Comparing Antemortem CT–Angiography Data with Autopsy Findings in Regard to Anterior Communicating Artery Aneurysms
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Determinants for the Presence of Ruptured and Unruptured AcoA Aneurysms
3.2. Correlations Between External Diameters of AcoA Aneurysms and Anatomical Variants of Circle of Willis
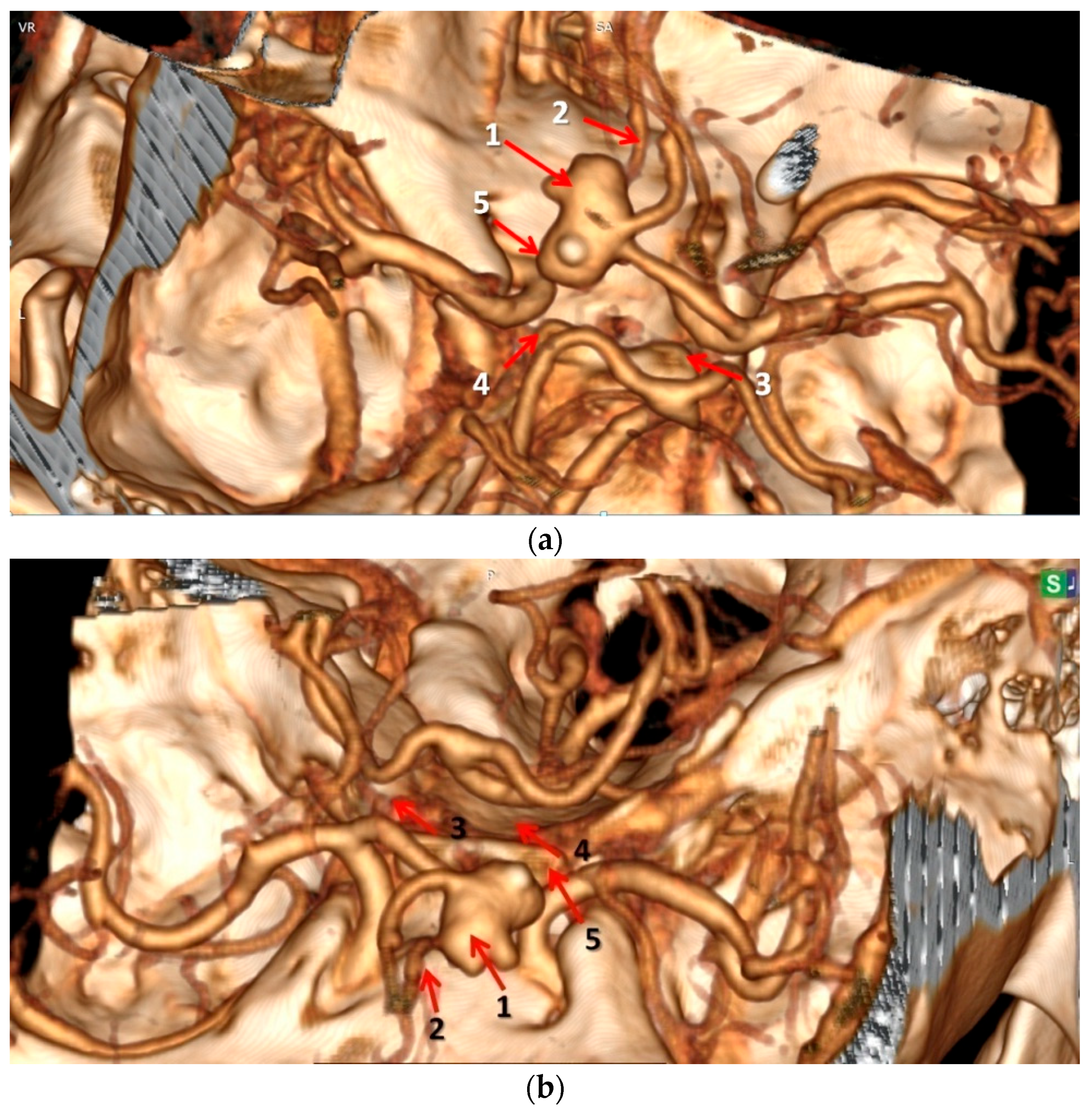
3.3. Agreement Between Autopsy Data and CT–Angiography Regarding the Presence of AcoA Aneurysm and Anatomical Variants of the Constituting Arteries of Circle of Willis at Initial Analysis and Postmortem Re-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACA | Anterior Cerebral Artery(ies) |
| AcoA | Anterior Communicating Artery |
| CW | Circle of Willis |
| CTA | Computed Tomography–Angiography |
| ICA | Internal Carotid Artery |
| PCA | Posterior Cerebral Artery(ies) |
| PcoA | Posterior Communicating Artery(ies) |
| SD | Standard Deviation (SD) |
References
- Chen, J.; Li, M.; Zhu, X.; Chen, Y.; Zhang, C.; Shi, W.; Chen, Q.; Wang, Y. Anterior communicating artery aneurysms: Anatomical considerations and microsurgical strategies. Front. Neurol. 2020, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, L.; Nesvick, C.L.; Rabinstein, A.A.; Lanzino, G. Differences in size between unruptured and ruptured saccular intracranial aneurysms by location. World Neurosurg. 2020, 133, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, K.; Przepiorka, L.; Kujawski, S.; Marchel, A.; Kunert, P. Unruptured anterior communicating artery aneurysms: Management strategy and results of a single-center experience. J. Clin. Med. 2023, 12, 4619. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.M.; Cobzaru, R.G.; Dima-Cozma, L.C.; Costea, C.F.; Chiran, D.A.; Rîpă, C.V.; Cucu, A.I.; Slănină, A.M.; Rădulescu, A.M.; Dobre, C.E.; et al. Circle of Willis Variants and Intracranial Aneurysm Risk: Evidence from an Autopsy-Based Study. BRAIN Broad Res. Artif. Intell. Neurosci. 2025, 16, 66–83. [Google Scholar] [CrossRef]
- Wollschlaeger, G.; Wollschlaeger, T.P.B.; Lucas, F.V.; Lopez, V.F. Experience and result with postmortem cerebral angiography performed as routine procedure of the autopsy. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1967, 101, 68–87. Available online: https://www.ajronline.org/doi/epdf/10.2214/ajr.101.1.68 (accessed on 25th April 2025). [CrossRef]
- Papantchev, V.; Stoinova, V.; Aleksandrov, A.; Todorova-Papantcheva, D.; Hristov, S.; Petkov, D.; Nachev, G.; Ovtscharoff, W. The role of Willis circle variations during unilateral selective cerebral perfusion: A study of 500 circles. Eur. J. Cardiothorac. Surg. 2013, 44, 743–753. [Google Scholar] [CrossRef]
- Jeong, S.H.; Hong, H.S.; Park, S.I.; Kim, D.H.; Lee, H.K. Congenital Absence of the Internal Carotid Artery. J. Soonchunhyang Med. Sci. 2010, 16, 9–15. Available online: https://jsms.sch.ac.kr/upload/pdf/16(1)%20p.9~16.pdf (accessed on 10th May 2025).
- Iqbal, S. A comprehensive study of the anatomical variations of the circle of Willis in adult human brains. J. Clin. Diagn. Res. 2013, 7, 2423–2427. [Google Scholar] [CrossRef]
- Nagawa, E.; Mwaka, E.; Kalungi, S. Bilateral hypoplasia of the posterior communicating artery: A morphological case report. Anat. Physiol. 2017, 7, 276. [Google Scholar] [CrossRef]
- Davidoiu, A.M.; Mincă, D.I.; Rusu, M.C.; Hostiuc, S.; Toader, C. The Fetal Type of Posterior Cerebral Artery. Medicina 2023, 59, 231. [Google Scholar] [CrossRef]
- Shaban, A.; Albright, K.C.; Boehme, A.K.; Martin-Schild, S. Circle of Willis Variants: Fetal PCA. Stroke Res. Treat. 2013, 2013, 105937. [Google Scholar] [CrossRef]
- Șahin, H.; Pekçevik, Y. Anatomical Variations of the Circle of Willis: Evaluation with CT Angiography. Anatomy 2018, 12, 20–26. Available online: https://dergipark.org.tr/tr/download/article-file/482887 (accessed on 10th May 2025). [CrossRef]
- Hassanin, B.G.; Abdelhameid, A.K.; Abbas, M.A.; Mohamed, R.B.; Schebesch, K.-M. Predictive value of the anterior communicating artery (ACoA) complex variations for the incidence and rupture of ACoA aneurysms. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 158. [Google Scholar] [CrossRef]
- Jiménez-Sosa, M.S.; Cantu-Gonzalez, J.R.; Morales-Avalos, R.; de la Garza-Castro, O.; Quiroga-Garza, A.; Pinales-Razo, R.; Elizondo-Riojas, G.; Elizondo-Omaña, R.E.; Guzmán-López, S. Anatomical variants of anterior cerebral arterial circle. A study by multidetector computerized 3D tomographic angiography. Int. J. Morphol. 2017, 35, 1121–1128. [Google Scholar] [CrossRef]
- He, J.; Liu, H.; Huang, B.; Chi, C. Investigation of morphology and anatomic variations of circle of Willis and measurement of diameter of cerebral arteries by 3D-TOF angiography. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2007, 24, 39–44. [Google Scholar] [PubMed]
- Shadad, M.; Balaha, A.; Ganna, A. Anterior communicating artery complex anatomy and its correlation with aneurysm formation. Med. J. Cairo Univ. 2021, 89, 2397–2402. [Google Scholar] [CrossRef]
- Rilianto, B.; Prasetyo, B.T.; Kurniawan, R.G.; Gotama, K.T.; Windiani, P.R.; Arham, A.; Kusdiansah, M. Clinical and morphological factors for ruptured anterior communicating artery aneurysms. Vasc. Health Risk Manag. 2023, 19, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.M.; Eva, L.; Costea, C.F.; Stan, C.I.; Mihai, B.M.; Stârcea, I.M.; Gavrilescu, C.M.; Sava, A. The significance of lifestyle, associated diseases, and anatomical variants of the circle of Willis in the formation of Anterior Communicating Artery aneurysms. Rev. Med. Chir. Soc. Med. Nat. Iaşi 2023, 127, 243–253. [Google Scholar] [CrossRef]
- Popa, E.; Traian, M.G.; Bacusca, A.I.; Slanina, A.M.; Boanca, M.; Macovei, I.; Popa, A.; Coman, A.E. Évaluation de la tension artérielle et de la pression du pouls dans le syndrome métabolique. Arch. Balk. Med. Union. 2018, 53, 393–400. [Google Scholar] [CrossRef]
- Popa, E.; Zugun-Eloae, F.; Zlei, M.; Jitaru, D.; Pintilie, O.M.; Coman, A.E.; Traian, M.; Ungureanu, D.A.; Carasevici, E. Flow Cytometry Analysis of PPARα Receptors in Metabolic Syndrome. Rev. Rom. Med. Lab. 2014, 22, 427–438. Available online: https://sciendo.com/article/10.2478/rrlm-2014-0036 (accessed on 15 May 2025).
- Nedelcu, A.H.; Hutanu, A.; Nedelcu, I.; Partene Vicoleanu, S.; Statescu, G.; Gavril, L.; Haliciu, A.M.; Ursaru, M.; Tarniceriu, C.C. The Prevalence and Morphology-Wise Demographic Distribution of Ponticulus Posticus on CT Scans—A Retrospective Observational Study. Medicina 2023, 59, 650. [Google Scholar] [CrossRef]
- Hurjui, L.L.; Tarniceriu, C.C.; Moraru, M.C.; Partene Vicoleanu, S.A.; Haliciu, A.M.; Statescu, G.; Maxim, R.R.; Nedelcu, A.H. Middle turbinate pneumatization—“concha bullosa”. A narrative literature review and comprehensive developmental concepts. Rom. J. Oral Rehabil. 2024, 16, 173–185. [Google Scholar] [CrossRef]
- Nedelcu, A.H.; Hurjui, L.L.; Stan, C.I.; Cumpat, C.M.; Ioniuc, I.; Tepordei, R.T.; Tarniceriu, C.C.; Moraru, M.C. Anatomo-morphological study and new original classification of concha bullosa. Rom. J. Oral Rehabil. 2024, 16, 46–61. [Google Scholar] [CrossRef]
- Nedelcu, A.H.; Lupu, V.V.; Lupu, A.; Tepordei, R.T.; Ioniuc, I.; Stan, C.I.; Partene Vicoleanu, S.A.; Haliciu, A.M.; Statescu, G.; Ursaru, M.; et al. Triangular fossa of the third cerebral ventricle—An original 3D model and morphometric study. Front. Neuroanat. 2024, 18, 1398858. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, A.M.; Eva, L.; Haba, D.; Cucu, A.I.; Dumitrescu, G.F.; Burduloi, V.M.; Dima-Cozma, L.C.; Vatavu, R.; Moroşanu, G.C.; Sava, A. Anatomical study of circle of Willis on fresh autopsied brains. A study of a Romanian population. Rom. J. Morphol. Embryol. 2022, 63, 395–406. [Google Scholar] [CrossRef]
- Bennett, P.; de Aguiar, G.B.; da Silva, R.C. The relationship between smoking and brain aneurysms: From formation to rupture. Rev. Assoc. Med. Bras. 2021, 67, 895–899. [Google Scholar] [CrossRef]
- Lin, H.; Yin, Y.; Li, J.; Liu, S.; Long, X.; Liao, Z. Exploring the causal links between cigarette smoking, alcohol consumption, and aneurysmal subarachnoid hemorrhage: A two-sample Mendelian randomization analysis. Front. Nutr. 2024, 11, 1397776. [Google Scholar] [CrossRef]
- Feng, X.; Qian, Z.; Zhang, B.; Guo, E.; Wang, L.; Liu, P.; Wen, X.; Xu, W.; Jiang, C.; Li, Y.; et al. Number of Cigarettes Smoked Per Day, Smoking Index, and Intracranial Aneurysm Rupture: A Case–Control Study. Front. Neurol. 2018, 9, 380. [Google Scholar] [CrossRef]
- Steklacova, A.; Bradac, O.; de Lacy, P.; Lacman, J.; Charvat, F.; Benes, V. “Coil mainly” policy in management of intracranial ACoA aneurysms: Single-centre experience with the systematic review of literature and meta-analysis. Neurosurg. Rev. 2018, 41, 825–839. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fukuda, H.; Yamada, D.; Kurosaki, Y.; Handa, A.; Lo, B.; Lo, B.; Yamagata, S. Association of perforator infarction with clinical courses and outcomes following surgical clipping of ruptured anterior communicating artery aneurysms. World Neurosurg. 2017, 107, 724–731. [Google Scholar] [CrossRef]
- International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms-risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Bijlenga, P.; Ebeling, C.; Jaegersberg, M.; Summers, P.; Rogers, A.; Waterworth, A.; Iavindrasana, J.; Macho, J.; Mendes Pereira, V.; Bukovics, P.; et al. Risk of rupture of small anterior communicating artery aneurysms is similar to posterior circulation aneurysms. Stroke 2013, 44, 3018–3026. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, N.K.; Velthuis, B.K.; Algra, A.; Rinkel, G.J.E. Configuration of the circle of Willis, direction of flow, and shape of the aneurysm as risk factors for rupture of intracranial aneurysms. J. Neurol. 2009, 256, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, M.A.; Ouyang, B.; Chen, M. The role of circle of Willis anomalies in cerebral aneurysm rupture. J. Neurointerv. Surg. 2012, 4, 22–26. [Google Scholar] [CrossRef]
- Feng, L.; Mao, H.-J.; Zhang, D.-D.; Zhu, Y.-C.; Han, F. Anatomical variations in the circle of Willis and the formation and rupture of intracranial aneurysms: A systematic review and meta-analysis. Front. Neurol. 2023, 13, 1098950. [Google Scholar] [CrossRef]
- Arrambide-Garza, F.J.; Alvarez-Lozada, L.A.; de León-Gutiérrez, H.; Villarreal-Silva, E.E.; Alvarez-Villalobos, N.A.; Quiroga-Garza, A.; Elizondo-Omaña, R.E.; Guzman-Lopez, S. Fetal-type posterior cerebral artery and association of rupture in posterior communicating artery aneurysms: A systematic review and meta-analysis. Clin. Neurol. Neurosurg. 2023, 231, 107815. [Google Scholar] [CrossRef]
- Tarulli, E.; Sneade, M.; Clarke, A.; Molyneux, A.J.; Fox, A.J. Effects of circle of Willis anatomic variations on angiographic and clinical outcomes of coiled anterior communicating artery aneurysms. AJNR Am. J. Neuroradiol. 2014, 35, 1551–1555. [Google Scholar] [CrossRef]
- Oberman, D.Z.; Akly, M.S.P.; Rabelo, N.N.; Elizondo, C.; Correa, J.L.A.; Ajler, P.; Baccanelli, M.M. Morphologic variations in the circle of Willis as a risk factor for aneurysm rupture in the anterior and posterior communicating arteries. World Neurosurg. 2021, 154, e155–e162. [Google Scholar] [CrossRef]
- Yang, F.; Li, H.; Wu, J.; Li, M.; Chen, X.; Jiang, P.; Li, Z.; Cao, Y.; Wang, S. Relationship of A1 segment hypoplasia with the radiologic and clinical outcomes of surgical clipping of anterior communicating artery aneurysms. World Neurosurg. 2017, 106, 806–812. [Google Scholar] [CrossRef]
- Rinaldo, L.; McCutcheon, B.A.; Murphy, M.E.; Bydon, M.; Rabinstein, A.A.; Lanzino, G. Relationship of A1 segment hypoplasia to anterior communicating artery aneurysm morphology and risk factors for aneurysm formation. J. Neurosurg. 2017, 127, 89–95. [Google Scholar] [CrossRef]
- Uyanik, S.A.; Uyanik, H.U.; Oguslu, U.; Dede, D. Intracranial arterial variations and their relation with cerebral aneurysms: Analysis of 640 patients. Ann. Med. Res. 2020, 27, 3093–3098. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, G.; Liu, W.; Gu, W.; Zhu, Y.; Meng, L.; Wei, L.; Li, M.; Lu, H.; Teng, G. Analysis of risk factors for anterior communicating artery aneurysm rupture: A single-center study. World Neurosurg. 2021, 153, e59–e65. [Google Scholar] [CrossRef]
- Negatie, H.M.; Kebede, M.A.; Abate, A.D.; Admassie, S.H.; Worku, A.B.; Ahmed, H.T.; Mesfine, Y.Y.; Melak, M.M. Association of circle of Willis variants with stroke and aneurysm: Insights from a tertiary hospital in Ethiopia. BMC Neurol. 2025, 25, 73. [Google Scholar] [CrossRef]
- Kaya, V.; Tahtabasi, M.; Yıldırım, I.O. Risk factors for the rupture of anterior communicating artery aneurysms: Coexistence of fetal-type posterior cerebral artery and A1 segment hypoplasia/agenesis. J. Clin. Neurosci. 2023, 110, 74–79. [Google Scholar] [CrossRef]
- Dumitrescu, A.M.; Sava, A.; Costea, C.F.; Dobrin, N.; Furnică, C.; Cucu, A.I.; Şufaru, R.F.; Turliuc, Ş. Aneurysm of the Anterior Communicating Artery Associated with Multiple Anatomical Variants of the Circle of Willis. A Case Report and Literature Review. Rom. J. Funct. Clin. Macro-Microsc. Anat. Anthropol. 2020, 19, 192–202. Available online: https://revanatomie.ro/pdf/2020_4_3.pdf (accessed on 20th May 2025).


| Descriptor | Ruptured AcoA Aneurysms (n = 14) | Unruptured AcoA Aneurysms (n = 2) | Total | p-Value |
|---|---|---|---|---|
| Gender, n (%) | 0.500 † | |||
| Male | 8 (57.1%) | 2 (100%) | 10 (62.5%) | |
| Female | 6 (42.9%) | 0 (0.0%) | 6 (37.5%) | |
| Age (mean ± SD), years | 63.29 ± 12.89 | 59.50 ± 13.43 | 62.81 ± 12.56 | 0.704 ‡ |
| Male | 58.50 ± 12.62 | 59.50 ± 13.43 | 58.70 ± 12.01 | |
| Female | 69.67 ± 11.11 | - | 69.67 ± 11.11 | |
| Age (years), n (%) | 1.000 † | |||
| ≥60 | 10 (71.4%) | 1 (50.0%) | 11 (68.8%) | |
| <60 | 4 (28.6%) | 1 (50.0%) | 5 (31.3%) | |
| Total | 14 (100%) | 2 (100%) | 16 (100%) |
| Descriptor | Ruptured AcoA Aneurysms (n = 14) | Unruptured AcoA Aneurysms (n = 2) | Total | p-Value |
|---|---|---|---|---|
| External diameters of AcoA aneurysms (mm) | 9.50 | 4.00 | 8.81 | <0.001 ‡ |
| No. of associated anatomical variants | 0.767 † | |||
| 1 | 2 (14.3%) | - | 2 (12.5%) | |
| 2 | 2 (14.3%) | - | 2 (12.5%) | |
| 3 | 7 (50.0%) | 1 (50.0%) | 8 (50.0%) | |
| 4 | 3 (21.4%) | 1 (50.0%) | 4 (25.0%) |
| Descriptor | Ruptured AcoA Aneurysms (n = 14) | Unruptured AcoA Aneurysms (n = 2) | Total | p-Value |
|---|---|---|---|---|
| Size of aneurysm | <0.001 † | |||
| <5 mm | - | 2 (100.0%) | 2 (12.5%) | |
| 5–9.9 mm | 8 (57.1%) | - | 8 (50.0%) | |
| ≥10 mm | 6 (42.9%) | - | 6 (37.5%) | |
| Type of anatomical variant | ||||
| 9 (64.3%) | 1 (50.0%) | 10 (62.5%) | 1.000 |
| - | - | - | - |
| 5 (35.7%) | 1 (50.0%) | 6 (37.5%) | 1.000 |
| Descriptor | AcoA Aneurysm External Diameter (Mean ± SD) | p-Value |
|---|---|---|
| No. of associated anatomical variants | 0.409 † | |
| 1 | 6.50 ± 2.12 | |
| 2 | 7.50 ± 0.70 | |
| 3 | 8.63 ± 2.77 | |
| 4 | 11.00 ± 4.83 |
| Type of Agreement | Antemortem Analysis (No. of Cases = 16) | Postmortem Analysis (No. of Cases = 16) | p-Value † |
|---|---|---|---|
| Complete agreement | - | 16 (100.0%) | <0.001 † |
| Incomplete agreement | 3 (18.8%) | - | |
| No agreement | 13 (81.3%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, A.M.; Chiran, D.A.; Stan, C.I.; Paraschiv, C.M.; Dobrin, N.; Chiriac, A.; Leon, M.M.; Dima-Cozma, L.C.; Dascalu, C.G.; Radulescu, A.M.; et al. Comparing Antemortem CT–Angiography Data with Autopsy Findings in Regard to Anterior Communicating Artery Aneurysms. NeuroSci 2025, 6, 81. https://doi.org/10.3390/neurosci6030081
Dumitrescu AM, Chiran DA, Stan CI, Paraschiv CM, Dobrin N, Chiriac A, Leon MM, Dima-Cozma LC, Dascalu CG, Radulescu AM, et al. Comparing Antemortem CT–Angiography Data with Autopsy Findings in Regard to Anterior Communicating Artery Aneurysms. NeuroSci. 2025; 6(3):81. https://doi.org/10.3390/neurosci6030081
Chicago/Turabian StyleDumitrescu, Ana Maria, Dragos Andrei Chiran, Cristinel Ionel Stan, Cringuta Mariana Paraschiv, Nicolaie Dobrin, Alexandru Chiriac, Maria Magdalena Leon, Lucia Corina Dima-Cozma, Cristina Gena Dascalu, Ana Marina Radulescu, and et al. 2025. "Comparing Antemortem CT–Angiography Data with Autopsy Findings in Regard to Anterior Communicating Artery Aneurysms" NeuroSci 6, no. 3: 81. https://doi.org/10.3390/neurosci6030081
APA StyleDumitrescu, A. M., Chiran, D. A., Stan, C. I., Paraschiv, C. M., Dobrin, N., Chiriac, A., Leon, M. M., Dima-Cozma, L. C., Dascalu, C. G., Radulescu, A. M., Gavril, R. F., & Sava, A. (2025). Comparing Antemortem CT–Angiography Data with Autopsy Findings in Regard to Anterior Communicating Artery Aneurysms. NeuroSci, 6(3), 81. https://doi.org/10.3390/neurosci6030081






