Organs-on-Chips: Revolutionizing Biomedical Research
Abstract
1. Introduction
2. Organ-on-a-Chip Technology: Mimicking Human Organ Structures
3. Personalized Applications and 3D Modeling in OoC Technology
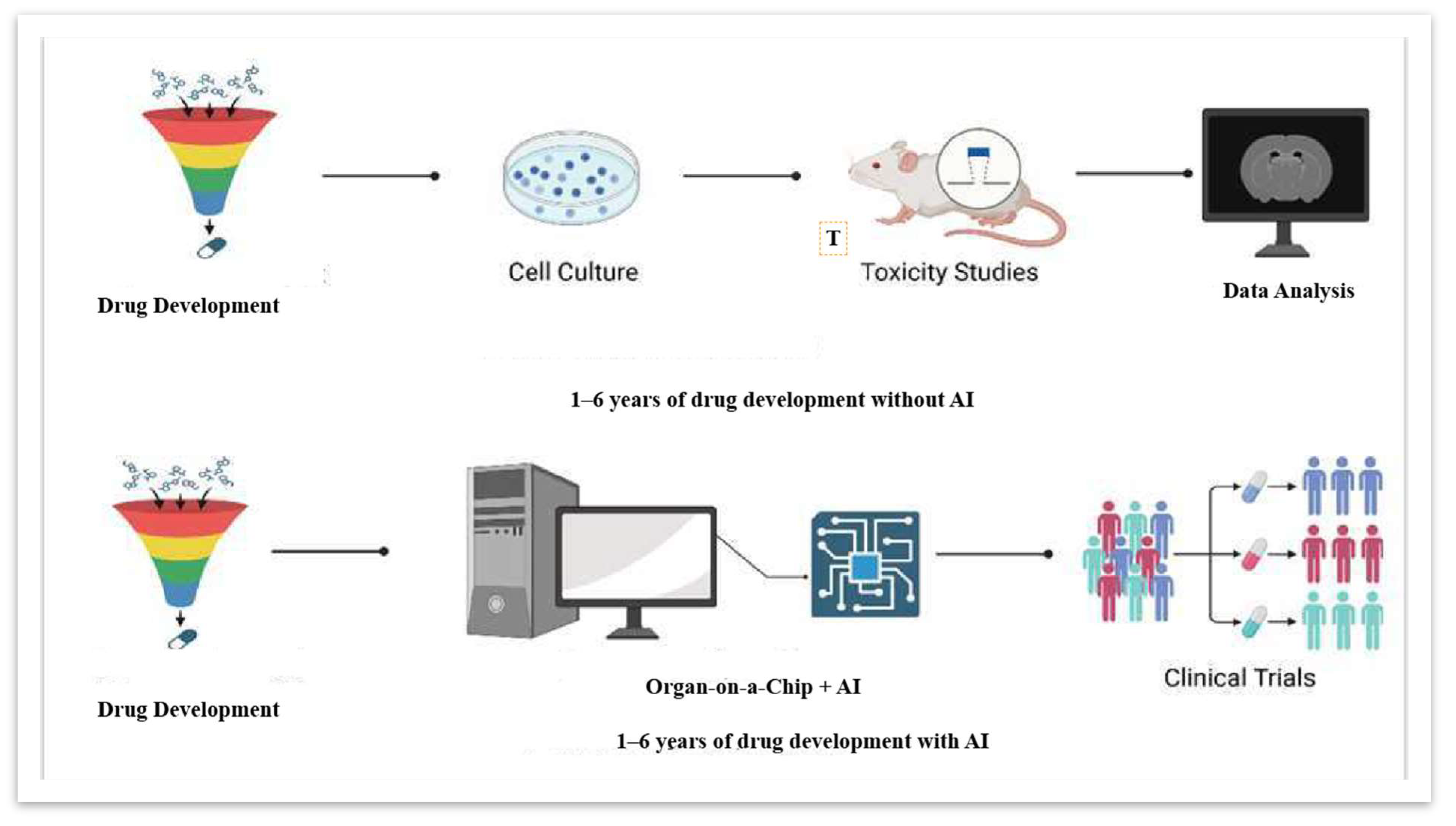
4. Integrating AI with Organ-on-a-Chip Technology
5. Organ-on-a-Chip Models: Advancements and Applications in Biomedical Research
5.1. Heart-on-a-Chip
5.2. Bone Marrow-on-a-Chip
5.3. Lung-on-a-Chip (LOC)
5.4. Liver-on-a-Chip (LiOC)
5.5. Gut-on-a-Chip (GOC)
5.6. Kidney-on-a-Chip
5.7. Central Neural Axis and Blood–Brain Barrier (BBB)-on-a-Chip
5.8. Immune System-on-a-Chip
5.9. Microfluidics in Cancer Immunotherapy and Tumor Modeling
5.10. Skin-on-a-Chip
6. Organ-on-Chip Technology and Specialized Skills and Data Privacy
7. Commercialization and Challenges of Organ-on-a-Chip Technology
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Landau, S.; Okhovatian, S.; Liu, C.; Lu, R.X.Z.; Lai, B.F.L.; Wu, Q.; Kieda, J.; Cheung, K.; Rajasekar, S.; et al. Integrating organoids and organ-on-a-chip devices. Nat. Rev. Bioeng. 2024, 7, 588–608. [Google Scholar] [CrossRef]
- Baig, M.A.A. Bridging the Gap: Integration of Artificial Intelligence with Organ-on-Chip (AI-OoC). IJLAI Trans. Sci. Eng. 2024, 2, 17–23. Available online: https://www.academia.edu/119822664/Bridging_the_Gap_Integration_of_Artificial_Intelligence_with_Organ_on_Chip_AI_OoC_ (accessed on 25 July 2024).
- Nithin, R.; Aggarwal, A. Organ-On-A-Chip: An Emerging Research Platform. Organogenesis 2023, 19, 2278236. [Google Scholar] [CrossRef]
- Cauli, E.; Polidoro, M.A.; Marzorati, S.; Bernardi, C.; Rasponi, M.; Lleo, A. Cancer-on-chip: A 3D model for the study of the tumor microenvironment. J. Biol. Eng. 2023, 17, 53. [Google Scholar] [CrossRef]
- Aydogmus, H.; Hu, M.; Ivancevic, L.; Frimat, J.P.; van den Maagdenberg, A.M.; Sarro, P.M.; Mastrangeli, M. An organ-on-chip device with integrated charge sensors and recording microelectrodes. Sci. Rep. 2023, 13, 8062. [Google Scholar] [CrossRef]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-chip: A new paradigm for drug development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.B.; Lee, J.; Kim, H.C.; Huh, D. Organomimetic microsystems technologies. Biomed. Eng. Lett. 2012, 2, 88–94. [Google Scholar] [CrossRef]
- Osório, L.A.; Silva, E.; Mackay, R.E. A review of biomaterials and scaffold fabrication for organ-on-a-chip (OOAC) systems. Bioengineering 2021, 8, 113. [Google Scholar] [CrossRef]
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Artifical Intelligence in Organoids and Organ-on-a-Chip Report. Available online: https://www.deep-pharma.tech/organoids-ai-2023 (accessed on 25 July 2024).
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005.e6. [Google Scholar] [CrossRef]
- Haddrick, M.; Simpson, P.B. Organ-on-a-chip technology: Turning its potential for clinical benefit into reality. Drug Discov. Today 2019, 24, 1217–1223. [Google Scholar] [CrossRef]
- Allwardt, V.; Ainscough, A.J.; Viswanathan, P.; Sherrod, S.D.; McLean, J.A.; Haddrick, M.; Pensabene, V. Translational Roadmap for the Organs-on-a-Chip Industry toward Broad Adoption. Bioengineering 2020, 7, 112. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. OnLine 2020, 19, 9. [Google Scholar] [CrossRef]
- Cho, C.H.; Park, J.; Tilles, A.W.; Berthiaume, F.; Toner, M.; Yarmush, M.L. Layered patterning of hepatocytes in co-culture systems using microfabricated stencils. Biotechniques 2018, 48, 47–52. [Google Scholar] [CrossRef]
- Peel, S.; Corrigan, A.M.; Ehrhardt, B.; Jang, K.J.; Caetano-Pinto, P.; Boeckeler, M.; Rubins, J.E.; Kodella, K.; Petropolis, D.B.; Ronxhi, J.; et al. Introducing an automated high content confocal imaging approach for organs-on-chips. Lab Chip 2019, 19, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Technology Networks. A Guide to Organ-on-a-Chip. Available online: https://www.technologynetworks.com/cell-science/articles/a-guide-to-organ-on-a-chip-381700 (accessed on 25 July 2024).
- Gao, W.; Wang, C.; Li, Q.; Zhang, X.; Yuan, J.; Li, D.; Sun, Y.; Chen, Z.; Gu, Z. Application of medical imaging methods and artificial intelligence in tissue engineering and organ-on-a-chip. Front. Bioeng. Biotechnol. 2022, 10, 985692. [Google Scholar] [CrossRef] [PubMed]
- Adam Kratz, S.R.; Höll, G.; Schuller, P.; Ertl, P.; Rothbauer, M. Latest trends in biosensing for microphysiological organs-on-a-chip and body-on-a-chip systems. Biosensors 2019, 9, 110. [Google Scholar]
- Kane, K.I.; Moreno, E.L.; Hachi, S.; Walter, M.; Jarazo, J.; Oliveira, M.A.; Hankemeier, T.; Vulto, P.; Schwamborn, J.C.; Thoma, M.; et al. Automated microfluidic cell culture of stem cell derived dopaminergic neurons. Sci. Rep. 2019, 9, 1796. [Google Scholar] [CrossRef]
- Trafton, A. Using “organs-on-a-chip” to model complicated diseases. MIT News. 18 March 2020. Available online: https://news.mit.edu/2020/organ-on-microfluidic-chip-0318 (accessed on 25 July 2024).
- Hinkson, I.V.; Madej, B.; Stahlberg, E.A. Accelerating Therapeutics for Opportunities in Medicine: A Paradigm Shift in Drug Discovery. Front. Pharmacol. 2020, 11, 770. [Google Scholar] [CrossRef]
- Guha, M.; Dove, R.; Gebeyehu, A.; Merenich, D.; Collier, P.; Crowgey, E.L.; Schaffer, M.; Shuey, D.; Macarrón, R.; Arjona, A.A. Establishment of Human Bone Marrow-on-Chip as a Preclinical Model to Evaluate Drug-Induced Toxicities and Myelofibrosis Patient-Specific Pathophysiology. Blood 2023, 142 (Suppl. S1), 5611. [Google Scholar] [CrossRef]
- Chou, D.B.; Frismantas, V.; Milton, Y.; David, R.; Pop-Damkov, P.; Ferguson, D.; MacDonald, A.; Vargel Bölükbaşı, Ö.; Joyce, C.E.; Moreira Teixeira, L.S.; et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat. Biomed. Eng. 2020, 4, 394–406. [Google Scholar] [CrossRef]
- Li, L.; Bo, W.; Wang, G.; Juan, X.; Xue, H.; Zhang, H. Progress and application of lung-on-a-chip for lung cancer. Front. Bioeng. Biotechnol. 2024, 12, 1378299. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Guo, Q.; Kuang, J.; Li, D.; Wu, M.; Mo, Y.; Zhang, T.; Gao, X.; Tan, J. Advanced lung organoids and lung-on-a-chip for cancer research and drug evaluation: A review. Front. Bioeng. Biotechnol. 2023, 11, 1299033. [Google Scholar] [CrossRef]
- Fu, J.; Qiu, H.; Tan, C.S. Microfluidic Liver-on-a-Chip for Preclinical Drug Discovery. Pharmaceutics 2023, 15, 1300. [Google Scholar] [CrossRef]
- George, E.; Velayudhan, S.; Anil Kumar, P.R. Liver-on-a-Chip. In Microfluidics and Multi Organs on Chip; Springer: Singapore, 2022; pp. 341–357. [Google Scholar] [CrossRef]
- Liu, J.; Du, Y.; Xiao, X.; Tan, D.; He, Y.; Qin, L. Construction of in vitro liver-on-a-chip models and application progress. Biomed. Eng. OnLine 2024, 23, 33. [Google Scholar] [CrossRef]
- Giampetruzzi, L.; Barca, A.; Francioso, L.; Verri, T.; Siciliano, P. Technology and Application of Gut-On-Chip Gut epithelial mimicking in integrated sensor platforms. GIT Lab. J. Eur. 2019, 2, 28–29. Available online: https://mlj.goums.ac.ir/index.php (accessed on 25 July 2024).
- Thomas, D.P.; Zhang, J.; Nguyen, N.T.; Ta, H.T. Microfluidic Gut-on-a-Chip: Fundamentals and Challenges. Biosensors 2023, 13, 136. [Google Scholar] [CrossRef]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y.; et al. Correction to: Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv. Healthc. Mater. 2018, 7, 1700506. [Google Scholar] [CrossRef]
- Fu, Z.; Gu, Q.; Wang, L.; Chen, L.; Zhou, L.; Jin, Q.; Li, T.; Zhao, Y.; Wu, S.; Luo, X.; et al. Cell-free fat extract regulates oxidative stress and alleviates Th2-mediated inflammation in atopic dermatitis. Front. Bioeng. Biotechnol. 2024, 12, 1373419. [Google Scholar] [CrossRef]
- Pastorino, B.; Touret, F.; Gilles, M.; De Lamballerie, X.; Charrel, R.N. Current status of pathogen handling in European laboratories: Focus on viral inactivation process. Front. Bioeng. Biotechnol. 2024, 12, 1422553. [Google Scholar] [CrossRef]
- Cho, S.; Lee, S.; Ahn, S.I. Design and engineering of organ-on-a-chip. Biomed. Eng. Lett. 2023, 13, 97–109. [Google Scholar] [CrossRef]
- Cell Culture and Diagnostic Virology Since the Discovery. Available online: https://slidetodoc.com/cell-culture-and-diagnostic-virology-since-the-discovery/ (accessed on 25 July 2024).
- Cui, M.; Wiraja, C.; Zheng, M.; Singh, G.; Yong, K.T.; Xu, C. Recent Progress in Skin-on-a-Chip Platforms. Adv. Ther. 2022, 5, 2100138. [Google Scholar] [CrossRef]
- Emulate. Emulate Closes $82 Million Series E Financing to Scale Amid Rapid Growth in Organ-on-a-Chip Market. Available online: https://emulatebio.com/press/emulate-closes-82-million-series-e-financing/ (accessed on 25 July 2024).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monga, A.; Jain, K.; Popli, H.; Telgote, P.; Kaur, G.; Rizwani, F.; Chauhan, R.; Kaur, D.; Chauhan, A.; Tuli, H.S. Organs-on-Chips: Revolutionizing Biomedical Research. Biophysica 2025, 5, 38. https://doi.org/10.3390/biophysica5030038
Monga A, Jain K, Popli H, Telgote P, Kaur G, Rizwani F, Chauhan R, Kaur D, Chauhan A, Tuli HS. Organs-on-Chips: Revolutionizing Biomedical Research. Biophysica. 2025; 5(3):38. https://doi.org/10.3390/biophysica5030038
Chicago/Turabian StyleMonga, Ankit, Khush Jain, Harvinder Popli, Prashik Telgote, Ginpreet Kaur, Fariah Rizwani, Ritu Chauhan, Damandeep Kaur, Abhishek Chauhan, and Hardeep Singh Tuli. 2025. "Organs-on-Chips: Revolutionizing Biomedical Research" Biophysica 5, no. 3: 38. https://doi.org/10.3390/biophysica5030038
APA StyleMonga, A., Jain, K., Popli, H., Telgote, P., Kaur, G., Rizwani, F., Chauhan, R., Kaur, D., Chauhan, A., & Tuli, H. S. (2025). Organs-on-Chips: Revolutionizing Biomedical Research. Biophysica, 5(3), 38. https://doi.org/10.3390/biophysica5030038







