The Impact of a Rosemary Containing Drink on Cognition and Mood: The Role of Eye Blink Dynamics
Abstract
1. Introduction
The Current Study
2. Methods
2.1. Participants
2.2. Treatment
3. Measures
3.1. The 3-Stimulus Odd-Ball Task
3.2. Electrooculograms (Eye Blinks)
3.3. Event-Related Potentials
3.4. Mood and Arousal Measures
4. Results
4.1. Subjective Arousal and Mood Ratings
4.2. Blink Metrics (Total Rate and Variability)
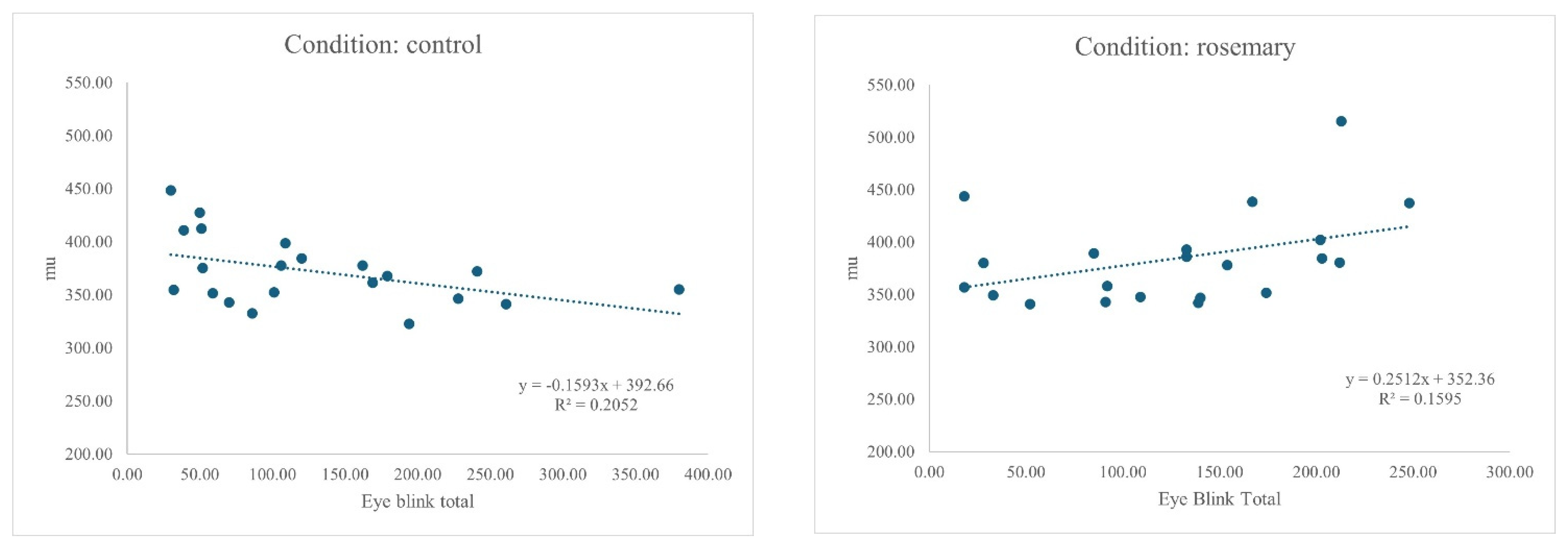
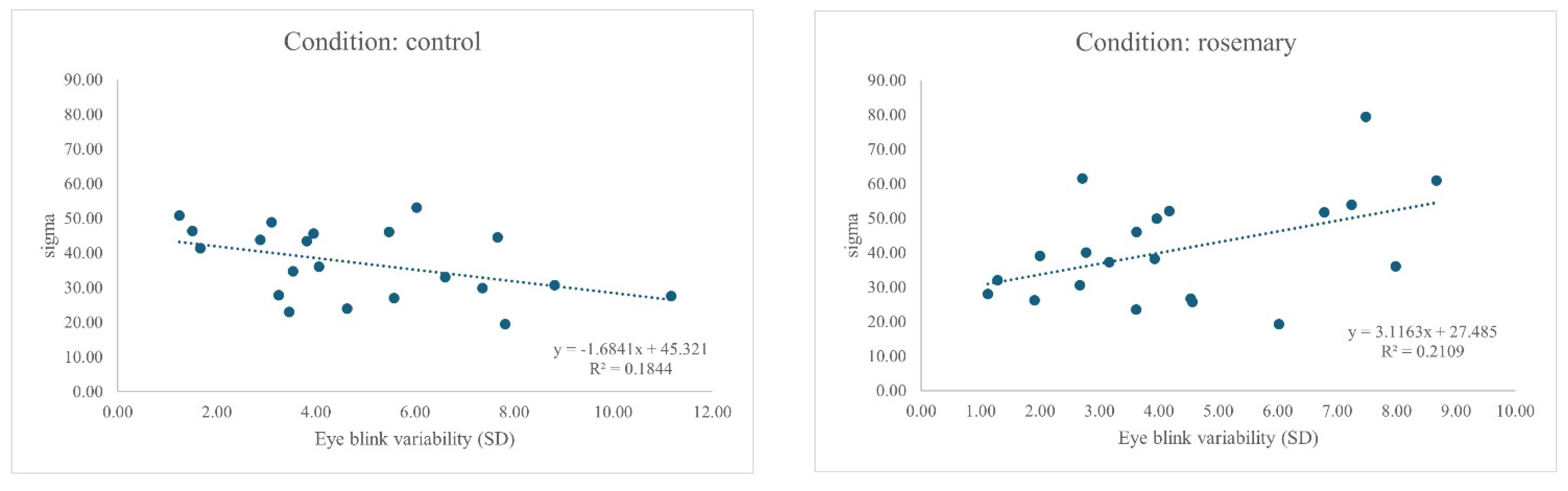
4.3. Bivariate Correlations
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Hanson, J.R. Rosemary, the beneficial chemistry of a garden herb. Sci. Prog. 2016, 99, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Shakespeare, W. Hamlet; Nicholas Ling and John Trundle: London, UK, 1603. [Google Scholar]
- Moss, M.; Cook, J.; Wesnes, K.; Duckett, P. Aromas of rosemary and lavender essential oils differentially affect cognition and mood in healthy adults. Int. J. Neurosci. 2003, 113, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K. Rosemary: An overview of potential health benefits. Nutr. Today 2016, 51, 102–112. [Google Scholar] [CrossRef]
- Hongratanaworakit, T. Simultaneous aromatherapy massage with rosemary oil on humans. Sci. Pharm. 2009, 77, 375–388. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Mesulam, M.-M. The cholinergic innervation of the human cerebral cortex. Prog. Brain Res. 2004, 145, 67–78. [Google Scholar]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef]
- Sarter, M.; Gehring, W.J.; Kozak, R. More attention must be paid: The neurobiology of attentional effort. Brain Res. Rev. 2006, 51, 145–160. [Google Scholar] [CrossRef]
- Savelev, S.; Okello, E.; Perry, N.; Wilkins, R.; Perry, E. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Duszkiewicz, A.J.; McNamara, C.G.; Takeuchi, T.; Genzel, L. Novelty and dopaminergic modulation of memory persistence: A tale of two systems. Trends Neurosci. 2019, 42, 102–114. [Google Scholar] [CrossRef]
- Matzel, L.D.; Sauce, B. A multi-faceted role of dual-state dopamine signaling in working memory, attentional control, and intelligence. Front. Behav. Neurosci. 2023, 17, 1060786. [Google Scholar] [CrossRef]
- Pitzianti, M.B.; Spiridigliozzi, S.; Bartolucci, E.; Esposito, S.; Pasini, A. New insights on the effects of methylphenidate in attention deficit hyperactivity disorder. Front. Psychiatry 2020, 11, 531092. [Google Scholar] [CrossRef]
- Stanzione, A.; Melchiori, F.M.; Costa, A.; Leonardi, C.; Scalici, F.; Caltagirone, C.; Carlesimo, G.A. Dopaminergic Treatment and Episodic Memory in Parkinson’s Disease: A Meta-analysis of the Literature. Neuropsychol. Rev. 2024, 34, 1148–1169. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III—The final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Jongkees, B.J.; Hommel, B.; Kühn, S.; Colzato, L.S. Effect of tyrosine supplementation on clinical and healthy populations under stress or cognitive demands—A review. J. Psychiatr. Res. 2015, 70, 50–57. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic Med. Sci. 2020, 23, 1100. [Google Scholar]
- Reddy, V.C.; Patel, S.V.; Hodge, D.O.; Leavitt, J.A. Corneal sensitivity, blink rate, and corneal nerve density in progressive supranuclear palsy and Parkinson disease. Cornea 2013, 32, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Riby, L.M.; Fenwick, S.K.; Kardzhieva, D.; Allan, B.; McGann, D. Unlocking the beat: Dopamine and eye blink response to classical music. NeuroSci 2023, 4, 152–163. [Google Scholar] [CrossRef]
- Müller, J.; Dreisbach, G.; Brocke, B.; Lesch, K.-P.; Strobel, A.; Goschke, T. Dopamine and cognitive control: The influence of spontaneous eyeblink rate, DRD4 exon III polymorphism and gender on flexibility in set-shifting. Brain Res. 2007, 1131, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.; Huette, S. Extracting blinks from continuous eye-tracking data in a mind wandering paradigm. Conscious. Cogn. 2022, 100, 103303. [Google Scholar] [CrossRef]
- Riby, L.M.; Marr, L.; Barron-Millar, L.; Greer, J.; Hamilton, C.J.; McGann, D.; Smallwood, J. Elevated blink rates predict mind wandering: Dopaminergic insights into attention and task focus. J. Integr. Neurosci. 2025, 24, 26508. [Google Scholar] [CrossRef] [PubMed]
- Parris, B.A.; Dienes, Z.; Hodgson, T.L. Application of the ex-Gaussian function to the effect of the word blindness suggestion on Stroop task performance suggests no word blindness. Front. Psychol. 2013, 4, 647. [Google Scholar] [CrossRef]
- Riby, L.M.; Edwards, S.; McDonald, H.; Moss, M. The impact of a rosemary containing drink on event-related potential neural markers of sustained attention. PLoS ONE 2023, 18, e0286113. [Google Scholar] [CrossRef]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef]
- Bond, A.; Lader, M. The use of analogue scales in rating subjective feelings. Br. J. Med. Psychol. 1974, 47, 211–218. [Google Scholar] [CrossRef]
- Sayorwan, W.; Ruangrungsi, N.; Piriyapunyporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Siripornpanich, V. Effects of inhaled rosemary oil on subjective feelings and activities of the nervous system. Sci. Pharm. 2012, 81, 531. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The effects of essential oils on the nervous system: A scoping review. Molecules 2023, 28, 3771. [Google Scholar] [CrossRef]
- Moss, M.; Hewitt, S.; Moss, L.; Wesnes, K. Modulation of cognitive performance and mood by aromas of peppermint and ylang-ylang. Int. J. Neurosci. 2008, 118, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Hawiset, T.; Sriraksa, N.; Somwang, P.; Inkaew, P. Effect of orange essential oil inhalation on mood and memory in female humans. J. Physiol. Biomed. Sci. 2016, 29, 5–11. [Google Scholar]
- Woo, C.C.; Miranda, B.; Sathishkumar, M.; Dehkordi-Vakil, F.; Yassa, M.A.; Leon, M. Overnight olfactory enrichment using an odorant diffuser improves memory and modifies the uncinate fasciculus in older adults. Front. Neurosci. 2023, 17, 1200448. [Google Scholar] [CrossRef]
- Riby, L.M. The impact of age and task domain on cognitive performance: A meta-analytic review of the glucose facilitation effect. Brain Impair. 2004, 5, 145–165. [Google Scholar] [CrossRef]
- Jongkees, B.J.; Colzato, L.S. Spontaneous eye blink rate as predictor of dopamine-related cognitive function—A review. Neurosci. Biobehav. Rev. 2016, 71, 58–82. [Google Scholar] [CrossRef]
- Dreisbach, G.; Müller, J.; Goschke, T.; Strobel, A.; Schulze, K.; Lesch, K.-P.; Brocke, B. Dopamine and cognitive control: The influence of spontaneous eyeblink rate and dopamine gene polymorphisms on perseveration and distractibility. Behav. Neurosci. 2005, 119, 483. [Google Scholar] [CrossRef]
- Callara, A.L.; Greco, A.; Scilingo, E.P.; Bonfiglio, L. Neuronal correlates of eyeblinks are an expression of primary consciousness phenomena. Sci. Rep. 2023, 13, 12617. [Google Scholar] [CrossRef]
- Ortega, J.; Plaska, C.R.; Gomes, B.A.; Ellmore, T.M. Spontaneous eye blink rate during the working memory delay period predicts task accuracy. Front. Psychol. 2022, 13, 788231. [Google Scholar] [CrossRef] [PubMed]
- Paprocki, R.; Lenskiy, A. What does eye-blink rate variability dynamics tell us about cognitive performance? Front. Hum. Neurosci. 2017, 11, 620. [Google Scholar] [CrossRef]
- Warren, C.V.; Kroll, C.F.; Kopp, B. Dopaminergic and norepinephrinergic modulation of endogenous event-related potentials: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 151, 105221. [Google Scholar] [CrossRef] [PubMed]
- Rac-Lubashevsky, R.; Slagter, H.A.; Kessler, Y. Tracking real-time changes in working memory updating and gating with the event-based eye-blink rate. Sci. Rep. 2017, 7, 2547. [Google Scholar] [CrossRef]
- Mark, G.; Iqbal, S.T.; Czerwinski, M.; Johns, P. Bored mondays and focused afternoons: The rhythm of attention and online activity in the workplace. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, 2014, Toronto, ON, Canada, 26 April–1 May 2014; pp. 3025–3034. [Google Scholar]
- Veltman, J.; Gaillard, A. Physiological workload reactions to increasing levels of task difficulty. Ergonomics 1998, 41, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Riby, L.M.; Greer, J.; Smallwood, J. Absorbed in thought: The effect of mind wandering on the processing of relevant and irrelevant events. Psychol. Sci. 2011, 22, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Kosmopoulou, D.; Lafara, M.P.; Adamantidi, T.; Ofrydopoulou, A.; Grabrucker, A.M.; Tsoupras, A. Neuroprotective benefits of Rosmarinus officinalis and its bioactives against Alzheimer’s and Parkinson’s Diseases. Appl. Sci. 2024, 14, 6417. [Google Scholar] [CrossRef]

| Measure | Control M (SD) | Rosemary M (SD) | Cohen’s d |
|---|---|---|---|
| Eye Blink Rate (EBR) | 120.24 ± 90.21 | 122.91 ± 67.77 | 0.03 |
| Blink Variability (EBV) | 4.69 ± 2.52 | 4.14 ± 2.21 | −0.23 |
| Alertness (Pre) | 35.11 ± 12.08 | 36.91 ± 11.56 | 0.15 |
| Calmness (Pre) | 30.91 ± 14.43 | 33.54 ± 17.75 | 0.17 |
| Contentedness (Pre) | 24.22 ± 9.48 | 26.67 ± 10.97 | 0.25 |
| Alertness (Post) | 38.32 ± 18.48 | 45.06 ± 18.01 | 0.37 |
| Calmness (Post) | 32.92 ± 13.99 | 32.87 ± 14.00 | −0.00 |
| Contentedness (Post) | 28.34 ± 10.94 | 30.97 ± 15.06 | 0.19 |
| Mu (μ) | 372.04 ± 32.40 | 383.98 ± 44.06 | 0.31 |
| Sigma (σ) | 37.01 ± 10.15 | 40.87 ± 15.24 | 0.30 |
| Tau (τ) | 33.86 ± 12.74 | 36.81 ± 10.61 | 0.25 |
| Hits | 75.75 ± 21.53 | 78.37 ± 14.67 | 0.13 |
| P3a Amplitude (Cz) | 4.79 ± 2.86 | 6.44 ± 2.89 | 0.63 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Eye blink total | 1 | 0.588 ** | 0.555 ** | 0.232 | 0.316 | 0.309 | 0.051 | 0.063 | −0.453 * | −0.078 | 0.042 | −0.281 | 0.176 |
| 2. Eye blink variability | 0.588 ** | 1 | 0.462 * | 0.164 | 0.302 | 0.416 * | 0.174 | 0.243 | −0.138 | −0.429 | −0.062 | 0.114 | −0.319 |
| 3. Alertness (pre) | 0.555 ** | 0.462 * | 1 | −0.117 | 0.249 | 0.692 ** | −0.132 | 0.266 | −0.296 | −0.428 | 0.396 | 0.857 | 0.498 |
| 4. Calmness (pre) | 0.232 | 0.164 | −0.117 | 1 | 0.237 | 0.048 | 0.220 | −0.049 | 0.084 | −0.063 | −0.342 | 0.162 | −0.086 |
| 5. Contentedness (pre) | 0.316 | 0.302 | 0.249 | 0.237 | 1 | 0.496 * | 0.265 | 0.267 | −0.312 | −0.480 * | −0.061 | −0.113 | −0.040 |
| 6. Alertness (post) | 0.309 | 0.416 * | 0.692 ** | 0.048 | 0.496 * | 1 | −0.133 | 0.572 ** | −0.146 | −0.234 | −0.330 | −0.036 | 0.424 |
| 7. Calmness (post) | 0.051 | 0.174 | −0.132 | 0.220 | 0.265 | −0.133 | 1 | 0.511 * | −0.197 | −0.197 | −0.438 | 0.205 | 0.446 |
| 8. Contentedness (post) | 0.063 | 0.243 | 0.243 | 0.266 | 0.267 | 0.572 ** | 0.511 * | 1 | −0.269 | −0.104 | −0.400 | −0.314 | 0.436 |
| 9. Mu (μ) | −0.453 * | −0.138 | −0.296 | 0.084 | −0.312 | −0.146 | −0.197 | −0.269 | 1 | 0.242 | 0.136 | 0.448 * | −0.252 |
| 10. Sigma (σ) | −0.078 | −0.429 | −0.428 | −0.063 | −0.480 * | −0.234 | −0.197 | −0.104 | 0.242 | 1 | −0.002 | −0.250 | 0.262 |
| 11. Tau (τ) | 0.042 | −0.062 | 0.396 | −0.342 | −0.061 | −0.330 | −0.438 | −0.400 | 0.136 | −0.002 | 1 | 0.046 | −0.163 |
| 12. Hits | −0.281 | 0.114 | 0.857 | 0.162 | −0.113 | −0.036 | 0.205 | −0.314 | 0.448 * | −0.250 | 0.046 | 1 | −0.559 * |
| 13. P3a ERP | 0.176 | −0.319 | 0.498 | −0.086 | −0.040 | 0.424 | 0.446 | 0.436 | −0.252 | 0.262 | −0.163 | −0.559 * | 1 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Eye blink total | 1 | 0.7760 ** | 0.228 | 0.201 | 0.158 | 0.056 | 0.092 | 0.077 | 0.399 | 0.491 * | 0.118 | 0.001 | −0.227 |
| 2. Eye blink variability | 0.776 ** | 1 | 0.215 | 0.262 | 0.426 * | 0.221 | −0.049 | 0.306 | 0.388 | 0.459 * | 0.096 | 0.345 | −0.310 |
| 3. Alertness (pre) | 0.228 | 0.215 | 1 | 0.337 | 0.467 * | 0.465 * | −0.275 | 0.475 * | 0.242 | 0.388 | 0.104 | −0.091 | −0.251 |
| 4. Calmness (pre) | 0.201 | 0.262 | 0.337 | 1 | 0.826 ** | 0.375 | 0.498 * | 0.743 ** | 0.291 | 0.433 | 0.290 | 0.323 | 0.138 |
| 5. Contentedness (pre) | 0.158 | 0.426 * | 0.467 * | 0.826 ** | 1 | 0.469 * | 0.267 | 0.865 ** | 0.302 | 0.371 | 0.188 | 0.388 | −0.095 |
| 6. Alertness (post) | 0.056 | 0.221 | 0.465 * | 0.375 | 0.469 * | 1 | −0.043 | 0.739 ** | −0.038 | −0.134 | −0.117 | 0.195 | 0.015 |
| 7. Calmness (post) | 0.092 | −0.049 | −0.275 | 0.498 * | 0.267 | −0.043 | 1 | 0.241 | 0.041 | 0.155 | −0.149 | −0.221 | 0.275 |
| 8. Contentedness (post) | 0.077 | 0.306 | 0.475 * | 0.743 ** | 0.865 ** | 0.739 ** | 0.241 | 1 | 0.023 | 0.182 | 0.182 | 0.318 | 0.019 |
| 9. Mu (μ) | 0.399 | 0.388 | 0.242 | 0.291 | 0.302 | −0.038 | 0.155 | 0.182 | 1 | 0.691 ** | −0.004 | 0.181 | −0.026 |
| 10. Sigma (σ) | 0.491 * | 0.459 * | 0.388 | 0.433 | 0.371 | −0.134 | 0.155 | 0.182 | 0.691 ** | 1 | 0.108 | 0.258 | −0.032 |
| 11. Tau (τ) | 0.118 | 0.096 | 0.104 | 0.290 | 0.188 | −0.117 | −0.149 | 0.182 | −0.004 | 0.108 | 1 | 0.531 * | −0.247 |
| 12. Hits | 0.001 | 0.345 | −0.091 | 0.323 | 0.388 | 0.195 | −0.221 | 0.318 | 0.181 | 0.258 | 0.531 * | 1 | −0.120 |
| 13. P3a ERP | −0.227 | −0.310 | −0.251 | 0.138 | −0.095 | 0.015 | 0.275 | 0.019 | −0.026 | −0.032 | −0.247 | −0.120 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Riby, L.M.; Kardzhieva, D.; Fenwick, S.; Fowler, S.; Moss, M. The Impact of a Rosemary Containing Drink on Cognition and Mood: The Role of Eye Blink Dynamics. NeuroSci 2026, 7, 15. https://doi.org/10.3390/neurosci7010015
Riby LM, Kardzhieva D, Fenwick S, Fowler S, Moss M. The Impact of a Rosemary Containing Drink on Cognition and Mood: The Role of Eye Blink Dynamics. NeuroSci. 2026; 7(1):15. https://doi.org/10.3390/neurosci7010015
Chicago/Turabian StyleRiby, Leigh Martin, Dimana Kardzhieva, Sam Fenwick, Sophia Fowler, and Mark Moss. 2026. "The Impact of a Rosemary Containing Drink on Cognition and Mood: The Role of Eye Blink Dynamics" NeuroSci 7, no. 1: 15. https://doi.org/10.3390/neurosci7010015
APA StyleRiby, L. M., Kardzhieva, D., Fenwick, S., Fowler, S., & Moss, M. (2026). The Impact of a Rosemary Containing Drink on Cognition and Mood: The Role of Eye Blink Dynamics. NeuroSci, 7(1), 15. https://doi.org/10.3390/neurosci7010015







