The Probable Infectious Origin of Multiple Sclerosis
Abstract
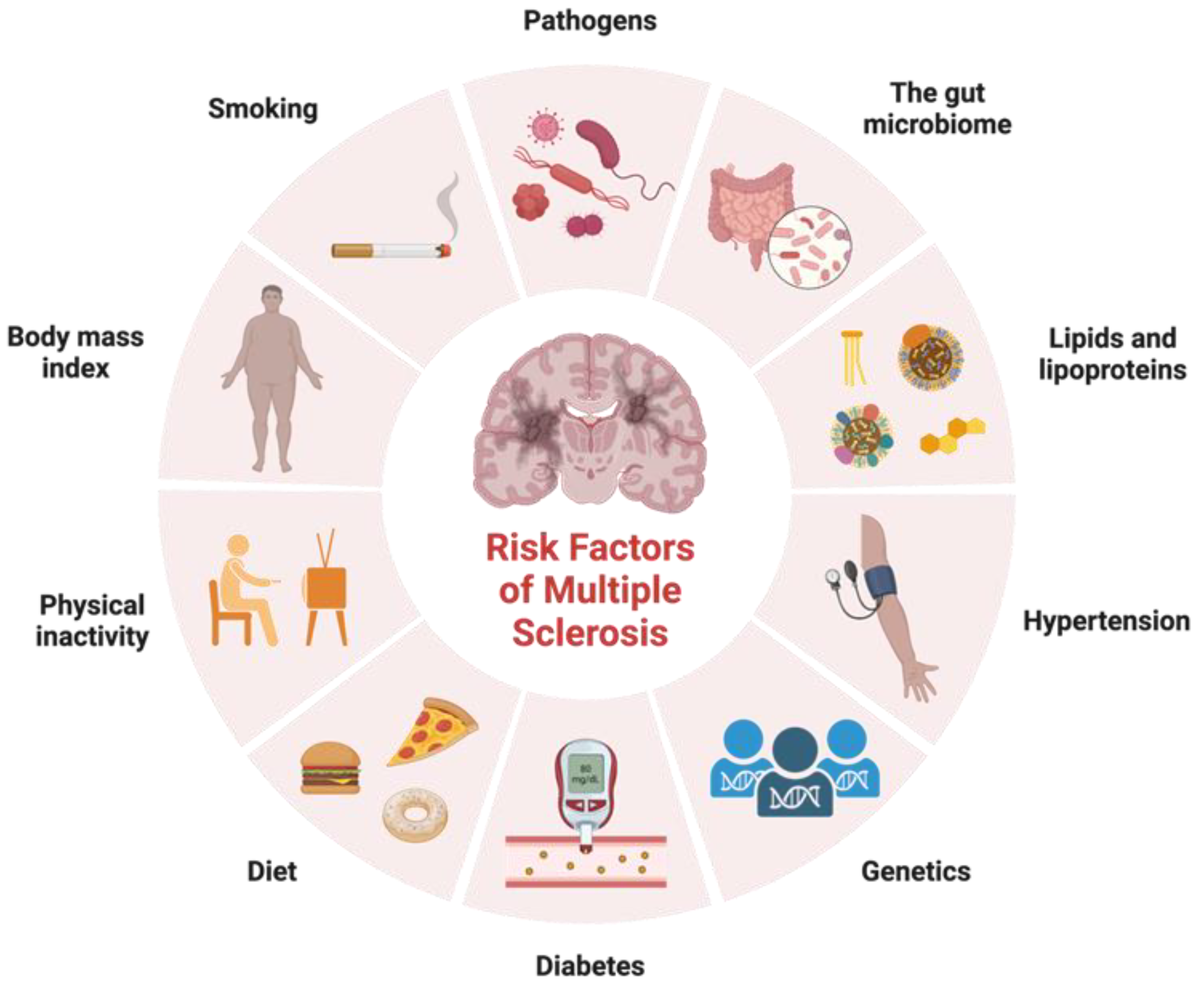
1. Introduction
2. Pathogenesis
2.1. Nature vs. Nurture
2.2. Microbes and Infectious Agents
2.3. Treatments
3. Viral Triggers
3.1. Epstein–Barr Virus
3.2. HHV-6
| Source | Year | Methods | Samples |
|---|---|---|---|
| Sola et al. [93] | 1993 | PCR, Southern blot, IFA | CSF, serum |
| Wilborn et al. [100] | 1994 | PCR, ELISA | Serum |
| Challoner et al. [99] | 1995 | Representational difference analysis | CNS tissue, PBL |
| Liedtke et al. [101] | 1995 | Nested PCR | CSF, serum |
| Sanders et al. [102] | 1996 | PCR | Tissue |
| Carrigan and Knox [103] | 1997 | -- | -- |
| Merelli et al. [95] | 1997 | PCR | PBMCs |
| Martin et al. [104] | 1997 | PCR, indirect immunofluorescent assay | CSF, serum |
| Soldan et al. [105] | 1997 | EIA, IFA | Serum |
| Ablashi et al. [106] | 1998 | PCR | CSF |
| Coates and Bell [107] | 1998 | PCR | Serum, CSF |
| Mayne et al. [108] | 1998 | PCR, nested PCR | Blood |
| Friedman et al. [109] | 1999 | PCR, immunohistochemistry | Tissue, CSF |
| Ongradi et al. [110] | 1999 | ELISA | CSF |
| Rotola et al. [111] | 1999 | Nested PCR | PBMCs |
| Ablashi et al. [112] | 2000 | PCR | CSF, sera, plasma, blood |
| Akhyani et al. [113] | 2000 | Nested PCR | PBL, serum, saliva, urine |
| Kim et al. [114] | 2000 | PCR | PBMC |
| Knox et al. [115] | 2000 | IFA, rapid HHV-6 culture assay | CNS tissue, blood |
| Alvarez-Lafuente et al. [116] | 2002 | qRT-PCR | Blood |
| Berti et al. [117] | 2002 | Nested PCR | Blood |
| Tejada-Simon et al. [118] | 2002 | PCR, nested PCR, Southern hybridization | CSF, blood |
| Xu et al. [119] | 2002 | ELISA | Serum |
| Al-Shammari et al. [120] | 2003 | Nested PCR, PCR | Serum |
| Cermelli et al. [121] | 2003 | Nested PCR | Tissue |
| Chapenko et al. [122] | 2003 | PCR, nested PCR | PBMCs, blood |
| Alvarez-Lafuente et al. [123] | 2004 | Quantitative RT-PCR | Blood, serum |
| Rotola et al. [124] | 2004 | Nested PCR | CSF, PBMCs |
| Derfuss et al. [125] | 2005 | ELISA | CSF/Serum, PBMCs |
| Fogdell-Hahn et al. [126] | 2005 | PCR | Blood, CSF |
| Höllsberg et al. [127] | 2005 | RT-PCR | Blood, saliva |
| Alvarez-Lafuente et al. [128] | 2006 | qRT-PCR | Serum |
| Alvarez-Lafuente et al. [129] | 2007 | RT-PCR | Serum, PBMCs |
| Virtanen et al. [130] | 2007 | Immunofluorescence avidity assays, multiplex PCR | Serum, CSF |
| Kuusisto et al. [131] | 2008 | PCR, ELISA | Serum, CSF |
| Alvarez-Lafuente et al. [132] | 2009 | qRT-PCR | PBMCs, serum |
| Mancuso et al. [133] | 2010 | RT-PCR | CSF |
| Behzad-Behbahani et al. [134] | 2011 | Nested PCR | Serum |
| Garcia-Montojo et al. [135] | 2011 | Quantitative RT-PCR | Blood, serum |
| Nora-Krukle et al. [136] | 2011 | Nested PCR, RT-PCR, ELISA | Plasma, serum |
| Virtanen et al. [137] | 2011 | Isoelectric focusing and immunofixation, affinity-driven immunoblot | Serum, CSF |
| Dominguez-Mozo et al. [138] | 2012 | qRT-PCR | Blood, serum |
| Ramroodi et al. [139] | 2013 | qRT-PCR | PMBCs, serum, saliva |
| Alenda et al. [140] | 2014 | SDS-PAGE, MALDI-TOF MS | CSF |
| Hon et al. [77] | 2014 | PCR | CSF, blood |
| Ortega-Madueño et al. [141] | 2014 | ELISA | Serum |
| Simpson et al. [142] | 2014 | qRT-PCR | Serum, CSF |
| Kofahi et al. [143] | 2020 | ELISA | Blood |
| Tao et al. [97] | 2022 | PCR, indirect immunofluorescence assay | Blood, serum |
3.3. Varicella-Zoster Virus
3.4. Cytomegalovirus
4. Bacterial Triggers
4.1. H. pylori
4.2. C. pneumoniae
4.3. Borrelia burgdorferi
4.4. Mycobacterium Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef]
- McKay, K.A.; Kwan, V.; Duggan, T.; Tremlett, H. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: A systematic review. Biomed Res. Int. 2015, 2015, 817238. [Google Scholar] [CrossRef]
- Krajnc, N.; Berger, T.; Bsteh, G. Measuring Treatment Response in Progressive Multiple Sclerosis-Considerations for Adapting to an Era of Multiple Treatment Options. Biomolecules 2021, 11, 1342. [Google Scholar] [CrossRef] [PubMed]
- Hemond, C.C.; Bakshi, R. Magnetic Resonance Imaging in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028969. [Google Scholar] [CrossRef]
- Rammohan, K.W. Cerebrospinal fluid in multiple sclerosis. Ann. Indian Acad. Neurol. 2009, 12, 246–253. [Google Scholar] [CrossRef]
- Popescu, B.F.; Pirko, I.; Lucchinetti, C.F. Pathology of multiple sclerosis: Where do we stand? Continuum 2013, 19, 901–921. [Google Scholar] [CrossRef]
- Barcelos, I.P.; Troxell, R.M.; Graves, J.S. Mitochondrial Dysfunction and Multiple Sclerosis. Biology 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Linker, R.A. Inflammation in multiple sclerosis. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211007687. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Patti, F. Gray matters in multiple sclerosis: Cognitive impairment and structural MRI. Mult. Scler. Int. 2014, 2014, 609694. [Google Scholar] [CrossRef]
- Eshaghi, A.; Young, A.L.; Wijeratne, P.A.; Prados, F.; Arnold, D.L.; Narayanan, S.; Guttmann, C.R.G.; Barkhof, F.; Alexander, D.C.; Thompson, A.J.; et al. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat. Commun. 2021, 12, 2078. [Google Scholar] [CrossRef]
- Cook, S.D. Multiple sclerosis and viruses. Mult. Scler. 1997, 3, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Jersild, C.; Dupont, B.; Fog, T.; Platz, P.J.; Svejgaard, A. Histocompatibility determinants in multiple sclerosis. Transplant. Rev. 1975, 22, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Libbey, J.E.; McCoy, L.L.; Fujinami, R.S. Molecular mimicry in multiple sclerosis. Int. Rev. Neurobiol. 2007, 79, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Liblau, R.; Gautam, A.M. HLA, molecular mimicry and multiple sclerosis. Rev. Immunogenet. 2000, 2, 95–104. [Google Scholar]
- Ruhl, G.; Niedl, A.G.; Patronov, A.; Siewert, K.; Pinkert, S.; Kalemanov, M.; Friese, M.A.; Attfield, K.E.; Antes, I.; Hohlfeld, R.; et al. Multiple sclerosis: Molecular mimicry of an antimyelin HLA class I restricted T-cell receptor. Neurol. Neuroimmunol. Neuroinflamm. 2016, 3, e241. [Google Scholar] [CrossRef]
- Rojas, M.; Restrepo-Jimenez, P.; Monsalve, D.M.; Pacheco, Y.; Acosta-Ampudia, Y.; Ramirez-Santana, C.; Leung, P.S.C.; Ansari, A.A.; Gershwin, M.E.; Anaya, J.M. Molecular mimicry and autoimmunity. J. Autoimmun. 2018, 95, 100–123. [Google Scholar] [CrossRef]
- Cotsapas, C.; Mitrovic, M. Genome-wide association studies of multiple sclerosis. Clin. Transl. Immunol. 2018, 7, e1018. [Google Scholar] [CrossRef]
- Galarza-Munoz, G.; Briggs, F.B.S.; Evsyukova, I.; Schott-Lerner, G.; Kennedy, E.M.; Nyanhete, T.; Wang, L.; Bergamaschi, L.; Widen, S.G.; Tomaras, G.D.; et al. Human Epistatic Interaction Controls IL7R Splicing and Increases Multiple Sclerosis Risk. Cell 2017, 169, 72–84.e13. [Google Scholar] [CrossRef]
- Hoe, E.; McKay, F.C.; Schibeci, S.D.; Gandhi, K.; Heard, R.N.; Stewart, G.J.; Booth, D.R. Functionally significant differences in expression of disease-associated IL-7 receptor alpha haplotypes in CD4 T cells and dendritic cells. J. Immunol. 2010, 184, 2512–2517. [Google Scholar] [CrossRef]
- Gregory, S.G.; Schmidt, S.; Seth, P.; Oksenberg, J.R.; Hart, J.; Prokop, A.; Caillier, S.J.; Ban, M.; Goris, A.; Barcellos, L.F.; et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 2007, 39, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Patsopoulos, N.A. Genetics of Multiple Sclerosis: An Overview and New Directions. Cold Spring Harb. Perspect. Med. 2018, 8, a028951. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Nishanth, K.; Tariq, E.; Nzvere, F.P.; Miqdad, M.; Cancarevic, I. Role of Smoking in the Pathogenesis of Multiple Sclerosis: A Review Article. Cureus 2020, 12, e9564. [Google Scholar] [CrossRef] [PubMed]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Multiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Kadowaki, A.; Quintana, F.J. The Gut-CNS Axis in Multiple Sclerosis. Trends Neurosci. 2020, 43, 622–634. [Google Scholar] [CrossRef]
- Boussamet, L.; Rajoka, M.S.R.; Berthelot, L. Microbiota, IgA and Multiple Sclerosis. Microorganisms 2022, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood-Brain Barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The Role of Short-Chain Fatty Acids of Gut Microbiota Origin in Hypertension. Front. Microbiol. 2021, 12, 730809. [Google Scholar] [CrossRef] [PubMed]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2022, 76, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhu, H.; Zhang, J.; Wan, D. The Pivotal Role of Microbiota in Modulating the Neuronal-Glial-Epithelial Unit. Infect. Drug Resist. 2021, 14, 5613–5628. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 517–528. [Google Scholar] [CrossRef]
- Mechelli, R.; Romano, S.; Romano, C.; Morena, E.; Buscarinu, M.C.; Bigi, R.; Bellucci, G.; Renie, R.; Pellicciari, G.; Salvetti, M.; et al. MAIT Cells and Microbiota in Multiple Sclerosis and Other Autoimmune Diseases. Microorganisms 2021, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Gargano, F.; Guerrera, G.; Piras, E.; Serafini, B.; Di Paola, M.; Rizzetto, L.; Buscarinu, M.C.; Annibali, V.; Vuotto, C.; De Bardi, M.; et al. Proinflammatory mucosal-associated invariant CD8+ T cells react to gut flora yeasts and infiltrate multiple sclerosis brain. Front. Immunol. 2022, 13, 890298. [Google Scholar] [CrossRef]
- Schnell, A.; Huang, L.; Singer, M.; Singaraju, A.; Barilla, R.M.; Regan, B.M.L.; Bollhagen, A.; Thakore, P.I.; Dionne, D.; Delorey, T.M.; et al. Stem-like intestinal Th17 cells give rise to pathogenic effector T cells during autoimmunity. Cell 2021, 184, 6281–6298.e23. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yang, X.O.; Chung, Y.; Fukunaga, A.; Nurieva, R.; Pappu, B.; Martin-Orozco, N.; Kang, H.S.; Ma, L.; Panopoulos, A.D.; et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J. Immunol. 2008, 181, 8391–8401. [Google Scholar] [CrossRef]
- Gacias, M.; Casaccia, P. Epigenetic Mechanisms in Multiple Sclerosis. Rev. Esp. Escler. Mult. 2014, 6, 25–35. [Google Scholar]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef]
- Lanata, C.M.; Chung, S.A.; Criswell, L.A. DNA methylation 101: What is important to know about DNA methylation and its role in SLE risk and disease heterogeneity. Lupus Sci. Med. 2018, 5, e000285. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Scicluna, B.P.; van der Poll, T. The Role of Host Cell DNA Methylation in the Immune Response to Bacterial Infection. Front. Immunol. 2021, 12, 696280. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Farez, M.F.; Gaitan, M.I. Environmental factors influencing multiple sclerosis in Latin America. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317715049. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Kanneganti, T.D. Immune responses against protozoan parasites: A focus on the emerging role of Nod-like receptors. Cell. Mol. Life Sci. 2016, 73, 3035–3051. [Google Scholar] [CrossRef]
- Nutman, T.B. Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol. 2015, 37, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Higuera, L.; Carlin, C.S.; Anderson, S. Adherence to Disease-Modifying Therapies for Multiple Sclerosis. J. Manag. Care Spec. Pharm. 2016, 22, 1394–1401. [Google Scholar] [CrossRef]
- Robertson, D.; Moreo, N. Disease-Modifying Therapies in Multiple Sclerosis: Overview and Treatment Considerations. Fed. Pract. 2016, 33, 28–34. [Google Scholar]
- Voge, N.V.; Alvarez, E. Monoclonal Antibodies in Multiple Sclerosis: Present and Future. Biomedicines 2019, 7, 20. [Google Scholar] [CrossRef]
- Filipi, M.; Jack, S. Interferons in the Treatment of Multiple Sclerosis: A Clinical Efficacy, Safety, and Tolerability Update. Int. J. MS Care 2020, 22, 165–172. [Google Scholar] [CrossRef]
- Yaldizli, O.; Putzki, N. Natalizumab in the treatment of multiple sclerosis. Ther. Adv. Neurol. Disord. 2009, 2, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, B.; Einarsson, H.B.; Vorup-Jensen, T. Glatiramer acetate in treatment of multiple sclerosis: A toolbox of random co-polymers for targeting inflammatory mechanisms of both the innate and adaptive immune system? Int. J. Mol. Sci. 2012, 13, 14579–14605. [Google Scholar] [CrossRef]
- Prosperini, L.; Haggiag, S.; Ruggieri, S.; Tortorella, C.; Gasperini, C. Dimethyl Fumarate or Teriflunomide for Relapsing-Remitting Multiple Sclerosis: A Meta-analysis of Post-marketing Studies. Neurotherapeutics 2023, 20, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, L.; Pontecorvo, S. Dimethyl fumarate in the management of multiple sclerosis: Appropriate patient selection and special considerations. Ther. Clin. Risk Manag. 2016, 12, 339–350. [Google Scholar] [CrossRef]
- Groves, A.; Kihara, Y.; Chun, J. Fingolimod: Direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J. Neurol. Sci. 2013, 328, 9–18. [Google Scholar] [CrossRef]
- Sgarlata, E.; Chisari, C.G.; Toscano, S.; Finocchiaro, C.; Lo Fermo, S.; Millefiorini, E.; Patti, F. Changes in John Cunningham Virus Index in Multiple Sclerosis Patients Treated with Different Disease-Modifying Therapies. Curr. Neuropharmacol. 2022, 20, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, S.; Gholizadeh, O.; Yasamineh, S.; Akbarzadeh, S.; Amini, P.; Favakehi, P.; Afkhami, H.; Firouzi-Amandi, A.; Pahlevan, D.; Eslami, M.; et al. Comprehensive Investigations Relationship between Viral Infections and Multiple Sclerosis Pathogenesis. Curr. Microbiol. 2022, 80, 15. [Google Scholar] [CrossRef]
- Tselis, A. Evidence for viral etiology of multiple sclerosis. Semin. Neurol. 2011, 31, 307–316. [Google Scholar] [CrossRef]
- Arnadottir, T.; Reunanen, M.; Meurman, O.; Salmi, A.; Panelius, M.; Halonen, P. Measles and rubella virus antibodies in patients with multiple sclerosis. A longitudinal study of serum and CSF specimens by radioimmunoassay. Arch. Neurol. 1979, 36, 261–265. [Google Scholar] [CrossRef]
- Ferrante, P.; Castellani, P.; Barbi, M.; Bergamini, F. The Italian Cooperative Multiple Sclerosis case-control study: Preliminary results on viral antibodies. Ital. J. Neurol. Sci. 1987, Suppl. S6, 45–50. [Google Scholar]
- Wong, Y.; Meehan, M.T.; Burrows, S.R.; Doolan, D.L.; Miles, J.J. Estimating the global burden of Epstein-Barr virus-related cancers. J. Cancer Res. Clin. Oncol. 2022, 148, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef]
- Dunmire, S.K.; Hogquist, K.A.; Balfour, H.H. Infectious Mononucleosis. Curr. Top. Microbiol. Immunol. 2015, 390, 211–240. [Google Scholar] [CrossRef]
- Bar-Or, A.; Pender, M.P.; Khanna, R.; Steinman, L.; Hartung, H.P.; Maniar, T.; Croze, E.; Aftab, B.T.; Giovannoni, G.; Joshi, M.A. Epstein-Barr Virus in Multiple Sclerosis: Theory and Emerging Immunotherapies. Trends Mol. Med. 2020, 26, 296–310. [Google Scholar] [CrossRef]
- Albanese, M.; Tagawa, T.; Hammerschmidt, W. Strategies of Epstein-Barr virus to evade innate antiviral immunity of its human host. Front. Microbiol. 2022, 13, 955603. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Ascherio, A.; Munch, M. Epstein-Barr virus and multiple sclerosis. Epidemiology 2000, 11, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Almohmeed, Y.H.; Avenell, A.; Aucott, L.; Vickers, M.A. Systematic review and meta-analysis of the sero-epidemiological association between Epstein Barr virus and multiple sclerosis. PLoS ONE 2013, 8, e61110. [Google Scholar] [CrossRef]
- Bar-Or, A.; Banwell, B.; Berger, J.R.; Lieberman, P.M. Guilty by association: Epstein-Barr virus in multiple sclerosis. Nat. Med. 2022, 28, 904–906. [Google Scholar] [CrossRef]
- Banwell, B.; Krupp, L.; Kennedy, J.; Tellier, R.; Tenembaum, S.; Ness, J.; Belman, A.; Boiko, A.; Bykova, O.; Waubant, E.; et al. Clinical features and viral serologies in children with multiple sclerosis: A multinational observational study. Lancet Neurol. 2007, 6, 773–781. [Google Scholar] [CrossRef]
- Pakpoor, J.; Disanto, G.; Gerber, J.E.; Dobson, R.; Meier, U.C.; Giovannoni, G.; Ramagopalan, S.V. The risk of developing multiple sclerosis in individuals seronegative for Epstein-Barr virus: A meta-analysis. Mult. Scler. 2013, 19, 162–166. [Google Scholar] [CrossRef]
- Lunemann, J.D.; Huppke, P.; Roberts, S.; Bruck, W.; Gartner, J.; Munz, C. Broadened and elevated humoral immune response to EBNA1 in pediatric multiple sclerosis. Neurology 2008, 71, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Pohl, D.; Krone, B.; Rostasy, K.; Kahler, E.; Brunner, E.; Lehnert, M.; Wagner, H.J.; Gartner, J.; Hanefeld, F. High seroprevalence of Epstein-Barr virus in children with multiple sclerosis. Neurology 2006, 67, 2063–2065. [Google Scholar] [CrossRef] [PubMed]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022, 603, 321–327. [Google Scholar] [CrossRef]
- Afrasiabi, A.; Parnell, G.P.; Swaminathan, S.; Stewart, G.J.; Booth, D.R. The interaction of Multiple Sclerosis risk loci with Epstein-Barr virus phenotypes implicates the virus in pathogenesis. Sci. Rep. 2020, 10, 193. [Google Scholar] [CrossRef]
- Holden, D.W.; Gold, J.; Hawkes, C.H.; Giovannoni, G.; Saxton, J.M.; Carter, A.; Sharrack, B. Epstein Barr virus shedding in multiple sclerosis: Similar frequencies of EBV in saliva across separate patient cohorts. Mult. Scler. Relat. Disord. 2018, 25, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Hon, G.M.; Erasmus, R.T.; Matsha, T. Low prevalence of human herpesvirus-6 and varicella zoster virus in blood of multiple sclerosis patients, irrespective of inflammatory status or disease progression. J. Clin. Neurosci. 2014, 21, 1437–1440. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.W.; Hatfield, L.M.; Crawford, M.P.; Patel, S. Quantitative PCR for Epstein-Barr virus DNA and RNA in multiple sclerosis. Mult. Scler. 2009, 15, 153–158. [Google Scholar] [CrossRef]
- Veroni, C.; Serafini, B.; Rosicarelli, B.; Fagnani, C.; Aloisi, F. Transcriptional profile and Epstein-Barr virus infection status of laser-cut immune infiltrates from the brain of patients with progressive multiple sclerosis. J. Neuroinflamm. 2018, 15, 18. [Google Scholar] [CrossRef]
- Willis, S.N.; Stadelmann, C.; Rodig, S.J.; Caron, T.; Gattenloehner, S.; Mallozzi, S.S.; Roughan, J.E.; Almendinger, S.E.; Blewett, M.M.; Bruck, W.; et al. Epstein-Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 2009, 132, 3318–3328. [Google Scholar] [CrossRef]
- Hassani, A.; Corboy, J.R.; Al-Salam, S.; Khan, G. Epstein-Barr virus is present in the brain of most cases of multiple sclerosis and may engage more than just B cells. PLoS ONE 2018, 13, e0192109. [Google Scholar] [CrossRef]
- Sargsyan, S.A.; Shearer, A.J.; Ritchie, A.M.; Burgoon, M.P.; Anderson, S.; Hemmer, B.; Stadelmann, C.; Gattenlohner, S.; Owens, G.P.; Gilden, D.; et al. Absence of Epstein-Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 2010, 74, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, R.; Serafini, B.; Rosicarelli, B.; Chiappetta, G.; Veroni, C.; Reynolds, R.; Aloisi, F. B-cell enrichment and Epstein-Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J. Neuropathol. Exp. Neurol. 2013, 72, 29–41. [Google Scholar] [CrossRef]
- Robinson, W.H.; Steinman, L. Epstein-Barr virus and multiple sclerosis. Science 2022, 375, 264–265. [Google Scholar] [CrossRef] [PubMed]
- Ihira, M.; Yoshikawa, T.; Ishii, J.; Nomura, M.; Hishida, H.; Ohashi, M.; Enomoto, Y.; Suga, S.; Iida, K.; Saito, Y.; et al. Serological examination of human herpesvirus 6 and 7 in patients with coronary artery disease. J. Med. Virol. 2002, 67, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.A.; Weber, J.M. An enzyme-linked immunosorbent assay for the detection of IgG and IgM antibodies to human herpesvirus type 6. J. Virol. Methods 1993, 41, 265–275. [Google Scholar] [CrossRef]
- Baillargeon, J.; Piper, J.; Leach, C.T. Epidemiology of human herpesvirus 6 (HHV-6) infection in pregnant and nonpregnant women. J. Clin. Virol. 2000, 16, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Saxinger, C.; Polesky, H.; Eby, N.; Grufferman, S.; Murphy, R.; Tegtmeir, G.; Parekh, V.; Memon, S.; Hung, C. Antibody reactivity with HBLV (HHV-6) in U.S. populations. J. Virol. Methods 1988, 21, 199–208. [Google Scholar] [CrossRef]
- Sibley, W.A.; Bamford, C.R.; Clark, K. Clinical viral infections and multiple sclerosis. Lancet 1985, 1, 1313–1315. [Google Scholar] [CrossRef]
- Voumvourakis, K.I.; Fragkou, P.C.; Kitsos, D.K.; Foska, K.; Chondrogianni, M.; Tsiodras, S. Human herpesvirus 6 infection as a trigger of multiple sclerosis: An update of recent literature. BMC Neurol. 2022, 22, 57. [Google Scholar] [CrossRef]
- Yao, K.; Crawford, J.R.; Komaroff, A.L.; Ablashi, D.V.; Jacobson, S. Review part 2: Human herpesvirus-6 in central nervous system diseases. J. Med. Virol. 2010, 82, 1669–1678. [Google Scholar] [CrossRef] [PubMed]
- Hogestyn, J.M.; Mock, D.J.; Mayer-Proschel, M. Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology. Neural Regen Res. 2018, 13, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Sola, P.; Merelli, E.; Marasca, R.; Poggi, M.; Luppi, M.; Montorsi, M.; Torelli, G. Human herpesvirus 6 and multiple sclerosis: Survey of anti-HHV-6 antibodies by immunofluorescence analysis and of viral sequences by polymerase chain reaction. J. Neurol. Neurosurg. Psychiatry 1993, 56, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Azimi, T.; Falah, F.; Faghihloo, E. Relationship of human herpes virus 6 and multiple sclerosis: A systematic review and meta-analysis. J. Cell Physiol. 2018, 233, 2850–2862. [Google Scholar] [CrossRef]
- Merelli, E.; Bedin, R.; Sola, P.; Barozzi, P.; Mancardi, G.L.; Ficarra, G.; Franchini, G. Human herpes virus 6 and human herpes virus 8 DNA sequences in brains of multiple sclerosis patients, normal adults and children. J. Neurol. 1997, 244, 450–454. [Google Scholar] [CrossRef]
- Liedtke, W.; Malessa, R.; Faustmann, P.M.; Eis-Hubinger, A.M. Human herpesvirus 6 polymerase chain reaction findings in human immunodeficiency virus associated neurological disease and multiple sclerosis. J. Neurovirol. 1995, 1, 253–258. [Google Scholar] [CrossRef]
- Tao, C.; Simpson-Yap, S.; Taylor, B.; Blizzard, L.; Lucas, R.; Ponsonby, A.L.; Broadley, S.; van der Mei, I.; AusLong/Ausimmune Investigators, G. Markers of Epstein-Barr virus and Human Herpesvirus-6 infection and multiple sclerosis clinical progression. Mult. Scler. Relat. Disord. 2022, 59, 103561. [Google Scholar] [CrossRef]
- Opsahl, M.L.; Kennedy, P.G. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 2005, 128, 516–527. [Google Scholar] [CrossRef]
- Challoner, P.B.; Smith, K.T.; Parker, J.D.; MacLeod, D.L.; Coulter, S.N.; Rose, T.M.; Schultz, E.R.; Bennett, J.L.; Garber, R.L.; Chang, M.; et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. USA 1995, 92, 7440–7444. [Google Scholar] [CrossRef]
- Wilborn, F.; Brinkmann, V.; Schmidt, C.A.; Neipel, F.; Gelderblom, H.; Siegert, W. Herpesvirus type 6 in patients undergoing bone marrow transplantation: Serologic features and detection by polymerase chain reaction. Blood 1994, 83, 3052–3058. [Google Scholar] [CrossRef]
- Liedtke, W.; Trubner, K.; Schwechheimer, K. On the role of human herpesvirus 6 in viral latency in nervous tissue and in cerebral lymphoma. J. Neurol. Sci. 1995, 134, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Sanders, V.J.; Felisan, S.; Waddell, A.; Tourtellotte, W.W. Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. J. Neurovirol. 1996, 2, 249–258. [Google Scholar] [CrossRef]
- Carrigan, D.R.; Knox, K.K. Human herpesvirus six and multiple sclerosis. Mult. Scler. 1997, 3, 390–394. [Google Scholar] [CrossRef]
- Martin, C.; Enbom, M.; Soderstrom, M.; Fredrikson, S.; Dahl, H.; Lycke, J.; Bergstrom, T.; Linde, A. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol. Scand. 1997, 95, 280–283. [Google Scholar] [CrossRef]
- Soldan, S.S.; Berti, R.; Salem, N.; Secchiero, P.; Flamand, L.; Calabresi, P.A.; Brennan, M.B.; Maloni, H.W.; McFarland, H.F.; Lin, H.C.; et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: Increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 1997, 3, 1394–1397. [Google Scholar] [CrossRef]
- Ablashi, D.V.; Lapps, W.; Kaplan, M.; Whitman, J.E.; Richert, J.R.; Pearson, G.R. Human Herpesvirus-6 (HHV-6) infection in multiple sclerosis: A preliminary report. Mult. Scler. 1998, 4, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Coates, A.R.; Bell, J. HHV-6 and multiple sclerosis. Nat. Med. 1998, 4, 537–538. [Google Scholar] [CrossRef]
- Mayne, M.; Krishnan, J.; Metz, L.; Nath, A.; Auty, A.; Sahai, B.M.; Power, C. Infrequent detection of human herpesvirus 6 DNA in peripheral blood mononuclear cells from multiple sclerosis patients. Ann. Neurol. 1998, 44, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.E.; Lyons, M.J.; Cu, G.; Ablashl, D.V.; Whitman, J.E.; Edgar, M.; Koskiniemi, M.; Vaheri, A.; Zabriskie, J.B. The association of the human herpesvirus-6 and MS. Mult. Scler. 1999, 5, 355–362. [Google Scholar] [CrossRef]
- Ongradi, J.; Rajda, C.; Marodi, C.L.; Csiszar, A.; Vecsei, L. A pilot study on the antibodies to HHV-6 variants and HHV-7 in CSF of MS patients. J. Neurovirol. 1999, 5, 529–532. [Google Scholar] [CrossRef]
- Rotola, A.; Cassai, E.; Tola, M.R.; Granieri, E.; Di Luca, D. Human herpesvirus 6 is latent in peripheral blood of patients with relapsing-remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1999, 67, 529–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ablashi, D.V.; Eastman, H.B.; Owen, C.B.; Roman, M.M.; Friedman, J.; Zabriskie, J.B.; Peterson, D.L.; Pearson, G.R.; Whitman, J.E. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J. Clin. Virol. 2000, 16, 179–191. [Google Scholar] [CrossRef]
- Akhyani, N.; Berti, R.; Brennan, M.B.; Soldan, S.S.; Eaton, J.M.; McFarland, H.F.; Jacobson, S. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: Increased prevalence of HHV-6A in patients with multiple sclerosis. J. Infect. Dis. 2000, 182, 1321–1325. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, K.S.; Park, J.H.; Kim, M.Y.; Shin, W.S. Detection of human herpesvirus 6 variant A in peripheral blood mononuclear cells from multiple sclerosis patients. Eur. Neurol. 2000, 43, 170–173. [Google Scholar] [CrossRef]
- Knox, K.K.; Brewer, J.H.; Henry, J.M.; Harrington, D.J.; Carrigan, D.R. Human herpesvirus 6 and multiple sclerosis: Systemic active infections in patients with early disease. Clin. Infect. Dis. 2000, 31, 894–903. [Google Scholar] [CrossRef]
- Alvarez-Lafuente, R.; Martin-Estefania, C.; de Las Heras, V.; Castrillo, C.; Picazo, J.J.; Varela de Seijas, E.; Gonzalez, R.A. Active human herpesvirus 6 infection in patients with multiple sclerosis. Arch. Neurol. 2002, 59, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Berti, R.; Soldan, S.S.; Akhyani, N.; McFarland, H.F.; Jacobson, S. Extended observations on the association of HHV-6 and multiple sclerosis. J. Neurovirol. 2000, 6 (Suppl. S2), S85–S87. [Google Scholar]
- Tejada-Simon, M.V.; Zang, Y.C.; Hong, J.; Rivera, V.M.; Killian, J.M.; Zhang, J.Z. Detection of viral DNA and immune responses to the human herpesvirus 6 101-kilodalton virion protein in patients with multiple sclerosis and in controls. J. Virol. 2002, 76, 6147–6154. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Schillinger, J.A.; Sternberg, M.R.; Johnson, R.E.; Lee, F.K.; Nahmias, A.J.; Markowitz, L.E. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988–1994. J. Infect. Dis. 2002, 185, 1019–1024. [Google Scholar] [CrossRef]
- Al-Shammari, S.; Nelson, R.F.; Voevodin, A. HHV-6 DNAaemia in patients with multiple sclerosis in Kuwait. Acta Neurol. Scand. 2003, 107, 122–124. [Google Scholar] [CrossRef]
- Cermelli, C.; Berti, R.; Soldan, S.S.; Mayne, M.; D’Ambrosia, J.M.; Ludwin, S.K.; Jacobson, S. High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J. Infect. Dis. 2003, 187, 1377–1387. [Google Scholar] [CrossRef]
- Chapenko, S.; Millers, A.; Nora, Z.; Logina, I.; Kukaine, R.; Murovska, M. Correlation between HHV-6 reactivation and multiple sclerosis disease activity. J. Med. Virol. 2003, 69, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lafuente, R.; De Las Heras, V.; Bartolome, M.; Picazo, J.J.; Arroyo, R. Beta-interferon treatment reduces human herpesvirus-6 viral load in multiple sclerosis relapses but not in remission. Eur. Neurol. 2004, 52, 87–91. [Google Scholar] [CrossRef]
- Rotola, A.; Merlotti, I.; Caniatti, L.; Caselli, E.; Granieri, E.; Tola, M.R.; Di Luca, D.; Cassai, E. Human herpesvirus 6 infects the central nervous system of multiple sclerosis patients in the early stages of the disease. Mult. Scler. 2004, 10, 348–354. [Google Scholar] [CrossRef]
- Derfuss, T.; Hohlfeld, R.; Meinl, E. Intrathecal antibody (IgG) production against human herpesvirus type 6 occurs in about 20% of multiple sclerosis patients and might be linked to a polyspecific B-cell response. J. Neurol. 2005, 252, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Fogdell-Hahn, A.; Soldan, S.S.; Shue, S.; Akhyani, N.; Refai, H.; Ahlqvist, J.; Jacobson, S. Co-purification of soluble membrane cofactor protein (CD46) and human herpesvirus 6 variant A genome in serum from multiple sclerosis patients. Virus Res. 2005, 110, 57–63. [Google Scholar] [CrossRef]
- Hollsberg, P.; Kusk, M.; Bech, E.; Hansen, H.J.; Jakobsen, J.; Haahr, S. Presence of Epstein-Barr virus and human herpesvirus 6B DNA in multiple sclerosis patients: Associations with disease activity. Acta Neurol. Scand. 2005, 112, 395–402. [Google Scholar] [CrossRef]
- Alvarez-Lafuente, R.; Garcia-Montojo, M.; De las Heras, V.; Bartolome, M.; Arroyo, R. Clinical parameters and HHV-6 active replication in relapsing-remitting multiple sclerosis patients. J. Clin. Virol. 2006, 37 (Suppl. S1), S24–S26. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lafuente, R.; de las Heras, V.; Garcia-Montojo, M.; Bartolome, M.; Arroyo, R. Human herpesvirus-6 and multiple sclerosis: Relapsing-remitting vs. secondary progressive. Mult. Scler. 2007, 13, 578–583. [Google Scholar] [CrossRef]
- Virtanen, J.O.; Farkkila, M.; Multanen, J.; Uotila, L.; Jaaskelainen, A.J.; Vaheri, A.; Koskiniemi, M. Evidence for human herpesvirus 6 variant A antibodies in multiple sclerosis: Diagnostic and therapeutic implications. J. Neurovirol. 2007, 13, 347–352. [Google Scholar] [CrossRef]
- Kuusisto, H.; Hyoty, H.; Kares, S.; Kinnunen, E.; Elovaara, I. Human herpes virus 6 and multiple sclerosis: A Finnish twin study. Mult. Scler. 2008, 14, 54–58. [Google Scholar] [CrossRef]
- Alvarez-Lafuente, R.; Garcia-Montojo, M.; De Las Heras, V.; Dominguez-Mozo, M.I.; Bartolome, M.; Arroyo, R. CD46 expression and HHV-6 infection in patients with multiple sclerosis. Acta Neurol. Scand. 2009, 120, 246–250. [Google Scholar] [CrossRef]
- Mancuso, R.; Hernis, A.; Cavarretta, R.; Caputo, D.; Calabrese, E.; Nemni, R.; Ferrante, P.; Delbue, S.; Clerici, M. Detection of viral DNA sequences in the cerebrospinal fluid of patients with multiple sclerosis. J. Med. Virol. 2010, 82, 1051–1057. [Google Scholar] [CrossRef]
- Behzad-Behbahani, A.; Mikaeili, M.H.; Entezam, M.; Mojiri, A.; Pour, G.Y.; Arasteh, M.M.; Rahsaz, M.; Banihashemi, M.; Khadang, B.; Moaddeb, A.; et al. Human herpesvirus-6 viral load and antibody titer in serum samples of patients with multiple sclerosis. J. Microbiol. Immunol. Infect. 2011, 44, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montojo, M.; De Las Heras, V.; Dominguez-Mozo, M.; Bartolome, M.; Garcia-Martinez, M.A.; Arroyo, R.; Alvarez-Lafuente, R.; HHV-6 and Multiple Sclerosis Study Group. Human herpesvirus 6 and effectiveness of interferon beta1b in multiple sclerosis patients. Eur. J. Neurol. 2011, 18, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Nora-Krukle, Z.; Chapenko, S.; Logina, I.; Millers, A.; Platkajis, A.; Murovska, M. Human herpesvirus 6 and 7 reactivation and disease activity in multiple sclerosis. Medicina 2011, 47, 527–531. [Google Scholar] [CrossRef]
- Virtanen, J.O.; Pietilainen-Nicklen, J.; Uotila, L.; Farkkila, M.; Vaheri, A.; Koskiniemi, M. Intrathecal human herpesvirus 6 antibodies in multiple sclerosis and other demyelinating diseases presenting as oligoclonal bands in cerebrospinal fluid. J. Neuroimmunol. 2011, 237, 93–97. [Google Scholar] [CrossRef]
- Dominguez-Mozo, M.I.; Garcia-Montojo, M.; De Las Heras, V.; Garcia-Martinez, A.; Arias-Leal, A.M.; Casanova, I.; Arroyo, R.; Alvarez-Lafuente, R. MHC2TA mRNA levels and human herpesvirus 6 in multiple sclerosis patients treated with interferon beta along two-year follow-up. BMC Neurol. 2012, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Ramroodi, N.; Sanadgol, N.; Ganjali, Z.; Niazi, A.A.; Sarabandi, V.; Moghtaderi, A. Monitoring of active human herpes virus 6 infection in Iranian patients with different subtypes of multiple sclerosis. J. Pathog. 2013, 2013, 194932. [Google Scholar] [CrossRef]
- Alenda, R.; Alvarez-Lafuente, R.; Costa-Frossard, L.; Arroyo, R.; Mirete, S.; Alvarez-Cermeno, J.C.; Villar, L.M. Identification of the major HHV-6 antigen recognized by cerebrospinal fluid IgG in multiple sclerosis. Eur. J. Neurol. 2014, 21, 1096–1101. [Google Scholar] [CrossRef]
- Ortega-Madueno, I.; Garcia-Montojo, M.; Dominguez-Mozo, M.I.; Garcia-Martinez, A.; Arias-Leal, A.M.; Casanova, I.; Arroyo, R.; Alvarez-Lafuente, R. Anti-human herpesvirus 6A/B IgG correlates with relapses and progression in multiple sclerosis. PLoS ONE 2014, 9, e104836. [Google Scholar] [CrossRef]
- Simpson, S., Jr.; Taylor, B.; Burrows, J.; Burrows, S.; Dwyer, D.E.; Taylor, J.; Ponsonby, A.L.; Blizzard, L.; Dwyer, T.; Pittas, F.; et al. EBV & HHV6 reactivation is infrequent and not associated with MS clinical course. Acta Neurol. Scand. 2014, 130, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Kofahi, R.M.; Kofahi, H.M.; Sabaheen, S.; Qawasmeh, M.A.; Momani, A.; Yassin, A.; Alhayk, K.; El-Salem, K. Prevalence of seropositivity of selected herpesviruses in patients with multiple sclerosis in the North of Jordan. BMC Neurol. 2020, 20, 397. [Google Scholar] [CrossRef] [PubMed]
- Arvin, A.M. Varicella-zoster virus. Clin. Microbiol. Rev. 1996, 9, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Manouchehrinia, A.; Tanasescu, R.; Kareem, H.; Jerca, O.P.; Jabeen, F.; Shafei, R.; Breuer, J.; Neal, K.; Irving, W.; Constantinescu, C.S. Prevalence of a history of prior varicella/herpes zoster infection in multiple sclerosis. J. Neurovirol. 2017, 23, 839–844. [Google Scholar] [CrossRef]
- Ciancia, S.; Crisafi, A.; Fontana, I.; De Fanti, A.; Amarri, S.; Iughetti, L. Encephalitis due to herpes zoster without rash in an immunocompetent 12-year-old girl: Case report and review of the literature. BMC Pediatr. 2020, 20, 348. [Google Scholar] [CrossRef]
- Ross, R.T.; Cheang, M.; Landry, G.; Klassen, L.; Doerksen, K. Herpes zoster and multiple sclerosis. Can. J. Neurol. Sci. 1999, 26, 29–32. [Google Scholar] [PubMed]
- Ross, R.T. The varicella-zoster virus and multiple sclerosis. J. Clin. Epidemiol. 1998, 51, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Barron, A.L.; Olszewski, W.A.; Milgrom, F. Antibody titers by mixed agglutination to varicella-zoster, herpes simplex and vaccinia viruses in patients with multiple sclerosis. Proc. Soc. Exp. Biol. Med. 1975, 149, 835–839. [Google Scholar] [CrossRef]
- Ordonez, G.; Martinez-Palomo, A.; Corona, T.; Pineda, B.; Flores-Rivera, J.; Gonzalez, A.; Chavez-Munguia, B.; Sotelo, J. Varicella zoster virus in progressive forms of multiple sclerosis. Clin. Neurol. Neurosurg. 2010, 112, 653–657. [Google Scholar] [CrossRef]
- Dudek, M.I.R.; Thies, K.; Kammenhuber, S.; Bosel, J.; Rosche, J. HSV-2-encephalitis in a patient with multiple sclerosis treated with ocrelizumab. J. Neurol. 2019, 266, 2322–2323. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.; Ordonez, G.; Pineda, B. Varicella-zoster virus at relapses of multiple sclerosis. J. Neurol. 2007, 254, 493–500. [Google Scholar] [CrossRef]
- Sotelo, J.; Corona, T. Varicella zoster virus and relapsing remitting multiple sclerosis. Mult. Scler. Int. 2011, 2011, 214763. [Google Scholar] [CrossRef]
- Sotelo, J.; Ordonez, G.; Pineda, B.; Flores, J. The participation of varicella zoster virus in relapses of multiple sclerosis. Clin. Neurol. Neurosurg. 2014, 119, 44–48. [Google Scholar] [CrossRef]
- Sotelo, J.; Martinez-Palomo, A.; Ordonez, G.; Pineda, B. Varicella-zoster virus in cerebrospinal fluid at relapses of multiple sclerosis. Ann. Neurol. 2008, 63, 303–311. [Google Scholar] [CrossRef]
- Marrie, R.A.; Wolfson, C. Multiple sclerosis and varicella zoster virus infection: A review. Epidemiol. Infect. 2001, 127, 315–325. [Google Scholar] [CrossRef]
- Pfender, N.; Jelcic, I.; Linnebank, M.; Schwarz, U.; Martin, R. Reactivation of herpesvirus under fingolimod: A case of severe herpes simplex encephalitis. Neurology 2015, 84, 2377–2378. [Google Scholar] [CrossRef]
- Perini, P.; Rinaldi, F.; Puthenparampil, M.; Marcon, M.; Perini, F.; Gallo, P. Herpes simplex virus encephalitis temporally associated with dimethyl fumarate-induced lymphopenia in a multiple sclerosis patient. Mult. Scler. Relat. Disord. 2018, 26, 68–70. [Google Scholar] [CrossRef]
- Staras, S.A.; Dollard, S.C.; Radford, K.W.; Flanders, W.D.; Pass, R.F.; Cannon, M.J. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin. Infect. Dis. 2006, 43, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Dowd, J.B.; Aiello, A.E.; Alley, D.E. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol. Infect. 2009, 137, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Ghane, M.; Poortahmasebi, V.; Jazayeri, S.M.; Yousefzadeh-Chabok, S. Prevalence of Cytomegalovirus in Patients with Multiple Sclerosis: A Case-Control Study in Northern Iran. Jundishapur J. Microbiol. 2016, 9, e36582. [Google Scholar] [CrossRef] [PubMed]
- Sanadgol, N.; Ramroodi, N.; Ahmadi, G.A.; Komijani, M.; Moghtaderi, A.; Bouzari, M.; Rezaei, M.; Kardi, M.T.; Dabiri, S.; Moradi, M.; et al. Prevalence of cytomegalovirus infection and its role in total immunoglobulin pattern in Iranian patients with different subtypes of multiple sclerosis. New Microbiol. 2011, 34, 263–274. [Google Scholar] [PubMed]
- Clerico, M.; De Mercanti, S.; Artusi, C.A.; Durelli, L.; Naismith, R.T. Active CMV infection in two patients with multiple sclerosis treated with alemtuzumab. Mult. Scler. 2017, 23, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Vanheusden, M.; Stinissen, P.; t Hart, B.A.; Hellings, N. Cytomegalovirus: A culprit or protector in multiple sclerosis? Trends Mol. Med. 2015, 21, 16–23. [Google Scholar] [CrossRef]
- Zivadinov, R.; Nasuelli, D.; Tommasi, M.A.; Serafin, M.; Bratina, A.; Ukmar, M.; Pirko, I.; Johnson, A.J.; Furlan, C.; Pozzi-Mucelli, R.S.; et al. Positivity of cytomegalovirus antibodies predicts a better clinical and radiological outcome in multiple sclerosis patients. Neurol. Res. 2006, 28, 262–269. [Google Scholar] [CrossRef]
- Waubant, E.; Mowry, E.M.; Krupp, L.; Chitnis, T.; Yeh, E.A.; Kuntz, N.; Ness, J.; Chabas, D.; Strober, J.; McDonald, J.; et al. Common viruses associated with lower pediatric multiple sclerosis risk. Neurology 2011, 76, 1989–1995. [Google Scholar] [CrossRef]
- Sundqvist, E.; Bergstrom, T.; Daialhosein, H.; Nystrom, M.; Sundstrom, P.; Hillert, J.; Alfredsson, L.; Kockum, I.; Olsson, T. Cytomegalovirus seropositivity is negatively associated with multiple sclerosis. Mult. Scler. 2014, 20, 165–173. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Wu, J.; Lucas, R.; Smith, J.; Gonzales, E.; Amezcua, L.; Haraszti, S.; Chen, L.H.; Quach, H.; James, J.A.; et al. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: A multiethnic study. Neurology 2017, 89, 1330–1337. [Google Scholar] [CrossRef]
- Alari-Pahissa, E.; Moreira, A.; Zabalza, A.; Alvarez-Lafuente, R.; Munteis, E.; Vera, A.; Arroyo, R.; Alvarez-Cermeno, J.C.; Villar, L.M.; Lopez-Botet, M.; et al. Low cytomegalovirus seroprevalence in early multiple sclerosis: A case for the ‘hygiene hypothesis’? Eur. J. Neurol. 2018, 25, 925–933. [Google Scholar] [CrossRef]
- Pakpoor, J.; Pakpoor, J.; Disanto, G.; Giovannoni, G.; Ramagopalan, S.V. Cytomegalovirus and multiple sclerosis risk. J. Neurol. 2013, 260, 1658–1660. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Claeys, D.; Faller, G.; Appelmelk, B.J.; Negrini, R.; Kirchner, T. The gastric H+,K+-ATPase is a major autoantigen in chronic Helicobacter pylori gastritis with body mucosa atrophy. Gastroenterology 1998, 115, 340–347. [Google Scholar] [CrossRef]
- Kountouras, J.; Deretzi, G.; Zavos, C.; Karatzoglou, P.; Touloumis, L.; Nicolaides, T.; Chatzopoulos, D.; Venizelos, I. Association between Helicobacter pylori infection and acute inflammatory demyelinating polyradiculoneuropathy. Eur. J. Neurol. 2005, 12, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kountouras, J.; Zavos, C.; Chatzopoulos, D. A concept on the role of Helicobacter pylori infection in autoimmune pancreatitis. J. Cell Mol. Med. 2005, 9, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Sokratous, M.; Tsouris, Z.; Siokas, V.; Mentis, A.A.; Aloizou, A.M.; Michalopoulou, A.; Bogdanos, D.P.; Xiromerisiou, G.; Deretzi, G.; et al. Association between Helicobacter pylori infection and Guillain-Barre Syndrome: A meta-analysis. Eur. J. Clin. Investig. 2020, 50, e13218. [Google Scholar] [CrossRef]
- Moran, A.P.; Prendergast, M.M. Molecular mimicry in Campylobacter jejuni and Helicobacter pylori lipopolysaccharides: Contribution of gastrointestinal infections to autoimmunity. J. Autoimmun. 2001, 16, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, D.; Abdollahi, A.; Ardekani, A.; Razavian, I.; Razavian, E.; Sartip, B.; Mahjour, S.; Parsa, H.; Kyvanani, N.A.; Marhoommirzabak, E.; et al. Helicobacter pylori infection and risk of multiple sclerosis: An updated meta-analysis. Helicobacter 2022, 27, e12927. [Google Scholar] [CrossRef]
- Lopes, A.I.; Vale, F.F.; Oleastro, M. Helicobacter pylori infection—Recent developments in diagnosis. World J. Gastroenterol. 2014, 20, 9299–9313. [Google Scholar] [CrossRef]
- Bordin, D.S.; Voynovan, I.N.; Andreev, D.N.; Maev, I.V. Current Helicobacter pylori Diagnostics. Diagnostics 2021, 11, 1458. [Google Scholar] [CrossRef]
- Gavalas, E.; Kountouras, J.; Deretzi, G.; Boziki, M.; Grigoriadis, N.; Zavos, C.; Venizelos, I. Helicobacter pylori and multiple sclerosis. J. Neuroimmunol. 2007, 188, 187–189; author reply 190. [Google Scholar] [CrossRef]
- Li, W.; Minohara, M.; Piao, H.; Matsushita, T.; Masaki, K.; Matsuoka, T.; Isobe, N.; Su, J.J.; Ohyagi, Y.; Kira, J. Association of anti-Helicobacter pylori neutrophil-activating protein antibody response with anti-aquaporin-4 autoimmunity in Japanese patients with multiple sclerosis and neuromyelitis optica. Mult. Scler. 2009, 15, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Zarkesh, H.; Mostajeran, M.; Etemadifar, M.; Maghzi, A.H. The relationship between multiple sclerosis and Helicobacter pylori infection. In Proceedings of the Multiple Sclerosis; SAGE: Thousand Oaks, CA, USA, 2009. [Google Scholar]
- Sanadgol, N.; Shahraki, E.; Estakhr, J. Relationship between Helicobacter pylori (H. pylori) infection and Multiple sclerosis (MS) in southeast of Iran. Afr. J. Microbiol. Res. 2012, 6, 1411–1421. [Google Scholar]
- Long, Y.; Gao, C.; Qiu, W.; Hu, X.; Shu, Y.; Peng, F.; Lu, Z. Helicobacter pylori infection in Neuromyelitis Optica and Multiple Sclerosis. Neuroimmunomodulation 2013, 20, 107–112. [Google Scholar] [CrossRef]
- Mohebi, N.; Mamarabadi, M.; Moghaddasi, M. Relation of Helicobacter pylori infection and multiple sclerosis in Iranian patients. Neurol. Int. 2013, 5, 31–33. [Google Scholar] [CrossRef]
- Yoshimura, S.; Isobe, N.; Matsushita, T.; Yonekawa, T.; Masaki, K.; Sato, S.; Kawano, Y.; Kira, J.; South Japan Multiple Sclerosis Genetics, C. Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J. Neurol. Neurosurg. Psychiatry 2013, 84, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.W.; Crooks, J.; Hussain, K.; O’Brien, K.; Braitch, M.; Kareem, H.; Constantinescu, C.S.; Robinson, K.; Gran, B. Helicobacter pylori infection reduces disease severity in an experimental model of multiple sclerosis. Front. Microbiol. 2015, 6, 52. [Google Scholar] [CrossRef]
- Gavalas, E.; Kountouras, J.; Boziki, M.; Zavos, C.; Polyzos, S.A.; Vlachaki, E.; Venizelos, I.; Tsiptsios, D.; Deretzi, G. Relationship between Helicobacter pylori infection and multiple sclerosis. Ann. Gastroenterol. 2015, 28, 353–356. [Google Scholar]
- Malli, C.; Pandit, L.; D’Cunha, A.; Mustafa, S. Environmental factors related to multiple sclerosis in Indian population. PLoS ONE 2015, 10, e0124064. [Google Scholar] [CrossRef]
- Pedrini, M.J.; Seewann, A.; Bennett, K.A.; Wood, A.J.; James, I.; Burton, J.; Marshall, B.J.; Carroll, W.M.; Kermode, A.G. Helicobacter pylori infection as a protective factor against multiple sclerosis risk in females. J. Neurol. Neurosurg. Psychiatry 2015, 86, 603–607. [Google Scholar] [CrossRef]
- Riskind, P.N.; Stoicov, C.; Houghton, J.; Cooper, C.; Kane, K.; Weaver, J.; Beyroutey, R.; O’Leary, K. Prevalence of Helicobacter pylori seropositivity (HP plus) in American patients with multiple sclerosis. Mult. Scler. 2010, 16, 1008. [Google Scholar]
- Efthymiou, G.; Dardiotis, E.; Liaskos, C.; Marou, E.; Tsimourtou, V.; Rigopoulou, E.I.; Scheper, T.; Daponte, A.; Meyer, W.; Sakkas, L.I.; et al. Immune responses against Helicobacter pylori-specific antigens differentiate relapsing remitting from secondary progressive multiple sclerosis. Sci. Rep. 2017, 7, 7929. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Karampoor, S.; Jalilian, F.A. The protective effect of Helicobacter pylori infection on the susceptibility of multiple sclerosis. J. Neuroimmunol. 2019, 337, 577069. [Google Scholar] [CrossRef] [PubMed]
- Kiani, S.; Vakilian, A.; Kamiab, Z.; Shamsizadeh, A. Correlation of Dietary Intake and Helicobacter pylori Infection with Multiple Sclerosis, a Case-Control Study in Rafsanjan, Iran, 2017–18. Qatar Med. J. 2020, 2020, 45. [Google Scholar] [CrossRef] [PubMed]
- Mirmosayyeb, O.; Barzegar, M.; Nehzat, N.; Najdaghi, S.; Ansari, B.; Shaygannejad, V. Association of Helicobacter pylori with multiple sclerosis: Protective or risk factor? Curr. J. Neurol. 2020, 19, 59–66. [Google Scholar] [CrossRef]
- Zahedi, M.J.; Shafiee, K.; Abbasi, M.M.H.; Shafieipour, S.; Afshar, R.M.; Rohani, M.; Pooshani, A.; Robati, F.K.; Dehgani, A. Patients with Newly Diagnosed Multiple Sclerosis Are Less Seropositive for Helicobacter pylori Infection: A Case Control Study in Iran. Govaresh 2021, 26, 113–117. [Google Scholar]
- Gaydos, C.A. Chlamydia pneumoniae and its proposed link to multiple sclerosis: To be or not to be? Neurology 2001, 56, 1126–1127. [Google Scholar] [CrossRef]
- Porritt, R.A.; Crother, T.R. Chlamydia pneumoniae Infection and Inflammatory Diseases. For. Immunopathol. Dis. Ther. 2016, 7, 237–254. [Google Scholar] [CrossRef]
- Landry, R.L.; Embers, M.E. Does Dementia Have a Microbial Cause? NeuroSci 2022, 3, 262–283. [Google Scholar] [CrossRef]
- Perlmutter, L.J.; Darvish, M. Possible relationship of Chlamydia to multiple sclerosis. Med. Hypotheses 1983, 12, 95–98. [Google Scholar] [CrossRef]
- Sriram, S.; Mitchell, W.; Stratton, C. Multiple sclerosis associated with Chlamydia pneumoniae infection of the CNS. Neurology 1998, 50, 571–572. [Google Scholar] [CrossRef]
- Sriram, S.; Stratton, C.W.; Yao, S.; Tharp, A.; Ding, L.; Bannan, J.D.; Mitchell, W.M. Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis. Ann. Neurol. 1999, 46, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Layh-Schmitt, G.; Bendl, C.; Hildt, U.; Dong-Si, T.; Juttler, E.; Schnitzler, P.; Grond-Ginsbach, C.; Grau, A.J. Evidence for infection with Chlamydia pneumoniae in a subgroup of patients with multiple sclerosis. Ann. Neurol. 2000, 47, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Krametter, D.; Niederwieser, G.; Berghold, A.; Birnbaum, G.; Strasser-Fuchs, S.; Hartung, H.P.; Archelos, J.J. Chlamydia pneumoniae in multiple sclerosis: Humoral immune responses in serum and cerebrospinal fluid and correlation with disease activity marker. Mult. Scler. 2001, 7, 13–18. [Google Scholar] [CrossRef]
- Fainardi, E.; Castellazzi, M.; Seraceni, S.; Granieri, E.; Contini, C. Under the microscope: Focus on Chlamydia pneumoniae infection and multiple sclerosis. Curr. Neurovasc. Res. 2008, 5, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Stratton, C.W.; Wheldon, D.B. Multiple sclerosis: An infectious syndrome involving Chlamydophila pneumoniae. Trends Microbiol. 2006, 14, 474–479. [Google Scholar] [CrossRef]
- Hammerschlag, M.R.; Ke, Z.; Lu, F.; Roblin, P.; Boman, J.; Kalman, B. Is Chlamydia pneumoniae present in brain lesions of patients with multiple sclerosis? J. Clin. Microbiol. 2000, 38, 4274–4276. [Google Scholar] [CrossRef]
- Ke, Z.; Lu, F.; Roblin, P.; Boman, J.; Hammerschlag, M.R.; Kalman, B. Lack of detectable Chlamydia pneumoniae in brain lesions of patients with multiple sclerosis. Ann. Neurol. 2000, 48, 400. [Google Scholar] [CrossRef]
- Morre, S.A.; De Groot, C.J.; Killestein, J.; Meijer, C.J.; Polman, C.H.; Van der Valk, P.; Van den Brule, A.J. Is Chlamydia pneumoniae present in the central nervous system of multiple sclerosis patients? Ann. Neurol. 2000, 48, 399. [Google Scholar] [CrossRef]
- Pucci, E.; Taus, C.; Cartechini, E.; Morelli, M.; Giuliani, G.; Clementi, M.; Menzo, S. Lack of Chlamydia infection of the central nervous system in multiple sclerosis. Ann. Neurol. 2000, 48, 399–400. [Google Scholar] [CrossRef]
- Sotgiu, S.; Piana, A.; Pugliatti, M.; Sotgiu, A.; Deiana, G.A.; Sgaramella, E.; Muresu, E.; Rosati, G. Chlamydia pneumoniae in the cerebrospinal fluid of patients with multiple sclerosis and neurological controls. Mult. Scler. 2001, 7, 371–374. [Google Scholar] [CrossRef]
- Gieffers, J.; Pohl, D.; Treib, J.; Dittmann, R.; Stephan, C.; Klotz, K.; Hanefeld, F.; Solbach, W.; Haass, A.; Maass, M. Presence of Chlamydia pneumoniae DNA in the cerebral spinal fluid is a common phenomenon in a variety of neurological diseases and not restricted to multiple sclerosis. Ann. Neurol. 2001, 49, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.L.; Peeling, R.W.; Hernan, M.A.; Chasan-Taber, L.; Olek, M.J.; Hankinson, S.E.; Hunter, D.; Ascherio, A. Infection with Chlamydia pneumoniae and risk of multiple sclerosis. Epidemiology 2003, 14, 141–147. [Google Scholar] [CrossRef]
- Bashir, K.; Kaslow, R.A. Chlamydia pneumoniae and multiple sclerosis: The latest etiologic candidate. Epidemiology 2003, 14, 133–134. [Google Scholar] [CrossRef]
- Contini, C.; Seraceni, S.; Cultrera, R.; Castellazzi, M.; Granieri, E.; Fainardi, E. Chlamydophila pneumoniae Infection and Its Role in Neurological Disorders. Interdiscip. Perspect. Infect. Dis. 2010, 2010, 273573. [Google Scholar] [CrossRef]
- Contini, C.; Seraceni, S.; Castellazzi, M.; Granieri, E.; Fainardi, E. Chlamydophila pneumoniae DNA and mRNA transcript levels in peripheral blood mononuclear cells and cerebrospinal fluid of patients with multiple sclerosis. Neurosci. Res. 2008, 62, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Contini, C. International conference on chlamydial and Mycoplasma human infections. Future Microbiol. 2007, 2, 373–376. [Google Scholar] [CrossRef]
- Pachner, A.R. Neurologic manifestations of Lyme disease, the new “great imitator”. Rev. Infect. Dis. 1989, 11 (Suppl. S6), S1482–S1486. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.K.; Blacklock, J.W.S.; M’Cluskie, J.A.W. Spirochetes in the ventricular fluid of monkeys inoculated from cases of disseminated sclerosis. J. Pathol. Bacteriol. 1925, 28, 117–118. [Google Scholar] [CrossRef]
- Steiner, G. Acute plaques in multiple sclerosis, their pathogenetic significance and the role of spirochetes as etiological factor. J Neuropathol. Exp. Neurol. 1952, 11, 343–372. [Google Scholar] [CrossRef]
- Ichelson, R.R. Cultivation of spirochaetes from spinal fluids of multiple sclerosis cases and negative controls. Proc. Soc. Exp. Biol. Med. 1957, 95, 57–58. [Google Scholar] [CrossRef]
- Newman, H.W.; Purdy, C.; Rantz, L.; Hill, F.C., Jr. The spirochete and multiple sclerosis. Calif. Med. 1958, 89, 387–389. [Google Scholar]
- Nocton, J.J.; Bloom, B.J.; Rutledge, B.J.; Persing, D.H.; Logigian, E.L.; Schmid, C.H.; Steere, A.C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in Lyme neuroborreliosis. J. Infect. Dis. 1996, 174, 623–627. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Iranpour, S.; Adineh, H.A.; Aliyari, R. Antibiotic use and multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2023, 75, 104765. [Google Scholar] [CrossRef]
- Schmutzhard, E.; Pohl, P.; Stanek, G. Borrelia burgdorferi antibodies in patients with relapsing/remitting form and chronic progressive form of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1215–1218. [Google Scholar] [CrossRef]
- Marshall, V. Multiple sclerosis is a chronic central nervous system infection by a spirochetal agent. Med. Hypotheses 1988, 25, 89–92. [Google Scholar] [CrossRef]
- Coyle, P.K. Borrelia burgdorferi antibodies in multiple sclerosis patients. Neurology 1989, 39, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Monco, J.C.; Miro Jornet, J.; Fernandez Villar, B.; Benach, J.L.; Guerrero Espejo, A.; Berciano, J.A. Multiple sclerosis or Lyme disease? a diagnosis problem of exclusion. Med. Clin. 1990, 94, 685–688. [Google Scholar]
- Heller, J.; Holzer, G.; Schimrigk, K. Immunological differentiation between neuroborreliosis and multiple sclerosis. J. Neurol. 1990, 237, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Coyle, P.K.; Krupp, L.B.; Doscher, C. Significance of reactive Lyme serology in multiple sclerosis. Ann. Neurol. 1993, 34, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Lana-Peixoto, M.A. Multiple sclerosis and positive Lyme serology. Arq. Neuropsiquiatr. 1994, 52, 566–571. [Google Scholar] [CrossRef]
- Chmielewska-Badora, J.; Cisak, E.; Dutkiewicz, J. Lyme borreliosis and multiple sclerosis: Any connection? A seroepidemic study. Ann. Agric. Environ. Med. 2000, 7, 141–143. [Google Scholar]
- Cheema, J.; Huynh, A.C.; Prat, S.S. Multiple Sclerosis and psychosis: A case report. Mult. Scler. Relat. Disord. 2019, 34, 158–161. [Google Scholar] [CrossRef]
- MacLean, G.; Cook, P.; Lindsay, L.R.; Hatchette, T.F.; Webster, D. Low Seroprevalence of Lyme Disease Among Multiple Sclerosis Patients in New Brunswick. Can. J. Neurol. Sci. 2020, 47, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Stricker, R.B.; Johnson, L. ‘Rare’ infections mimicking multiple sclerosis: Consider Lyme disease. Clin. Neurol. Neurosurg. 2011, 113, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Vatne, A.; Mygland, A.; Ljostad, U. Multiple sclerosis in Vest-Agder County, Norway. Acta Neurol. Scand. 2011, 123, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.D.; Kugeler, K.J.; Perea, A.E.; Pastula, D.M.; Mead, P.S. No Geographic Correlation between Lyme Disease and Death Due to 4 Neurodegenerative Disorders, United States, 2001–2010. Emerg. Infect. Dis. 2015, 21, 2036–2039. [Google Scholar] [CrossRef]
- Cossu, D.; Cocco, E.; Paccagnini, D.; Masala, S.; Ahmed, N.; Frau, J.; Marrosu, M.G.; Sechi, L.A. Association of Mycobacterium avium subsp. paratuberculosis with multiple sclerosis in Sardinian patients. PLoS ONE 2011, 6, e18482. [Google Scholar] [CrossRef]
- Cossu, D.; Masala, S.; Frau, J.; Mameli, G.; Marrosu, M.G.; Cocco, E.; Sechi, L.A. Antigenic epitopes of MAP2694 homologous to T-cell receptor gamma-chain are highly recognized in multiple sclerosis Sardinian patients. Mol. Immunol. 2014, 57, 138–140. [Google Scholar] [CrossRef]
- Cossu, D.; Yokoyama, K.; Hattori, N. Conflicting Role of Mycobacterium Species in Multiple Sclerosis. Front. Neurol. 2017, 8, 216. [Google Scholar] [CrossRef]
- Ristori, G.; Buzzi, M.G.; Sabatini, U.; Giugni, E.; Bastianello, S.; Viselli, F.; Buttinelli, C.; Ruggieri, S.; Colonnese, C.; Pozzilli, C.; et al. Use of Bacille Calmette-Guerin (BCG) in multiple sclerosis. Neurology 1999, 53, 1588–1589. [Google Scholar] [CrossRef]
- Covian, C.; Fernandez-Fierro, A.; Retamal-Diaz, A.; Diaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; Gonzalez, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef] [PubMed]
| References | Year | Diagnostic Method | Number of MS Patients | H. pylori Positive | Number of Controls | H. pylori Positive |
|---|---|---|---|---|---|---|
| Gavalas et al. [180] | 2007 | Histology | 29 | 24 | 25 | 12 |
| Li et al. [181] | 2009 | ELISA | 162 | 67 | 85 | 36 |
| Zarkesh et al. [182] | 2009 | ELISA | 210 | 11 | 200 | 9 |
| Ramroodi et al. [183] | 2012 | Western blot | 78 | 20 | 123 | 27 |
| Long et al. [184] | 2013 | Immunofluorescence | 42 | 31 | 27 | 16 |
| Mohebi et al. [185] | 2013 | ELISA | 163 | 88 | 150 | 110 |
| Yoshimura et al. [186] | 2013 | ELISA | 71 | 15 | 42 | 14 |
| Cook et al. [187] | 2015 | Histology | 44 | 38 | 20 | 10 |
| Gavalas et al. [188] | 2015 | ELISA | 139 | 31 | 139 | 64 |
| Malli et al. [189] | 2015 | ELISA | 550 | 73 | 299 | 64 |
| Pedrini et al. [190] | 2015 | ELISA | 149 | 34 | 150 | 49 |
| Riskind et al. [191] | 2016 | Latex agglutination | 139 | 60 | 68 | 33 |
| Efthymiou et al. [192] | 2017 | ELISA | 386 | 188 | 420 | 298 |
| Ranjbar et al. [193] | 2019 | ELISA | 92 | 66 | 91 | 78 |
| Kiani et al. [194] | 2020 | ELISA | 154 | 74 | 39 | 11 |
| Mirmosayyeb et al. [195] | 2020 | ELISA | 127 | 44 | 177 | 74 |
| Zahedi et al. [196] | 2021 | ELISA | 71 | 44 | 145 | 111 |
| Source | Conclusions |
|---|---|
| Adams et al., 1925 [219] | Researchers inoculated rhesus monkeys with material from MS patients. Spirochetes emerged in CSF after several months. |
| Steiner, 1952 [220] | Reported the presence of spirochetes in plaques obtained from autopsied MS patients. |
| Ichelson, 1957 [221] | New culture medium allowed for growth of spirochetes from CSF of MS cases. |
| Newman et al., 1958 [222] | Replicated Ichelson’s culture method and detected spirochetes in spinal fluid in 18.5% of MS patients. |
| Schmutzhard, 1988 [225] | There is no etiologic association between Borrelia and the relapsing/remitting form of multiple sclerosis. |
| Marshall, 1988 [226] | Medical practitioners and researchers should consider using antibiotics as treatment for MS in patients who do not respond to treatment. |
| Coyle, 1989 [227] | Infection with B. burgdorferi is rare in MS and unlikely to play a significant role in MS. |
| Garcia-Monco et al., 1990 [228] | Researchers evaluated 55 patients with a definite diagnosis of multiple sclerosis and found Lyme disease infection in 3. |
| Heller et al., 1990 [229] | ELISA assay can substantiate the diagnosis of neuroborreliosis to rule it out in MS patients with positive Borrelia serology. |
| Coyle et al., 1993 [230] | Lyme serology in MS patients with no suggestive features was unlikely to indicate neurological Lyme disease. |
| Lana-Peixoto, 1994 [231] | A 45-year-old MS patient was infected with Borrelia burgdorferi, confirmed by ELISA and Western blotting. The relationship between spirochetal infection and neurological disease could not be ascertained. |
| Chmielewska-Badora et al., 2000 [232] | A statistically significant relationship was found between clinically confirmed diagnosis of MS and positive serologic reaction with Borrelia antigen. |
| Cheema et al., 2019 [233] | Case report of co-occurrence of MS and psychiatric features of Lyme borreliosis. |
| MacLean et al., 2020 [234] | No positive serological evidence of Lyme disease was identified in MS patients in Atlantic Canada. |
| Stricker and Johnson, 2011 [235] | Serology or CSF testing may lead to oversight of a considerable proportion of patients with neuroborreliosis, often resulting in failure to diagnose and address a condition that resembles MS. |
| Vatne et al., 2011 [236] | Researchers did not detect a higher frequency of Bb antibodies in serum from patients with MS compared to controls. |
| Forrester et al., 2015 [237] | No geographic correlation between Lyme disease and deaths due to multiple sclerosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landry, R.L.; Embers, M.E. The Probable Infectious Origin of Multiple Sclerosis. NeuroSci 2023, 4, 211-234. https://doi.org/10.3390/neurosci4030019
Landry RL, Embers ME. The Probable Infectious Origin of Multiple Sclerosis. NeuroSci. 2023; 4(3):211-234. https://doi.org/10.3390/neurosci4030019
Chicago/Turabian StyleLandry, Remi L., and Monica E. Embers. 2023. "The Probable Infectious Origin of Multiple Sclerosis" NeuroSci 4, no. 3: 211-234. https://doi.org/10.3390/neurosci4030019
APA StyleLandry, R. L., & Embers, M. E. (2023). The Probable Infectious Origin of Multiple Sclerosis. NeuroSci, 4(3), 211-234. https://doi.org/10.3390/neurosci4030019






