Cyanobacterial UV Pigments Evolved to Optimize Photon Dissipation Rather than Photoprotection
Abstract
1. Introduction
2. Properties of Cyanobacterial UV-Absorbing Pigments
2.1. Mycosporine-like Amino Acids (MAAs)
2.1.1. Physicochemical Properties of MAAs
2.1.2. MAAs Spatial Distribution Within the Biosphere
2.1.3. Extant Biosynthesis of MAAs
2.1.4. Function of MAAs: Traditional Protective View vs. Thermodynamic View
2.2. Scytonemins
2.2.1. Physicochemical Properties of Scytonemins
2.2.2. Scytonemin Spatial Distribution Within the Biosphere
2.2.3. Extant Biosynthesis of Scytonemins
2.2.4. Function of Scytonemins: Traditional Protective View vs. Thermodynamic View
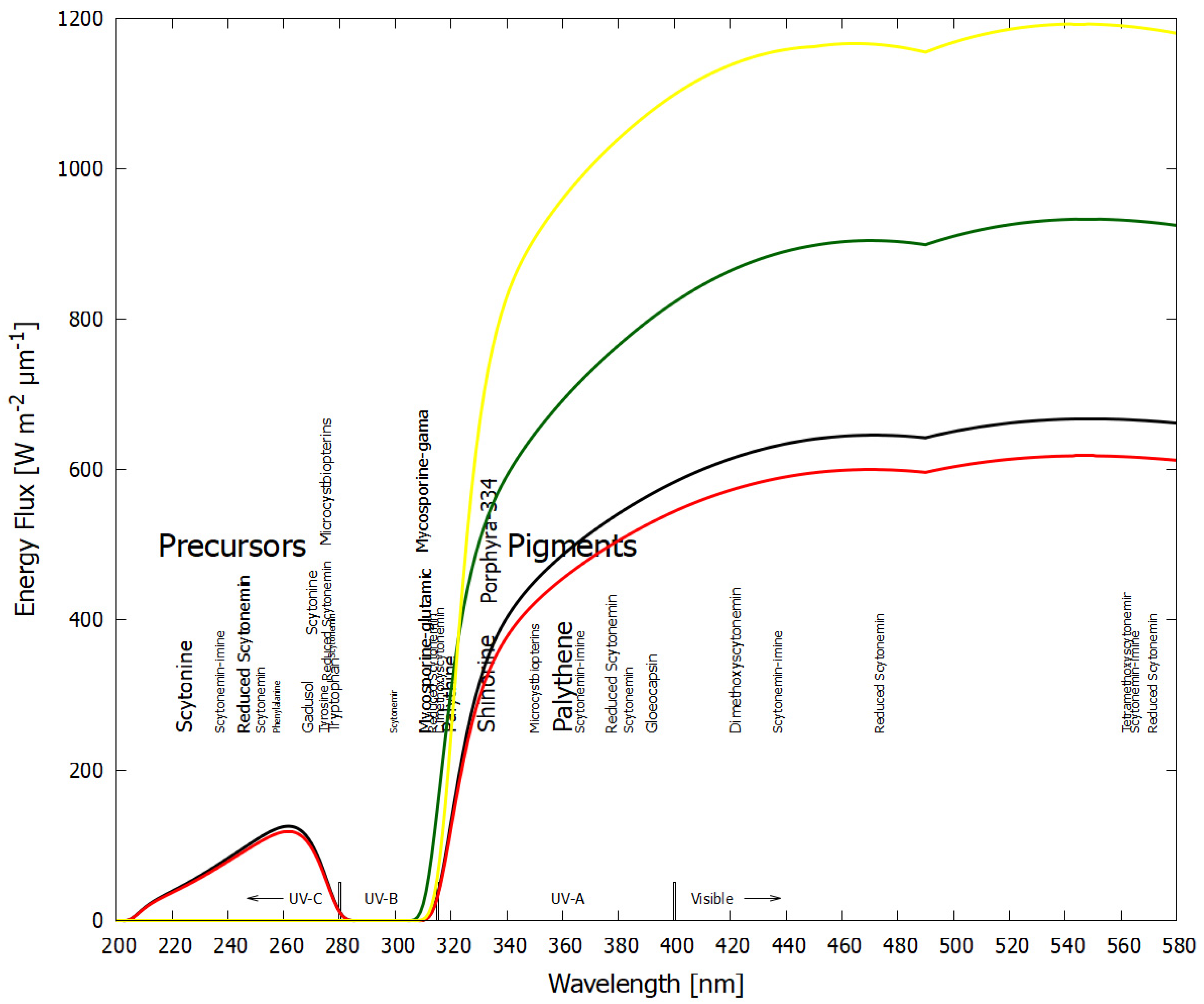
- Inability to explain the strong visible absorption bands of scytonemin-imine, where photosynthetic pigments absorb. The question is raised by Grant and Louda [83].“The absorption spectrum (max 237, 366, 437, 564 nm in vitro), extending from the ultraviolet (UVB & UVA) into the blue and green of the visible, appears to indicate a photoprotective role beyond shielding only UVR. That is, going on the premise that evolution generates and retains only advantageous secondary metabolites, then what is the role of the visible bands in this case?”
- Inability to explain the production of the strongly UV-C/UV-B-absorbing methoxyscytonemins and scytonine, in spite of the absence of UV-C wavelengths and the low intensity of UV-B in today’s surface solar spectrum. The question is raised by Varnali and Edwards [81].“The realization that scytonemin is the parent molecule of perhaps a whole family of related molecules is important in that an analytical challenge is generated to detect these family members in admixture and in the presence of each other naturally, and also the question is raised about the role of these molecules in the survival strategy processes involving scytonemin; what subtle changes to the radiation absorption process require molecular modification of what apparently is already a highly successful radiation protectant, especially when the molecular syntheses are accomplished in energy-poor situations?”
- Soule et al. [171] developed a scytoneminless mutant of the cyanobacterium Nostoc punctiforme, which proved to have an indistinguishable growth rate from the wild type after both were subjected to UV-A irradiation. The conclusion of the authors was that other photoprotective mechanisms can fully accommodate the absence of scytonemin in the mutant.
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AAAs | Aromatic amino acids |
| CDOM | Chromophoric dissolved organic matter |
| DOM | Dissolved organic matter |
| EPSs | Extracellular polymeric substances |
| Ga | Giga (1000 million) years ago |
| IC | Internal Conversion |
| MAAs | Mycosporine-like amino acids |
| NPQ | Non-photochemical quenching |
| PAR | Photosynthetically active radiation |
| ROS | Reactive oxygen species |
| SML | Sea-surface microlayer |
| UV | Ultraviolet |
| UV-A | Ultraviolet A 315–400 nm |
| UV-B | Ultraviolet B 280–315 nm |
| UV-C | Ultraviolet C 100–280 nm |
| UVR | Ultraviolet radiation |
References
- Prigogine, I. Thermodynamics of Irreversible Processes; Wiley: New York, NY, USA, 1967. [Google Scholar]
- Glansdorff, P.; Prigogine, I. Thermodynamic Theory of Structure, Stability, and Fluctuations; Wiley-Interscience: London, UK, 1971. [Google Scholar]
- Michaelian, K. Thermodynamic origin of life. arXiv 2009, arXiv:0907.0042. Available online: https://arxiv.org/abs/0907.0042 (accessed on 25 July 2015).
- Michaelian, K. Thermodynamic dissipation theory for the origin of life. Earth Syst. Dyn. 2011, 2, 37–51. [Google Scholar] [CrossRef]
- Michaelian, K. The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine. Entropy 2021, 23, 217. [Google Scholar] [CrossRef]
- Michaelian, K. The Non-Equilibrium Thermodynamics of Natural Selection: From Molecules to the Biosphere. Entropy 2023, 25, 1059. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. The Pigment World: Life’s Origins as Photon-Dissipating Pigments. Life 2024, 14, 912. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of light harvesting in green plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Berera, R.; Ilioaia, C.; van Stokkum, I.H.; Kennis, J.T.; Pascal, A.A.; van Amerongen, H.; Robert, B.; Horton, P.; van Grondelle, R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 2007, 450, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Staleva, H.; Komenda, J.; Shukla, M.K.; Šlouf, V.; Kaňa, R.; Polívka, T.; Sobotka, R. Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nat. Chem. Biol. 2015, 11, 287–291. [Google Scholar] [CrossRef]
- Gupta, S.; Guttman, M.; Leverenz, R.L.; Zhumadilova, K.; Pawlowski, E.G.; Petzold, C.J.; Lee, K.K.; Ralston, C.Y.; Kerfeld, C.A. Local and global structural drivers for the photoactivation of the orange carotenoid protein. Proc. Natl. Acad. Sci. USA 2015, 112, E5567–E5574. [Google Scholar] [CrossRef]
- Son, M.; Pinnola, A.; Gordon, S.C.; Bassi, R.; Schlau-Cohen, G.S. Observation of dissipative chlorophyll-to-carotenoid energy transfer in light-harvesting complex II in membrane nanodiscs. Nat. Commun. 2020, 11, 1295. [Google Scholar] [CrossRef]
- Murchie, E.H.; Ruban, A.V. Dynamic non-photochemical quenching in plants: From molecular mechanism to productivity. Plant J. 2020, 101, 885–896. [Google Scholar] [CrossRef]
- Michaelian, K. HESS Opinions “Biological catalysis of the hydrological cycle: Life’s thermodynamic function”. Hydrol. Earth Syst. Sci. 2012, 16, 2629–2645. [Google Scholar] [CrossRef]
- Michaelian, K.; Simeonov, A. Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum. Biogeosciences 2015, 12, 4913–4937. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Junge, W. On the origin of photosynthesis as inferred from sequence analysis. Photosynth. Res. 1997, 51, 27–42. [Google Scholar] [CrossRef]
- Wynn-Williams, D.D.; Edwards, H.G.M.; Newton, E.M.; Holder, J.M. Pigmentation as a survival strategy for ancient and modern photosynthetic microbes under high ultraviolet stress on planetary surfaces. Int. J. Astrobiol. 2002, 1, 39–49. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Cherepanov, D.A.; Galperin, M.Y. Survival of the fittest before the beginning of life: Selection of the first oligonucleotide-like polymers by UV light. BMC Evol. Biol. 2003, 3, 12. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial responses to UV radiation. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 481–499. [Google Scholar] [CrossRef]
- Fuentes-Tristan, S.; Parra-Saldivar, R.; Iqbal, H.M.N.; Carrillo-Nieves, D. Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. J. Photochem. Photobiol. B Biol. 2019, 201, 111684. [Google Scholar] [CrossRef] [PubMed]
- Moisan, T.A.; Mitchell, B.G. UV absorption by mycosporine-like amino acids in Phaeocystis antarctica Karsten induced by photosynthetically available radiation. Mar. Biol. 2001, 138, 217–227. [Google Scholar] [CrossRef]
- Sagan, C. Ultraviolet selection pressure on the earliest organisms. J. Theor. Biol. 1973, 39, 195–200. [Google Scholar] [CrossRef]
- Garcia-Pichel, F. Solar ultraviolet and the evolutionary history of cyanobacteria. Orig. Life Evol. Biosph. 1998, 28, 321–347. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. Microscopic dissipative structuring and proliferation at the origin of life. Heliyon 2017, 3, e00424. [Google Scholar] [CrossRef]
- Walter, M.R.; Buick, R.; Dunlop, J.S.R. Stromatolites 3400–3500 Myr old from the North Pole area, Western Australia. Nature 1980, 284, 443–445. [Google Scholar] [CrossRef]
- Awramik, S.M.; Schopf, J.W.; Walter, M.R. Filamentous fossil bacteria from the Archean of Western Australia. Precambrian Res. 1983, 20, 357–374. [Google Scholar] [CrossRef]
- Schopf, J.W.; Packer, B.M. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science 1987, 237, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Schopf, J.W. Microfossils of the Early Archean Apex chert: New evidence of the antiquity of life. Science 1993, 260, 640–646. [Google Scholar] [CrossRef]
- Schopf, J.W.; Kudryavtsev, A.B.; Agresti, D.G.; Wdowiak, T.J.; Czaja, A.D. Laser-Raman imagery of Earth’s earliest fossils. Nature 2002, 416, 73–76. [Google Scholar] [CrossRef]
- Tice, M.M.; Lowe, D.R. Photosynthetic microbial mats in the 3,416-Myr-old ocean. Nature 2004, 431, 549–552. [Google Scholar] [CrossRef]
- Van Kranendonk, M.J.; Philippot, P.; Lepot, K.; Bodorkos, S.; Pirajno, F. Geological setting of Earth’s oldest fossils in the c. 3.5 Ga Dresser Formation, Pilbara craton, Western Australia. Precambr. Res. 2008, 167, 93–124. [Google Scholar] [CrossRef]
- Hickman-Lewis, K.; Cavalazzi, B.; Giannoukos, K.; D’Amico, L.; Vrbaski, S.; Saccomano, G.; Dreossi, D.; Tromba, G.; Foucher, F.; Brownscombe, W.; et al. Advanced two- and three-dimensional insights into Earth’s oldest stromatolites (ca. 3.5 Ga): Prospects for the search for life on Mars. Geology 2023, 51, 33–38. [Google Scholar] [CrossRef]
- Westall, F.; de Ronde, C.E.J.; Southam, G.; Grassineau, N.; Colas, M.; Cockell, C.; Lammer, H. Implications of a 3.472-3.333 Gyr-old subaerial microbial mat from the Barberton greenstone belt, South Africa for the UV environmental conditions on the early Earth. Philos. Trans. R. Soc. B 2006, 361, 1857–1875. [Google Scholar] [CrossRef] [PubMed]
- Westall, F. Life on an anaerobic planet. Science 2009, 323, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N.N. Recent advances in the discovery of novel marine natural products and mycosporine-like amino acid UV-absorbing compounds. Appl. Microbiol. Biotechnol. 2021, 105, 7053–7067. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, W.C.; Chalker, B.E. Identification and quantitation of near-UV absorbing compounds (S-320) in a hermatypic scleractinian. Coral Reefs 1986, 5, 155–159. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Daleo, G.; De Marco, S.G. Occurrence of mycosporine-like amino acids in the red-tide dinoflagellate Alexandrium excavatum: UV-photoprotective compounds? J. Plankton Res. 1990, 12, 909–921. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-like amino acids from coral dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, N.; Zhou, J.; Dong, S.; Zhang, X.; Guo, L.; Guo, G. Distribution, Contents, and Types of Mycosporine-Like Amino Acids (MAAs) in Marine Macroalgae and a Database for MAAs Based on These Characteristics. Mar. Drugs 2020, 18, 43. [Google Scholar] [CrossRef]
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV protective functions in aquatic organisms. Annu. Rev. Plant Physiol. 2002, 64, 223–262. [Google Scholar] [CrossRef]
- Řezanka, T.; Temina, M.; Tolsikov, A.G.; Dembitsky, V.M. Natural microbial UV radiation filters–mycosporine-like amino acids. Folia Microbiol. 2004, 49, 339–352. [Google Scholar] [CrossRef]
- Geraldes, V.; Pinto, E. Mycosporine-Like Amino Acids (MAAs): Biology, Chemistry and Identification features. Pharmaceuticals 2021, 14, 63. [Google Scholar] [CrossRef]
- Leach, C.M. Ultraviolet absorbing substances associated with light-induced sporulation in fungi. Can. J. Bot. 1965, 43, 185–200. [Google Scholar] [CrossRef]
- Trione, E.J.; Leach, C.M. Light-induced sporulation and sporogenic substances in fungi. Phytopathology 1969, 59, 1077–1083. [Google Scholar]
- Favre-Bonvin, J.; Arpin, N.; Brevard, C. Structure de la mycosporine (P310). Can. J. Chem. 1976, 54, 1105–1113. [Google Scholar] [CrossRef]
- Ito, S.; Hirata, Y. Isolation and structure of a mycosporine from the zoanthidian Palythoa tuberculosa. Tetrahedron Lett. 1977, 28, 2429–2430. [Google Scholar] [CrossRef]
- Arpin, N.; Curt, R.; Favre-Bonvin, J. Mycosporines: Mise au point et donées nouvelles concernant leurs structures, leur distribution, leur localisation et leur biogenèse. Rev. Mycol. 1979, 43, 247–257. [Google Scholar]
- Karentz, D. Chemical defenses of marine organisms against solar radiation exposure: UV absorbing mycosporine-like amino acids and scytonemin. In Marine Chemical Ecology; McClintock, J.B., Baker, B.J., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 481–519. [Google Scholar]
- Bandaranayake, W.M. Mycosporines: Are they nature’s sunscreens? Nat. Prod. Rep. 1998, 15, 159–171. [Google Scholar] [CrossRef]
- Carreto, J.I.; Roy, S.; Whitehead, K.; Llewellyn, C.A.; Carignan, M.O. UV-absorbing ‘pigments’: Mycosporine-like amino acids. In Phytoplankton Pigments: Characterisation, Chemotaxonomy and Applications in Oceanography; Roy, S., Llewellyn, C.A., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 412–441. [Google Scholar]
- Moliné, M.; Libkind, D.; de Garcia, V.; Giraudo, M.R. Production of Pigments and Photo-Protective Compounds by Cold-Adapted Yeasts. In Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 193–224. [Google Scholar]
- Miyamoto, K.T.; Komatsu, M.; Ikeda, H. Discovery of gene cluster for mycosporine-like amino acid biosynthesis from Actinomycetales microorganisms and production of a novel mycosporine-like amino acid by heterologous expression. Appl. Environ. Microbiol. 2014, 80, 5028–5036. [Google Scholar] [CrossRef]
- Korbee, N.; Figueroa, F.L.; Aguilera, J. Accumulation of mycosporine-like amino acids (MAAs): Biosynthesis, photocontrol and ecophysiological functions. Rev. Chil. Hist. Nat. 2006, 79, 119–132. [Google Scholar]
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. J. Photochem. Photobiol. B Biol. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Javalkote, V.S.; Mazmouz, R.; Pickford, R.; Puranik, P.R.; Neilan, B.A. Comparative profiling and discovery of novel glycosylated mycosporine-like amino acids in two strains of the cyanobacterium Scytonema cf. crispum. Appl. Environ. Microbiol. 2016, 82, 5951–5959. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Zülli, F. UV-A sunscreen from red algae for protection against premature skin aging. Cosmet. Toilet. Manuf. Worldw. 2000, 115, 139–143. [Google Scholar]
- Dunlap, W.C.; Shick, J.M. Ultraviolet radiation absorbing mycosporine-like amino acids in coral reef organisms: A biochemical and environmental environmental perspective. J. Phycol. 1998, 34, 418–430. [Google Scholar] [CrossRef]
- Carreto, J.I.; Carignan, M.O.; Montoya, N.G. A high-resolution reverse-phase liquid chromatography method for the analysis of mycosporine-like amino acids (MAAs) in marine organisms. Mar. Biol. 2005, 146, 237–252. [Google Scholar] [CrossRef]
- Gao, Q.; Garcia-Pichel, F. Microbial ultraviolet sunscreens. Nat. Rev. Microbiol. 2011, 9, 791–802. [Google Scholar] [CrossRef]
- Singh, A.; Čížková, M.; Bišová, K.; Vítová, M. Exploring Mycosporine-Like Amino Acids (MAAs) as Safe and Natural Protective Agents against UV-Induced Skin Damage. Antioxidants 2021, 10, 683. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Madamwar, D. Cyanobacteria Synthesize their own UV-Sunscreens for Photoprotection. Bioenergetics 2016, 5, 138. [Google Scholar] [CrossRef]
- Karentz, D. Ultraviolet tolerance mechanisms in Antarctic marine organisms. Antarct. Res. Ser. 1994, 62, 93–110. [Google Scholar]
- Carroll, A.K.; Shick, J.M. Dietary accumulation of UV-absorbing mycosporine-like amino acids (MAAs) by the green sea urchin (Strongylocentrotus droebachiensis). Mar. Biol. 1996, 124, 561–569. [Google Scholar] [CrossRef]
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.P. Genome mining of mycosporine-like amino acid (MAA) synthesizing and non-synthesizing cyanobacteria: A bioinformatics study. Genomics 2010, 95, 120–128. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-Like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Portwich, A.; Garcia-Pichel, F. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium Chlorogloeopsis sp. strain PCC 6912. Phycologia 2003, 42, 384–392. [Google Scholar] [CrossRef]
- Rosic, N.N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef]
- Uemura, D.; Katayama, C.; Wada, A.; Hirata, Y. Crystal and molecular structure of palythene possessing a novel 360 nm chromophore. Chem. Lett. 1980, 6, 755–756. [Google Scholar] [CrossRef]
- Würfel, P.; Ruppel, W. The flow equilibrium of a body in a radiation field. J. Phys. C Solid State Phys. 1985, 18, 2987–3000. [Google Scholar] [CrossRef]
- Michaelian, K. Thermodynamic Dissipation Theory of the Origin and Evolution of Life: Salient Characteristics of RNA and DNA and Other Fundamental Molecules Suggest an Origin of Life Driven by UV-C Light; CreateSpace: Mexico City, Mexico, 2016; ISBN 978-1541317482. [Google Scholar]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B Biol. 2000, 56, 139–144. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. Experimental study of excited-state properties and photostability of the mycosporine-like amino acid palythine in water solution. Photochem. Photobiol. Sci. 2007, 6, 669–674. [Google Scholar] [CrossRef]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatakeyama, M.; Nakamura, S. “Button-on-a-String” Mechanism in Water, the Ultrafast UV-to-Heat Conversion by Mycosporine-like Amino Acid Porphyra-334 of Natural Sunscreen Compound. ACS Phys. Chem. Au 2025, 5, 274–282. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Arbeloa, E.M.; Bertolotti, S.G.; Churio, M.S. Photophysics and Reductive Quenching Reactivity of Gadusol in Solution. Photochem. Photobiol. Sci. 2011, 10, 133–142. [Google Scholar] [CrossRef]
- Varnali, T.; Edwards, H.G.M. Raman spectroscopic identification of scytonemin and its derivatives as key biomarkers in stressed environments. Phil. Trans. R. Soc. A 2014, 372, 20140197. [Google Scholar] [CrossRef]
- Balskus, E.P.; Case, R.J.; Walsh, C.T. The biosynthesis of cyanobacterial sunscreen scytonemin in intertidal microbial mat communities. FEMS Microbiol. Ecol. 2011, 77, 322–332. [Google Scholar] [CrossRef]
- Grant, C.S.; Louda, J.W. Scytonemin-imine, a mahogany-colored UV/Vis sunscreen of cyanobacteria exposed to intense solar radiation. Org. Geochem. 2013, 65, 29–36. [Google Scholar] [CrossRef]
- Storme, J.Y.; Golubic, S.; Wilmotte, A.; Kleinteich, J.; Velázquez, D.; Javaux, E.J. Raman Characterization of the UV-Protective Pigment Gloeocapsin and Its Role in the Survival of Cyanobacteria. Astrobiology 2015, 15, 843–857. [Google Scholar] [CrossRef]
- Lifshits, M.; Kovalerchik, D.; Carmeli, S. Microcystbiopterins A–E, five O-methylated biopterin glycosides from two Microcystis spp. bloom biomasses. Phytochemistry 2016, 123, 69–74. [Google Scholar] [CrossRef]
- Sampedro, D. Computational exploration of natural sunscreens. Phys. Chem. Chem. Phys. 2011, 13, 5584–5586. [Google Scholar] [CrossRef]
- Schermann, J.-P. Spectroscopy and Modeling of Biomolecular Building Blocks; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 2008. [Google Scholar]
- Rastogi, R.P.; Richa, S.R.P.; Singh, S.P.; Häder, D.P. Photoprotective compounds from marine organisms. J. Ind. Microbiol. Biotechnol. 2010, 37, 537–558. [Google Scholar] [CrossRef]
- Karsten, U. Defense strategies of algae and cyanobacteria against solar ultraviolet radiation. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Garcia-Pichel, F.; Castenholz, R.W. Occurrence of UV absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl. Environ. Microbiol. 1993, 59, 163–169. [Google Scholar] [CrossRef]
- Jain, S.; Prajapat, G.; Abrar, M.; Ledwani, L.; Singh, A.; Agrawal, A. Cyanobacteria as efficient producers of mycosporine-like amino acids. J. Basic Microbiol. 2017, 57, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Lembcke, S.; Schumann, R. The effect of ultraviolet radiation on photosynthetic performance, growth and sunscreen compound in aeroterrestrial biofilm algae isolated from building facades. Planta 2007, 225, 991–1000. [Google Scholar] [CrossRef]
- Ingalls, A.E.; Whitehead, K.; Bridoux, M.C. Tinted windows: The presence of the UV absorbing compounds called mycosporine-like amino acids embedded in the frustules of marine diatoms. Geochim. Cosmochim. Acta 2010, 74, 104–115. [Google Scholar] [CrossRef]
- Favre-Bonvin, J.; Bernillon, J.; Salin, N.; Arpin, N. Biosynthesis of mycosporines: Mycosporine glutaminol in Trichothecium roseum. Phytochemistry 1987, 26, 2509–2514. [Google Scholar] [CrossRef]
- Shick, J.M.; Romaine-Lioud, S.; Ferrier-Pagès, C.; Gattuso, J.P. Ultraviolet-B radiation stimulates shikimate pathway-dependent accumulation of mycosporine-like amino acids in the coral Stylophora pistillata despite decreases in its population of symbiotic dinoflagellates. Limnol. Oceanogr. 1999, 44, 1667–1682. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef]
- Pope, M.A.; Spence, E.; Seralvo, V.; Gacesa, R.; Heidelberger, S.; Weston, A.J.; Dunlap, W.C.; Shick, J.M.; Long, P.F. O-Methyltransferase is shared between the pentose phosphate and shikimate pathways and is essential for mycosporine-like amino acid biosynthesis in Anabaena variabilis ATCC 29413. Chembiochem 2015, 16, 320–327. [Google Scholar] [CrossRef]
- Arsın, S.; Delbaje, E.; Jokela, J.; Wahlsten, M.; Farrar, Z.M.; Permi, P.; Fewer, D. A plastic biosynthetic pathway for the production of structurally distinct microbial sunscreens. ACS Chem. Biol. 2023, 18, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.N. Microbial Biochemistry, 3rd ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 85–90, 415–440. [Google Scholar]
- Richards, T.A.; Dacks, J.B.; Campbell, S.A.; Blanchard, J.L.; Foster, P.G.; McLeod, R.; Roberts, C.W. Evolutionary origins of the eukaryotic shikimate pathway: Gene fusions, horizontal gene transfer, and endosymbiotic replacements. Eukaryot Cell 2006, 5, 1517–1531. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.A.; Turchyn, A.V.; Ralser, M. Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean ocean. Mol. Syst. Biol. 2014, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, Y.; Kakegawa, T.; Furukawa, Y. Hexose phosphorylation for a non-enzymatic glycolysis and pentose phosphate pathway on early Earth. Sci. Rep. 2024, 14, 264. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; Björn, L.O.; Bornman, J.F.; Gaberscik, A.; Häder, D.P.; Trost, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R.P.; et al. The role of UV-B radiation in aquatic and terrestrial ecosystems-an experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B Biol. 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Waller, R.F.; Slamovits, C.H.; Keeling, P.J. Lateral gene transfer of a multigene region from cyanobacteria to dinoflagellates resulting in a novel plastid-targeted fusion protein. Mol. Biol. Evol. 2006, 23, 1437–1443. [Google Scholar] [CrossRef]
- Starcevic, A.; Akthar, S.; Dunlap, W.C.; Shick, J.M.; Hranueli, D.; Cullum, J.; Long, P.F. Enzymes of the shikimic acid pathway encoded in the genome of a basal metazoan, Nematostella vectensis, have microbial origins. Proc. Natl. Acad. Sci. USA 2008, 105, 2533–2537. [Google Scholar] [CrossRef]
- Singh, S.P.; Häder, D.P.; Sinha, R.P. Bioinformatics evidence for the transfer of mycosporine-like amino acid core (4-deoxygadusol) synthesizing gene from cyanobacteria to dinoflagellates and an attempt to mutate the same gene (YP_324358) in Anabaena variabilis PCC 7937. Gene 2012, 500, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Han, M.; Teng, X.; Ding, J.; Chen, L.; Wu, B. Recent advances and future prospects of mycosporine-like amino acids. Mar. Drugs 2023, 21, 354. [Google Scholar] [CrossRef]
- Kinzie, R.A., III. Effects of ambient levels of solar ultraviolet radiation on zooxanthellae and photosynthesis of the reef coral Montipora verrucosa. Mar. Biol. 1993, 116, 319–327. [Google Scholar] [CrossRef]
- Karentz, D.; Spero, H.J. Response of natural Phaeocystis population to ambient fluctuations of UVB radiation caused by Antarctic ozone depletion. J. Plankton Res. 1995, 17, 1771–1789. [Google Scholar] [CrossRef]
- Lesser, M.P. Acclimation of phytoplankton to UV-B radiation: Oxidative stress and photo inhibition of photosynthesis are not prevented by UV-absorbing compounds in the dinoflagellate Prorocentrum micans. Mar. Ecol. Prog. Ser. 1996, 132, 287–297. [Google Scholar] [CrossRef]
- Quesada, A.; Vincent, W.F. Strategies of adaptation by Antarctic cyanobacteria to ultraviolet radiation. Eur. J. Phycol. 1997, 32, 335–342. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Gröniger, A.; Häder, D.-P. Mycosporine-like amino acids in the marine red alga Gracilaria cornea—Effects of UV and heat. Environ. Exp. Bot. 2000, 43, 33–43. [Google Scholar] [CrossRef]
- Ryan, K.G.; McMinn, A.; Mitchell, K.A.; Trenerry, L. Mycosporine-like amino acids in Antarctic sea ice algae, and their response to UVB radiation. Z. Naturforschung C J. Biosci. 2002, 57, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Dummermuth, A.; Hoyer, K.; Wiencke, C. Interactive effects of ultraviolet radiation and salinity on the ecophysiology of two Arctic red algae from shallow waters. Polar Biol. 2003, 26, 249–258. [Google Scholar] [CrossRef]
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporinelike amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10. [Google Scholar] [CrossRef]
- Zwerger, M.J.; Hammerle, F.; Siewert, B.; Ganzera, M. Application of feature-based molecular networking in the field of algal research with special focus on mycosporine-like amino acids. J. Appl. Phycol. 2023, 35, 1377–1392. [Google Scholar] [CrossRef]
- Michaelian, K.; Simeonov, A. Thermodynamic Explanation for the Cosmic Ubiquity of Organic Pigments. J. Astrobiol. Outreach 2017, 5, 156. [Google Scholar] [CrossRef]
- Morel, A. Optical modeling of the upper ocean in relation to its biogenous matter content (case I waters). J. Geophys. Res. 1988, 93, 10749–10768. [Google Scholar] [CrossRef]
- Kahru, M.; Leppanen, J.M.; Rud, O. Cyanobacterial blooms cause heating of the sea surface. Mar. Ecol. Prog. Ser. 1993, 101, 1–7. [Google Scholar] [CrossRef]
- Jones, I.; George, G.; Reynolds, C. Quantifying effects of phytoplankton on the heat budgets of two large limnetic enclosures. Freshw. Biol. 2005, 50, 1239–1247. [Google Scholar] [CrossRef]
- Patara, L.; Vichi, M.; Masina, S.; Fogli, P.G.; Manzini, E. Global response to solar radiation absorbed by phytoplankton in a coupled climate model. Clim. Dyn. 2012, 39, 1951–1968. [Google Scholar] [CrossRef]
- Wurl, O.; Bird, K.; Cunliffe, M.; Landing, W.M.; Miller, U.; Mustaffa, N.I.H.; Ribas-Ribas, M.; Witte, C.; Zappa, C.J. Warming and Inhibition of Salinization at the Ocean’s Surface by Cyanobacteria. Geophys. Res. Lett. 2018, 45, 4230–4237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soppa, M.A.; Pefanis, V.; Hellmann, S.; Losa, S.N.; Hölemann, J.; Martynov, F.; Heim, B.; Janout, M.A.; Dinter, T.; Rozanov, V.; et al. Assessing the Influence of Water Constituents on the Radiative Heating of Laptev Sea Shelf Waters. Front. Mar. Sci. 2019, 6, 221. [Google Scholar] [CrossRef]
- Gnanadesikan, A.; Emanuel, K.; Vecchi, G.A.; Anderson, W.G.; Hallberg, R. How ocean color can steer Pacific tropical cyclones. Geophys. Res. Lett. 2010, 37, L18802. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A. The global distribution and dynamics of chromophoric dissolved organic matter. Ann. Rev. Mar. Sci. 2013, 5, 447–476. [Google Scholar] [CrossRef]
- Logozzo, L.; Tzortziou, M.; Neale, P.; Clark, J.B. Photochemical and microbial degradation of chromophoric dissolved organic matter exported from tidal marshes. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005744. [Google Scholar] [CrossRef]
- Siegel, D.A.; Maritorena, S.; Nelson, N.B.; Hansell, D.A.; Lorenzi-Kayser, M. Global distribution and dynamics of colored dissolved and detrital organic materials. J. Geophys. Res. 2002, 107, 3228. [Google Scholar] [CrossRef]
- Babin, M.; Stramski, D.; Ferrari, G.M.; Claustre, H.; Bricaud, A.; Obolensky, G.; Hoepffner, N. Variations in the light absorption coefficients of phyto-plankton, nonalgal particles, and dissolved organic matter in coastal waters around Europe. J. Geophys. Res. 2003, 108, 3211. [Google Scholar]
- Bricaud, A.; Babin, M.; Claustre, H.; Ras, J.; Tieche, F. Light absorption properties and absorption budget of Southeast Pacific waters. J. Geophys. Res. 2010, 115, C08009. [Google Scholar] [CrossRef]
- Organelli, E.; Bricaud, A.; Antoine, D.; Matsouka, A. Seasonal dynamics of light absorption by chromophoric dissolved organic matter (CDOM) in the NW Mediterranean Sea (BOUSSOLE site). Deep Sea Res. Part I Oceanogr. Res. Pap. 2014, 91, 72–85. [Google Scholar] [CrossRef]
- Röttgers, R.; Koch, B.P. Spectroscopic detection of a ubiquitous dissolved pigment degradation product in subsurface waters of the global ocean. Biogeosciences 2012, 9, 2585–2596. [Google Scholar] [CrossRef]
- Carlson, D.J.; Mayer, L.M. Enrichment of dissolved phenolic material in the surface microlayer of coastal waters. Nature 1980, 286, 482–483. [Google Scholar] [CrossRef]
- Galgani, L.; Engel, A. Changes in optical characteristics of surface microlayers hint to photochemically and microbially mediated DOM turnover in the upwelling region off the coast of Peru. Biogeosciences 2016, 13, 2453–2473. [Google Scholar] [CrossRef]
- Yamashita, Y.; Tanoue, E. Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids. Mar. Chem. 2003, 82, 255–271. [Google Scholar] [CrossRef]
- Whitehead, K.; Vernet, M. Influence of mycosporine-like amino acids (MAAs) on UV absorption by particulate and dissolved organic matter in La Jolla Bay. Limnol. Oceanogr. 2000, 45, 1788–1796. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Nelson, N.; Carlson, C.A.; Prusak, A.C. Production of chromophoric dissolved organic matter (CDOM) in the open ocean by zooplankton and the colonial cyanobacterium Trichodesmium spp. Mar. Ecol. Prog. Ser. 2004, 267, 45–56. [Google Scholar] [CrossRef]
- Tilstone, G.; Airs, R.; Martinez-Vicente, V.; Widdicombe, C.; Llewellyn, C. High concentrations of Mycosporine-like amino acids and coloured dissolved organic material in the sea surface microlayer off the Iberian Peninsula. Limnol. Oceanogr. 2010, 55, 1835–1850. [Google Scholar] [CrossRef]
- Clementson, L.A.; Oubelkheir, K.; Ford, P.W.; Blondeau-Patissier, D. Distinct Peaks of UV-Absorbing Compounds in CDOM and Particulate Absorption Spectra of Near-Surface Great Barrier Reef Coastal Waters, Associated with the Presence of Trichodesmium spp. (NE Australia). Remote Sens. 2022, 14, 3686. [Google Scholar] [CrossRef]
- Nelson, N.B.; Siegel, D.A.; Michaels, A.F. Seasonal dynamics of colored dissolved material in the Sargasso Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 1998, 45, 931–957. [Google Scholar] [CrossRef]
- Romera-Castillo, C.; Sarmento, H.; Álvarez-Salgado, X.A.; Gasol, J.M.; Marrasé, C. Production of chromophoric dissolved organic matter by marine phyto-plankton. Limnol. Oceanogr. 2010, 55, 446–454. [Google Scholar] [CrossRef]
- Fukuzaki, K.; Imai, I.; Fukushima, K.; Ishii, K.-I.; Sawayama, S.; Yoshioka, T. Fluorescent characteristics of dissolved organic matter produced by bloom-forming coastal phytoplankton. J. Plankton Res. 2014, 36, 685–694. [Google Scholar] [CrossRef]
- Subramaniam, A.; Carpenter, E.J.; Karentz, D.; Falkowski, P.G. Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnol. Oceanogr. 1999, 44, 608–617. [Google Scholar] [CrossRef]
- Vernet, M.; Whitehead, K. Release of ultraviolet-absorbing compounds by the red-tide dinoflagellate Lingulodinium polyedra. Mar Biol. 1996, 127, 35–44. [Google Scholar] [CrossRef]
- Llewellyn, C.A.; Greig, C.; Silkina, A.; Kultschar, B.; Hitchings, M.D.; Farnham, G. Mycosporine-like amino acid and aromatic amino acid transcriptome response to UV and far-red light in the cyanobacterium Chlorogloeopsis fritschii PCC 6912. Sci. Rep. 2020, 10, 20638. [Google Scholar] [CrossRef] [PubMed]
- Nägeli, C. Gattungen Einzelliger Algen, Physiologisch Und Systematisch Bearbeitet; Friedrich Schulthess: Zurich, Switzerland, 1849; Volume 10, pp. 1–138. [Google Scholar]
- Nägeli, C.; Schwenderer, S. Das Mikroskop, 2nd ed.; Willhelm Engelmann: Leipzig, Germany, 1877; p. 505. [Google Scholar]
- Garcia-Pichel, F.; Castenholz, R.W. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J. Phycol. 1991, 27, 395–409. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H.; Garcia-Pichel, F.; Castenholz, R. The structure of scytonemin, an ultraviolet sunscreen pigment from the sheaths of cyanobacteria. Experientia 1993, 49, 825–829. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kajiyama, S.; Inawaka, K.; Kanzaki, H.; Kawazu, K. Nostodione A, a novel mitotic spindle poison from a blue-green alga Nostoc commune. Z. Naturforschung-Sect. C J. Biosci. 1994, 49, 464–470. [Google Scholar] [CrossRef]
- Bultel-Poncé, V.; Felix-Theodose, F.; Sarlhou, C.; Ponge, J.F.; Bodo, B. New pigments from the terrestrial cyanobacterium Scytonema sp. collected on the Mitakara Inselberg, French Guyana. J. Nat. Prod. 2004, 67, 678–681. [Google Scholar] [CrossRef]
- Orellana, G.; Gómez-Silva, B.; Urrutia, M.; Galetović, A. UV-A Irradiation Increases Scytonemin Biosynthesis in Cyanobacteria Inhabiting Halites at Salar Grande, Atacama Desert. Microorganisms 2020, 8, 1690. [Google Scholar] [CrossRef]
- Gao, X.; Jing, X.; Liu, X.; Lindblad, P. Biotechnological production of the sunscreen pigment scytonemin in cyanobacteria: Progress and strategy. Mar. Drugs 2021, 19, 129. [Google Scholar] [CrossRef]
- Sinha, R.P.; Klisch, M.; Vaishampayan, A.; Häder, D.P. Biochemical and spectroscopic characterization of the cyanobacterium Lyngbya sp. Inhabiting Mango (Mangifera indica) trees: Presence of an ultraviolet-absorbing pigment, scytonemin. Acta Protozool. 1999, 38, 291–298. [Google Scholar]
- Garcia-Pichel, F.; Sherry, N.D.; Castenholz, R.W. Evidence for a UV sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochem. Photobiol. 1992, 56, 17–23. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. Partial characterization, UV-induction and photoprotective function of sunscreen pigment, scytonemin from Rivularia sp. HKAR-4. Chemosphere 2013, 93, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz, S.; Castenholz, R.W. Long-term effects of UV and visible irradiance on natural populations of a scytonemin-containing cyanobacterium (Calothrix sp.). FEMS Microbiol. Ecol. 1997, 24, 343–352. [Google Scholar]
- Fleming, E.D.; Castenholz, R.W. Effects of periodic desiccation on the synthesis of the UV-screening compound, scytonemin, in cyanobacteria. Environ. Microbiol. 2007, 9, 1448–1455. [Google Scholar] [CrossRef]
- Fulton, J.M.; Arthur, M.A.; Freeman, K.H. Subboreal aridity and scytonemin in the Holocene Black Sea. Org. Geochem. 2012, 49, 47–55. [Google Scholar] [CrossRef]
- Lepot, K.; Deremiens, L.; Namsaraev, Z.; Compère, P.; Gérard, E.; Verleyen, E.; Tavernier, I.; Hodgson, D.A.; Wilmotte, A.; Javaux, E.J. Organo-mineral imprints in fossil cyanobacterial mats of an Antarctic lake. Geobiology 2014, 12, 424–450. [Google Scholar] [CrossRef]
- Ploutno, A.; Carmeli, S. Prenostodione, a novel UV-absorbing metabolite from a natural bloom of the cyanobacterium Nostoc species. J. Nat. Prod. 2001, 64, 544–545. [Google Scholar] [CrossRef]
- Soule, T.; Palmer, K.; Gao, Q.; Potrafka, R.M.; Stout, V.; Garcia-Pichel, F. A comparative genomics approach to understanding the biosynthesis of the sunscreen scytonemin in cyanobacteria. BMC Genom. 2009, 10, 336. [Google Scholar] [CrossRef]
- Pathak, J.; Rajneesh, R.; Sonker, A.S.; Kannaujiya, V.K.; Sinha, R.P. Cyanobacterial extracellular polysaccharide sheath pigment, scytonemin: A novel multipurpose pharmacophore. In Marine Glycobiology: Principles and Applications, 1st ed.; Se-Kwon, K., Ed.; Taylor & Francis Group: Boca Raton, FL, USA; CRC Press: Boca Raton, FL, USA, 2016; pp. 323–337. [Google Scholar]
- Sinha, R.P.; Häder, D.P. UV-protectants in cyanobacteria. Plant Sci. 2008, 174, 278–289. [Google Scholar] [CrossRef]
- Büdel, B.; Karsten, U.; Garcia-Pichel, F. Ultraviolet absorbing scytonemin and mycosporine-like amino acid derivatives in exposed, rock-inhabiting cyanobacterial lichens. Oecologia 1997, 112, 165–172. [Google Scholar] [CrossRef]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; de Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef]
- Kehr, J.C.; Dittmann, E. Biosynthesis and function of extracellular glycans in cyanobacteria. Life 2015, 5, 164–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ehling-Schulz, M.; Bilger, W.; Scherer, S. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 1997, 179, 1940–1945. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Scherer, S. UV protection in cyanobacteria. Eur. J. Phycol. 1999, 34, 329–338. [Google Scholar] [CrossRef]
- Karsten, U.; Maier, J.; Garcia-Pichel, F. Seasonality in UV absorbing compounds of cyanobacterial mat communities from an intertidal mangrove flat. Aquat. Microb. Ecol. 1998, 16, 37–44. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Al Kharusi, S.; Schramm, A.; Robinson, M.D. Bacterial diversity, pigments and nitrogen fixation of biological desert crusts from the Sultanate of Oman. FEMS Microbiol. Ecol. 2010, 72, 418–428. [Google Scholar] [CrossRef]
- Soule, T.; Stout, V.; Swingley, W.D.; Meeks, J.C.; Garcia-Pichel, F. Molecular genetics and genomic analysis of scytonemin biosynthesis in Nostoc punctiforme ATCC29133. J. Bacteriol. 2007, 189, 4465–4472. [Google Scholar] [CrossRef]
- Soule, T.; Garcia-Pichel, F.; Stout, V. Gene expression patterns associated with the biosynthesis of the sunscreen scytoneminin Nostoc punctiforme ATCC29133 in response to UVA radiation. J. Bacteriol. 2009, 191, 4639–4646. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. Investigating the initial steps in the biosynthesis of cyanobacterial sunscreen scytonemin. J. Am. Chem. Soc. 2008, 130, 15260–15261. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. An enzymatic cyclopentyl[b]indole formation involved in scytonemin biosynthesis. J. Am. Chem. Soc. 2009, 131, 14648–14649. [Google Scholar] [CrossRef]
- Sorrels, C.M.; Proteau, P.J.; Gerwick, W.H. Organization, evolution, and expression analysis of the biosynthetic gene cluster for scytonemin, a cyanobacterial UV-absorbing pigment. Appl. Environ. Microbiol. 2009, 75, 4861–4869. [Google Scholar] [CrossRef]
- Knaggs, A.R. The biosynthesis of shikimate metabolites. Nat. Prod. Rep. 2003, 20, 119–136. [Google Scholar] [CrossRef]
- Tang, Q.; Li, Z.; Chen, N.; Luo, X.; Zhao, Q. Natural pigments derived from plants and microorganisms: Classification, biosynthesis, and applications. Plant Biotechnol. J. 2025, 23, 592–614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ekebergh, A.; Sandin, P.; Mårtensson, J. On the photostability of scytonemin, analogues thereof and their monomeric counterparts. Photochem. Photobiol. Sci. 2015, 14, 2179–2186. [Google Scholar] [CrossRef]
- Ručová, D.; Vilková, M.; Sovová, S.; Vargová, Z.; Kostecká, Z.; Frenák, R.; Routray, D.; Bačkor, M. Photoprotective and antioxidant properties of scytonemin isolated from Antarctic cyanobacterium Nostoc commune Vaucher ex Bornet & Flahault and its potential as sunscreen ingredient. J. Appl. Phycol. 2023, 35, 2839–2850. [Google Scholar] [CrossRef]
- Wynn-Williams, D.D.; Edwards, H.G.M.; Garcia-Pichel, F. Functional biomolecules of Antarctic stromatolitic and endolithic cyanobacterial communities. Eur. J. Phycol. 1999, 34, 381–391. [Google Scholar] [CrossRef]
- Hunsucker, S.W.; Tissue, B.M.; Potts, M.; Helm, R.F. Screening protocol for the ultraviolet-photoprotective pigment scytonemin. Analyt. Biochem. 2001, 288, 227–230. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sinha, R.P.; Moh, S.H.; Lee, T.K.; Kottuparambil, S.; Kim, Y.J.; Rhee, J.S.; Choi, E.M.; Brown, M.T.; Häder, D.P.; et al. Ultraviolet radiation and cyanobacteria. J. Photochem. Photobiol. B Biol. 2014, 141, 154–169. [Google Scholar] [CrossRef]
- Dillon, J.G.; Tatsumi, C.M.; Tandingan, P.G.; Castenholz, R.W. Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.). Arch. Microbiol. 2002, 177, 322–331. [Google Scholar] [CrossRef]
- Matsui, K.; Nazifi, E.; Hirai, Y.; Wada, N.; Matsugo, S.; Sakamoto, T. The cyanobacterial UV-absorbing pigment scytonemin displays radical scavenging activity. J. Gen. Appl. Microbiol. 2012, 58, 137–144. [Google Scholar] [CrossRef]
- Dillon, J.G.; Castenholz, R.W. Scytonemin, a cyanobacterial sheath pigment, protects against UVC radiation: Implications for early photosynthetic life. J. Phycol. 1999, 35, 673–681. [Google Scholar] [CrossRef]
- Häder, D.P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Aquatic ecosystems: Effects of solar ultraviolet radiation and interactions with other climatic change factors. Photochem. Photobiol. Sci. 2003, 2, 39–50. [Google Scholar] [CrossRef]
- Tamre, E.; Fournier, G.P. Inferred ancestry of scytonemin biosynthesis proteins in cyanobacteria indicates a response to Paleoproterozoic oxygenation. Geobiology 2022, 20, 764–775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Edwards, H.G.M.; Jorge Villar, S.E.; Pullan, D.; Hofmann, B.A.; Hargreaves, M.D.; Westall, F. Morphological biosignatures from relict fossilized sedimentary geological specimens: A Raman spectroscopic study. J. Raman Spectros. 2007, 38, 1352–1361. [Google Scholar] [CrossRef]
- Pullan, D.; Westall, F.; Hofmann, B.A.; Parnell, J.; Cockell, C.S.; Edwards, H.G.; Villar, S.E.; Schröder, C.; Cressey, G.; Marinangeli, L.; et al. Identification of morphological biosignatures in Martian analogue field specimens using in situ planetary instrumentation. Astrobiology 2008, 8, 119–156. [Google Scholar] [CrossRef]
- Hofmann, H.J. Precambrian microflora, Belcher Islands, Canada: Significance and systematic. J. Paleontol. 1976, 50, 1040–1073. [Google Scholar]
- Golubic, S.; Hofmann, H.J. Comparison of Holocene and mid-Precambrian Entophysalidaceae (Cyanophyta) in stromatolitic algal mats: Cell division and degradation. J. Paleontol. 1976, 50, 1074–1082. [Google Scholar]
- Golubic, S.; Abed, R.M.M. Entophysalis mats as environmental regulators. In Microbial Mats, Modern and Ancient Microorganisms in Stratified Systems; Seckbach, J., Oren, A., Eds.; Springer: Heidelberg, Germany, 2010; pp. 237–251. [Google Scholar]
- Demoulin, C.F.; Lara, Y.J.; Cornet, L.; François, C.; Baurain, D.; Wilmotte, A.; Javaux, E.J. Cyanobacteria evolution: Insight from the fossil record. Free Radic. Biol. Med. 2019, 140, 206–223. [Google Scholar] [CrossRef]
- Castenholz, R.W.; Garcia-Pichel, F. Cyanobacterial responses to UV-radiation. In The Ecology of Cyanobacteria: Their Diversity in Time and Space; Whitton, B.A., Potts, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 591–611, 669. [Google Scholar]
- Singh, V.K.; Jha, S.; Rana, P.; Mishra, S.; Kumari, N.; Singh, S.C.; Anand, S.; Upadhye, V.; Sinha, R.P. Resilience and Mitigation Strategies of Cyanobacteria under Ultraviolet Radiation Stress. Int. J. Mol. Sci. 2023, 24, 12381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michaelian, K. A non-linear irreversible thermodynamic perspective on organic pigment proliferation and biological evolution. J. Phys. Conf. Ser. 2013, 475, 012010. [Google Scholar] [CrossRef]
- Rowan, K.S. Photosynthetic Pigments of Algae; Cambridge University Press: Cambridge, UK, 1989; pp. 112–210. [Google Scholar]
- Ustin, S.L.; Valko, P.G.; Kefauver, S.C.; Santos, M.J.; Zimpfer, J.F.; Smith, S.D. Remote sensing of biological soil crust under simulated climate change manipulations in the Mojave Desert. Remote Sens. Environ. 2009, 113, 317–328. [Google Scholar] [CrossRef]
- Mahmud, T.; Moore, D.C.; Dergachev, I.D.; Sun, H.; Varganov, S.A.; Tucker, M.J.; Jeffrey, C.S. Cortical polysaccharides play a crucial role in lichen UV protection. Cell Rep. Phys. Sci. 2024, 5, 102242. [Google Scholar] [CrossRef]
- Evans, R.D.; Johansen, J.R. Microbiotic crusts and ecosystem processes. Crit. Rev. Plant Sci. 1999, 18, 183–225. [Google Scholar] [CrossRef]
- Belnap, J.; Lange, O.L. Biological Soil Crusts: Structure, Function, and Management; Springer: Berlin, Germany, 2001. [Google Scholar]
- Lan, S.; Elliott, D.R.; Chamizo, S.; Felde, V.J.M.N.L.; Thomas, A.D. Editorial: Biological soil crusts: Spatio-temporal development and ecological functions of soil surface microbial communities across different scales. Front. Microbiol. 2024, 15, 1447058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beraldi-Campesi, H.; Farmer, J.D.; Garcia-Pichel, F. Modern terrestrial sedimentary biostructures and their fossil analogs in mesoproterozoic subaerial deposits. Palaios 2014, 29, 45–54. [Google Scholar] [CrossRef]
- Wellman, C.H.; Strother, P.K.; Servais, T. The terrestrialization process: A palaeobotanical and palynological perspective. Annu. Rev. Earth Planet. Sci. 2021, 49, 423–451. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Paulsen, B.S.; Klaveness, D. Studies on Polysaccharides from Three Edible Species of Nostoc (Cyanobacteria) with Different Colony Morphologies: Comparison of Monosaccharide Compositions and Viscosities of Polysaccharides from Field Colonies and Suspension Cultures. J. Phycol. 1998, 34, 962–968. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, D.; Huang, Z.; Liu, Y. The vertical microdistribution of cyanobacteria and green algae within desert crusts and the development of the algal crusts. Plant Soil. 2003, 257, 97–111. [Google Scholar] [CrossRef]
- Roeselers, G.; Norris, T.B.; Castenholz, R.W.; Rysgaard, S.; Glud, R.N.; Kühl, M.; Muyzer, G. Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environ. Microbiol. 2007, 9, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Potrafka, R.M.; Garcia-Pichel, F.; De Philippis, R. The role of the exopolysaccharides in enhancing hydraulic conductivity of biological soil crusts. Soil Biol. Biochem. 2012, 46, 33–40. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. Metabolites Facilitating Adaptation of Desert Cyanobacteria to Extremely Arid Environments. Plants 2022, 11, 3225. [Google Scholar] [CrossRef] [PubMed]
- Mager, D.M.; Thomas, A.D. Carbohydrates in cyanobacterial soil crusts as a source of carbon in the southwest Kalahari, Botswana. Soil Biol. Biochem. 2010, 42, 313–318. [Google Scholar] [CrossRef]
- Karunakaran, E.; Mukherjee, J.; Ramalingam, B.; Biggs, C.A. “Biofilmology”: A multidisciplinary review of the study of microbial biofilms. Appl. Microbiol. Biotechnol. 2011, 90, 1869–1881. [Google Scholar] [CrossRef] [PubMed]
- Colica, G.; Li, H.; Rossi, F.; Li, D.; Liu, Y.; de Philippis, R. Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol. Biochem. 2014, 68, 62–70. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef]
- Pokorny, J.; Brom, J.; Cermak, J.; Hesslerova, P.; Huryna, H.; Nadezhdina, N.; Rejskova, A. Solar energy dissipation and temperature control by water and plants. Int. J. Water 2010, 5, 4. [Google Scholar] [CrossRef]
- Maes, W.H.; Pashuysen, T.; Trabucco, A.; Veroustraete, F.; Muys, B. Does energy dissipation increase with ecosystem succession? Testing the ecosystem exergy theory combining theoretical simulations and thermal remote sensing observations. Ecol. Model. 2011, 222, 3917–3941. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, T.J.; Zhang, P.; Peng, C.L. Pigment patterns and photoprotection of anthocyanins in the young leaves of four dominant subtropical forest tree species in two successional stages under contrasting light conditions. Tree Physiol. 2016, 36, 1092–1104. [Google Scholar] [CrossRef]
- Couradeau, E.; Karaoz, U.; Lim, H.C.; Nunes da Rocha, U.; Northern, T.; Brodie, E.; Garcia-Pichel, F. Bacteria increase arid-land soil surface temperature through the production of sunscreens. Nat. Commun. 2016, 7, 10373. [Google Scholar] [CrossRef]
- Mejía Morales, J.; Michaelian, K. Photon Dissipation as the Origin of Information Encoding in RNA and DNA. Entropy 2020, 22, 940. [Google Scholar] [CrossRef]
- Wehbi, S.; Wheeler, A.; Morel, B.; Manepalli, N.; Minh, B.Q.; Lauretta, D.S.; Masel, J. Order of amino acid recruitment into the genetic code resolved by last universal common ancestor’s protein domains. Proc. Natl. Acad. Sci. USA 2024, 121, e2410311121. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.; Rajendran, C.; Heyn, K.; Schlee, S.; Merkl, R.; Sterner, R. Ancestral Tryptophan Synthase Reveals Functional Sophistication of Primordial Enzyme Complexes. Cell Chem. Biol. 2016, 23, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Losantos, R.; Churio, M.S.; Sampedro, D. Computational Exploration of the Photoprotective Potential of Gadusol. ChemistryOpen 2015, 4, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Losantos, R.; Sampedro, D.; Churio, M.S. Photochemistry and photophysics of mycosporine-like amino acids and gadusols, nature’s ultraviolet screens. Pure Appl. Chem. 2015, 87, 979–996. [Google Scholar] [CrossRef]
- Holland, H.D. The Geologic History of Seawater. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003; Volume 6, pp. 583–625. ISBN 0-08-044341-9. [Google Scholar]
- Krissansen-Totton, J.; Arney, G.N.; Catling, D.C. Constraining the climate and ocean pH of the early Earth with a geological carbon cycle model. Proc. Natl. Acad. Sci. USA 2018, 115, 4105–4110. [Google Scholar] [CrossRef]
- Matsumi, Y.; Kawasaki, M. Photolysis of Atmospheric Ozone in the Ultraviolet Region. Chem. Rev. 2003, 103, 4767–4781. [Google Scholar] [CrossRef]
- Stanley, S.M. Earth System History, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 2008; pp. 263–287. [Google Scholar]
- Ferroni, L.; Klisch, M.; Pancaldi, S.; Häder, D.P. Complementary UV-Absorption of Mycosporine-like Amino Acids and Scytonemin is Responsible for the UV-Insensitivity of Photosynthesis in Nostoc flagelliforme. Mar. Drugs 2010, 8, 106–121. [Google Scholar] [CrossRef]
- Zepp, R.G.; Schlotzhauer, P.F.; Sink, R.M. Photochemical transformation of aquatic humic substances: Photoproduction of hydroxyl radicals. Environ. Sci. Technol. 1987, 21, 443–450. [Google Scholar] [CrossRef]
- Majerfeld, I.; Yarus, M. A diminutive and specific RNA binding site for L-tryptophan. Nucleic Acids Res. 2005, 33, 5482–5493. [Google Scholar] [CrossRef]
- Yarus, M.; Widmann, J.J.; Knight, R. RNA-amino acid binding: A stereochemical era for the genetic code. J. Mol. Evol. 2009, 69, 406–429. [Google Scholar] [CrossRef] [PubMed]
- Lara, Y.J.; McCann, A.; Malherbe, C.; François, C.; Demoulin, C.F.; Sforna, M.C.; Eppe, G.; De Pauw, E.; Wilmotte, A.; Jacques, P.; et al. Characterization of the Halochromic Gloeocapsin Pigment, a Cyanobacterial Biosignature for Paleobiology and Astrobiology. Astrobiology 2022, 22, 735–754. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Burgess, G.; Yamada, N.; Komatsu, K.; Yoshida, S.; Wachi, Y. An ultraviolet (UV-A) absorbing biopterin glucoside from the marine planktonic cyanobacterium Oscillatoria sp. Appl. Microbiol. Biotechnol. 1993, 39, 250–253. [Google Scholar] [CrossRef]
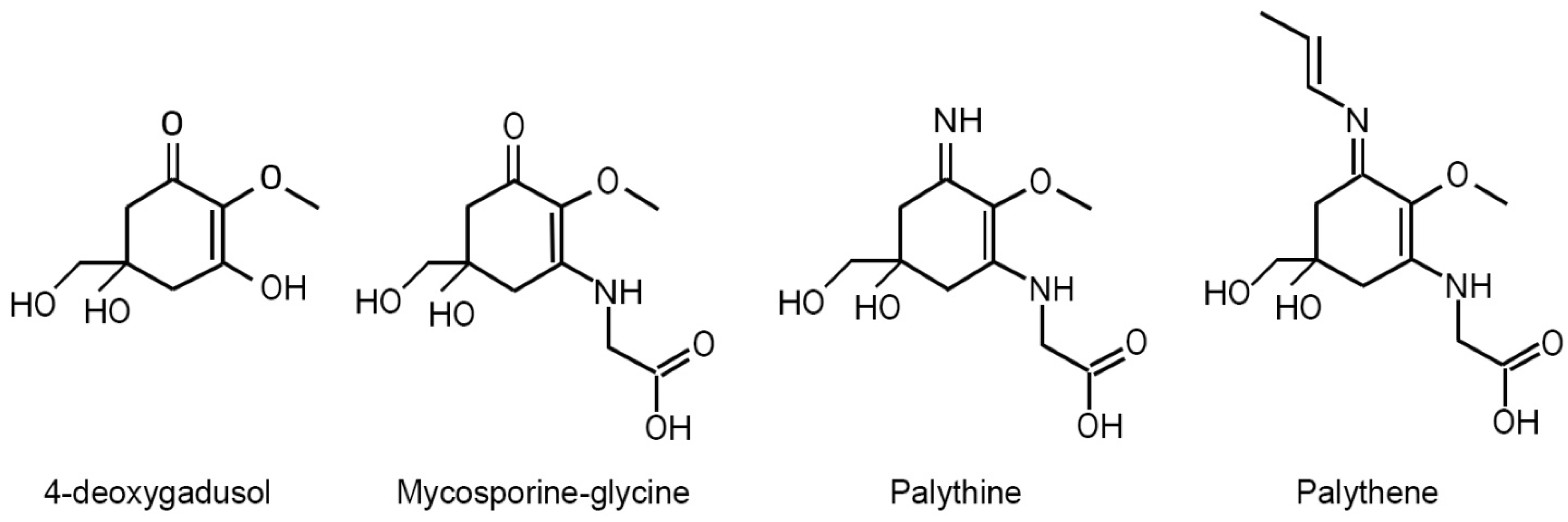
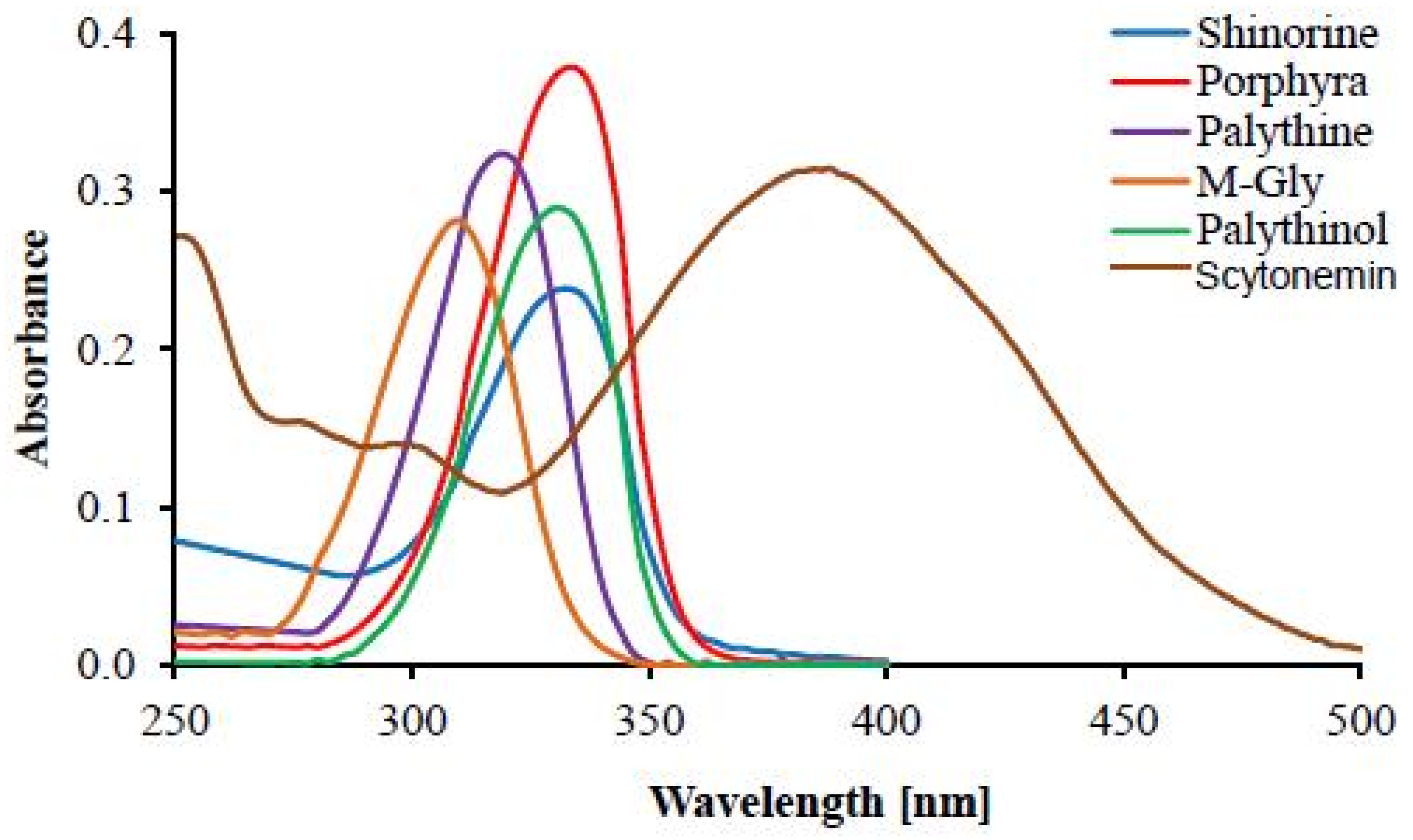
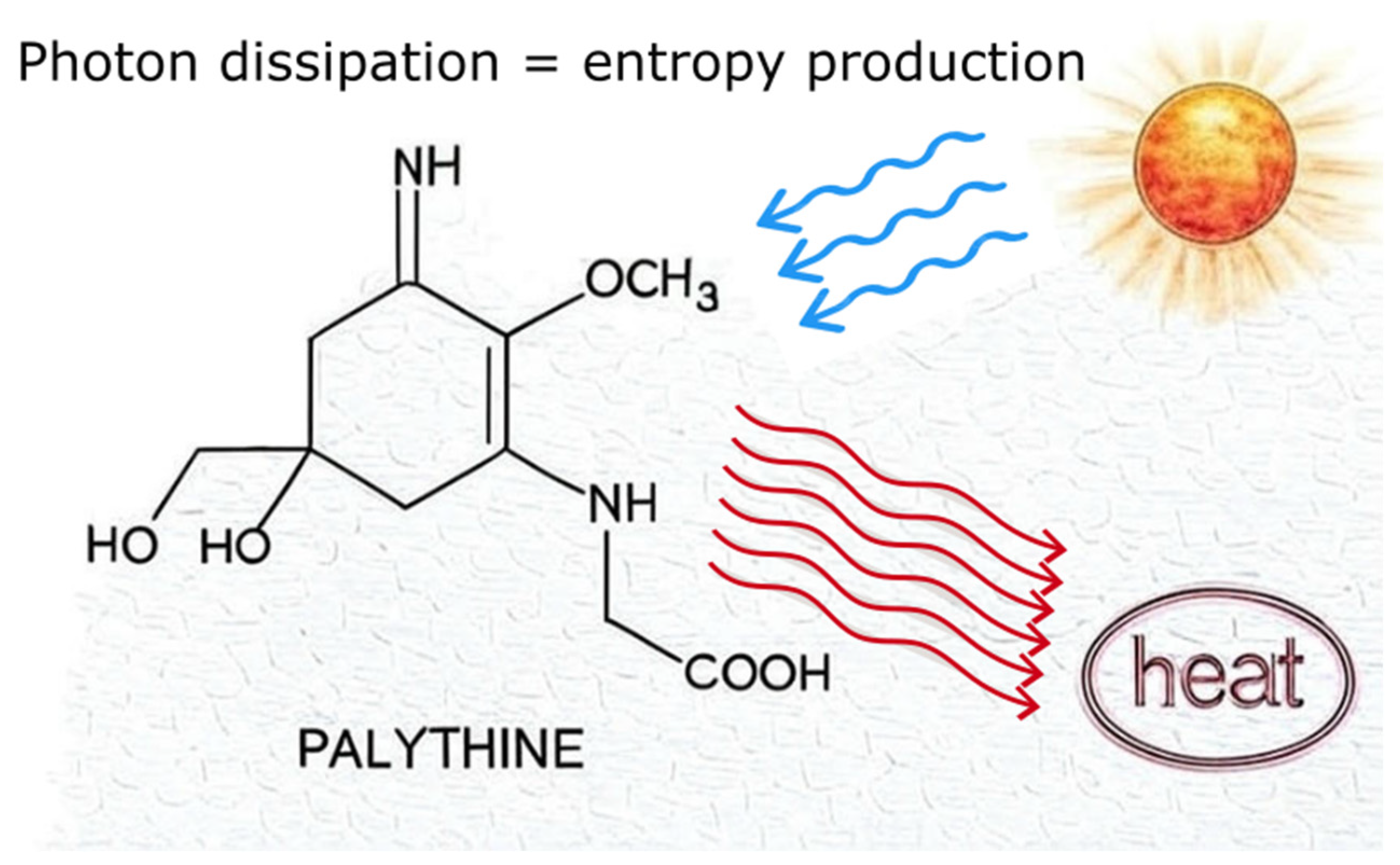

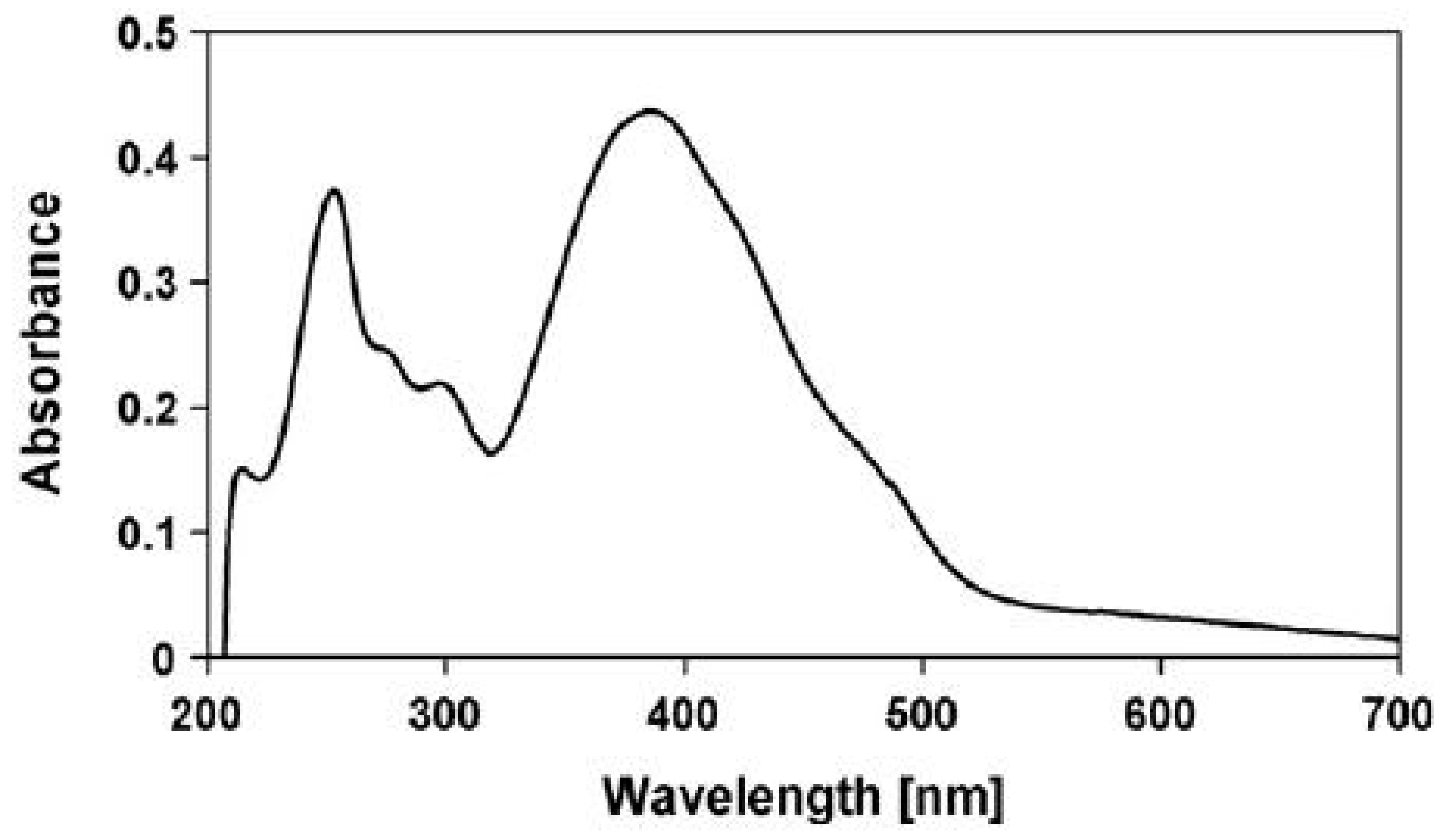
| UV Bio-Pigments | (nm) | (M−1 cm−1) | Excited State Lifetime (ns) | Fluorescence Quantum Yield () |
|---|---|---|---|---|
| Aromatic amino acids | ||||
| Phenylalanine a | 257 | 195 | 7.5 | 0.024 |
| Tyrosine a | 274 | 1405 | 2.5 | 0.14 |
| Tryptophan a | 278 | 5579 | 3.03 | 0.13 |
| Mycosporines and MAAs | ||||
| Gadusol b | 296 | 21,800 | / | non-fluorescent |
| Mycosporine-γ-aminobutyric acid c | 310 | 28,900 | / | / |
| Mycosporine-glutamic acid c | 311 | 20,900 | / | / |
| Palythine b,c | 320 | 36,200 | / | non-fluorescent |
| Shinorine b | 333 | 44,700 | 0.35 | 0.002 |
| Porphyra-334 b | 334 | 42,300 | 0.4 | 0.0016 |
| Palythene c | 360 | 50,000 | / | / |
| Scytonemins | ||||
| Scytonemin d,e | 212 252 278 300 384 | / / / / 16,200 | / | non-fluorescent |
| Reduced Scytonemin d | 246 276 314 378 474 572 | 30,000 14,000 15,000 22,000 14,000 7600 | / | / |
| Scytonemin-imine f | 237 366 437 564 | / / / / | / | / |
| Dimethoxyscytonemin d | 215 316 422 | 60,354 18,143 23,015 | / | / |
| Tetramethoxy-scytonemin d | 212 562 | 35,928 5944 | / | / |
| Scytonine d | 207 225 270 | 38,948 37,054 22,484 | / | / |
| Other poorly characterized cyanobacterial UV-absorbing pigments | ||||
| Gloeocapsin g | 392 | / | / | / |
| Microcystbiopterins h | ~275 ~350 | 10,000 3500 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simeonov, A.; Michaelian, K. Cyanobacterial UV Pigments Evolved to Optimize Photon Dissipation Rather than Photoprotection. Biophysica 2025, 5, 23. https://doi.org/10.3390/biophysica5020023
Simeonov A, Michaelian K. Cyanobacterial UV Pigments Evolved to Optimize Photon Dissipation Rather than Photoprotection. Biophysica. 2025; 5(2):23. https://doi.org/10.3390/biophysica5020023
Chicago/Turabian StyleSimeonov, Aleksandar, and Karo Michaelian. 2025. "Cyanobacterial UV Pigments Evolved to Optimize Photon Dissipation Rather than Photoprotection" Biophysica 5, no. 2: 23. https://doi.org/10.3390/biophysica5020023
APA StyleSimeonov, A., & Michaelian, K. (2025). Cyanobacterial UV Pigments Evolved to Optimize Photon Dissipation Rather than Photoprotection. Biophysica, 5(2), 23. https://doi.org/10.3390/biophysica5020023








