Effects of Haloperidol on Cardiac Histamine H2 Receptors and β-Adrenoceptors in Isolated Mouse and Human Atrial Preparations
Abstract
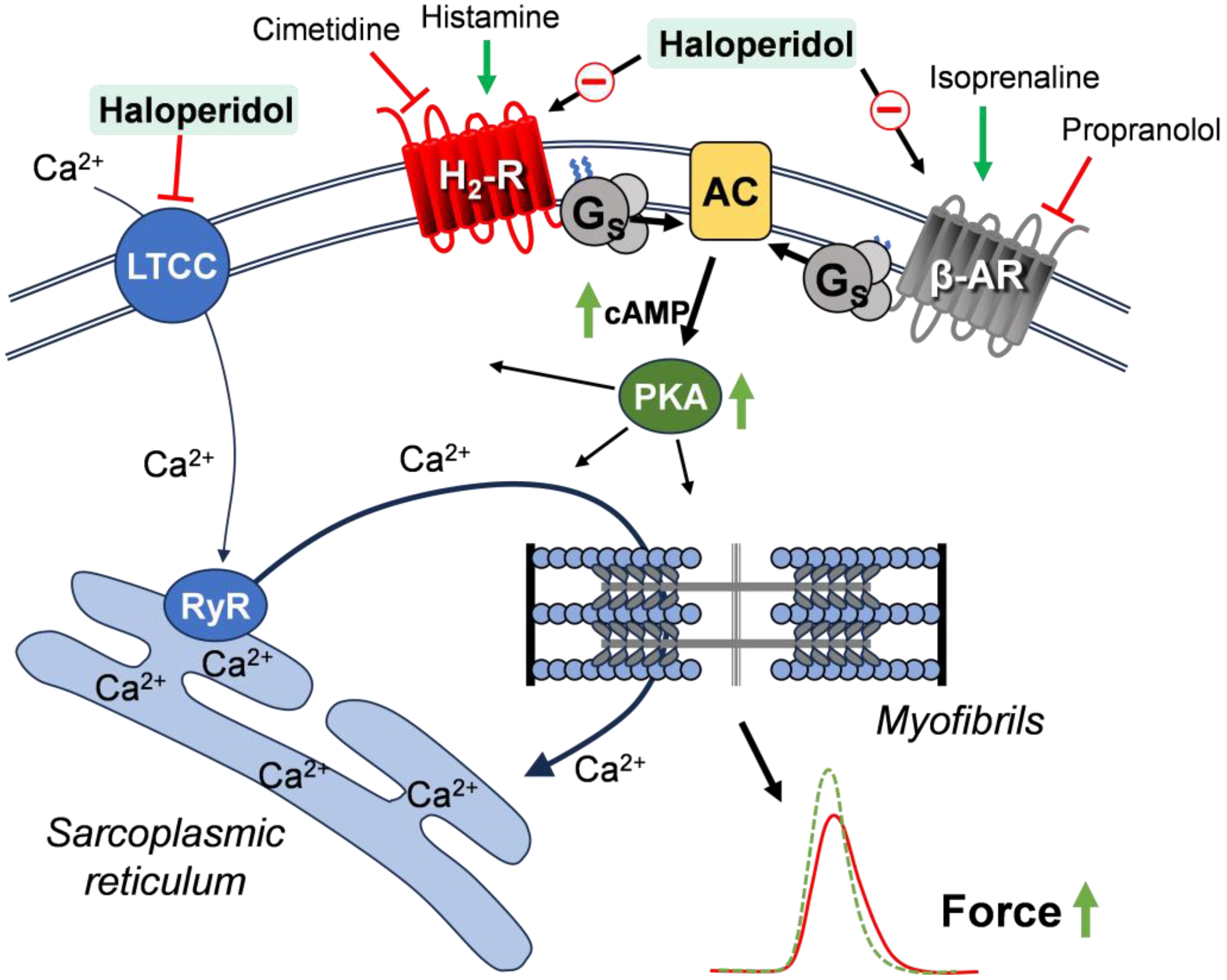
1. Introduction
2. Materials and Methods
2.1. Transgenic Mice
2.2. Contractile Studies in Mice
- (a)
- Propranolol (0.4 µM), followed by histamine (0.1 µM), followed by a haloperidol concentration–response curve (1–10 µM).
- (a)
- Incubation with 30 nM or 1 µM isoprenaline (time control);
- (b)
- Preincubation with 30 nM or 1 µM isoprenaline, followed by a haloperidol concentration–response curve (1–10 µM).
2.3. Contractile Studies on Human Preparations
- (a)
- The histamine concentration–response curve (10−8–10−4 M).
- (b)
- Preincubation with 1 or 3 or 10 µM haloperidol, followed by the histamine concentration–response curve (10−7–10−4 M).
- (c)
- The histamine concentration–response curve (10−8–10−4 M), followed by the haloperidol concentration–response curve (1–10 µM), followed by 10 µM cimetidine.
- (d)
- Incubation with 1 µM isoprenaline (time control).
- (e)
- Preincubation with 1 µM isoprenaline, followed by 10 µM haloperidol.
- (f)
- Preincubation with 50 mM KCl, followed by 1 µM isoprenaline, followed by 10 µM haloperidol.
2.4. Data Analysis
2.5. Drugs and Materials
3. Results
3.1. Interaction of Haloperidol and Histamine in Human Atrial Preparations
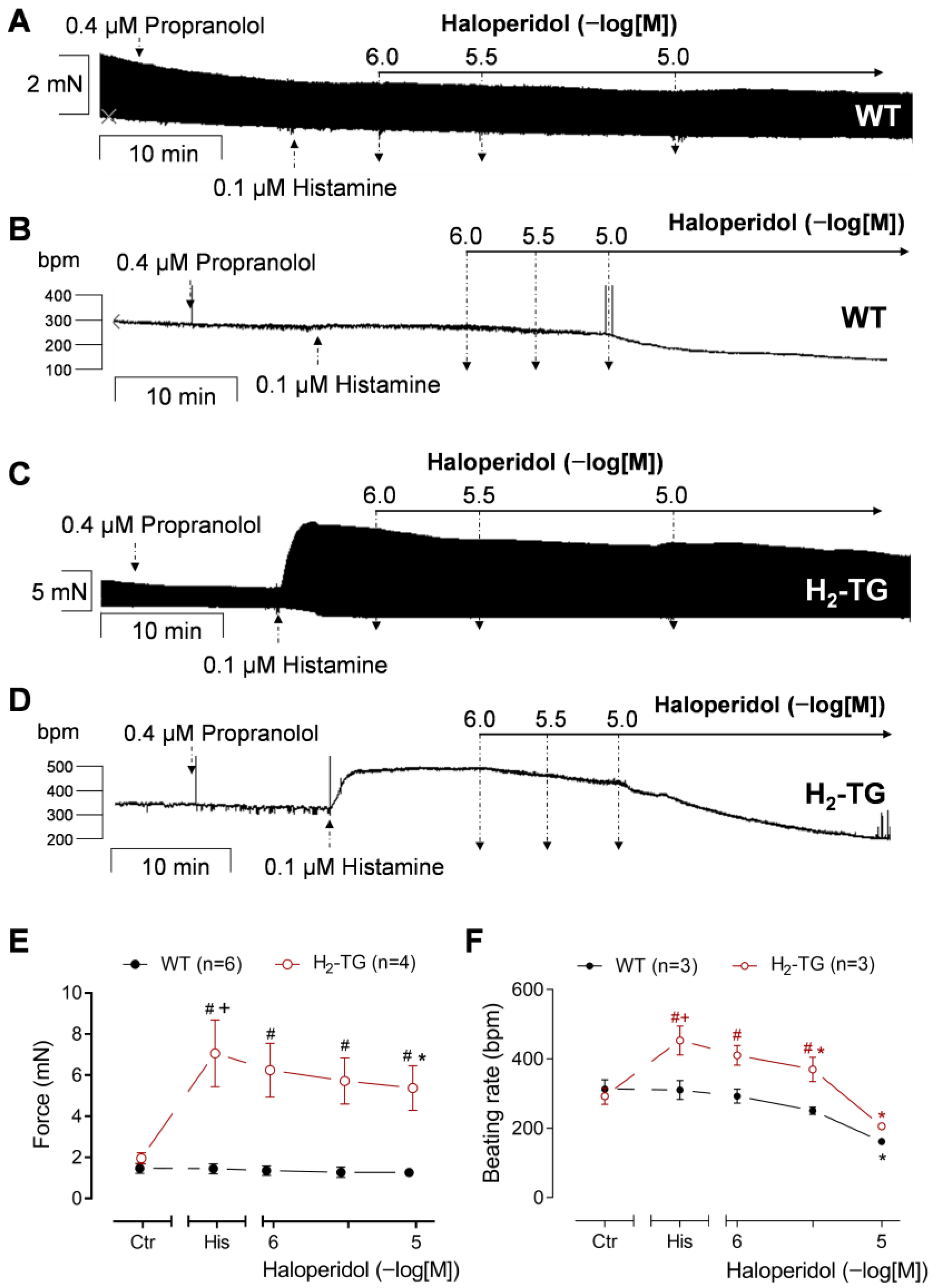
3.2. Interaction of Haloperidol and Histamine in Mouse Atrial Preparations
3.3. Interaction of Haloperidol and Isoprenaline in Human Atrial Preparations
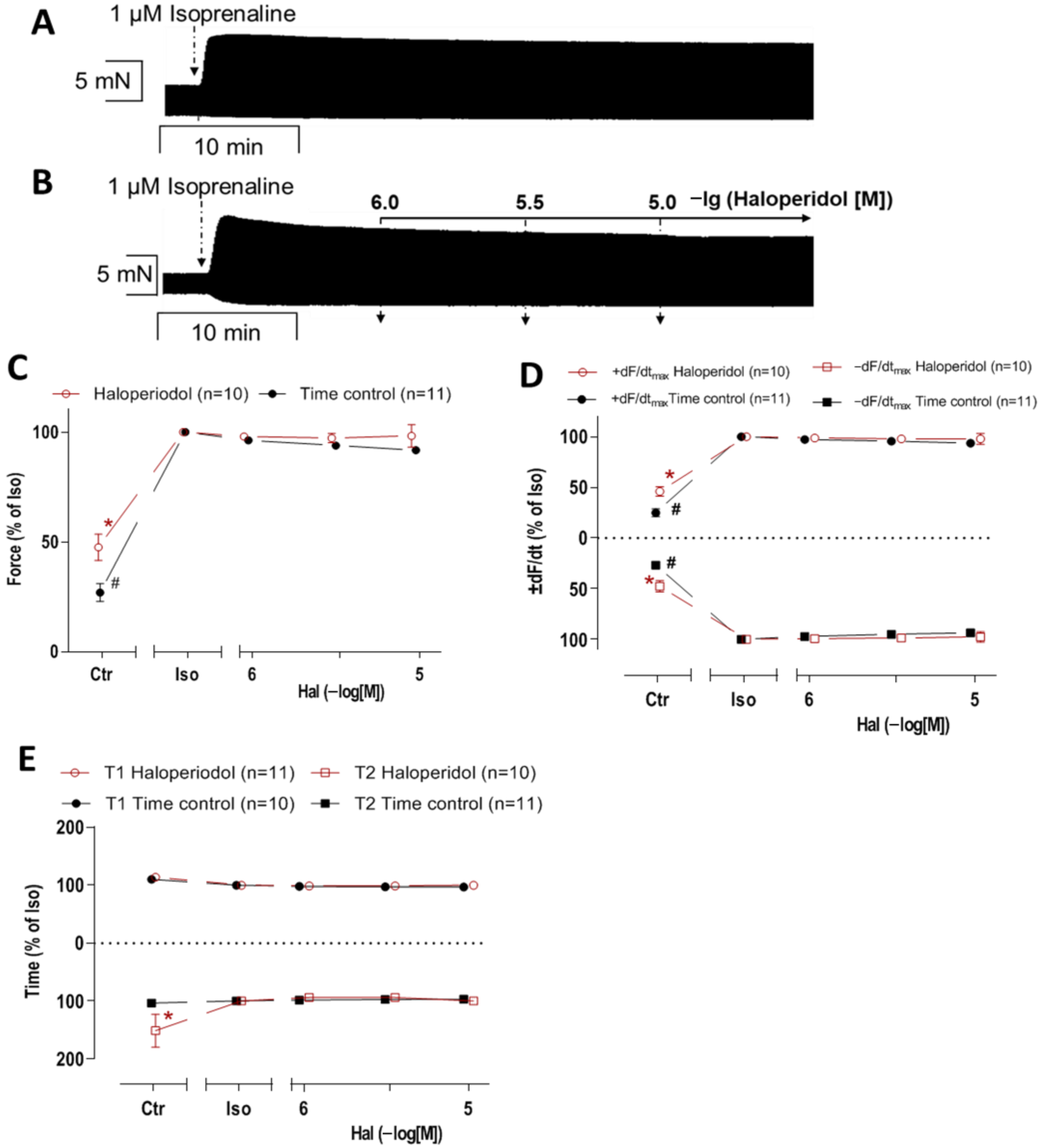
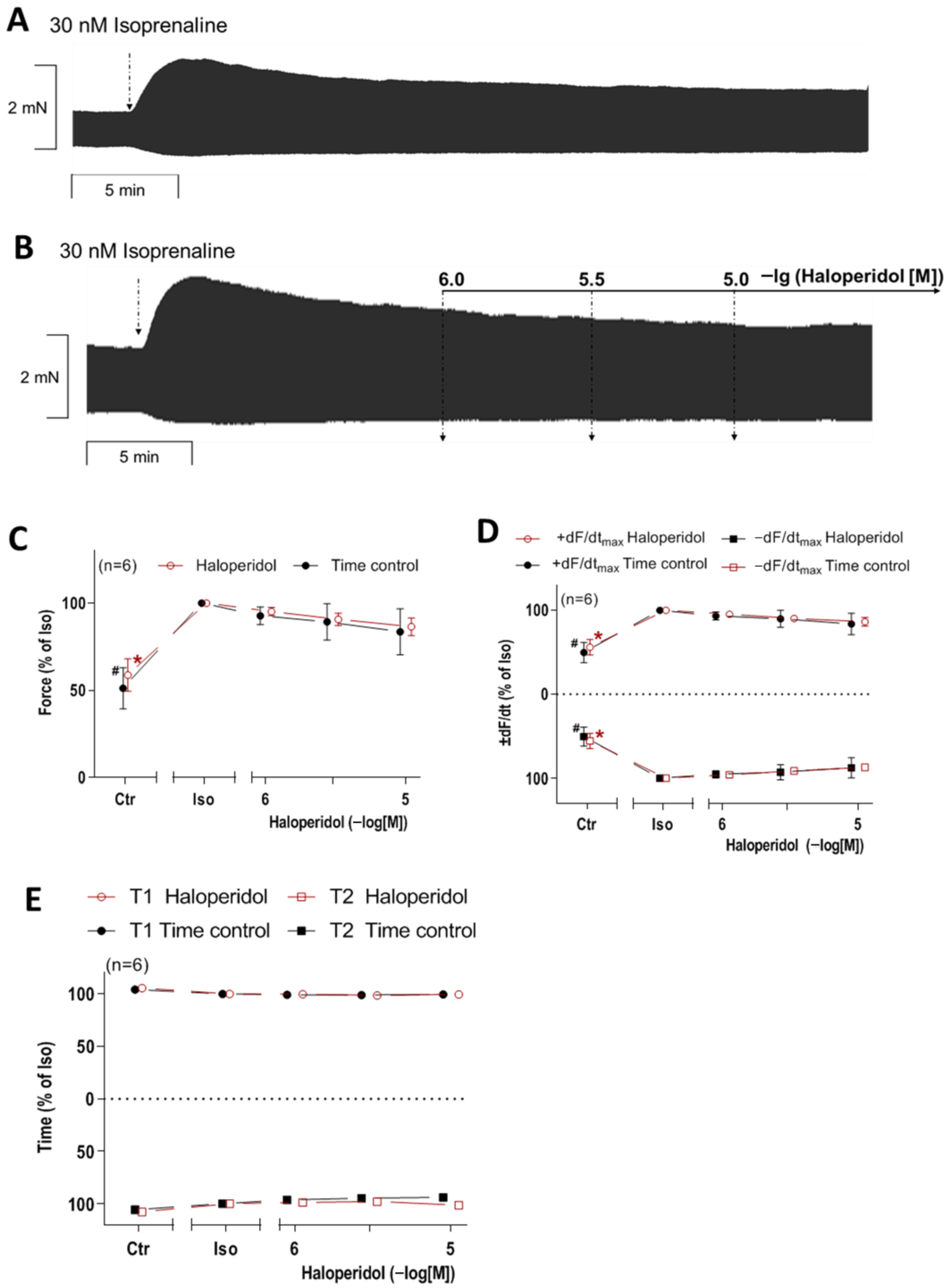
3.4. Interaction of Haloperidol and Isoprenaline in Mouse Atrial Preparations
3.5. Influence of the LTCC on the Interaction of Haloperidol and Isoprenaline in the Human Atrial Preparations
4. Discussion
Clinical Relevance and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FOC | Force of contraction |
| HAP | Human atrial preparation |
| LA | Left atrial preparation |
| PIE | Positive inotropic effect |
| RA | Right atrial preparation |
| WT | Wild-type mouse |
Appendix A

References
- Brunton, L.L.; Knollmann, B.C. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 14th ed.; McGraw-Hill Education: New York, NY, USA, 2022; ISBN 978-1-264-25807-9. [Google Scholar]
- Granger, B.; Albu, S. The Haloperidol Story. Ann. Clin. Psychiatry 2005, 17, 137–140. [Google Scholar] [CrossRef]
- Robinson, E. Psychopharmacology: From serendipitous discoveries to rationale design, but what next? Brain Neurosci. Adv. 2018, 2, 2398212818812629. [Google Scholar] [CrossRef]
- Marinho, E. Clozapine: A special case of an atypical antipsychotic. Eur. J. Med. Chem. Rep. 2024, 10, 100140. [Google Scholar] [CrossRef]
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef]
- Roldan, C.J.; Rowland, J.W.; Ye, A.L. Haloperidol for Pain Management: A Narrative Review. Pharmaceuticals 2024, 17, 1096. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Gunther, M. A Critical Reappraisal of Haloperidol for Delirium Management in the Intensive Care Unit: Perspective from Psychiatry. J. Clin. Med. 2025, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Web Annex A: World Health Organization Model List of Essential Medicines—23rd List, 2023. In The Selection and Use of Essential Medicines 2023, Proceedings of the Executive Summary of the Report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines, Geneva, Switzerland, 24–28 April 2023; World Health Organization: Geneva, Switzerland, 2023.
- Garcia, M.C.; Anderson, M.; Li, M.; Zewdu, M.; Schneider, T.; Matos-Silva, J.; Ramnarine, G.; Rong, K.; Mbuagbaw, L.; Holbrook, A. Major adverse cardiac events with haloperidol: A meta-analysis. PLoS ONE 2025, 20, e0326804. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Marwaha, R. Haloperidol. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560892/ (accessed on 11 July 2025).
- Appl, H.; Holzammer, T.; Dove, S.; Haen, E.; Strasser, A.; Seifert, R. Interactions of recombinant human histamine H1R, H2R, H3R, and H4R receptors with 34 antidepressants and antipsychotics. Naunyn Schmiedebergs Arch. Pharmacol. 2012, 385, 145–170. [Google Scholar] [CrossRef]
- Tarabová, B.; Nováková, M.; Lacinová, L. Haloperidol moderately inhibits cardiovascular L-type calcium current. Gen. Physiol. Biophys. 2009, 28, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Hatip-Al-Khatib, I.; Bolukbaşi-Hatip, F. Modulation of the negative inotropic effect of haloperidol by drugs with positive inotropic effects in isolated rabbit heart. Pharmacology 2002, 66, 19–25. [Google Scholar] [CrossRef]
- Hsu, S.-S.; Liang, W.-Z. Ca2+ signaling as a mechanism of haloperidol-induced cytotoxicity in human astrocytes and assessing the protective role of a Ca2+ chelator. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2117–2127. [Google Scholar] [CrossRef]
- Brown, L.; Lorenz, B.; Erdmann, E. The inotropic effects of dopamine and its precursor levodopa on isolated human ventricular myocardium. Klin. Wochenschr. 1985, 63, 1117–1123. [Google Scholar] [CrossRef]
- Panula, P.; Chazot, P.L.; Cowart, M.; Gutzmer, R.; Leurs, R.; Liu, W.L.S.; Stark, H.; Thurmond, R.L.; Haas, H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015, 67, 601–655. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Kirchhefer, U.; Dhein, S.; Hofmann, B.; Gergs, U. Cardiac role of histamine and histamine receptors. In Horizons in World Cardiovascular Research; Bennington, E.H., Ed.; Nova Science Publishers: New York, NY, USA, 2022; Volume 22, pp. 1–75. ISBN 9781685075682. [Google Scholar]
- Neumann, J.; Kirchhefer, U.; Dhein, S.; Hofmann, B.; Gergs, U. The Roles of Cardiovascular H2-Histamine Receptors Under Normal and Pathophysiological Conditions. Front. Pharmacol. 2021, 12, 732842. [Google Scholar] [CrossRef] [PubMed]
- Kou, E.; Zhang, X.; Dong, B.; Wang, B.; Zhu, Y. Combination of H1 and H2 Histamine Receptor Antagonists: Current Knowledge and Perspectives of a Classic Treatment Strategy. Life 2024, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, R.; Bristow, M.R.; Stinson, E.B.; Harrison, D.C. Histamine receptors in the human heart. Life Sci. 1980, 26, 2245–2249. [Google Scholar] [CrossRef]
- Levi, R.; Malm, J.R.; Bowman, F.O.; Rosen, M.R. The arrhythmogenic actions of histamine on human atrial fibers. Circ. Res. 1981, 49, 545–550. [Google Scholar] [CrossRef]
- Genovese, A.; Gross, S.S.; Sakuma, I.; Levi, R. Adenosine promotes histamine H1-mediated negative chronotropic and ino-tropic effects on human atrial myocardium. J. Pharmacol. Exp. Ther. 1988, 247, 844–849. [Google Scholar] [CrossRef]
- Zerkowski, H.R.; Broede, A.; Kunde, K.; Hillemann, S.; Schäfer, E.; Vogelsang, M.; Michel, M.C.; Brodde, O.E. Comparison of the positive inotropic effects of serotonin, histamine, angiotensin II, endothelin and isoprenaline in the isolated human right atrium. Naunyn Schmiedebergs Arch. Pharmacol. 1993, 347, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.; Lynham, J.A.; Kaumann, A.J. Chronic beta 1-adrenoceptor blockade sensitises the H1 and H2 receptor systems in human atrium: Rôle of cyclic nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 1996, 353, 661–670. [Google Scholar] [CrossRef]
- Gergs, U.; Bernhardt, G.; Buchwalow, I.B.; Edler, H.; Fröba, J.; Keller, M.; Kirchhefer, U.; Köhler, F.; Mißlinger, N.; Wache, H.; et al. Initial Characterization of Transgenic Mice Overexpressing Human Histamine H2 Receptors. J. Pharmacol. Exp. Ther. 2019, 369, 129–141. [Google Scholar] [CrossRef]
- Neumann, J.; Binter, M.B.; Fehse, C.; Marušáková, M.; Büxel, M.L.; Kirchhefer, U.; Hofmann, B.; Gergs, U. Amitriptyline functionally antagonizes cardiac H2 histamine receptors in transgenic mice and human atria. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1251–1262. [Google Scholar] [CrossRef]
- Schlicht, J.; Hofmann, B.; Gergs, U.; Neumann, J. Haloperidol, clozapine and mirtazapine are functional antagonists at histamine receptors in human atrial preparations. In Proceedings of the 51th Annual Meeting, Torino, Italy, 23–25 May 2024; European Histamine Research Society: Brussels, Belgium, 2024; p. 50. [Google Scholar]
- National Academies Press (US). Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011; ISBN 9780309154000. [Google Scholar]
- Schlicht, J.M.A.; Ahlrep, U.; Hofmann, B.; Kirchhefer, U.; Neumann, J.; Gergs, U. Clozapine is a functional antagonist at cardiac human H2-histamine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 6691–6706. [Google Scholar] [CrossRef]
- Gergs, U.; Weisgut, J.; Griethe, K.; Mißlinger, N.; Kirchhefer, U.; Neumann, J. Human histamine H2 receptors can initiate cardiac arrhythmias in a transgenic mouse. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Ginsburg, R.; Minobe, W.; Cubicciotti, R.S.; Sageman, W.S.; Lurie, K.; Billingham, M.E.; Harrison, D.C.; Stinson, E.B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 1982, 307, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Bremner, R.M.; Greengrass, P.M.; Morville, M.; Blackburn, K.J. Effect of tolamolol and other beta-adrenoceptor blocking drugs on 3Hhaloperidol binding to rat striatal membrane preparations. J. Pharm. Pharmacol. 1978, 30, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Li, J.; McCormick, P.J.; Huang, C.L.; Jeevaratnam, K. Is the sigma-1 receptor a potential pharmacological target for cardiac pathologies? A systematic review. Int. J. Cardiol. Heart Vasc. 2020, 26, 100449. [Google Scholar] [CrossRef]
- Stracina, T.; Novakova, M. Cardiac sigma receptors—An update. Physiol. Res. 2018, 67, S561–S576. [Google Scholar] [CrossRef]
- Kudo, S.; Ishizaki, T. Pharmacokinetics of haloperidol: An update. Clin. Pharmacokinet. 1999, 37, 435–456. [Google Scholar] [CrossRef]
- Elkayam, U.; Shotan, A.; Mehra, A.; Ostrzega, E. Calcium channel blockers in heart failure. J. Am. Coll. Cardiol. 1993, 22, 139A–144A. [Google Scholar] [CrossRef]
- Cruickshank, J.M. Beta-blockers and heart failure. Indian Heart J. 2010, 62, 101–110. [Google Scholar]
- Jedrusiak, J.; Brus, R.; Kostrzewa, R.M.; Słowiński, Z. Dopaminergic neuronal systems modulate the central cardiovascular effects of TRH in rats. Pol. J. Pharmacol. 1995, 47, 43–52. [Google Scholar]
- Hennig, J.; Rzepka, U.; Mai, B.; Netter, P. Suppression of HPA-axis activity by haloperidol after experimentally induced heat stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 603–614. [Google Scholar] [CrossRef]
- Iqbal, M.M.; Aneja, A.; Rahman, A.; Megna, J.; Freemont, W.; Shiplo, M.; Nihilani, N.; Lee, K. The potential risks of commonly prescribed antipsychotics: During pregnancy and lactation. Psychiatry 2005, 2, 36–44. [Google Scholar] [PubMed]
- Shinoda, Y.; Tagashira, H.; Bhuiyan, M.S.; Hasegawa, H.; Kanai, H.; Fukunaga, K. Haloperidol aggravates transverse aortic constriction-induced heart failure via mitochondrial dysfunction. J. Pharmacol. Sci. 2016, 131, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Nalepa, I.; Kreiner, G.; Kowalska, M.; Sanak, M.; Vetulani, J. Adrenergic receptors’ responsiveness after acute and chronic treatment with haloperidol in the presence of calcium channel blockade. Pol. J. Pharmacol. 1999, 51, 377–383. [Google Scholar] [PubMed]
- Hommers, L.; Scherf-Clavel, M.; Stempel, R.; Roth, J.; Falter, M.; Deckert, J.; Mattheisen, M.; Unterecker, S.; Gawlik, M. Anti-psychotics in routine treatment are minor contributors to QT prolongation compared to genetics and age. J. Psychopharmacol. 2021, 35, 1127–1133. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlicht, J.M.A.; Hofmann, B.; Kirchhefer, U.; Neumann, J.; Gergs, U. Effects of Haloperidol on Cardiac Histamine H2 Receptors and β-Adrenoceptors in Isolated Mouse and Human Atrial Preparations. NeuroSci 2025, 6, 91. https://doi.org/10.3390/neurosci6030091
Schlicht JMA, Hofmann B, Kirchhefer U, Neumann J, Gergs U. Effects of Haloperidol on Cardiac Histamine H2 Receptors and β-Adrenoceptors in Isolated Mouse and Human Atrial Preparations. NeuroSci. 2025; 6(3):91. https://doi.org/10.3390/neurosci6030091
Chicago/Turabian StyleSchlicht, Jonas M. A., Britt Hofmann, Uwe Kirchhefer, Joachim Neumann, and Ulrich Gergs. 2025. "Effects of Haloperidol on Cardiac Histamine H2 Receptors and β-Adrenoceptors in Isolated Mouse and Human Atrial Preparations" NeuroSci 6, no. 3: 91. https://doi.org/10.3390/neurosci6030091
APA StyleSchlicht, J. M. A., Hofmann, B., Kirchhefer, U., Neumann, J., & Gergs, U. (2025). Effects of Haloperidol on Cardiac Histamine H2 Receptors and β-Adrenoceptors in Isolated Mouse and Human Atrial Preparations. NeuroSci, 6(3), 91. https://doi.org/10.3390/neurosci6030091





