Romantic Partners with Mismatched Relationship Satisfaction Showed Greater Interpersonal Neural Synchrony When Co-Viewing Emotive Videos: An Exploratory Pilot fNIRS Hyperscanning Study
Abstract
1. Introduction
1.1. Interpersonal Neural Synchrony as a Measure of Emotional Attunement
1.2. Association Between Emotional Attunement and Relationship Satisfaction
1.3. Emotion Attunement and Emotional Valence
1.4. Aim and Hypothesis of Present Study
- (a)
- There would be differences in the interpersonal neural synchrony of the brain activity of the two partners in a romantic relationship during the shared co-viewing activity across the positive emotion condition, the negative emotion condition and the baseline condition.
- (b)
- The smaller the difference in the romantic partners’ relationship satisfaction levels (regardless of the extent of relationship satisfaction levels themselves), the greater the interpersonal neural synchrony in the brain activity of the two partners in a romantic relationship during the shared co-viewing activity.
2. Materials and Methods
2.1. Participants
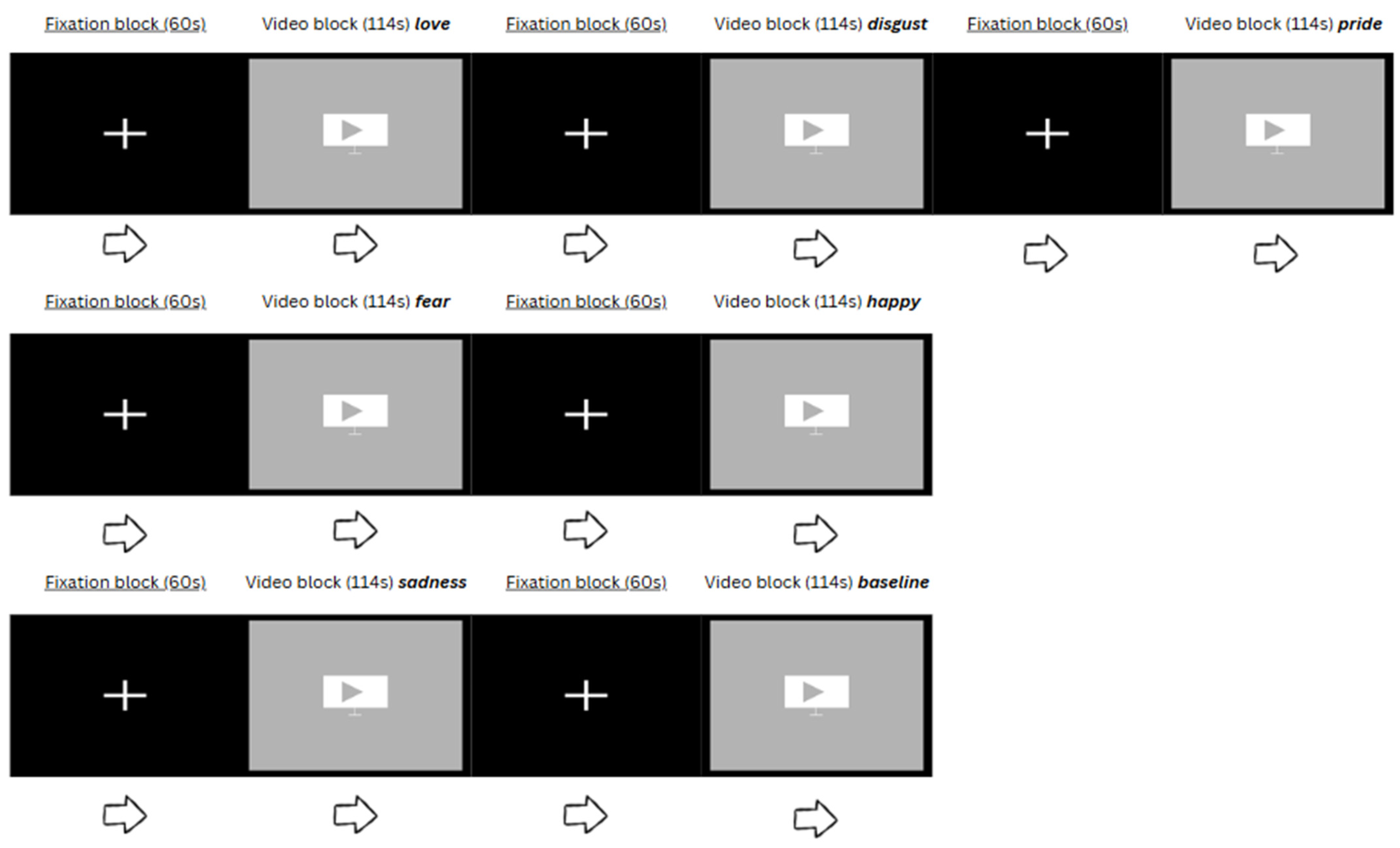
2.2. Study Design
2.3. Video Stimuli
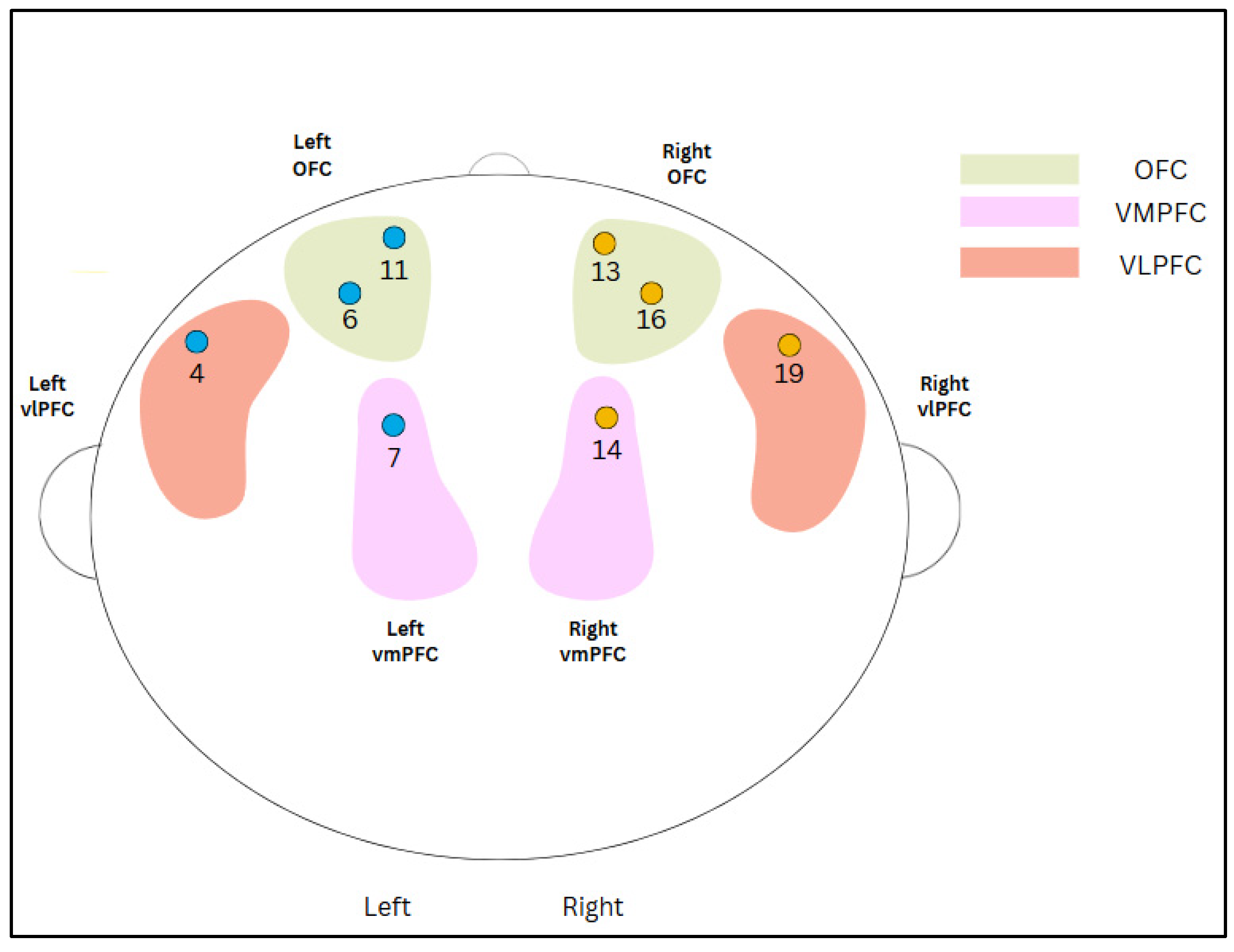
2.4. Functional Near-Infrared Spectroscopy (fNIRS) Data Preprocessing and Analysis
2.5. Analytical Plan
3. Results
3.1. Difference in Interpersonal Neural Synchrony Across Conditions
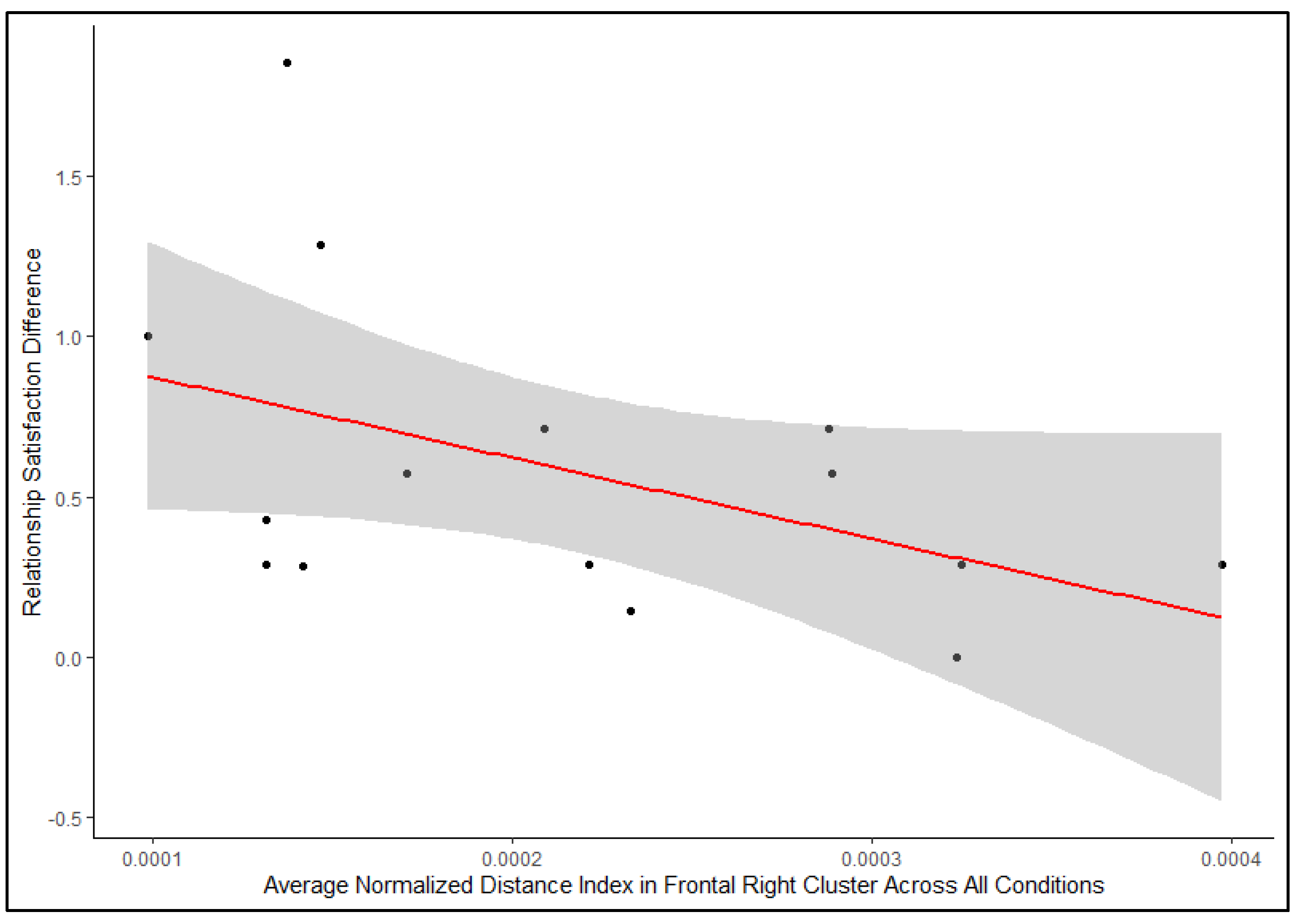
3.2. Relationship Between Relationship Satisfaction and Interpersonal Neural Synchrony
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butler, E.A.; Randall, A.K. Emotional coregulation in close relationships. Emot. Rev. 2013, 5, 202–210. [Google Scholar] [CrossRef]
- Gottman, J.M. The Science of Trust: Emotional Attunement for Couples; W. W. Norton & Co.: New York, NY, USA, 2011. [Google Scholar]
- Butner, J.; Diamond, L.M.; Hicks, A.M. Attachment style and two forms of affect coregulation between romantic partners. Pers. Relatsh. 2007, 14, 431–455. [Google Scholar] [CrossRef]
- Fu, W.; Wang, C.; Chai, H.; Xue, R. Correction: Examining the relationship of empathy, social support, and prosocial behavior of adolescents in China: A structural equation modeling approach. Humanit. Soc. Sci. Commun. 2020, 9, 269. [Google Scholar] [CrossRef]
- Lakin, J.L.; Chartrand, T.L. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol. Sci. 2003, 14, 334–339. [Google Scholar] [CrossRef]
- Chartrand, T.L.; Van Baaren, R. Chapter 5 Human mimicry. Adv. Exp. Soc. Psychol. 2009, 41, 219–274. [Google Scholar]
- Azhari, A.; Bizzego, A.; Esposito, G. Father-child dyads exhibit unique inter-subject synchronisation during co-viewing of animation video stimuli. Soc. Neurosci. 2021, 16, 522–533. [Google Scholar] [CrossRef]
- Azhari, A.; Bizzego, A.; Balagtas, J.P.M.; Leng, K.S.H.; Esposito, G. Asymmetric prefrontal cortex activation associated with mutual gaze of mothers and children during shared play. Symmetry 2022, 14, 998. [Google Scholar] [CrossRef]
- Azhari, A.; Lim, M.Y.; Bizzego, A.; Gabrieli, G.; Bornstein, M.H.; Esposito, G. Physical presence of spouse enhances brain-to-brain synchrony in co-parenting couples. Sci. Rep. 2020, 10, 7569. [Google Scholar] [CrossRef]
- Lu, K.; Hao, N. When do we fall in neural synchrony with others? Soc. Cogn. Affect. Neurosci. 2019, 14, 253–261. [Google Scholar] [CrossRef]
- Prochazkova, E.; Kret, M.E. Connecting minds and sharing emotions through mimicry: A neurocognitive model of emotional contagion. Neurosci. Biobehav. Rev. 2017, 80, 99–114. [Google Scholar] [CrossRef]
- Muller, M. Dynamic Time Warping. In Information Retrieval for Music and Motion; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Saxbe, D.; Repetti, R.L. For better or worse? Coregulation of couples’ cortisol levels and mood states. J. Personal. Soc. Psychol. 2010, 98, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Schoebi, D. The coregulation of daily affect in marital relationships. J. Fam. Psychol. 2008, 22, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, E.; Cacioppo, J.T.; Rapson, R.L. Susceptibility to emotional contagion. Emot. Contag. 1994, 14, 147–182. [Google Scholar] [CrossRef]
- Azhari, A.; Gabrieli, G.; Bizzego, A.; Bornstein, M.H.; Esposito, G. Probing the association between maternal anxious attachment style and mother-child brain-to-brain coupling during passive co-viewing of visual stimuli. Attach. Hum. Dev. 2020, 25, 19–34. [Google Scholar] [CrossRef]
- Li, L.; Huang, X.; Xiao, J.; Zheng, Q.; Shan, X.; He, C.; Liao, W.; Chen, H.; Menon, V.; Duan, X. Neural synchronization predicts marital satisfaction. Proc. Natl. Acad. Sci. USA 2022, 119, e2202515119. [Google Scholar] [CrossRef]
- Suziki, Y.; Tanah, S.C. Functions of the ventromedial prefrontal cortex in emotion regulation under stress. Nature 2021, 11, 18225. [Google Scholar] [CrossRef]
- Li, S.; Xie, H.; Zheng, Z.; Chen, W.; Xu, F.; Hu, X.; Zhang, D. The causal role of the bilateral ventrolateral prefrontal cortices on emotion regulation of social feedback. Hum. Brain Mapp. 2022, 43, 2898–2910. [Google Scholar] [CrossRef]
- Yu, W.; Li, Y.; Cao, X.; Mo, L.; Chen, Y.; Zhang, D. The role of ventrolateral prefrontal cortex on voluntary emotion regulation of social pain. Hum. Brain Mapp. 2023, 43, 4710–4721. [Google Scholar] [CrossRef]
- Golkar, A.; Lonsdorf, T.B.; Olsson, A.; Lindstrom, K.M.; Berrebi, J.; Fransson, P.; Schalling, M.; Ingvar, M.; Ohman, A. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS ONE 2012, 7, e48107. [Google Scholar] [CrossRef]
- Watson, D.; Klohnen, E.C.; Casillas, A.; Simms, E.N.; Haig, J.; Berry, D.S. Match makers and deal breakers: Analyses of assortative mating in newlywed couples. J. Personal. 2004, 72, 1029–1068. [Google Scholar] [CrossRef]
- Hendrick, S.S. A generic measure of relationship satisfaction. J. Marriage Fam. 1988, 50, 93–98. [Google Scholar] [CrossRef]
- Funk, J.L.; Rogge, R.D. Testing the ruler with item response theory: Increasing precision of measurement for relationship satisfaction with the Couples Satisfaction Index. J. Fam. Psychol. 2007, 21, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Rusbult, C.E. Commitment and satisfaction in romantic associations: A test of the investment model. J. Exp. Soc. Psychol. 1980, 16, 172–186. [Google Scholar] [CrossRef]
- Rosand, G.B.; Slinning, K.; Eberhard-Gran, M.; Roysamb, E.; Kristian, T. The buffering effect of relationship satisfaction on emotional distress in couples. BMC Public Health 2012, 12, 66. [Google Scholar] [CrossRef]
- Kinkead, A.; Sanduvete-Chaves, S.; Chacon-Moscoso, S.; Salas, C.E. Couples extrinsic emotion regulation questionnaire: Psychometric validation in a Chilean population. PLoS ONE 2021, 16, e0252329. [Google Scholar] [CrossRef]
- Gvirts, H.Z.; Perlmutter, R. What Guides Us to Neurally and Behaviorally Align with Anyone Specific? A Neurobiological Model Based on fNIRS Hyperscanning Studies. Neuroscientist 2019, 26, 108–116. [Google Scholar] [CrossRef]
- Arimoto, Y.; Okanoya, K. Emotional synchrony and covariation of behavioral/physiological reactions between interlocutors. In In Proceedings of the 17th Oriental Chapter of the International Committee for the Co-ordination and Standardization of Speech Databases and Assessment Techniques (COCOSDA), Phuket, Thailand, 10–12 September; 2014. [Google Scholar] [CrossRef]
- Coutinho, J.; Pereira, A.; Oliveira-Silva, P.; Meier, D.; Lourenço, V.; Tschacher, W. When our hearts beat together: Cardiac synchrony as an entry point to understand dyadic co-regulation in couples. Psychophysiology 2020, 58, e13739. [Google Scholar] [CrossRef]
- Coutinho, J.; Oliveira-Silva, P.; Fernandes, E.; Gonçalves, O.F.; Correia, D.; Perrone Mc-Govern, K.; Tschacher, W. Psychophysiological synchrony during verbal interaction in romantic relationships. Fam. Process 2018, 58, 716–733. [Google Scholar] [CrossRef]
- Lin, D.; Zhu, T.; Wang, Y. Emotion contagion and physiological synchrony: The more intimate relationships, the more contagion of positive emotions. Physiol. Behav. 2024, 275, 114434. [Google Scholar] [CrossRef]
- Scarapicchia, V.; Brown, C.; Mayo, C.; Gawryluk, J.R. Functional magnetic resonance imaging and functional near-infrared spectroscopy: Insights from combined recording studies. Front. Hum. Neurosci. 2017, 11, 419. [Google Scholar] [CrossRef]
- Balconi, M.; Molteni, E. Past and future of near-infrared spectroscopy in studies of emotion and social neuroscience. J. Cogn. Psychol. 2015, 28, 129–146. [Google Scholar] [CrossRef]
- Long, Y.; Chen, C.; Wu, K.; Zhou, S.; Zhou, F.; Zheng, L.; Zhao, H.; Zhai, Y.; Lu, C. Interpersonal conflict increases interpersonal neural synchronization in romantic couples. Cereb. Cortex 2021, 32, 3254–3268. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cheng, X.; Zhang, Z.; Li, X.; Hu, Y. Cooperation in lovers: An fNIRS-based hyperscanning study. Hum. Brain Mapp. 2016, 38, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Zhang, X.; Wu, Y.; Zhang, W.; Sun, B. Increased interpersonal brain synchronization in romantic couples is associated with higher honesty: An fNIRS hyperscanning study. Brain Sci. 2023, 13, 833. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, X.; Wang, C.; Cao, M.; Deng, S.; Du, X.; Dai, Y.; Geng, S.; Fan, Y.; Cui, L.; et al. Different strategies, distinguished cooperation efficiency, and brain synchronization for couples: An fNIRS-based hyperscanning study. Brain Behav. 2020, 10, e01768. [Google Scholar] [CrossRef]
- Cacioppo, S.; Zhou, H.; Monteleone, G.; Majka, E.A.; Quinn, K.A.; Ball, A.B.; Norman, G.J.; Semin, G.R.; Cacioppo, J.T. You are in sync with me: Neural correlates of interpersonal synchrony with a partner. Neuroscience 2014, 277, 842–858. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Zhang, R.; Li, X. Experiencing happiness together facilitates dyadic coordination through the enhanced interpersonal neural synchronization. Soc. Cogn. Affect. Neurosci. 2021, 17, 447–460. [Google Scholar] [CrossRef]
- Vaughn, M.J.; Baier, M.E.M. Reliability and validity of the relationship assessment scale. Am. J. Fam. Ther. 1999, 27, 137–147. [Google Scholar] [CrossRef]
- Hendrick, S.S.; Dicke, A.; Hendrick, C. The Relationship Assessment Scale. J. Soc. Pers. Relatsh. 1998, 15, 137–142. [Google Scholar] [CrossRef]
- Azhari, A.; Leck, W.Q.; Gabrieli, G.; Bizzego, A.; Rigo, P.; Setoh, P.; Bornstein, M.H.; Esposito, G. Parenting stress undermines mother-child brain-to-brain synchrony: A hyperscanning study. Sci. Rep. 2019, 9, 11407. [Google Scholar] [CrossRef]
- Li, X.; Xiong, G.; Dong, Z.; Cai, S.; Zhao, J.; She, Z.; Guo, Y. Causal role of the right dorsolateral prefrontal cortex in organizational fairness perception: Evidence from a transcranial direct current stimulation study. Front. Behav. Neurosci. 2020, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Terenzi, D.; Liu, L.; Bellucci, G.; Park, S.Q. Determinants and modulators of human social decisions. Neurosci. Biobehav. Rev. 2021, 128, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Reinero, D.A.; Dikker, S.; Van Bavel, J.J. Inter-brain synchrony in teams predicts collective performance. Soc. Cogn. Affect. Neurosci. 2020, 16, 43–57. [Google Scholar] [CrossRef]
- Azhari, A.; Bizzego, A.; Esposito, G. Parent–child dyads with greater parenting stress exhibit less synchrony in posterior areas and more synchrony in frontal areas of the prefrontal cortex during shared play. Soc. Neurosci. 2022, 17, 520–531. [Google Scholar] [CrossRef]
- Kinreich, S.; Djalovski, A.; Kraus, L.; Yoram, L.; Feldman, R. Brain-to-Brain Synchrony during Naturalistic Social Interactions. Sci. Rep. 2017, 7, 17060. [Google Scholar] [CrossRef]
- Zhou, C.; Jiang, X.; Chen, Y.; Ge, C.; Ao, N.; Du, F. Brain-to-brain synchrony increased during interpersonal touch in romantic lovers: An EEG-based hyperscanning study. BMC Psychol. 2024, 12, 560. [Google Scholar] [CrossRef]
| 7-Item Relationship Satisfaction Questionnaire, Using 7-Point Likert Scale |
|---|
|
|
|
|
|
|
|
| Sequence | Order of Video Clips |
|---|---|
| A | Love, Disgust, Pride, Fear, Happy, Sadness, Baseline |
| B | Sadness, Love, Baseline, Pride, Disgust, Fear, Happy |
| C | Baseline, Sadness, Disgust, Happy, Fear, Pride, Love |
| Emotion | Video Description |
|---|---|
| Happiness | A self-filmed video of a Korean child giving innocent answers to her parents’ questions on safety issues—it was intended to showcase the innocence of a young child |
| Love | A chewing-gum advertisement depicting how a couple’s romantic relationship evolved and strengthened through a period |
| Pride | A video of the 100 m butterfly men’s swimming event at the 2016 Summer Olympics, where a swimmer clinched Singapore’s first Gold medal–a significant event for Singaporean viewers |
| Sadness | A video which started with a girl happily celebrating her birthday, but progressed to war times in her hometown, where she gradually lost the things around her and lost her smile |
| Fear | A video of a woman fearing noises and flickering lights while alone at home in the dark, ending with an unexpected creature in her room at the end of the video |
| Disgust | A short documentary on the unexpected contents of McNuggets, including less-preferred parts of a chicken (e.g., cartilage and fats) and non-food (e.g., wire bits) |
| Neutral | A video of calm and repetitive ocean currents |
| (a) | ||||
|---|---|---|---|---|
| Variable | Estimate | Standard Error | t-Value | p-Value |
| (Intercept) | 0.0003 | 0.00003 | 8.49 | <0.001 |
| Romantic partners’ relationship satisfaction score difference | −0.00009 | 0.00004 | −2.31 | 0.028 * |
| Type of emotion (positive) | −0.00003 | 0.00004 | −0.81 | 0.42 |
| (b) | ||||
| Variable | Estimate | Standard error | t-value | p-value |
| (Intercept) | 0.0003 | 0.00003 | 9.19 | <0.001 |
| Romantic partners’ relationship satisfaction score difference | −0.00005 | 0.00003 | −1.63 | 0.11 |
| Type of emotion (positive) | −0.00006 | 0.00003 | −1.75 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heng, W.X.; Ng, L.Y.; Goh, Z.Z.; Esposito, G.; Azhari, A. Romantic Partners with Mismatched Relationship Satisfaction Showed Greater Interpersonal Neural Synchrony When Co-Viewing Emotive Videos: An Exploratory Pilot fNIRS Hyperscanning Study. NeuroSci 2025, 6, 55. https://doi.org/10.3390/neurosci6020055
Heng WX, Ng LY, Goh ZZ, Esposito G, Azhari A. Romantic Partners with Mismatched Relationship Satisfaction Showed Greater Interpersonal Neural Synchrony When Co-Viewing Emotive Videos: An Exploratory Pilot fNIRS Hyperscanning Study. NeuroSci. 2025; 6(2):55. https://doi.org/10.3390/neurosci6020055
Chicago/Turabian StyleHeng, Wen Xiu, Li Ying Ng, Zen Ziyi Goh, Gianluca Esposito, and Atiqah Azhari. 2025. "Romantic Partners with Mismatched Relationship Satisfaction Showed Greater Interpersonal Neural Synchrony When Co-Viewing Emotive Videos: An Exploratory Pilot fNIRS Hyperscanning Study" NeuroSci 6, no. 2: 55. https://doi.org/10.3390/neurosci6020055
APA StyleHeng, W. X., Ng, L. Y., Goh, Z. Z., Esposito, G., & Azhari, A. (2025). Romantic Partners with Mismatched Relationship Satisfaction Showed Greater Interpersonal Neural Synchrony When Co-Viewing Emotive Videos: An Exploratory Pilot fNIRS Hyperscanning Study. NeuroSci, 6(2), 55. https://doi.org/10.3390/neurosci6020055






