. Appetite 1993, 21, 41–51. [Google Scholar] [CrossRef]
- Başkent, E.Z.; Keleş, S.; Kadıoğulları, A.İ.; Bingöl, Ö. Quantifying the Effects of Forest Management Strategies on the Production of Forest Values: Timber, Carbon, Oxygen, Water, and Soil. Environ. Model. Assess. 2011, 16, 145–152. [Google Scholar] [CrossRef]
- Pawar, K.; Rothkar, R.V. Forest conservation & environmental awareness. Procedia Earth Planet. Sci. 2015, 11, 212–215. [Google Scholar]
- Yang, J.; McBride, J.; Zhou, J.; Sun, Z. The urban forest in Beijing and its role in air pollution reduction. Urban For. Urban Green. 2005, 3, 65–78. [Google Scholar] [CrossRef]
- Nisbet, T.R. The role of forest management in controlling diffuse pollution in UK forestry. For. Ecol. Manag. 2001, 143, 215–226. [Google Scholar] [CrossRef]
- Kweku, D.; Bismark, O.; Maxwell, A.; Desmond, K.; Danso, K.; Oti-Mensah, E.; Quachie, A.; Adormaa, B. Greenhouse Effect: Greenhouse Gases and Their Impact on Global Warming. J. Sci. Res. Rep. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Guo, L.; Li, Y.; Su, M.; Lin, Y.; de Perthuis, C.; Ju, X.; Lin, E.; Moran, D. Greenhouse gas intensity of three main crops and implications for low-carbon agriculture in China. Clim. Chang. 2014, 128, 57–70. [Google Scholar] [CrossRef]
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal control knob governing Earth’s temperature. Science 2010, 330, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.D.; McManus, B.; Urbanski, S.; Herndon, S.; Zahniser, M.S. High precision measurements of atmospheric nitrous oxide and methane using thermoelectrically cooled mid-infrared quantum cascade lasers and detectors. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 3325–3335. [Google Scholar] [CrossRef]
- Kolstad, C.D. Learning and Stock Effects in Environmental Regulation: The Case of Greenhouse Gas Emissions. J. Environ. Econ. Manag. 1996, 31, 1–18. [Google Scholar] [CrossRef]
- Cai, W.; Ng, B.; Wang, G.; Santoso, A.; Wu, L.; Yang, K. Increased ENSO sea surface temperature variability under four IPCC emission scenarios. Nat. Clim. Chang. 2022, 12, 228–231. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Havran, V.; Duduković, M.P.; Lo, C.S. Conversion of Methane and Carbon Dioxide to Higher Value Products. Ind. Eng. Chem. Res. 2011, 50, 7089–7100. [Google Scholar] [CrossRef]
- Broucek, J. Production of Methane Emissions from Ruminant Husbandry: A Review. J. Environ. Prot. 2014, 5, 1482–1493. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Ungerfeld, E.M.; Eckard, R.J.; Wang, M. Review: Fifty years of research on rumen methanogenesis: Lessons learned and future challenges for mitigation. Animal 2020, 14, S2–S16. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Dijkstra, J.; Kebreab, E.; Bannink, A.; Odongo, N.E.; McBride, B.W.; France, J. Aspects of rumen microbiology central to mechanistic modelling of methane production in cattle. J. Agric. Sci. 2008, 146, 213–233. [Google Scholar] [CrossRef]
- Boadi, D.; Benchaar, C.; Chiquette, J.; Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: Update review. Can. J. Anim. Sci. 2004, 84, 319–335. [Google Scholar] [CrossRef]
- Honan, M.; Feng, X.; Tricarico, J.M.; Kebreab, E. Feed additives as a strategic approach to reduce enteric methane production in cattle: Modes of action, effectiveness and safety. Anim. Prod. Sci. 2021, 62, 1303–1317. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional management for enteric methane abatement: A review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- McAllister, T.A.; Cheng, K.J.; Okine, E.K.; Mathison, G.W. Dietary, environmental and microbiological aspects of methane production in ruminants. Can. J. Anim. Sci. 1996, 76, 231–243. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Joblin, K.N. Ruminal acetogens and their potential to lower ruminant methane emissions. Aust. J. Agric. Res. 1999, 50, 1307–1314. [Google Scholar] [CrossRef]
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.P.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, R.; Dicksved, J.; Sun, L.; Gonda, H.; Muller, B.; Schnurer, A.; Bertilsson, J. Methane Production in Dairy Cows Correlates with Rumen Methanogenic and Bacterial Community Structure. Front. Microbiol. 2017, 8, 226. [Google Scholar] [CrossRef] [PubMed]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, E.M. Inhibition of Rumen Methanogenesis and Ruminant Productivity: A Meta-Analysis. Front. Vet. Sci. 2018, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a unique biological group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef]
- Lopez, S.; McIntosh, F.M.; Wallace, R.J.; Newbold, C.J. Effect of adding acetogenic bacteria on methane production by mixed rumen microorganisms. Anim. Feed Sci. Technol. 1999, 78, 1–9. [Google Scholar] [CrossRef]
- Asanuma, N.; Iwamoto, M.; Hino, T. Effect of the addition of fumarate on methane production by ruminal microorganisms in vitro. J. Dairy Sci. 1999, 82, 780–787. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Invited review: Enteric methane in dairy cattle production: Quantifying the opportunities and impact of reducing emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Buddle, B.M.; Denis, M.; Attwood, G.T.; Altermann, E.; Janssen, P.H.; Ronimus, R.S.; Pinares-Patino, C.S.; Muetzel, S.; Neil Wedlock, D. Strategies to reduce methane emissions from farmed ruminants grazing on pasture. Vet. J. 2011, 188, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N. Dietary manipulation: A sustainable way to mitigate methane emissions from ruminants. J. Anim. Sci. Technol. 2018, 60, 15. [Google Scholar] [CrossRef] [PubMed]
- Tarazkar, M.H.; Kargar Dehbidi, N.; Ansari, R.A.; Pourghasemi, H.R. Factors affecting methane emissions in OPEC member countries: Does the agricultural production matter? Environ. Dev. Sustain. 2020, 23, 6734–6748. [Google Scholar] [CrossRef]
- Dalby, F.R.; Hafner, S.D.; Petersen, S.O.; VanderZaag, A.C.; Habtewold, J.; Dunfield, K.; Chantigny, M.H.; Sommer, S.G. Understanding methane emission from stored animal manure: A review to guide model development. J. Environ. Qual. 2021, 50, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.O.; Blanchard, M.; Chadwick, D.; Del Prado, A.; Edouard, N.; Mosquera, J.; Sommer, S.G. Manure management for greenhouse gas mitigation. Animal 2013, 7 (Suppl. S2), 266–282. [Google Scholar] [CrossRef] [PubMed]
- Osada, T.; Kuroda, K.; Yonaga, M. Determination of nitrous oxide, methane, and ammonia emissions from a swine waste composting process. J. Mater. Cycles Waste Manag. 2000, 2, 51–56. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Li, H. Long-term microbial community succession and mechanisms of regulation of dissolved organic matter derivation in livestock manure fermentation system. Chemosphere 2023, 329, 138588. [Google Scholar] [CrossRef]
- Kim, S.Y.; Pramanik, P.; Bodelier, P.L.; Kim, P.J. Cattle Manure Enhances Methanogens Diversity and Methane Emissions Compared to Swine Manure under Rice Paddy. PLoS ONE 2014, 9, e113593. [Google Scholar] [CrossRef]
- Li, Y.; Achinas, S.; Zhao, J.; Geurkink, B.; Krooneman, J.; Willem Euverink, G.J. Co-digestion of cow and sheep manure: Performance evaluation and relative microbial activity. Renew. Energy 2020, 153, 553–563. [Google Scholar] [CrossRef]
- Sommer, S.G.; Petersen, S.O.; Søgaard, H.T. Greenhouse Gas Emission from Stored Livestock Slurry. J. Environ. Qual. 2000, 29, 744–751. [Google Scholar] [CrossRef]
- Kettunen, R.H.; Rintala, J.A. The effect of low temperature (5-29 degrees C) and adaptation on the methanogenic activity of biomass. Appl. Microbiol. Biotechnol. 1997, 48, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Elsgaard, L.; Olsen, A.B.; Petersen, S.O. Temperature response of methane production in liquid manures and co-digestates. Sci. Total Environ. 2016, 539, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.G.; Varel, V.H.; Chen, Y.R. Ultimate methane yield from beef cattle manure: Effect of temperature, ration constituents, antibiotics and manure age. Agric. Wastes 1981, 3, 241–256. [Google Scholar] [CrossRef]
- Massé, D.I.; Masse, L.; Claveau, S.; Benchaar, C.; Thomas, O. Methane Emissions from Manure Storages. Trans. ASABE 2008, 51, 1775–1781. [Google Scholar] [CrossRef]
- Sommer, S.G.; Olesen, J.E.; Petersen, S.O.; Weisbjerg, M.R.; Valli, L.; Rodhe, L.; BÉLine, F. Region-specific assessment of greenhouse gas mitigation with different manure management strategies in four agroecological zones. Glob. Chang. Biol. 2009, 15, 2825–2837. [Google Scholar] [CrossRef]
- Sharma, R.; Ryan, K.; Hao, X.; Larney, F.J.; McAllister, T.A.; Topp, E. Real-time quantification of mcrA, pmoA for methanogen, methanotroph estimations during composting. J. Environ. Qual. 2011, 40, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Wilshusen, J.H.; Hettiaratchi, J.P.; De Visscher, A.; Saint-Fort, R. Methane oxidation and formation of EPS in compost: Effect of oxygen concentration. Environ. Pollut. 2004, 129, 305–314. [Google Scholar] [CrossRef]
- Fan, Y.; Lei, Z.; Yang, X.; Kobayashi, M.; Adachi, Y.; Zhang, Z.; Shimizu, K. Effect of nano-bubble water on high solid anaerobic digestion of pig manure: Focus on digestion stability, methanogenesis performance and related mechanisms. Bioresour. Technol. 2020, 315, 123793. [Google Scholar] [CrossRef]
- Eggleston, H.S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Institute for Global Environmental Strategies (IGES): Hayama, Japan, 2006. [Google Scholar]
- Schulze, E.D.; Luyssaert, S.; Ciais, P.; Freibauer, A.; Janssens, I.A.; Soussana, J.F.; Smith, P.; Grace, J.; Levin, I.; Thiruchittampalam, B.; et al. Importance of methane and nitrous oxide for Europe’s terrestrial greenhouse-gas balance. Nat. Geosci. 2009, 2, 842–850. [Google Scholar] [CrossRef]
- Crutzen, P.J. The Influence of Nitrogen Oxides on Atmospheric Ozone Content. In Paul J. Crutzen: A Pioneer on Atmospheric Chemistry and Climate Change in the Anthropocene; Crutzen, P.J., Brauch, H.G., Eds.; Springer Briefs on Pioneers in Science and Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 108–116. [Google Scholar]
- Badr, O.; Probert, S.D. Environmental impacts of atmospheric nitrous oxide. Appl. Energy 1993, 44, 197–231. [Google Scholar] [CrossRef]
- Reay, D.S.; Davidson, E.A.; Smith, K.A.; Smith, P.; Melillo, J.M.; Dentener, F.; Crutzen, P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Chang. 2012, 2, 410–416. [Google Scholar] [CrossRef]
- Lu, Q.; Wu, J.; Wang, M.; Zhou, C.; Han, X.; Odongo, E.N.; Tan, Z.; Tang, S. Effects of dietary addition of cellulase and a Saccharomyces cerevisiae fermentation product on nutrient digestibility, rumen fermentation and enteric methane emissions in growing goats. Arch. Anim. Nutr. 2016, 70, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Houlton, B.Z.; Zheng, Y.; Zhou, F.; Ma, L.; Li, B.; Liu, X.; Li, G.; Lu, H.; Quan, F.; et al. Policy-enabled stabilization of nitrous oxide emissions from livestock production in China over 1978–2017. Nat. Food 2022, 3, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Rotz, C.A. Management to reduce nitrogen losses in animal production. J. Anim. Sci. 2004, 82, E119–E137. [Google Scholar]
- Jia, Z.; Conrad, R. Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 2009, 11, 1658–1671. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Otawa, K.; Nakai, Y. Diversity and abundance of ammonia-oxidizing bacteria and ammonia-oxidizing archaea during cattle manure composting. Microb. Ecol. 2010, 60, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Wallenstein, M.D.; Myrold, D.D.; Firestone, M.; Voytek, M. Environmental controls on denitrifying communities and denitrification rates: Insights from molecular methods. Ecol. Appl. 2006, 16, 2143–2152. [Google Scholar] [CrossRef]
- Petersen, S.O.; Nielsen, A.L.; Haarder, K.; Henriksen, K. Factors controlling nitrification and denitrification: A laboratory study with gel-stabilized liquid cattle manure. Microb. Ecol. 1992, 23, 239–255. [Google Scholar] [CrossRef]
- Husted, S.; Jensen, L.S.; Jørgensen, S.S. Reducing ammonia loss from cattle slurry by the use of acidifying additives: The role of the buffer system. J. Sci. Food Agric. 1991, 57, 335–349. [Google Scholar] [CrossRef]
- Baggs, E.M.; Smales, C.L.; Bateman, E.J. Changing pH shifts the microbial sourceas well as the magnitude of N2O emission from soil. Biol. Fertil. Soils 2010, 46, 793–805. [Google Scholar] [CrossRef]
- Heinke, J.; Lannerstad, M.; Gerten, D.; Havlík, P.; Herrero, M.; Notenbaert, A.M.O.; Hoff, H.; Müller, C. Water Use in Global Livestock Production—Opportunities and Constraints for Increasing Water Productivity. Water Resour. Res. 2020, 56, e2019WR026995. [Google Scholar] [CrossRef]
- Bogardi, J.J.; Dudgeon, D.; Lawford, R.; Flinkerbusch, E.; Meyn, A.; Pahl-Wostl, C.; Vielhauer, K.; Vörösmarty, C. Water security for a planet under pressure: Interconnected challenges of a changing world call for sustainable solutions. Curr. Opin. Environ. Sustain. 2012, 4, 35–43. [Google Scholar] [CrossRef]
- Halla, C.; Blöthe, J.H.; Tapia Baldis, C.; Trombotto Liaudat, D.; Hilbich, C.; Hauck, C.; Schrott, L. Ice content and interannual water storage changes of an active rock glacier in the dry Andes of Argentina. Cryosphere 2021, 15, 1187–1213. [Google Scholar] [CrossRef]
- Ran, Y.; Lannerstad, M.; Herrero, M.; Van Middelaar, C.E.; De Boer, I.J.M. Assessing water resource use in livestock production: A review of methods. Livest. Sci. 2016, 187, 68–79. [Google Scholar] [CrossRef]
- Boulay, A.-M.; Drastig, K.; Amanullah; Chapagain, A.; Charlon, V.; Civit, B.; DeCamillis, C.; De Souza, M.; Hess, T.; Hoekstra, A.Y.; et al. Building consensus on water use assessment of livestock production systems and supply chains: Outcome and recommendations from the FAO LEAP Partnership. Ecol. Indic. 2021, 124, 107391. [Google Scholar] [CrossRef]
- Kebebe, E.G.; Oosting, S.J.; Haileslassie, A.; Duncan, A.J.; de Boer, I.J. Strategies for improving water use efficiency of livestock production in rain-fed systems. Animal 2015, 9, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Ridoutt, B.G.; Sanguansri, P.; Freer, M.; Harper, G.S. Water footprint of livestock: Comparison of six geographically defined beef production systems. Int. J. Life Cycle Assess. 2011, 17, 165–175. [Google Scholar] [CrossRef]
- Pesti, G.M.; Amato, S.V.; Minear, L.R. Water consumption of broiler chickens under commercial conditions. Poult. Sci. 1985, 64, 803–808. [Google Scholar] [CrossRef]
- Ncho, C.M.; Goel, A.; Gupta, V.; Jeong, C.M.; Choi, Y.H. Effect of in ovo feeding of gamma-aminobutyric acid combined with embryonic thermal manipulation on hatchability, growth, and hepatic gene expression in broilers. Anim. Biosci. 2023, 36, 284–294. [Google Scholar] [CrossRef]
- Ncho, C.M.; Gupta, V.; Choi, Y.H. Effects of Dietary Glutamine Supplementation on Heat-Induced Oxidative Stress in Broiler Chickens: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 570. [Google Scholar] [CrossRef]
- Ncho, C.M.; Gupta, V.; Goel, A. Effect of thermal conditioning on growth performance and thermotolerance in broilers: A systematic review and meta-analysis. J. Therm. Biol. 2021, 98, 102916. [Google Scholar] [CrossRef] [PubMed]
- Ncho, C.M.; Jeong, C.; Gupta, V.; Goel, A. The effect of gamma-aminobutyric acid supplementation on growth performances, immune responses, and blood parameters of chickens reared under stressful environment: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2021, 28, 45019–45028. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Ncho, C.M.; Choi, Y.H. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechnol. 2021, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Schlink, A.C.; Nguyen, M.L.; Viljoen, G.J. Water requirements for livestock production: A global perspective. Rev. Sci. Tech. 2010, 29, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Thornton, P.; Herrero, M. The Inter-Linkages between Rapid Growth in Livestock Production, Climate Change, and the Impacts on Water Resources, Land Use, and Deforestation; World Bank Policy Research Working Papers; The World Bank: Washington, DC, USA, 2010. [Google Scholar]
- Alkhamisi, S.A.; Abdelrahman, H.A.; Ahmed, M.; Goosen, M.F.A. Assessment of reclaimed water irrigation on growth, yield, and water-use efficiency of forage crops. Appl. Water Sci. 2011, 1, 57–65. [Google Scholar] [CrossRef]
- Manceron, S.; Ben-Ari, T.; Dumas, P. Feeding proteins to livestock: Global land use and foodvs.feed competition. OCL 2014, 21, D408. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Re-defining efficiency of feed use by livestock. Animal 2011, 5, 1014–1022. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Rounsevell, M.D.A. Human appropriation of land for food: The role of diet. Glob. Environ. Chang. 2016, 41, 88–98. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, E.-Y.; Ncho, C.M.; Kim, C.-J.; Son, Y.-M.; Hwang, Y.-H.; Joo, S.-T. Quality Characteristics of Meat Analogs through the Incorporation of Textured Vegetable Protein: A Systematic Review. Foods 2022, 11, 1242. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Lee, M.R.F. Review: Use of human-edible animal feeds by ruminant livestock. Animal 2018, 12, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of feeding plant by-products: A review of the implications for ruminant meat production. Anim. Feed Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Reid, R.S.; Galvin, K.A.; Kruska, R.S. Global significance of extensive grazing lands and pastoral societies: An introduction. In Fragmentation in Semi-Arid and Arid Landscapes: Consequences for Human and Natural Systems; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–24. [Google Scholar]
- Asner, G.P.; Elmore, A.J.; Olander, L.P.; Martin, R.E.; Harris, A.T. Grazing Systems, Ecosystem Responses, and Global Change. Annu. Rev. Environ. Resour. 2004, 29, 261–299. [Google Scholar] [CrossRef]
- Pei, S.; Fu, H.; Wan, C. Changes in soil properties and vegetation following exclosure and grazing in degraded Alxa desert steppe of Inner Mongolia, China. Agric. Ecosyst. Environ. 2008, 124, 33–39. [Google Scholar] [CrossRef]
- Filazzola, A.; Brown, C.; Dettlaff, M.A.; Batbaatar, A.; Grenke, J.; Bao, T.; Peetoom Heida, I.; Cahill, J.F., Jr. The effects of livestock grazing on biodiversity are multi-trophic: A meta-analysis. Ecol. Lett. 2020, 23, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. J. Environ. Qual. 2018, 47, 758–765. [Google Scholar] [CrossRef]
- Bai, Y.; Cotrufo, M.F. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, L.E.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Conant, R.T.; Cerri, C.E.; Osborne, B.B.; Paustian, K. Grassland management impacts on soil carbon stocks: A new synthesis. Ecol. Appl. 2017, 27, 662–668. [Google Scholar] [CrossRef]
- Eze, S.; Palmer, S.M.; Chapman, P.J. Soil organic carbon stock in grasslands: Effects of inorganic fertilizers, liming and grazing in different climate settings. J. Environ. Manag. 2018, 223, 74–84. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; He, Y.; Shao, J.; Hu, Z.; Liu, R.; Zhou, H.; Hosseinibai, S. Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta-analysis. Glob. Chang. Biol. 2017, 23, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, J.; Rozelle, S.; Uchida, E. Cultivated land conversion and potential agricultural productivity in China. Land Use Policy 2006, 23, 372–384. [Google Scholar] [CrossRef]
- Michalk, D.L.; Kemp, D.R.; Badgery, W.B.; Wu, J.; Zhang, Y.; Thomassin, P.J. Sustainability and future food security—A global perspective for livestock production. Land Degrad. Dev. 2018, 30, 561–573. [Google Scholar] [CrossRef]
- van Zanten, H.H.E.; Mollenhorst, H.; Klootwijk, C.W.; van Middelaar, C.E.; de Boer, I.J.M. Global food supply: Land use efficiency of livestock systems. Int. J. Life Cycle Assess. 2015, 21, 747–758. [Google Scholar] [CrossRef]
- Naylor, R.; Steinfeld, H.; Falcon, W.; Galloway, J.; Smil, V.; Bradford, E.; Alder, J.; Mooney, H. Agriculture. Losing the links between livestock and land. Science 2005, 310, 1621–1622. [Google Scholar] [CrossRef] [PubMed]
- Chazdon, R.L.; Brancalion, P.H.; Laestadius, L.; Bennett-Curry, A.; Buckingham, K.; Kumar, C.; Moll-Rocek, J.; Vieira, I.C.; Wilson, S.J. When is a forest a forest? Forest concepts and definitions in the era of forest and landscape restoration. Ambio 2016, 45, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Penna, I. Understanding the FAO’s’ Wood Supply from Planted Forests’ Projections; Centre for Environmental Management, University of Ballarat: Ballarat, VIC, Australia, 2010; Volume 2010. [Google Scholar]
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M.C. Forest Contribution to Climate Change Mitigation: Management Oriented to Carbon Capture and Storage. Climate 2020, 8, 21. [Google Scholar] [CrossRef]
- Bernal, B.; Murray, L.T.; Pearson, T.R.H. Global carbon dioxide removal rates from forest landscape restoration activities. Carbon Balance Manag. 2018, 13, 22. [Google Scholar] [CrossRef]
- Waring, B.; Neumann, M.; Prentice, I.C.; Adams, M.; Smith, P.; Siegert, M. Forests and Decarbonization—Roles of Natural and Planted Forests. Front. For. Glob. Chang. 2020, 3, 58. [Google Scholar] [CrossRef]
- Krug, J.; Koehl, M.; Kownatzki, D. Revaluing unmanaged forests for climate change mitigation. Carbon Balance Manag. 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Muluneh, M.G.; Worku, B.B. Carbon storages and sequestration potentials in remnant forests of different patch sizes in northern Ethiopia: An implication for climate change mitigation. Agric. Food Secur. 2022, 11, 57. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.M.; Ahmad, Z.; Siddiq, Z.; Ullah, A.; Yoo, S.; Han, H.; Raposo, A. Carbon sequestration potential of different forest types in Pakistan and its role in regulating services for public health. Front. Public Health 2022, 10, 1064586. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, S.M.; Siddiq, Z.; Ahmad, Z.; Ahmad, K.S.; Abdullah, A.; Hashem, A.; Al-Arjani, A.-B.F.; Abd_Allah, E.F. Carbon sequestration potential of reserve forests present in the protected Margalla Hills National Park. J. King Saud. Univ.-Sci. 2022, 34, 101978. [Google Scholar] [CrossRef]
- Díaz, S.; Hector, A.; Wardle, D.A. Biodiversity in forest carbon sequestration initiatives: Not just a side benefit. Curr. Opin. Environ. Sustain. 2009, 1, 55–60. [Google Scholar] [CrossRef]
- Hu, Y.; Su, Z.; Li, W.; Li, J.; Ke, X. Influence of Tree Species Composition and Community Structure on Carbon Density in a Subtropical Forest. PLoS ONE 2015, 10, e0136984. [Google Scholar] [CrossRef] [PubMed]
- Baul, T.K.; Chakraborty, A.; Nandi, R.; Mohiuddin, M.; Kilpelainen, A.; Sultana, T. Effects of tree species diversity and stand structure on carbon stocks of homestead forests in Maheshkhali Island, Southern Bangladesh. Carbon Balance Manag. 2021, 16, 11. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, X.; Yi, H.; Li, Y.; Xu, X.; He, J.; He, L. Plant diversity drives soil carbon sequestration: Evidence from 150 years of vegetation restoration in the temperate zone. Front. Plant Sci. 2023, 14, 1191704. [Google Scholar] [CrossRef]
- Alexandrov, G.A. Carbon stock growth in a forest stand: The power of age. Carbon Balance Manag. 2007, 2, 4. [Google Scholar] [CrossRef]
- De Villiers, C.; Chen, S.; Jin, C.; Zhu, Y. Carbon sequestered in the trees on a university campus: A case study. Sustain. Account. Manag. Policy J. 2014, 5, 149–171. [Google Scholar] [CrossRef]
- Kohl, M.; Neupane, P.R.; Lotfiomran, N. The impact of tree age on biomass growth and carbon accumulation capacity: A retrospective analysis using tree ring data of three tropical tree species grown in natural forests of Suriname. PLoS ONE 2017, 12, e0181187. [Google Scholar] [CrossRef] [PubMed]
- Udawatta, R.P.; Walter, D.; Jose, S. Carbon sequestration by forests and agroforests: A reality check for the United States. Carbon Footpr. 2022, 1, 2. [Google Scholar] [CrossRef]
- Ngaba, M.J.Y.; Uwiragiye, Y.; Zhou, J. Patterns and controlling factors of soil carbon sequestration in nitrogen-limited and -rich forests in China—A meta-analysis. PeerJ 2023, 11, e14694. [Google Scholar] [CrossRef] [PubMed]
- Haase, D. Urban wetlands and Riparian forests as a nature-based solution for climate change adaptation in cities and their surroundings. In Nature Based Solutions to Climate Change Adaptation in Urban Areas: Linkages between Science, Policy and Practice; Springer: Berlin/Heidelberg, Germany, 2017; pp. 111–121. [Google Scholar]
- Cheng, S.H.; Costedoat, S.; Sterling, E.J.; Chamberlain, C.; Jagadish, A.; Lichtenthal, P.; Nowakowski, A.J.; Taylor, A.; Tinsman, J.; Canty, S.W.J.; et al. What evidence exists on the links between natural climate solutions and climate change mitigation outcomes in subtropical and tropical terrestrial regions? A systematic map protocol. Environ. Evid. 2022, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Donatti, C.I.; Andrade, A.; Cohen-Shacham, E.; Fedele, G.; Hou-Jones, X.; Robyn, B. Ensuring that nature-based solutions for climate mitigation address multiple global challenges. One Earth 2022, 5, 493–504. [Google Scholar] [CrossRef]
- Petersson, H.; Ellison, D.; Appiah Mensah, A.; Berndes, G.; Egnell, G.; Lundblad, M.; Lundmark, T.; Lundström, A.; Stendahl, J.; Wikberg, P.E. On the role of forests and the forest sector for climate change mitigation in Sweden. GCB Bioenergy 2022, 14, 793–813. [Google Scholar] [CrossRef]
- Agrawal, A.; Nepstad, D.; Chhatre, A. Reducing Emissions from Deforestation and Forest Degradation. Annu. Rev. Environ. Resour. 2011, 36, 373–396. [Google Scholar] [CrossRef]
- Kruid, S.; Macedo, M.N.; Gorelik, S.R.; Walker, W.; Moutinho, P.; Brando, P.M.; Castanho, A.; Alencar, A.; Baccini, A.; Coe, M.T. Beyond deforestation: Carbon emissions from land grabbing and forest degradation in the Brazilian Amazon. Front. For. Glob. Chang. 2021, 4, 645282. [Google Scholar] [CrossRef]
- Plugge, D.; Baldauf, T.; Köhl, M. Reduced emissions from deforestation and forest degradation (REDD): Why a robust and transparent monitoring, reporting and verification (MRV) system is mandatory. In Climate Change-Research and Technology for Adaptation and Mitigation; IntechOpen: London, UK, 2011; pp. 155–170. [Google Scholar]
- Janowiak, M.; Swanston, C.; Ontl, T. Carbon Benefits of Wood-Based Products and Energy. In Considering Forest and Grassland Carbon in Land Management; US Department of Agriculture: Washington, DC, USA, 2017. [Google Scholar]
- Howard, C.; Dymond, C.C.; Griess, V.C.; Tolkien-Spurr, D.; van Kooten, G.C. Wood product carbon substitution benefits: A critical review of assumptions. Carbon Balance Manag. 2021, 16, 9. [Google Scholar] [CrossRef]
- Brunet-Navarro, P.; Jochheim, H.; Cardellini, G.; Richter, K.; Muys, B. Climate mitigation by energy and material substitution of wood products has an expiry date. J. Clean. Prod. 2021, 303, 127026. [Google Scholar] [CrossRef]
- Hurmekoski, E.; Seppälä, J.; Kilpeläinen, A.; Kunttu, J. Contribution of wood-based products to climate change mitigation. In Forest Bioeconomy and Climate Change; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–149. [Google Scholar]
- Sharma, J.; Sharma, Y. Effect of forest ecosystems on soil properties—A review. Agric. Rev. 2004, 25, 16–28. [Google Scholar]
- Lu, D.; Moran, E.; Mausel, P. Linking Amazonian secondary succession forest growth to soil properties. Land Degrad. Dev. 2002, 13, 331–343. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Borchert, R. Soil and Stem Water Storage Determine Phenology and Distribution of Tropical Dry Forest Trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Cirelli, D.; Vinge, T.; Lieffers, V.J. Assisted lodgepole pine regeneration on reclamation sites using logging slash as both a mulch and natural seed source. Can. J. For. Res. 2016, 46, 1132–1137. [Google Scholar] [CrossRef]
- Adekalu, K.O.; Okunade, D.A.; Osunbitan, J.A. Compaction and mulching effects on soil loss and runoff from two southwestern Nigeria agricultural soils. Geoderma 2006, 137, 226–230. [Google Scholar] [CrossRef]
- Ingestad, T. New concepts on soil fertility and plant nutrition as illustrated by research on forest trees and stands. Geoderma 1987, 40, 237–252. [Google Scholar] [CrossRef]
- Menyailo, O.V.; Hungate, B.A.; Zech, W. The effect of single tree species on soil microbial activities related to C and N cycling in the Siberian artificial afforestation experiment. Plant Soil 2002, 242, 183–196. [Google Scholar] [CrossRef]
- Gates, D.M. Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 1968, 19, 211–238. [Google Scholar] [CrossRef]
- Rasolofoson, R.A.; Ferraro, P.J.; Ruta, G.; Rasamoelina, M.S.; Randriankolona, P.L.; Larsen, H.O.; Jones, J.P.G. Impacts of Community Forest Management on Human Economic Well-Being across Madagascar. Conserv. Lett. 2016, 10, 346–353. [Google Scholar] [CrossRef]
- Appiah, D.O. Personifying sustainable rural livelihoods in forest fringe communities in Ghana: A historic rhetoric. J. Food Agric. Environ. 2009, 7, 873–877. [Google Scholar]
- Suleiman, M.S.; Wasonga, V.O.; Mbau, J.S.; Suleiman, A.; Elhadi, Y.A. Non-timber forest products and their contribution to households income around Falgore Game Reserve in Kano, Nigeria. Ecol. Process. 2017, 6, 23. [Google Scholar] [CrossRef]
- Assefa, E.; Bork, H.R. Deforestation and forest management in southern Ethiopia: Investigations in the Chencha and Arbaminch areas. Environ. Manag. 2014, 53, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Grandón, A.; Donoso, P.J.; Gerding, V. Forest Degradation: When Is a Forest Degraded? Forests 2018, 9, 726. [Google Scholar] [CrossRef]
- Carter, D.R. Sustainable Forest Management: From Concept to Practice. J. For. 2017, 116, 87. [Google Scholar]
- Hickey, G.M.; Innes, J.L. Monitoring sustainable forest management in different jurisdictions. Environ. Monit. Assess. 2005, 108, 241–260. [Google Scholar] [CrossRef]
- Pukkala, T. Carbon forestry is surprising. For. Ecosyst. 2018, 5, 11. [Google Scholar] [CrossRef]
- Chaudhary, A.; Burivalova, Z.; Koh, L.P.; Hellweg, S. Impact of Forest Management on Species Richness: Global Meta-Analysis and Economic Trade-Offs. Sci. Rep. 2016, 6, 23954. [Google Scholar] [CrossRef]
- Atsbha, T.; Belayneh Desta, A.; Zewdu, T. Carbon sequestration potential of natural vegetation under grazing influence in Southern Tigray, Ethiopia: Implication for climate change mitigation. Heliyon 2019, 5, e02329. [Google Scholar] [CrossRef]
- Asbeck, T.; Sabatini, F.; Augustynczik, A.L.D.; Basile, M.; Helbach, J.; Jonker, M.; Knuff, A.; Bauhus, J. Biodiversity response to forest management intensity, carbon stocks and net primary production in temperate montane forests. Sci. Rep. 2021, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Condé, T.M.; Tonini, H.; Higuchi, N.; Higuchi, F.G.; Lima, A.J.N.; Barbosa, R.I.; dos Santos Pereira, T.; Haas, M.A. Effects of sustainable forest management on tree diversity, timber volumes, and carbon stocks in an ecotone forest in the northern Brazilian Amazon. Land Use Policy 2022, 119, 106145. [Google Scholar] [CrossRef]
- Yin, W.; Yin, M.; Zhao, L.; Yang, L. Research on the Measurement of Carbon Storage in Plantation Tree Trunks Based on the Carbon Storage Dynamic Analysis Method. Int. J. For. Res. 2012, 2012, 626149. [Google Scholar] [CrossRef]
- He, G.; Zhang, Z.; Zhu, Q.; Wang, W.; Peng, W.; Cai, Y. Estimating Carbon Sequestration Potential of Forest and Its Influencing Factors at Fine Spatial-Scales: A Case Study of Lushan City in Southern China. Int. J. Environ. Res. Public Health 2022, 19, 9184. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Agrawal, M.; Pandey, J.S. Carbon footprint: Current methods of estimation. Environ. Monit. Assess. 2011, 178, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Kubová, P.; Hájek, M.; Třebický, V. Carbon footprint measurement and management: Case study of the school forest enterprise. BioResources 2018, 13, 4521–4535. [Google Scholar] [CrossRef]
- Awanthi, M.G.G.; Navaratne, C.M. Carbon Footprint of an Organization: A Tool for Monitoring Impacts on Global Warming. Procedia Eng. 2018, 212, 729–735. [Google Scholar] [CrossRef]
- Stavropoulos, P.; Panagiotopoulou, V.C. Carbon Footprint of Manufacturing Processes: Conventional vs. Non-Conventional. Processes 2022, 10, 1858. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhang, Z.; Wang, J. Development of an evaluating method for carbon emissions of manufacturing process plans. Discret. Dyn. Nat. Soc. 2015, 2015, 784751. [Google Scholar] [CrossRef]
- Jandl, R.; Bauhus, J.; Bolte, A.; Schindlbacher, A.; Schüler, S. Effect of Climate-Adapted Forest Management on Carbon Pools and Greenhouse Gas Emissions. Curr. For. Rep. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Shumi, G.; Rodrigues, P.; Hanspach, J.; Härdtle, W.; Hylander, K.; Senbeta, F.; Fischer, J.; Schultner, J. Woody plant species diversity as a predictor of ecosystem services in a social–ecological system of southwestern Ethiopia. Landsc. Ecol. 2021, 36, 373–391. [Google Scholar] [CrossRef]
- Ontl, T.A.; Janowiak, M.K.; Swanston, C.W.; Daley, J.; Handler, S.; Cornett, M.; Hagenbuch, S.; Handrick, C.; McCarthy, L.; Patch, N. Forest Management for Carbon Sequestration and Climate Adaptation. J. For. 2020, 118, 86–101. [Google Scholar] [CrossRef]
- Manaye, A.; Tesfamariam, B.; Tesfaye, M.; Worku, A.; Gufi, Y. Tree diversity and carbon stocks in agroforestry systems in northern Ethiopia. Carbon Balance Manag. 2021, 16, 14. [Google Scholar] [CrossRef]
- Tavoni, M.; Sohngen, B.; Bosetti, V. Forestry and the carbon market response to stabilize climate. Energy Policy 2007, 35, 5346–5353. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomon, T.; Gupta, V.; Ncho, C.M. Balancing Livestock Environmental Footprints with Forestry-Based Solutions: A Review. Ecologies 2023, 4, 714-730. https://doi.org/10.3390/ecologies4040047
Solomon T, Gupta V, Ncho CM. Balancing Livestock Environmental Footprints with Forestry-Based Solutions: A Review. Ecologies. 2023; 4(4):714-730. https://doi.org/10.3390/ecologies4040047
Chicago/Turabian StyleSolomon, Tamirat, Vaishali Gupta, and Chris Major Ncho. 2023. "Balancing Livestock Environmental Footprints with Forestry-Based Solutions: A Review" Ecologies 4, no. 4: 714-730. https://doi.org/10.3390/ecologies4040047
APA StyleSolomon, T., Gupta, V., & Ncho, C. M. (2023). Balancing Livestock Environmental Footprints with Forestry-Based Solutions: A Review. Ecologies, 4(4), 714-730. https://doi.org/10.3390/ecologies4040047






