Abstract
This study investigated the content of 137Cs (a long-lived radioactive isotope of caesium) in various parts of Pinus sylvestris L. (Scotch pine) and Dicranum polysetum Sw. (rugose fork-moss) at three different sites within the exclusion zone of the Chornobyl nuclear power plant over two years. The Leliv site is located within the 10 km zone, while the Paryshiv and Dytiatky sampling sites are within the 30 km zone. Samples of different P. sylvestris organs were collected, including 1- and 2-year-old branches and needles and wood and outer bark, and the entire D. polysetum. Sampling was conducted every two weeks throughout the year during 2014 and 2015. The specific activity levels of 137Cs in the samples were measured using gamma spectrometry with a CANBERRA gamma spectrometer unit and a coaxial high-purity HPGe semiconductor detector. The study found that at the Leliv and Paryshiv sites, the highest content of 137Cs in living organs of P. sylvestris was found in the wood. At the Dytiatky site, the needles and branches of the first and second years had anomalously high concentrations of radiocaesium (137Cs). This could be due to a thin layer of forest litter (1.5 cm) at that site. The study also found significant changes in the specific activity levels of 137Cs in living pine organs throughout the year. The highest concentration was observed in pine branches and needles in summer, and the maximum values in wood were observed in winter. The study suggests that a constant circulation of 137Cs in the soil–plant system can cause seasonal changes in the content of 137Cs in living pine organs. Symbiotic mycorrhizal fungi can play an important role in the circulation of radiocaesium in forest ecosystems. The outer bark of P. sylvestris did not show any seasonal changes in the content of 137Cs. It may not be involved in radiocaesium redistribution inside the plant but can serve as a long-term source of this radionuclide entering the forest litter. The study found no seasonal changes in the accumulation of 137Cs by D. polysetum, which might be due to the physiological characteristics of this plant species. Based on the analysis of the conducted studies, the recommendation is to consider the seasonal changes in the content of 137Cs during monitoring activities and when using Scots pine in areas potentially contaminated with this radionuclide.
1. Introduction
Since the mid-20th century, long-term studies have examined the effects of radionuclides that contaminated the Earth due to global fallouts from nuclear weapons testing and nuclear accidents on different ecosystems. In the case of the Chornobyl Nuclear Power Plant (ChNPP) disaster, large areas of the northern hemisphere were contaminated with radiocaesium, with the most significant contamination occurring in Europe. The ongoing research into the effects of radiocaesium contamination highlights the importance of monitoring and the possibility of mitigating the impacts of nuclear accidents on the environment [1,2,3,4,5,6,7,8,9,10].
In the case of the Fukushima Dai-ichi nuclear disaster, the release of radiocaesium and other radionuclides resulted in the contamination of the surrounding areas, including air, water, and soil. This accident served as a reminder that the use of atomic energy by humans still carries the risk of releasing various radionuclides, including radiocaesium, into the environment. Ongoing studies continue to assess the long-term environmental impacts of this disaster [11,12,13,14,15,16,17,18,19,20,21,22].
Forests are a natural environmental ecosystem that surrounds us. When radionuclides are released into the environment, the forest ecosystems are inevitably polluted. Close attention was paid to the study of the patterns of accumulation of various radionuclides (including 137Cs) in forest ecosystems in general and in individual forest objects in particular (wood, moss, soil, fungi and others) [23,24,25,26,27,28,29,30,31,32,33].
Compared to agricultural land, radioactive contamination persists longer in forests. Research carried out in the mid-1980s in the United States revealed that in areas contaminated by nuclear weapons testing in the early 1960s, the dose rate was four times higher in forests than in adjacent agricultural lands. The authors attributed this phenomenon to the ability of forest litter to retain 137Cs, thereby preventing its penetration into deeper soil layers [34].
Forests are a vital source of various elements that people use daily, such as wood for heating, building materials and furniture, medicinal plants, berries and mushrooms. However, all these objects could contain the long-lived and biologically hazardous radionuclide 137Cs, which could enter the human body and remain there for a prolonged period. Studying the redistribution of radiocaesium in forest ecosystems could enable a more comprehensive understanding of this source of radioactive caesium, which persists for a longer time in forests compared to agricultural lands.
Studies carried out over many years after the accident at the Chornobyl nuclear power plant have allowed us to speak confidently about a gradual decrease in the levels of radiocaesium pollution in forest ecosystems [35]. Against this background, it is essential to research the regular changes in 137Cs concentration in the plants of forest ecosystems during the year, since the specific activity of this radionuclide in some objects in certain seasons of the year can exceed its average values by almost an order of magnitude [36].
There is a scarcity of literature that explores the changes in the levels of specific activity of 137Cs in various components of forest ecosystems throughout the year. Most studies have focused on the growing season, and the published results often present contradictory findings.
Salt and Mayes [37] found that three types of grass growing on peat-podzolic soil had a maximum content of 137Cs during the summer. In another study [38], it was reported that there was a gradual decrease in the concentration of this radionuclide throughout the growing season.
The activity of 137Cs increased notably in the autumn of 1987 and 1988 and to a lesser extent in the autumn of 1986 when the concentration of K (potassium) in the needles usually increases. According to the study’s authors [39], Chornobyl 137Cs has mixed with its chemical analogue—K—and recirculates in trees along with it.
Orlov and Dolin [40] investigated the seasonal redistribution of radiocaesium in mosses in the Ukrainian Polissya territory. They discovered that this radionuclide passes gradually from the terrestrial phytomass to the underground phytomass during the growing season.
The highest concentration of 137Cs in the soil phytomass of blueberries was recorded in July, according to a publication [41].
Seasonal changes in the accumulation of 137Cs in plants (male fern, bracken, lingonberry and blueberry) were examined [42]. The content of 137Cs in plants varies throughout the growing season and is influenced by the physiological characteristics of each plant.
According to an article [43], the concentration of 137Cs in the above-ground phytomass of forest ecosystems increases tenfold during winter compared to May. It was noted [44], that this radionuclide concentration in pine growth decreased from spring to autumn. The author of [45] discovered that the radiocaesium content decreased during the growing season in one-year-old needles and two-year-old branches. However, no seasonal changes in the concentration of this radionuclide in two- and three-year-old needles were detected.
The content of 137Cs in mushrooms of the same species can vary by an order of magnitude throughout the year. The highest level of specific activity of this radionuclide in mushrooms from June to December was observed in October [46].
In the radioecology of the forest, there is still no unambiguous explanation for the fluctuations in the values of the coefficients of radiocaesium transfer from the soil to various plants and mushrooms [25,26,44]. Significant sudden changes in the levels of 137Cs-specific activity in different forest objects during the study of long-term dynamics [47] have yet to be fully explored. Changes in weather conditions are most often indicated as the causes of these phenomena [48]. However, there may be other explanations. Sharp changes in the levels of specific activity of 137Cs in the studied objects in different years can be explained by seasonal fluctuations in the content of this radionuclide.
This study aimed to investigate whether there are seasonal changes in the concentration of the radioactive isotope 137Cs in plants within the forest surrounding the Chornobyl nuclear power plant and to interpret the implications of any observed changes. Seasonal changes in 137Cs concentration in plants could provide insight into how this radioactive material is transported and distributed within the ecosystem.
To achieve the study’s goal, sampling sites were laid in the Chornobyl NPP’s exclusion zone, and a sampling technique was developed at the established landfills. Samples of different parts of Scotch pine and Dicranum moss were collected following the proven methodology, and the content of 137Cs was measured in the samples. The research results were analysed, and a possible explanation was put forward. Finally, conclusions were drawn on the stated goal.
The findings may contribute to a better understanding of the behaviour of 137Cs in forest ecosystems and help to develop more effective strategies for mitigating the impact of radioactive contamination.
2. Materials and Methods
The author developed a unique methodology for studying the redistribution of 137Cs in forest ecosystems after the Chornobyl accident. The method involves the frequent and year-round sampling of cosmopolitan plant species, including living and dead plant parts. The selection of easily accessible plant parts for sampling and the careful laying of the test sites are additional features of the methodology.
The study was conducted almost 30 years after the accident, allowing the investigation of these processes’ long-term and chronic nature. Through the analysis of their results, the researcher could identify regular patterns of 137Cs redistribution in the studied plant species, which occurred throughout the year and could be assessed in terms of their frequency and scope. Overall, this study sheds light on the long-term effects of 137Cs contamination on forest ecosystems and how these ecosystems continue to be impacted over time.
However, the methodology may have limitations when applied to deciduous forests due to the annual fall of leaves, which reduces the volume of information gathered about the redistribution of 137Cs in living plant parts throughout the year.
Overall, the methodology appeared well-suited for studying the redistribution of 137Cs in forest ecosystems and was developed per methodological recommendations for monitoring work in the Chornobyl exclusion zone [49].
2.1. Sampling Sites
137Cs circulation in various organs of the Scots pine and mosses in the exclusion zone of the Chornobyl nuclear power plant (ChNPP) was researched. The sampling sites are located at various distances and directions from the Chornobyl NPP (Figure 1). The three chosen sampling sites in the Chornobyl Exclusion Zone are identified by the following coordinates: Dytiatky (30.12449 E, 51.13088 N, distance from ChNPP, 29.9 km), Paryshiv (30.32473 E, 51.30069 N, distance from ChNPP, 18.8 km), Leliv (30.16031 E, 51.32326 N, distance from ChNPP, 8.4 km). A sampling of 1st and 2nd branches and needles was conducted in 2014 and 2015, once every two weeks. He outer bark was sampled from February 2014 to December 2015, and moss from May 2014 to December 2015. Wood was sampled only from November 2014. The studied sampling sites had an area of approximately 30 m2 and a sod-podzolic soil. The vast majority of woody vegetation was the Scots pine (Pinus sylvestris L.).
Figure 1.
Sampling sites within the Chornobyl exclusion zone.
2.2. Sampling of Various Objects of Forest Ecosystems
The sampling regime was once every two weeks. Such organs of P. sylvestris as the wood, the outer bark, branches, one-year and two-year needles and moss Dicranum polysetum Sw. were chosen as the objects of the study. The main reasons for using P. sylvestris as the main object of research were:
- -
- in the forest massifs of the Chornobyl exclusion zone, pine forests occupy almost 60% of the total area of 91,565.0 ha [50], and this species is dominant;
- -
- samples of photosynthetic organs of this plant are available throughout the year;
- -
- the capacity to simultaneously sample branches and needles of different ages.
Samples of branches and needles were taken with a pruner. A sampling of the outer bark was carried out with the help of a knife. The wood was selected with the help of a 30 cm long Preissler drill. Before using the drill, a trunk part was cleaned of the outer and inner bark. After sampling, the places of the trunk with the bark removed were covered with a cut paste to prevent the tree’s death. Moss samples were taken using a 10 × 10 cm frame. Moss Dicranum was chosen as an object of research because this plant is evergreen, so the processes of redistribution of 137Cs can occur throughout the year.
Fifteen model trees were used in the study at each sampling site. Samples were taken each time in the following amount:
- -
- 15 one-year and 15 two-year branches with needles;
- -
- 10–12 pieces of bark;
- -
- 10–12 wood samples.
A moss sample was taken from the soil surface in triplicate at each sample site.
There were 6 samples of Scottish pine organs: needles of the first year, branches of the first year, needles of the second year, branches of the second year, bark, wood, and 1 sample of moss. The branches were detached from the needles in a stationary laboratory. Then, all samples were dried separately for 1–1.5 months at room temperature (18–20 °C), ground using a laboratory mill and placed in disposable calibrated plastic cups with a diameter of 9 cm and a height of 6 cm for gamma spectrometric studies.
2.3. Radiometry
The specific activity of 137Cs was measured using a CANBERRA gamma spectrometer unit with a coaxial high-purity HPGe semiconductor detector (GC6020 model). The detection unit was covered with a 100 mm lead screen, which allowed practical measurements of samples with relatively low radionuclide-specific activity. A schematic block diagram of the gamma spectrometer is shown in Figure 2. Figure 3 shows a photo of the gamma spectrometric setup used to determine the specific activity of 137Cs in the samples and the spectrum displayed on a computer screen.

Figure 2.
Schematic block diagram of the gamma spectrometer used in this study.

Figure 3.
Gamma spectrometric setup: (a)—general view, (b)—spectrum displayed on a computer screen.
The obtained graphs showed measurement data on the specific activity of 137Cs with measurement errors. The measurements were carried out for 600–14,400 s, depending on the specific activity of 137Cs. The measurement errors did not exceed 10% and were within 3–5% of radionuclide activity [51].
The study of the dynamics of the content of radiocaesium (the physical half-life of 137Cs is 30.05 years [52]) in an object requires the exclusion of the influence of its physical decay. April 26, 1986 was chosen as the “zero” date, on which the results of the studies were recalculated.
The concentrations of 137Cs in various objects of the forest ecosystems were calculated using the radioactive decay formula; the specific activity of 137Cs in the samples (dry weight) is expressed in Bq/kg.
2.4. Correlation Analysis
A correlation analysis was carried out to check the presence or absence of a relationship between the content of 137Cs in the various studied objects. Spearman’s rank correlation coefficients were calculated. This coefficient is used to identify and determine the magnitude of the relationship between data. The ranks of the compared values are used as data. No preliminary assumptions about the nature of the distribution of features in the general population are required to calculate the Spearman coefficient.
The following formula calculates the Spearman’s rank correlation coefficient:
where
- n—number of paired ranked signs (sample size);
- D—difference between the ranks of the conjugate values of the features;
- )—sum of the squared differences in ranks [53].
To assess the existence of a relationship between variables, the Chaddock scale is used, according to which, with a correlation coefficient from 0 to 0.3, the strength of the relationship is considered very weak; from 0.3 to 0.5, it is weak; from 0.5 to 0.7, it is average; from 0.7 to 0.9 it is high; from 0.9 to 1 it is very high.
With a negative correlation, the values of the strength of the connection between the variables are reversed.
2.5. Statistical Analysis
Statistical analysis and graphical visualisation were performed using GraphPad Prism 9.5.1 (GraphPad Software, San Diego, CA, USA). Statistics were performed using two-way ANOVA and Tukey’s multiple comparisons tests. The data are presented as means ± standard deviation (SD). A p-value ≤ 0.05 was considered statistically significant. In the graphs, the variables of significance are labelled with asterisks (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001).
3. Results
The research identified trends in the changes of 137Cs concentration in one- and two-year-old branches, needles, and wood of P. sylvestris. The value of the specific activity of radiocaesium in these pine organs on the territory of all sampling sites was found to depend on the year’s season (Figure 4, Figure 5 and Figure 6).
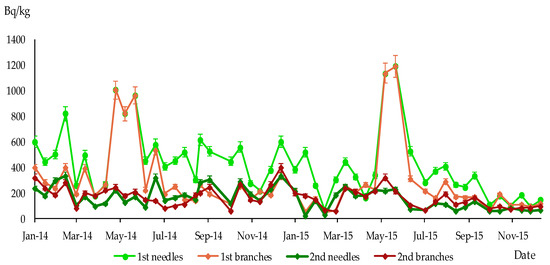
Figure 4.
Specific activity of 137Cs in 1st and 2nd branches and needles of P. sylvestris, sampling site Leliv, Bq/kg dry weight.
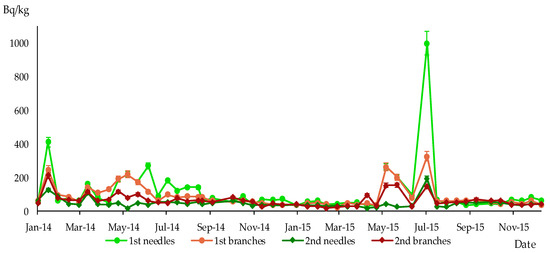
Figure 5.
Specific activity of 137Cs in 1st and 2nd branches and needles of P. sylvestris, sampling site Paryshiv, Bq/kg dry weight.
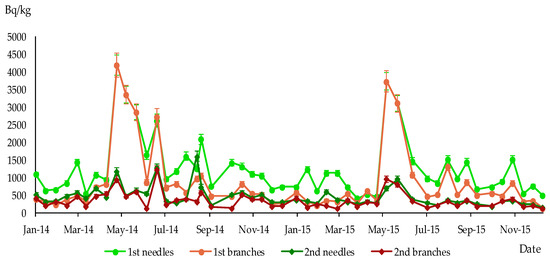
Figure 6.
Specific activity of 137Cs in 1st and 2nd branches and needles of P. sylvestris, sampling site Dytiatky, Bq/kg dry weight.
The specific activity level of 137Cs varied among branches and needles of different ages. On average, the specific activity was higher in one-year-old needles compared to two-year-old needles and one- and two-year-old branches. The lowest content of 137Cs was found in two-year-old needles at the Paryshiv and Leliv sampling sites, whereas at the Dytiatky site, the concentration of 137Cs in two-year-old needles was higher than in two-year-old branches (Table 1, Figure 7a,b).

Table 1.
Average concentrations of 137Cs in the researched organs of P. sylvestris during the study period (from January 2014 to December 2015), Bq/kg dry weight.
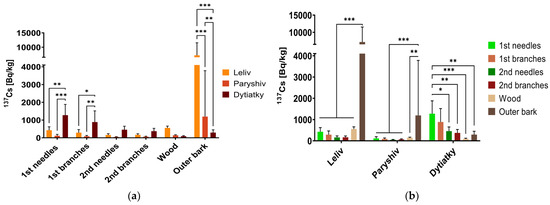
Figure 7.
Average concentrations of 137Cs in P. sylvestris (a) according to the organ of the tree and (b) according to the sampling site, Bq/kg dry weight. The variables of significance are labelled with asterisks (* p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001).
Table 1 and Figure 7 display the average 137Cs content in the wood and outer bark of P. sylvestris at the sampling sites. At the Leliv and Paryshiv sites, the highest 137Cs content among living tissues was observed in the wood, and the highest concentration among all studied pine organs was found in the dead outer bark. At the Dytiatky sampling site, the most contaminated part of P. sylvestris was one-year-old needles, with almost three times higher concentrations of radiocaesium compared to that at the Leliv site and 11 times higher than that at the Paryshiv site.
The performed statistical analysis (Figure 7) indicated the reliability of the differences in 137Cs accumulation at the three sampling sites in annual needles, annual branches and outer bark. For the rest of the studied pine organs, the differences in the concentration of this radionuclide at different sites were not statistically significant.
The maximum specific activity of 137Cs in one-year-old branches and needles was reached between May and August 2014 and 2015 in all sampling sites. The subsequent increase in specific activity levels of 137Cs in branches and needles was observed in the second year of their life during the next growing season (see Figure 4, Figure 5 and Figure 6).
The content of 137Cs in different parts of P. sylvestris was analysed, and the parameters’ correlation was determined using the Spearman method. The results, which can be found in Table 2, showed a strong direct relationship for the radiocaesium content between annual needles and annual branches, as indicated by Spearman’s correlation coefficients exceeding 0.7 in all sampling sites. Consequently, changes in 137Cs content in these pine organs occur almost simultaneously.

Table 2.
Spearman’s correlation coefficients for 137Cs concentrations in various organs of P. sylvestris.
Similarly, high correlation coefficients were observed for other pairs of objects, such as 1st branches–2nd branches, 1st needles–2nd needles, and 2nd needles–2nd branches, but this was not the case for all sampling sites. These results indicate that changes in the level of 137Cs in different organs of P. sylvestris during the study period often do not occur simultaneously.
Significant seasonal changes in the content of 137Cs in wood in all areas of the study could not be recorded. One can speak of a seasonal trend in the accumulation of this radionuclide in Leliv and Paryshiv (Figure 8). In the territory of these sampling sites, the highest values of 137Cs specific activity levels were observed from November 2014 to March 2015 and at the end of 2015. The lowest values of the specific activity of 137Cs in wood in Leliv and Paryshiv were observed from mid-summer to mid-autumn. In Dytiatky, the minimum values of the particular radiocaesium activity were noted in different seasons—winter, summer and autumn.
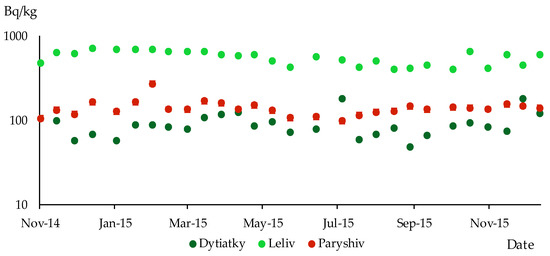
Figure 8.
Specific activity of 137Cs in the wood of P. sylvestris; different sampling sites in the research territory (logarithmic scale).
During the research period, low levels of specific activity in wood, which did not exceed 200 Bq/kg, were observed at two sampling sites—Paryshev and Dityatki. However, at the Leliv sampling site, the content of 137Cs in wood ranged from 400 to 703 Bq/kg.
The lowest values of 137Cs specific activity in wood at the Leliv and Paryshiv sampling sites were observed from mid-summer to mid-autumn. In contrast, at the Dytiatky site, the minimum specific activity of radiocaesium was noted during various seasons, including winter, summer and autumn. It was impossible to establish any seasonal trends in the accumulation of 137Cs in wood at this site.
Figure 9 shows the results of the measurements of the specific activity of 137Cs in the outer bark of P. sylvestris. Seasonal trends in the accumulation of radiocaesium in this pine organ were not observed at any of the sampling sites.
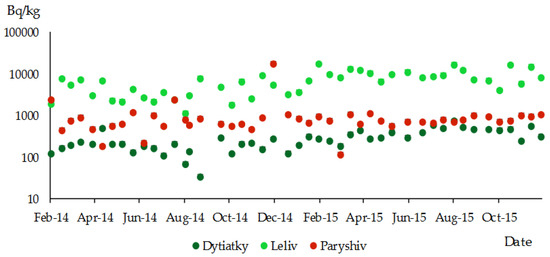
Figure 9.
Specific activity of 137Cs in the outer bark of P. sylvestris; different sampling sites in the research territory (logarithmic scale).
As a result of the research, seasonal trends in the specific activity levels of 137Cs in moss were not detected (Figure 10). During the year, there were increases and decreases in specific activity levels in moss at all sampling sites, which practically did not correlate with the seasonal changes in radiocaesium content in different pine organs.
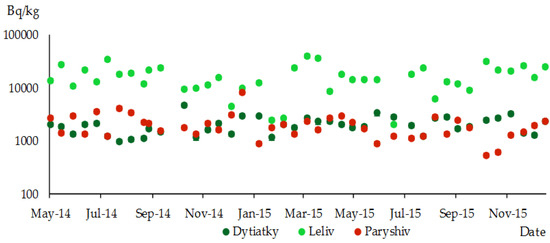
Figure 10.
Specific activity of 137Cs in moss; different sampling sites in the research territory (logarithmic scale).
4. Discussion
The concentration of 137Cs in plants in territories contaminated by nuclear accidents depends on the level of contamination [54,55]. The results obtained from the Leliv and Paryshiv sampling sites (see Figure 4, Figure 5 and Figure 8–10, Table 1) confirmed this regularity. The average radiocaesium content in various parts of the examined pine in Leliv, which is situated within the 10 km exclusion zone, was higher than that in Paryshiv. The decreasing order of the average 137Cs content in different pine organs at these sites is outer bark > wood > first-year needles > first-year branches > second-year branches > second-year needles.
At the Dytiatky sampling site (see Figure 6 and Table 1), a deviation from this regularity was observed, with needles of the first year showing higher levels of 137Cs compared to the other organs, followed by branches of the first year, needles of the second year, branches of the second year, outer bark and wood.
The peculiarities of 137Cs accumulation by various pine organs in Dytiatky cannot be explained by the magnitude of 137Cs contamination of the soil at this site because the content of this radionuclide in the soil at the Dytiatky sampling site was the lowest. The A0l layer in Leliv contained 137Cs 598 ± 321 Bq/kg, that in Paryshiv, 164 ± 80 Bq/kg, and that in Dytiatky, only 149 ± 107 Bq/kg. The concentration of 137Cs in A0f + A0h in Leliv was 49,171 ± 16,054, that in Paryshiv −6059 ± 2949, and that in Dytiatky −2490 ± 867 Bq/kg. An exception was the soil layer of 0–5 cm, in which the content of 137Cs was higher in Dytiatky than in Paryshiv. The radiocaesium content in the 0–5 cm soil layer in Leliv was 12,844 ± 5855, that in Paryshiv, −862 ± 428, and that in Dytiatky, −1034 ± 566 Bq/kg [56].
According to [57], the level of 137Cs accumulation by plants may depend on various factors, including the thickness of the litter layer. The thickness of the A0l + A0f + A0h soil layer at the Dytiatky sampling site is significantly different from those at other sites. At Dytiatky, the litter layer is only 1.5 cm thick, while at other sites, it is 7 cm thick. The A0l + A0f + A0h layer with less fungal mycelium and another soil biota can allow radiocaesium to penetrate plants more easily and its outflow into the soil. Symbiotic organisms can retain a significant portion of 137Cs from the soil, as evidenced by this radionuclide’s higher content in symbiotic fungi than symbiont plants [44,58]. It is likely that 137Cs first accumulates in shoots and needles before being redistributed into the wood and retained in the living bark once it enters a plant. At Dytiatky, the lower number of symbiotic organisms increases the availability of 137Cs for plants but also allows it to “leak” easily from pine trees into the soil, decreasing the possibility of its accumulation in wood and living bark, with the greater outflow of caesium from the pine.
The concentrations of radiocaesium in the living organs of P. sylvestris exhibited a seasonal trend, varying throughout the year. At all sampling sites, a significant increase in the levels of specific activity of 137Cs in the needles and branches of the first year was recorded since the beginning of their growth in late April to early May. This trend was also observed in two-year-old needles and branches (see Figure 4, Figure 5 and Figure 6). This suggests that as the summer progresses, the growth of young needles and branches slows down, leading to a decline in the concentration of 137Cs in these organs in the middle of summer.
The seasonal changes in the levels of specific activity of 137Cs in pine branches and needles can be explained by the existence of two differently directed processes occurring in the plant: accumulation and excretion of radiocaesium, i.e., the circulation of this radionuclide in the soil–plant system. These processes occur simultaneously, and the predominance of one of them leads to an increase or decrease in the content of 137Cs in the needles and branches of P. sylvestris during the year. A seasonal trend in the redistribution of 137Cs may indicate ascending and descending fluxes of this radionuclide in wood.
Based on the study of some tree species in Japan after the accident at the Fukushima Dai-ichi nuclear power plant, the authors of the work suggested that in these plants, there is both an inflow and an outflow of radiocaesium, and a decrease in its content in plants is a consequence of an excess of outflow over the entry of this radionuclide into them [59]. In [19], a downward flow of 137Cs in wood is indicated as a possible interpretation of the obtained research results. The constant circulation of the chemical analogue of caesium, potassium, in the soil–plant system was described [60,61]. Probably 137Cs also circulates in plants. With the beginning of the growing season, its additional entry into plants occurs. By the time the intensive growth stops, the outflow of radiocaesium from the needles and branches begins to prevail over its accumulation, and the levels of the specific activity of this radionuclide decrease.
The main radiocaesium fluxes in forest ecosystems may occur in the fungal symbiont–pine system rather than in the soil–pine system. In the soil–pine system, the circulation of 137Cs can be influenced by symbiotic soil organisms, particularly mycorrhiza-forming fungi, which are essential for P. sylvestris. Early studies after the Chornobyl accident suggested that the fungal mycelium can contain up to 63% of the total 137Cs reserve in the forest soil, making it a long-term depot for this radionuclide [62,63]. The symbiotic relationship between the pine tree and its fungal symbiont may allow the tree to receive additional radiocaesium from the mycelium during the growing season as the tree’s need for water increases, resulting in a higher volume of water passing through the fungal barrier and potentially transferring more radionuclides to the plant. In mid-autumn, the highest levels of 137Cs in mycorrhizal fungi suggest an outflow of this radionuclide from the plant to the fungi after the end of the growing season [46]. This means fungi could retain 137Cs until the following spring, making them a depot of this radionuclide in forest ecosystems.
No seasonal fluctuations in the specific activity of 137Cs were found in the outer dead bark. This suggests no radiocaesium transfer between the tree’s living parts and the outer bark. The movement of 137Cs within living parts of the tree may contribute to a gradual reduction in its concentration, unlike in the dead outer bark, where the decrease in concentration is solely due to the physical decay of this radioactive element (see Figure 9).
The studies that were carried out could not identify any seasonal fluctuations in the levels of 137Cs activity in moss. This may be due to the characteristics of this moss physiology, which lacks symbiotic mycorrhizal fungi. As a result, the content of 137Cs in moss experienced fluctuations throughout the year, as depicted in Figure 10.
5. Conclusions
As a result of this study, it was found that in all investigated parts of pines and in the moss, the content of 137Cs was not constant. Seasonal fluctuations in the levels of specific activity of this radionuclide were manifested only in the living parts of P. sylvestris—to a greater extent in the branches and needles and to a lesser extent in the wood. Seasonal changes in the concentration of 137Cs were not found in the dead outer bark of pine and moss. The absence of a symbiotic relationship between D. polysetum and mycorrhizal fungi may be the reason for the lack of seasonal fluctuations in 137Cs content in this plant.
The changes in the content of 137Cs in the living organs of the pine did not occur simultaneously. In summer, the maximum concentrations of this radionuclide in the branches and needles were observed, while in winter, the maximum values were observed in the wood.
In forest ecosystems, there is likely a constant circulation of 137Cs in the soil–plant chain. Radiocaesium enters the plant from the soil, and the fungal mycelium can be a source of an additional amount of this radionuclide during the intensive growth of branches and needles. Fungal mycelium is a depot of 137Cs in forest soil. An increase in the concentration of 137Cs in mycelium by mid-autumn may be due to the outflow of this radionuclide from the living organs of pine.
This study showed that the redistribution of 137Cs in plants largely depends on the peculiarities of the sampling site. The Dytiatky sampling site differs from the others in the low thickness of the forest litter layer, which may be the reason for the imbalance of the 137Cs ratios in different pine organs between this and other sampling sites. In addition, the low thickness of the forest litter in this area might be the reason for the anomalously high levels of 137Cs specific activity in the fast-growing organs of the pine, i.e., branches and needles.
An analysis of the results indicated that the seasonal changes in 137Cs specific activity levels in Scots pine are essential to consider for monitoring activities and in relation to the use of this plant by the population in radiocaesium-contaminated areas. Further research is needed to determine if these seasonal changes in 137Cs content are typical for other tree species. This highlights the need for continued research in this area, as it could have implications for monitoring and managing the impacts of radiocaesium contamination on the environment and human health.
Funding
This work was financed by the Science and Technology Center in Ukraine (STCU), project # 5954 (1 September 2013–31 August 2015), Donor Nations—the USA and the European Union.
Institutional Review Board Statement
The statement of the Institutional Review Board is not applicable.
Informed Consent Statement
The statement related to informed consent is not applicable.
Data Availability Statement
The author confirms that all relevant data are included in the manuscript.
Acknowledgments
The author thanks the Austrian Academy of Sciences for providing moral and financial support during the two most difficult months. This support made it possible to complete the work for the article. The author is grateful to Christian Koeberl (Department of Lithospheric Research, University of Vienna, Austria) for assistance in this work.
Conflicts of Interest
The author declares that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Bergan, T.D. Radioactive fallout in Norway from atmospheric nuclear weapons tests. J. Environ. Radioact. 2002, 60, 189–208. [Google Scholar] [CrossRef]
- Gwynn, J.P.; Nalbandyan, A.; Rudolfsen, G. 210Po, 210Pb, 40K and 137Cs in edible wild berries and mushrooms and ingestion doses to man from high consumption rates of these wild foods. J. Environ. Radioact. 2013, 116, 34–41. [Google Scholar] [CrossRef]
- Tracy, B.L.; Carini, F.; Barabash, S.; Berkovskyy, V.; Brittain, J.E.; Chouhan, S.; Eleftheriou, G.; Iosjpe, M.; Monte, L.; Psaltaki, M.; et al. The sensitivity of different environments to radioactive contamination. J. Environ. Radioact. 2013, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Khan, A.M.; Ahmad, M.; Akib, S.; Balkhair, K.S.; Bakar, N.K.A. Release, deposition and elimination of radiocesium (137Cs) in the terrestrial environment. Environ. Geochem. Health 2014, 36, 1165–1190. [Google Scholar] [CrossRef] [PubMed]
- Paller, M.H.; Jannik, G.T.; Baker, R.A. Effective Half-Life of Caesium-137 in Various Environmental Media at the Savannah River Site. J. Environ. Radioact. 2014, 131, 81–88. [Google Scholar] [CrossRef]
- Rosén, K.; Vinichuk, M. Potassium fertilization and 137Cs transfer from soil to grass and barley in Sweden after the Chernobyl fallout. J. Environ. Radioact. 2014, 130, 22–32. [Google Scholar] [CrossRef]
- Penrose, B.; Beresford, N.A.; Broadley, M.R.; Crout, N.M.J. Inter-varietal variation in caesium and strontium uptake by plants: A meta-analysis. J. Environ. Radioact. 2015, 139, 103–117. [Google Scholar] [CrossRef]
- Itthipoonthanakorn, T.; Dann, S.E.; Crout, N.M.J.; Shaw, G. Nuclear weapons fallout 137Cs in temperate and tropical pine forest soils, 50 years post-deposition. Sci. Total Environ. 2019, 660, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Holiaka, D.; Yoschenko, V.; Levchuk, S.; Kashparov, V. Distributions of 137Cs and 90Sr activity concentrations in trunk of Scots pine (Pinus sylvestris L.) in the Chernobyl zone. J. Environ. Radioact. 2020, 222, 106319. [Google Scholar] [CrossRef]
- Oloś, G.; Dołhańczuk-Śródka, A. Effective and environmental half-lives of radiocesium in game from Poland. J. Environ. Radioact. 2022, 248, 106870. [Google Scholar] [CrossRef]
- Hashimoto, S.; Ugawa, S.; Nanko, K.; Shichi, K. The total amounts of radioactively contaminated materials in forests in Fukushima, Japan. Sci. Rep. 2012, 2, 416. [Google Scholar] [CrossRef] [PubMed]
- Tagami, K.; Uchida, S.; Ishii, N.; Kagiya, S. Translocation of radiocesium from stems and leaves of plants and the effect on radiocesium concentrations in newly emerged plant tissues. J. Environ. Radioact. 2012, 111, 65–69. [Google Scholar] [CrossRef]
- Teramage, M.T.; Onda, Y.; Patin, J.; Kato, H.; Gomi, T.; Nam, S. Vertical distribution of radiocesium in coniferous forest soil after the Fukushima nuclear power plant accident. J. Environ. Radioact. 2014, 137, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Tamura, K.; Suda, T.; Matsumura, R.; Onda, Y. Vertical distribution and temporal changes of 137Cs in soil profiles under various land uses after the Fukushima Dai-ichi Nuclear Power Plant accident. J. Environ. Radioact. 2015, 139, 351–361. [Google Scholar] [CrossRef]
- Komatsu, M.; Kaneko, S.; Ohashi, S.; Kuroda, K.; Sano, T.; Ikeda, S.; Saito, S.; Kiyono, Y.; Tonosaki, M.; Miura, S.; et al. Characteristics of initial deposition and behavior of radiocesium in forest ecosystems of different locations and species affected by the Fukushima Daiichi Nuclear Power Plant accident. J. Environ. Radioact. 2016, 161, 2–10. [Google Scholar] [CrossRef]
- Niizato, T.; Abe, H.; Mitachi, K.; Sasaki, Y.; Ishii, Y.; Watanabe, T. Input and output budgets of radiocesium concerning the forest floor in the mountain forest of Fukushima released from the TEPCO’s Fukushima Dai-ichi nuclear power plant accident. J. Environ. Radioact. 2016, 161, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Nishina, K.; Hashimoto, S. Extensive analysis of radiocesium concentrations in wild mushrooms in eastern Japan affected by the Fukushima nuclear accident: Use of open accessible monitoring data. Environ. Poll. 2019, 255, 113236. [Google Scholar] [CrossRef]
- Kenzo, K.; Saito, S.; Araki, M.G.; Kajimoto, T. Vertical distribution of radiocesium concentrations among crown positions and year-to-year variation in four major tree species after the Fukushima Daiichi Nuclear Power Plant accident. J. Environ. Radioact. 2020, 225, 106447. [Google Scholar] [CrossRef]
- Ohashi, S.; Kuroda, K.; Fujiwara, T.; Takano, T. Tracing radioactive cesium in stem wood of three Japanese conifer species 3 years after the Fukushima Dai-ichi Nuclear Power Plant accident. J. Wood Sci. 2020, 66, 44. [Google Scholar] [CrossRef]
- Imamura, N.; Watanabe, M.; Manaka, T. Estimation of the rate of 137Cs root uptake into stemwood of Japanese cedar using an isotopic approach. Sci. Total Environ. 2021, 755, 142478. [Google Scholar] [CrossRef]
- Saidin, Z.H.; Levia, D.F.; Kato, H.; Kurihara, M.; Hudson, J.E.; Nanko, K.; Onda, Y. Vertical distribution and transport of radiocesium via branchflow and stemflow through the canopy of cedar and oak stands in the aftermath of the Fukushima Dai-ichi Nuclear Power Plant accident. Sci. Total Environ. 2022, 818, 151698. [Google Scholar] [CrossRef]
- Ota, M.; Koarashi, J. Contamination processes of tree components in Japanese forest ecosystems affected by the Fukushima Daiichi Nuclear Power Plant accident 137Cs fallout. Sci. Total Environ. 2022, 816, 142478. [Google Scholar] [CrossRef] [PubMed]
- Rafferty, B.; Brennan, M.; Dawson, D.; Dowding, D. Mechanisms of 137Cs migration in coniferous forest soils. J. Environ. Radioact. 2000, 48, 131–143. [Google Scholar] [CrossRef]
- Kruyts, N.; Delvaux, B. Soil organic horizons as a major source for radiocesium biorecycling in forest ecosystems. J. Environ. Radioact. 2002, 58, 175–190. [Google Scholar] [CrossRef]
- Kostiainen, E. 137Cs in Finnish wild berries, mushrooms and game meat in 2000–2005. Boreal Environ. Res. 2007, 12, 23–28. [Google Scholar]
- Bataitienė, I.P.; Butkus, D. Investigation of 137Cs and 90Sr transfer from sandy soil to Scots pine (Pinus sylvestris L.) rings. J. Environ. Eng. Landsc. Manag. 2010, 18, 281–287. [Google Scholar] [CrossRef]
- Škrkal, J.; Rulik, P.; Fantinova, K.; Burianova, J.; Helebrant, J. Long-term 137Cs activity monitoring of mushrooms in forest ecosystems of the Czech Republic. Radiat. Protect. Dosim. 2013, 157, 579–584. [Google Scholar] [CrossRef]
- Trappe, M.J.; Minc, L.D.; Kittredge, K.S.; Pinkd, J.W. Cesium radioisotope content of wild edible fungi, mineral soil, and surface litter in western North America after the Fukushima nuclear accident. Canad. J. For. Res. 2014, 44, 1441–1452. [Google Scholar] [CrossRef]
- Marčiulionienė, D.; Lukšienė, B.; Jefanova, O. Accumulation and translocation peculiarities of 137Cs and 40K in the soil-plant system. J. Environ. Radioact. 2015, 150, 86–92. [Google Scholar] [CrossRef]
- Huang, Y.; Kaneko, N.; Nakamori, T.; Miura, T.; Tanaka, Y.; Nonaka, M.; Takenaka, C. Radiocesium immobilization to leaf litter by fungi during first-year decomposition in a deciduous forest in Fukushima. J. Environ. Radioact. 2016, 152, 28–34. [Google Scholar] [CrossRef]
- Thiry, Y.; Garcia-Sanchez, L.; Hurtevent, P. Experimental quantification of radiocesium recycling in a coniferous tree after aerial contamination: Field loss dynamics, translocation and final partitioning. J. Environ. Radioact. 2016, 161, 42–50. [Google Scholar] [CrossRef]
- Tucakovića, I.; Barišića, D.; Graheka, Ž.; Kasap, A.; Širić, I. 137Cs in mushrooms from Croatia sampled 15–30 years after Chernobyl. J. Environ. Radioact. 2018, 181, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Strzałek, M.; Barczak, K.; Karwowska, J.; Królak, E. Activity of 137Cs and 40K Isotopes in Pine (Pinus sylvestris L.) and Birch (Betula pendula Roth) Stands of Different Ages in a Selected Area of Eastern Poland. Forests 2021, 12, 1205. [Google Scholar] [CrossRef]
- Miller, K.M.; Kuiper, J.L.; Heifer, I.K. 137Cs Fallout Depth Distributions in Forest Versus Field Sites: Implications for External Gamma Dose Rates. J. Environ. Radioact. 1990, 12, 23–47. [Google Scholar] [CrossRef]
- Orlov, O.O.; Krasnov, V.P. Long-term dynamics of 137Cs radioactive contamination of wild berries and mushrooms in Polissia of Ukraine. Sci. Bull. 2001, 46, 172–179. (In Ukrainian) [Google Scholar]
- Zarubina, N.Y. 137Cs circulation in forest ecosystems on the territory of the Chernobyl exclusion zone (Plant). Rep. NAS Ukrain. 2022, 2, 89–95. [Google Scholar] [CrossRef]
- Salt, C.A.; Mayes, R.W. Seasonal variations in radiocesium uptake by reseeded hill pasture grazed at different intensities by sheep. J. Appl. Ecol. 1991, 28, 947–962. [Google Scholar] [CrossRef]
- Bunzl, K.; Kracke, W. Transfer von 137Cs und 90Sr in Mehl, Kleie und Stroh von Weizen, Roggen, Gerste und Hafer in den Jahren 1982, 1986 (Reaktorunfall in Tschernobyl) und 1987 in Feldversuchen. Z. Lebensm Unters Forch. 1989, 188, 439–444. (In German) [Google Scholar] [CrossRef]
- Nygren, P.; Hari, P.; Raunemaa, T.; Kulmala, M.; Luokkanen, S.; Holmberg, M.; Nikinmaa, E. Behaviour of 137Cs from Chernobyl fallout in a Scots pine canopy in southern Finland. Can. J. For. Res. 1994, 24, 1210–1215. [Google Scholar] [CrossRef]
- Orlov, O.O.; Dolin, V.V. Biogeochemistry of 137Cs for Forest-Swamp Ecosystems of the Ukrainian Polissia; Naukova Dumka: Kyiv, Ukraine, 2010. (In Ukrainian) [Google Scholar]
- Krasnov, V.P.; Orlov, A.A. Radioecology of Berry Plants (In Ukraine); Zhytomyr: Volyn, Ukraine, 2004. [Google Scholar]
- Grabovskyi, V.; Dzendzelyuk, O. Seasonal changes of 137Cs content in some medical herbs and berry plants from Western Ukraine. Visnyk Lviv. University. Ser. Biol. 2012, 58, 175–184. (In Ukrainian) [Google Scholar]
- Mukhamedshin, K.D.; Chilimov, A.I.; Bezuglov, V.K.; Snytkin, G.V. Certification of forest resources based on radiation characteristics, as the basis for obtaining normative-clean forestry products in the territory contaminated with radionuclides. In Questions of Forest Radioecology; MSFU: Moscow, Russia, 2000. (In Russian) [Google Scholar]
- Shcheglov, A.I. Biogeochemistry of Technogenic Radionuclides in Forest Ecosystems; Nauka: Moscow, Russia, 2000. (In Russian) [Google Scholar]
- Perevolotsky, A.N. Distribution of 137Cs and 90Sr in Forest Biogeocenoses; RIIPE “Institute of Radiology”: Minsk, Belarus, 2006; ISBN 985-6765-18-8. (In Russian) [Google Scholar]
- Zarubina, N. The influence of biotic and abiotic factors on 137Cs accumulation in higher fungi after the accident at Chornobyl NPP. J. Environ. Radioact. 2016, 161, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, V.P.; Melnyk, V.V.; Courbet, T.V.; Zhukovsky, O.V.; Zborovska, O.V.; Orlov, O.O. Dynamics of the specific activity of 137Cs in (Convallaria majalis L.) in the forest of Ukrainian Pollissia after the accident on ChNPP. Nucl. Phys. At. Energ. 2019, 20, 278–284. (In Ukrainian) [Google Scholar] [CrossRef]
- Velasco, R.H.; Toso, J.P.; Belli, M.; Sansone, U. Radiocesium in the northeastern part of Italy after the chernobyl accident: Vertical soil transport and soil-to-plant transfer. J. Environ. Radioact. 1997, 37, 73–83. [Google Scholar] [CrossRef]
- Methodological Recommendations for Organizing and Conducting Radio-Ecological Monitoring of Forests in the Chornobyl Exclusion Zone; Chornobyl, Ukraine, 1997. (In Ukrainian)
- Project of Organization and Development of Forestry of the State Specialized Enterprise “NORTHERN PUSHCHA”; Ukrainian State Project Forest Management Production Association: Irpin, Ukraine, 2017. (In Ukrainian)
- Zarubina, N.Y. 137Cs and 40K in the needles and branches of Scotch pine (Pinus sylvetris L.) on the territory of Chornobyl exclusion zone. Nucl. Phys. Atom. En. 2019, 20, 51–59. (In Russian) [Google Scholar] [CrossRef]
- Bé, M.-M.; Chisté, V.; Dulieu, C.; Browne, E.; Baglin, C.; Chechev, V.; Kuzmenko, N.; Helmer, R.; Kondev, F.; MacMahon, D.; et al. Table of Radionuclides, Cs-137; Monographie BIPM-5; Bureau International des Poids et Mesures, Pavillon de Breteuil: Sèvres, France, 2006; Volume 3, ISBN 92-822-2218-7. [Google Scholar]
- Lakin, G.F. Boimetriya; Higher School: Moscow, Russia, 1990; 350p. (In Russian) [Google Scholar]
- Orlov, O. Evaluation of mosses and lichens as test-objects of monitoring of 137Cs contamination of pine forest biogeocenoses in Ukrainian Polissia. Geochem. Technog 2022, 7, 33–37. [Google Scholar] [CrossRef]
- Bal, S.Ş.; Kurşat, M.; Kuluöztürk, M.F.; Çelik, Ş.K.; Yilmaz, E. Soil to plant transfer of 226Ra, 232Th and 137Cs to some medicinal and aromatic plants growing in Bitlis (Turkey). J. Environ. Radioact. 2023, 257, 107089. [Google Scholar] [CrossRef]
- Zarubina, N.Y. 137Cs circulation in forest ecosystems on the territory of the Chornobyl exclusion zone (Soil). Rep. NAS Ukrain. 2020, 10, 85–92. [Google Scholar] [CrossRef]
- Butkus, D.; Konstantinova, M. Studies of 137Cs transfer in soil-fern system. J. Environ. Eng. Lands. Manag. 2005, 13, 97–102. [Google Scholar] [CrossRef]
- Arkhipov, M.P.; Kuchma, M.D.; Davydchuk, V.S.; Arkhipov, A.M. The role of natural factors in the fixation of radionuclides in the exclusion zone. Bul. Ecol. Cond. Excl. Zone Zone Uncon. (Compul.) Resettl. 2001, 17, 36–42. (In Ukrainian) [Google Scholar]
- Imamura, N.; Komatsu, M.; Ohashi, S.; Hashimoto, S.; Kajimoto, T.; Kaneko, S.; Takano, T. Temporal Changes in the Radiocesium Distribution in Forests over the Five Years after the Fukushima Daiichi Nuclear Power Plant Accident. Available online: http://www.nature.com/scientificreports/7:8179 (accessed on 15 August 2017).
- Libbert, E. Plant Physiology; Mir: Moscow, Russia, 1976. (In Russian) [Google Scholar]
- Kramer, P.D.; Kozlovsky, T.T. Physiology of Woody Plants Physiology; Publishing House “Lesnaia Promyshlennost”: Moscow, Russia, 1983. (In Russian) [Google Scholar]
- Olsen, R.A.; Joner, E.J.; Bakken, L.R. Soil fungi and the fate of radiocaesium in the soil ecosystem–a discussion of possible mechanisms involted in the radiocaesium accumulation in fungi, and the role of fungi as a Cs-sink in the soil. In Transfer of Radionuclides in Natural and Semi-Natural Environment; Desmet, G., Nassimbeni, P., Belli, M., Eds.; Elsivier Applied Science: London, UK; New York, NY, USA, 1990; pp. 657–663. ISBN 1-85166-539-0. [Google Scholar]
- Dahlberg, A.; Nikolova, I.; Johanson, K.-J. Intraspecific variation in Cs-137 activity concentration in sporocarps of Suillus variegatus in seven Swedish population. Mycol. Res. 1997, 101, 545–551. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).