The Neuroanatomical Correlates of Dyspnea: An Activation Likelihood Estimation Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Eligibility Criteria
2.3. Study Election and Data Extraction
2.4. Statistical Analysis
3. Results
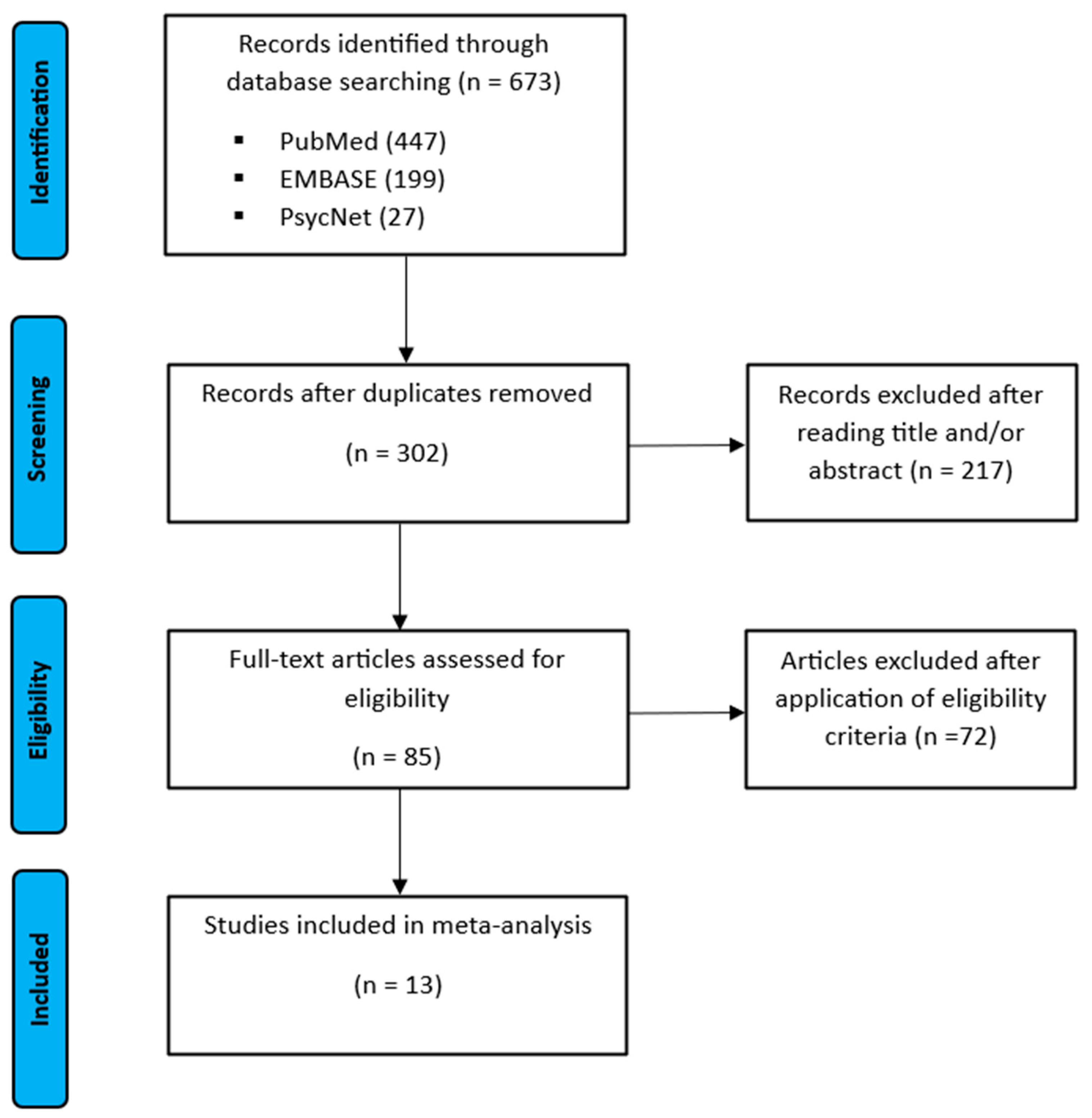
3.1. Study Selection
3.2. Within-Condition Analysis
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Anterior cingulate cortex |
| ALE | Activation likelihood estimation |
| BA | Brodmann area |
| fMRI | Functional magnetic resonance imaging |
| FWHM | Full width half maximum |
| MNI | Montreal Neurological Imaging |
| PCC | Posterior cingulate cortex |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| VMA | Visceromotor areas |
References
- Mahler, D.A.; O’Donnell, D.E. Neurobiology of dyspnea: An overview. In Dyspnea: Mechanisms, Measurement, and Management, 3rd ed.; CRC Press: London, UK, 2014; Volume 20, pp. 3–10. [Google Scholar] [CrossRef]
- Desbiens, N.A.; Mueller-Rizner, N.; Connors, A.F.; Wenger, N.S. The relationship of nausea and dyspnea to pain in seriously ill patients. Pain 1997, 71, 149–156. [Google Scholar] [CrossRef]
- Parshall, M.B.; Schwartzstein, R.M.; Adams, L.; Banzett, R.B.; Manning, H.L.; Bourbeau, J.; Calverley, P.M.; Gift, A.G.; Harver, A.; Lareau, S.C.; et al. An official American Thoracic Society statement: Update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012, 185, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, N.; Harrison, O.; Toohey, S.; Brændholt, M.; Legrand, N.; Correa, C.; Vejlø, M.; Jensen, M.S.; Fardo, F.; Allen, M. The respiratory resistance sensitivity task: An automated method for quantifying respiratory interoception and metacognition. Biol. Psychol. 2022, 170, 108325. [Google Scholar] [CrossRef] [PubMed]
- Lansing, R.W.; Gracely, R.H.; Banzett, R.B. The multiple dimensions of dyspnea: Review and hypotheses. Respir. Physiol. Neurobiol. 2008, 167, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef]
- Denton, D.A.; McKinley, M.J.; Farrell, M.; Egan, G.F. The role of primordial emotions in the evolutionary origin of consciousness. Conscious. Cogn. 2009, 18, 500–514. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2012; pp. 101–211. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012, 59, 2349–2361. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Prisma-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Banzett, R.B.; Mulnier, H.E.; Murphy, K.; Rosen, S.D.; Wise, R.J.; Adams, L. Breathlessness in humans activates insular cortex. Neuroreport 2000, 11, 2117–2120. [Google Scholar] [CrossRef] [PubMed]
- Liotti, M.; Brannan, S.; Egan, G.; Shade, R.; Madden, L.; Abplanalp, B.; Robillard, R.; Lancaster, J.; Zamarripa, F.E.; Fox, P.T.; et al. Brain responses associated with consciousness of breathlessness (air hunger). Proc. Natl. Acad. Sci. USA 2001, 98, 2035–2040. [Google Scholar] [CrossRef]
- Peiffer, C.; Poline, J.B.; Thivard, L.; Aubier, M.; Samson, Y. Neural substrates for the perception of acutely induced dyspnea. Am J. Respir. Crit. Care Med. 2001, 163, 951–957. [Google Scholar] [CrossRef]
- Evans, K.C.; Banzett, R.B.; Adams, L.; Mckay, L.; Frackowiak, R.S.; Corfield, D.R. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J. Neurophysiol. 2002, 88, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Macey, K.E.; Macey, P.M.; Woo, M.A.; Henderson, L.A.; Frysinger, R.C.; Harper, R.K.; Alger, J.R.; Yan-Go, F.; Harper, R.M. Inspiratory loading elicits aberrant fMRI signal changes in obstructive sleep apnea. Respir. Physiol. Neurobiol. 2006, 151, 44–60. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.C.; Adam, L.; Frackowiak, R.S.; Corfield, D.R. A bilateral cortico-bulbar network associated with breath holding in humans, determined by functional magnetic resonance imaging. Neuroimage 2008, 40, 1824–1832. [Google Scholar] [CrossRef]
- von Leupoldt, A.; Sommer, T.; Kegat, S.; Baumann, H.J.; Klose, H.; Dahme, B.; Büchel, C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. Am. J. Respir. Crit. Care Med. 2008, 177, 1026–1032. [Google Scholar] [CrossRef]
- Binks, A.P.; Evans, K.C.; Reed, J.D.; Moosavi, S.H.; Banzett, R.B. The time-course of cortico-limbic neural responses to air hunger. Respir. Physiol. Neurobiol. 2014, 204, 78–85. [Google Scholar] [CrossRef]
- Herigstad, M.; Hayen, A.; Evans, E.; Hardringe, F.M.; Davie, R.J.; Wiech, K.; Pattinson, K.T.S. Dyspnea-Related Cues Engage the Prefrontal Cortex Evidence From Functional Brain Imaging in COPD. Chest 2015, 148, 953–961. [Google Scholar] [CrossRef]
- Esser, R.W.; Stoeckel, M.C.; Kirsten, A.; Watz, H.; Taube, K.; Lehmann, K.; Magnussen, H.; Büchel, C.; von Leupoldt, A. Brain activation during perception and anticipation of dyspnea in chronic obstructive pulmonary disease. Front. Physiol. 2017, 8, 617. [Google Scholar] [CrossRef]
- Chan, P.Y.; Cheng, C.H.; Wu, Y.T.; Wu, C.W.; Liu, H.L.; Shaw, F.Z.; Liu, C.Y.; Davenport, P.W. Cortical and subcortical neural correlates for respiratory sensation in response to transient inspiratory occlusions in humans. Front. Physiol. 2018, 9, 1804. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.S.; Simmons, W.K.; Feinstein, J.S.; Luo, Q.; Lapidus, R.C.; Bodurka, J.; Paulus, M.P.; Khalsa, S.S. The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology 2018, 43, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.Y.; Wu, Y.T.; Hsu, A.L.; Li, C.W.; Wu, C.W.; von Leupoldt, A.; Hsu, S.C. The effect of anxiety on brain activation patterns in response to inspiratory occlusions: An fMRI study. Sci. Rep. 2019, 9, 15045. [Google Scholar] [CrossRef]
- von Leupoldt, A.; Dahme, B. Cortical substrates for the perception of dyspnea. Chest 2005, 128, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.W.; Vovk, A. Cortical and subcortical central neural pathways in respiratory sensations. Respir. Physiol. Neeurobiol. 2009, 167, 72–86. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and function of the human insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel? Interoception: The sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef]
- Flynn, F.G. Anatomy of the insula functional and clinical correlates. Aphasiology 1999, 13, 55–78. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef]
- Ungerleider, L.G.; Haxby, J.V. ‘What’ and ‘where’ in the human brain. Curr. Opin. Neurobiol. 1994, 4, 157–165. [Google Scholar] [CrossRef]
- Medford, N.; Critchley, H.D. Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct. Funct. 2010, 214, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Bailey, P.H. The dyspnea-anxiety-dyspnea cycle-COPD patients’ stories of breathlessness: “It’s scary/when you can’t breathe”. Qual. Health Res. 2004, 14, 760–778. [Google Scholar] [CrossRef]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Öhman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Barrett, L.F.; Simmons, W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Daunizeau, J.; Kilner, J.; Kiebel, S.J. Action and behavior: A free-energy formulation. Biol. Cybern. 2010, 102, 227–260. [Google Scholar] [CrossRef]
- Pezzulo, G.; Rigoli, F.; Friston, K.J. Active inference, homeostatic regulation and adaptive behavioural control. Prog. Neurobiol. 2015, 134, 17–35. [Google Scholar] [CrossRef]
- Damasio, A.; Carvalho, G.B. The nature of feelings: Evolutionary and neurobiological origins. Nat. Rev. Neurosci. 2013, 14, 143–152. [Google Scholar] [CrossRef]
- Nieuwenhuys, R.; Voogd, J.; van Huijzen, C. Telencephalon: Hippocampus and related structures. In The Human Central Nervous System; Springer: Berlin/Heidelberg, Germany, 2008; pp. 361–400. [Google Scholar] [CrossRef]

| Study ID | Population | Intervention | |||||
|---|---|---|---|---|---|---|---|
| Author (Year) | Journal | Study Design | Imaging Modality | Sample Size (% Female) | Age (Years) | Conditions | Foci |
| Banzett et al. (2000) [13] | Brain Imaging NeuroReport | Prospective interventional study | PET | 8 (0) | n.a. | Ventilation via nose mask or mouthpiece by positive pressure ventilation controlling for pCO2 constant at 40 Torr, 14 breaths/minute | 13 |
| Liotti et al. (2000) [14] | PNAS | Prospective interventional study | PET | 8 (n.a.) | n.a. | Administration of 8% CO2/92% O2 via face mask or mouth piece, dyspnea rating scale from 0 to 100 | 14 |
| Pfeiffer et al. (2001) [15] | Am J Respir Crit Care Med | Prospective interventional study | PET | 8 (0) | 33 ± 3 (mean ± SD) | Two loads that induce a sensation of either slight/moderate or severe dyspnea in more than 50% of runs, 10 s before data acquisition the load was added | 10 |
| Evans et al. (2002) [16] | Journal of Neurophysiology | Prospective interventional study | fMRI (2 T) | 6 (4) | 25–32 (range) | 13.2 breaths/min, pCO2 constant by raising inspired CO2, low Vt = 0.75 ± 0.21 L, high Vt = 1.38 ± 0.06 L | 23 |
| Macey et al. (2006) [17] | Resp Physiol Neurobiol | Prospective interventional study | fMRI (1.5 T) | 7 (0) OSAS, 12 (0) controls | 46 ± 5 (mean ± SD) 47 ± 3 (mean ± SD) | Nose clip and breathing via mouthpiece, each trial of 60 s unrestricted breathing, 90 s of inspiratory loading (−6 to 15 mmHg) | 11 |
| McKay et al. (2008) [18] | Neuroimage | Prospective interventional study | fMRI (2 T) | 8 (6) | 20–35 | Breath holds for 15 s at resting expiratory lung volume, raised inspiratory CO2 for 15 s to increase petCO2 by 7–8 mmHg | 19 |
| von Leupoldt et al. (2008) [19] | Am J Respir Crit Care Med | Prospective interventional study | fMRI (3 T) | 14 (7) | 26.6 ± 6.2 (mean ± SD) | 11 conditions of inspiratory loaded and unloaded breathing continuous for 24 s with a mean load of 8.22 kPa/L/s, loaded condition with dyspnea pictures | 9 |
| Study ID | Population | Intervention | |||||
|---|---|---|---|---|---|---|---|
| Author (Year) | Journal | Study Design | Imaging Modality | Sample Size (% Female) | Age (Years) | Conditions | Foci |
| Binks et al. (2014) [20] | Resp Physiol and Neurobiol | Prospective interventional study | fMRI (1.5 T) | 8 (3) | 23–31 (range) | Mechanical ventilation with constant mild hypercapnia (~45 mmHg), Vt alternating between 0.96 ± 0.23 L and 0.48 ± 0.08 L, air hunger on transient and steady state | 9 |
| Herigstad et al. (2015) [21] | Chest | Prospective interventional study | fMRI (3 T) | 41 (15) COPD 40 (16) controls | 68.0 ± 8.2 (mean ± SD) | Dyspnea related cues, rating on a scale from 0 to 100 | 13 |
| Esser et al. (2017) [22] | Frontiers in Physiology | Prospective interventional study | fMRI (3 T) | 17 (8) in COPD 21 (11) in controls | 65.6 ± 9.3 (mean ± SD) in COPD 63.4 ± 8.8 (mean ± SD) in controls | Inspiratory resistive loads of increasing magnitude, 10 blocks of mild dyspnea (smallest load), 10 blocks of severe dyspnea (≥5 of 10 on Borg Scale) each lasting for 24 s | 13 |
| Chan et al. (2018) [23] | Frontiers in Physiology | Prospective interventional study | fMRI (3 T) | 23 (13) | 23.7 ± 3.1 (mean ± SD) | Inspiratory occlusion of 150 ms at the onset of inspiration every 2–4 breaths, 12 min of acquisition time, at least 32 successfully occluded breaths, rating of level of breathlessness based on VAS from 0 to 100 | 8 |
| Hassanpour et al. (2018) [24] | Neuropharmacology | Prospective interventional study | MRI (3 T) | 22 (11) | 26 ± 6 (mean ± SD) | Intravenous infusion of isoproterenol hydrochlorid and normal saline as control, each dose (1 μg, 2 μg and normal saline) was repeated twice, intensity of breathing sensation (0 = none, 10 most ever) was reduced | 7 |
| Chan et al. (2019) [25] | Scientific Reports nature research | Prospective interventional study | MRI (3 T) | 34 (20) | 23 ± 3.4 (mean ± SD) | Breathing through face mask while respiration was repeatedly interrupted by inspiratory occlusion of 150 ms delivered every 2–4 breaths, at least 32 breaths were collected | 14 |
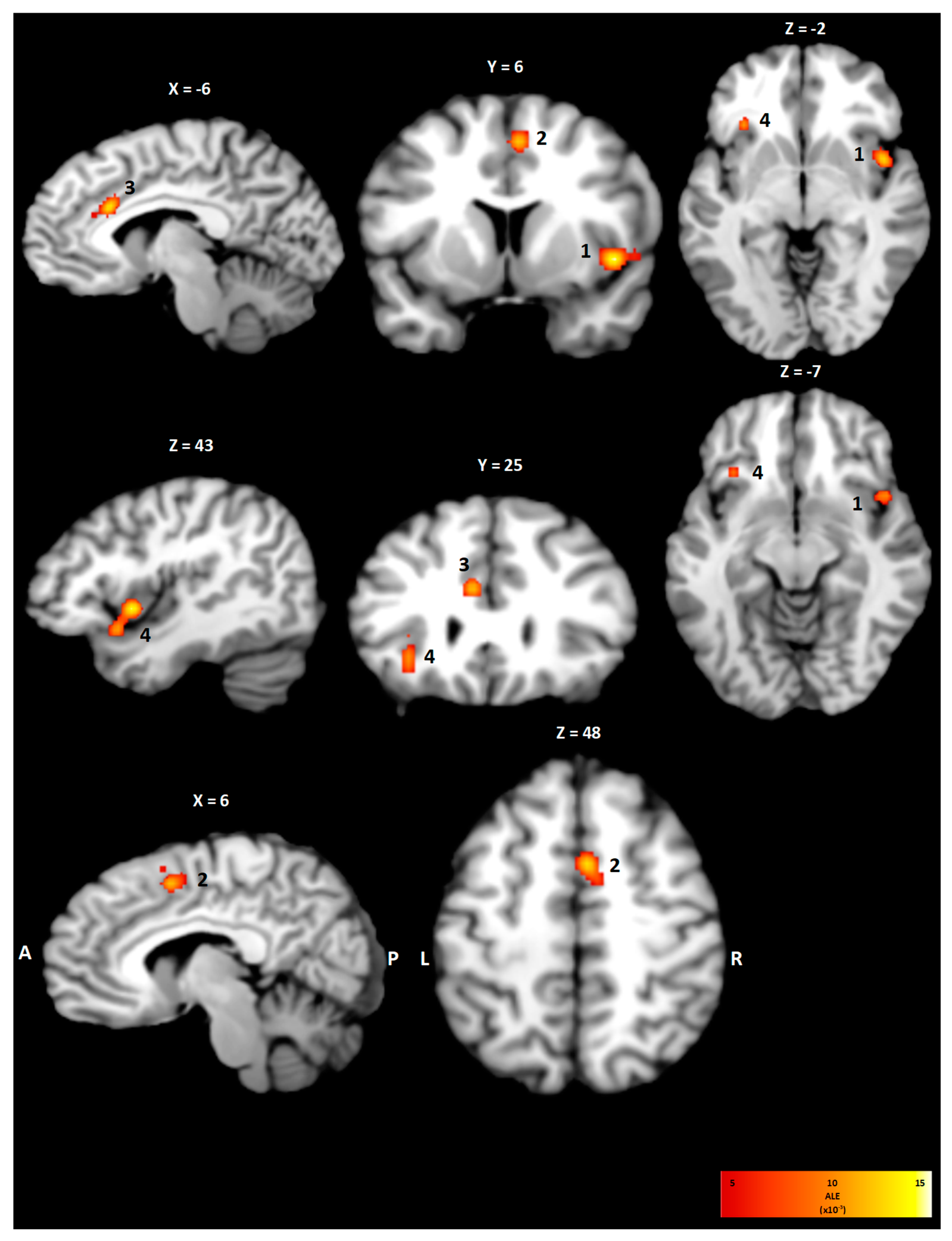
| Cluster | Brain Region | BA | x | y | z | Volume (mm3) | ALE (×10−3) |
|---|---|---|---|---|---|---|---|
| 1 | Right Insula | 13 | 44 | 9 | −4 | 1760 | 18.7 |
| 2 | Right Cingulate Cortex | 24 | 5 | 6 | 49 | 976 | 17.4 |
| 3 | Left Cingulate Cortex | 24 | −6 | 23 | 26 | 888 | 17.4 |
| 4 | Left Insula | 13 | −32 | 23 | 0 | 744 | 15.2 |
| Cluster | Brain Region | BA | x | y | z | Volume (mm3) | ALE (×10−3) |
|---|---|---|---|---|---|---|---|
| Restrained breathing | |||||||
| 1 | Left/Right Cingulate Cortex | 24 | 2 | 10 | 48 | 2448 | 16.9 |
| 2 | Left Cingulate Cortex | 32 | −3 | 28 | 23 | 1296 | 11.0 |
| Loaded Breathing | |||||||
| 3 | Right Postcentral gyrus | 1 | 63 | −7 | 14 | 560 | 13.4 |
| 4 | Left Superior Temporal Gyrus/Transverse Temporal Gyrus | 41 | −47 | −30 | 15 | 512 | 12.0 |
| 5 | Right Thalamus | - | 5 | −17 | 7 | 504 | 9.9 |
| Restrained and Loaded Breathing | |||||||
| 6 | Right Cingulate Cortex | 24 | 4 | 8 | 48 | 1800 | 17.4 |
| 7 | Right Insula | 13 | 43 | 6 | 1 | 1376 | 18.5 |
| 8 | Right Thalamus | 10 | −17 | 8 | 688 | 11.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, C.; Kerl, J.; Dellweg, D. The Neuroanatomical Correlates of Dyspnea: An Activation Likelihood Estimation Meta-Analysis. NeuroSci 2025, 6, 36. https://doi.org/10.3390/neurosci6020036
Müller C, Kerl J, Dellweg D. The Neuroanatomical Correlates of Dyspnea: An Activation Likelihood Estimation Meta-Analysis. NeuroSci. 2025; 6(2):36. https://doi.org/10.3390/neurosci6020036
Chicago/Turabian StyleMüller, Christoph, Jens Kerl, and Dominic Dellweg. 2025. "The Neuroanatomical Correlates of Dyspnea: An Activation Likelihood Estimation Meta-Analysis" NeuroSci 6, no. 2: 36. https://doi.org/10.3390/neurosci6020036
APA StyleMüller, C., Kerl, J., & Dellweg, D. (2025). The Neuroanatomical Correlates of Dyspnea: An Activation Likelihood Estimation Meta-Analysis. NeuroSci, 6(2), 36. https://doi.org/10.3390/neurosci6020036





