Localization of Thioredoxin-Interacting Protein in Aging and Alzheimer’s Disease Brains
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Brain Tissue Samples
2.2. Brain Tissue Preservation and Fixation
2.3. Neuropathological Diagnosis Criteria
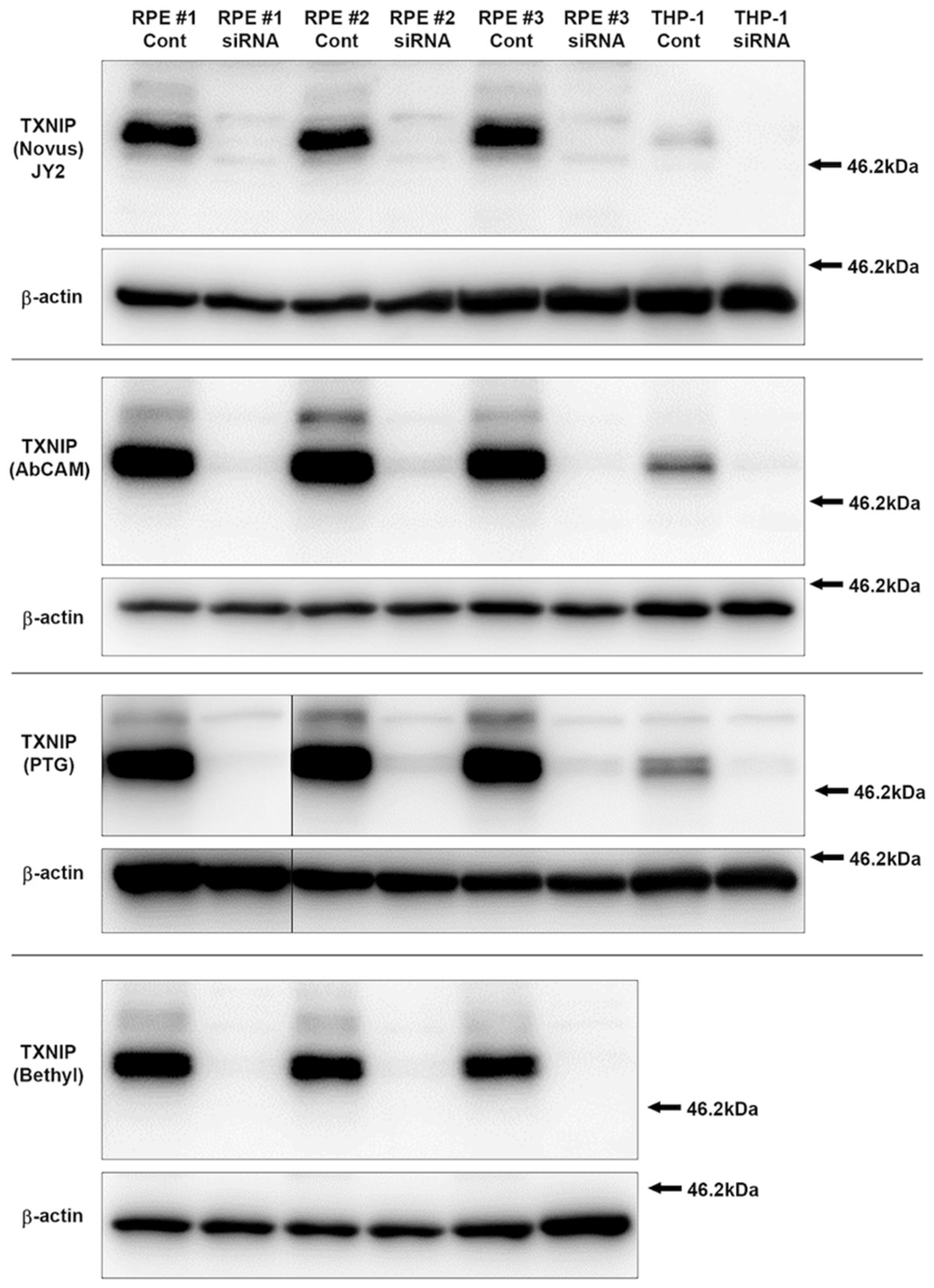
2.4. Antibodies
2.5. Immunohistochemistry
2.5.1. Peroxidase/Diaminobenzidine Immunohistochemistry
2.5.2. Laser Confocal Immunofluorescent Staining
2.6. Protein Extract Preparation
2.6.1. Si RNA transfected cells for verification of antibody
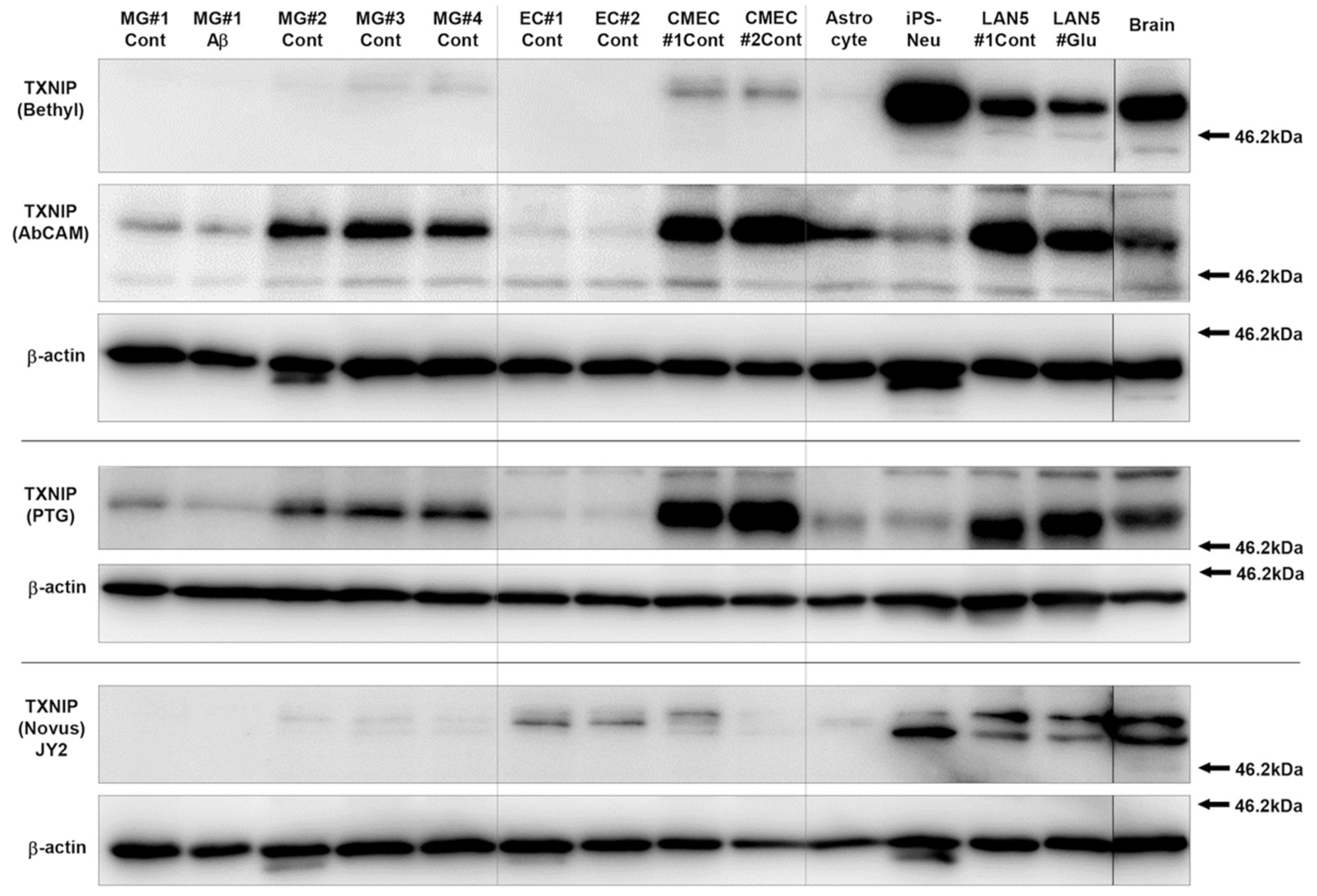
2.6.2. Cell-Type Specificity
2.7. Western Immunoblotting Analysis
2.8. Statistical Analyses
3. Results
3.1. Characterization and Validation of TXNIP Antibodies Used
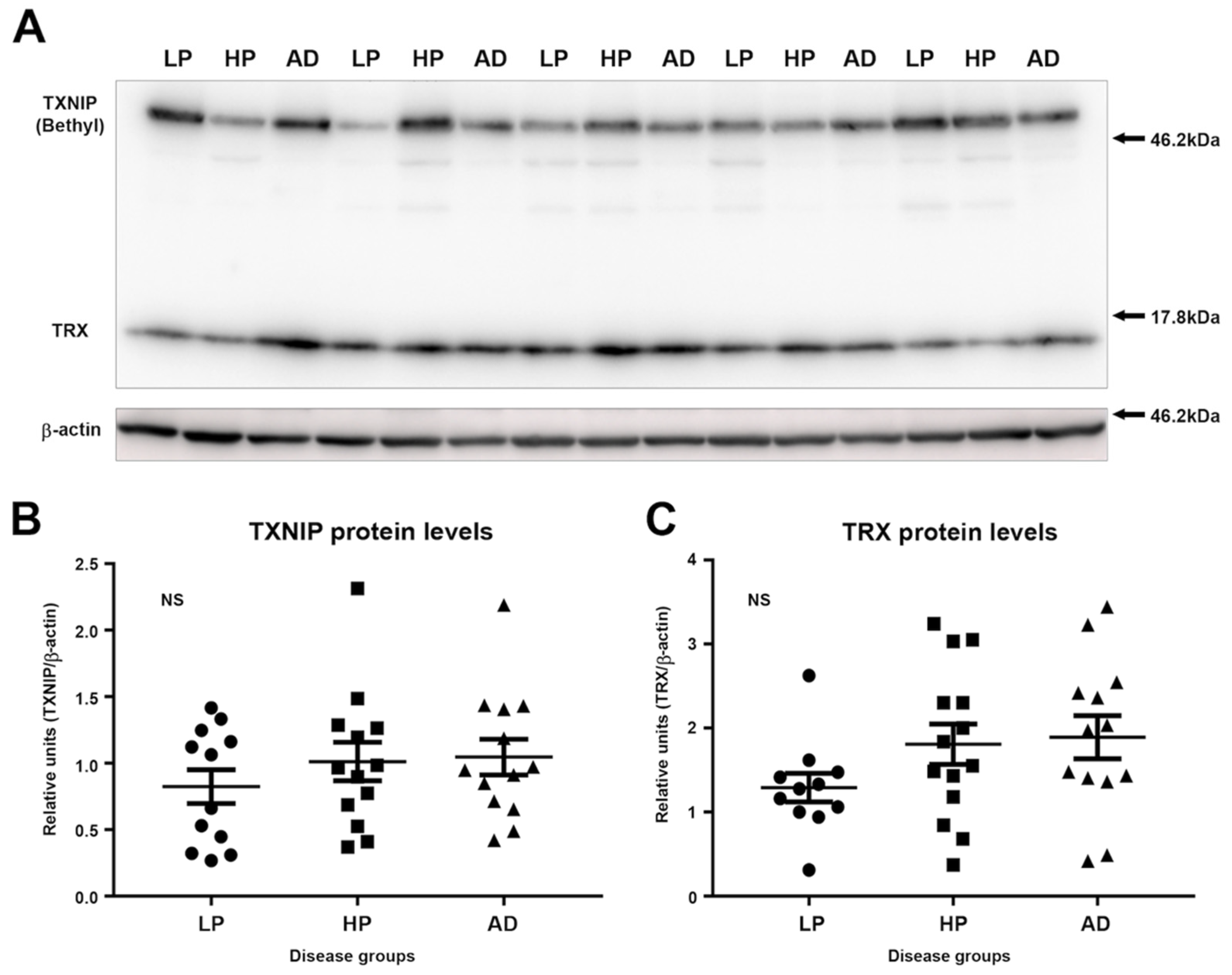
3.2. Immunoblot Measurements of TXNIP and TRX Levels in LP, HP, and AD MTG Brain Samples
3.3. Patterns of Expression of TXNIP in Aged and AD Brains
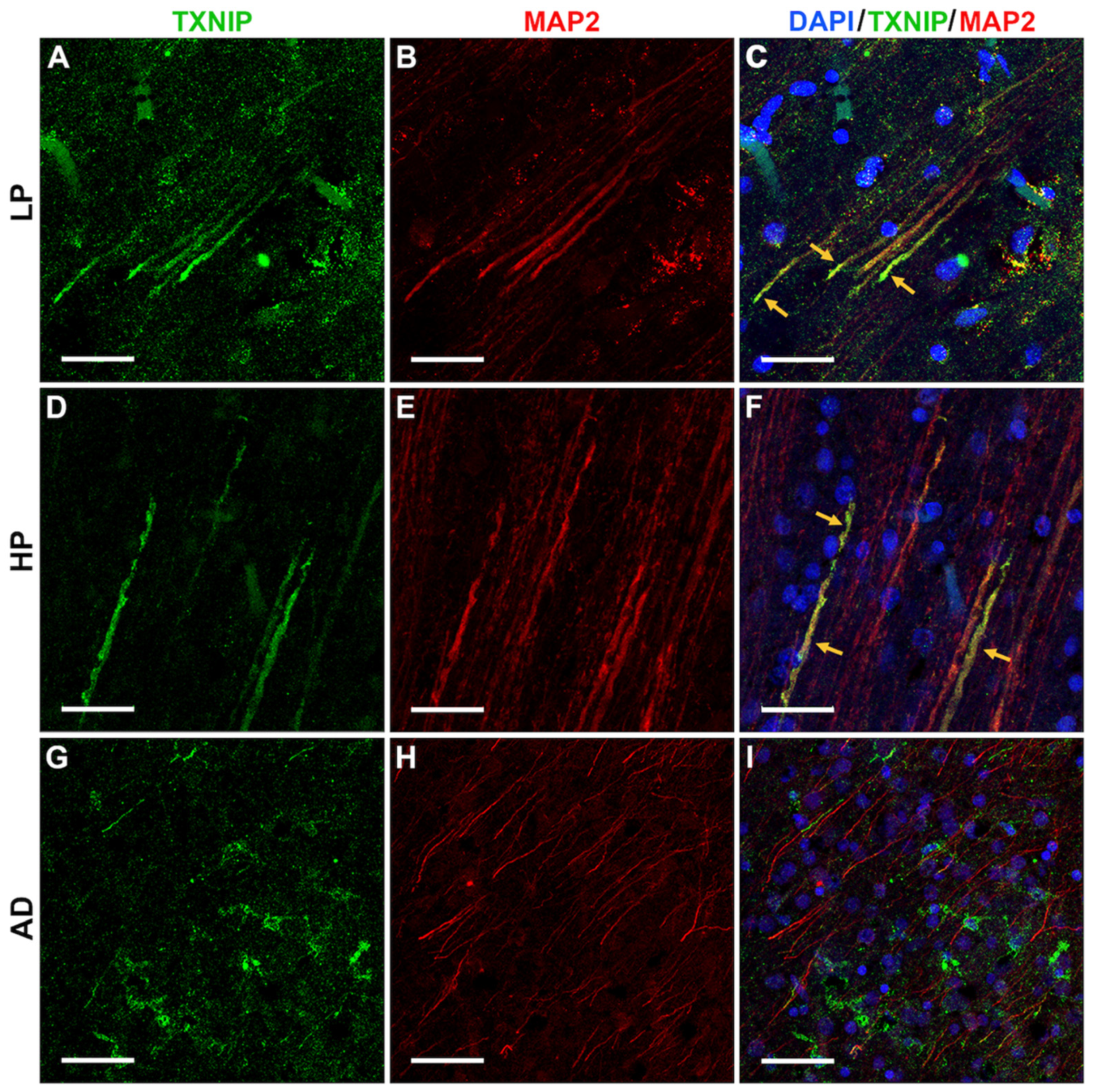
3.4. Patterns of Expression of TXNIP (Confocal Microscopy)
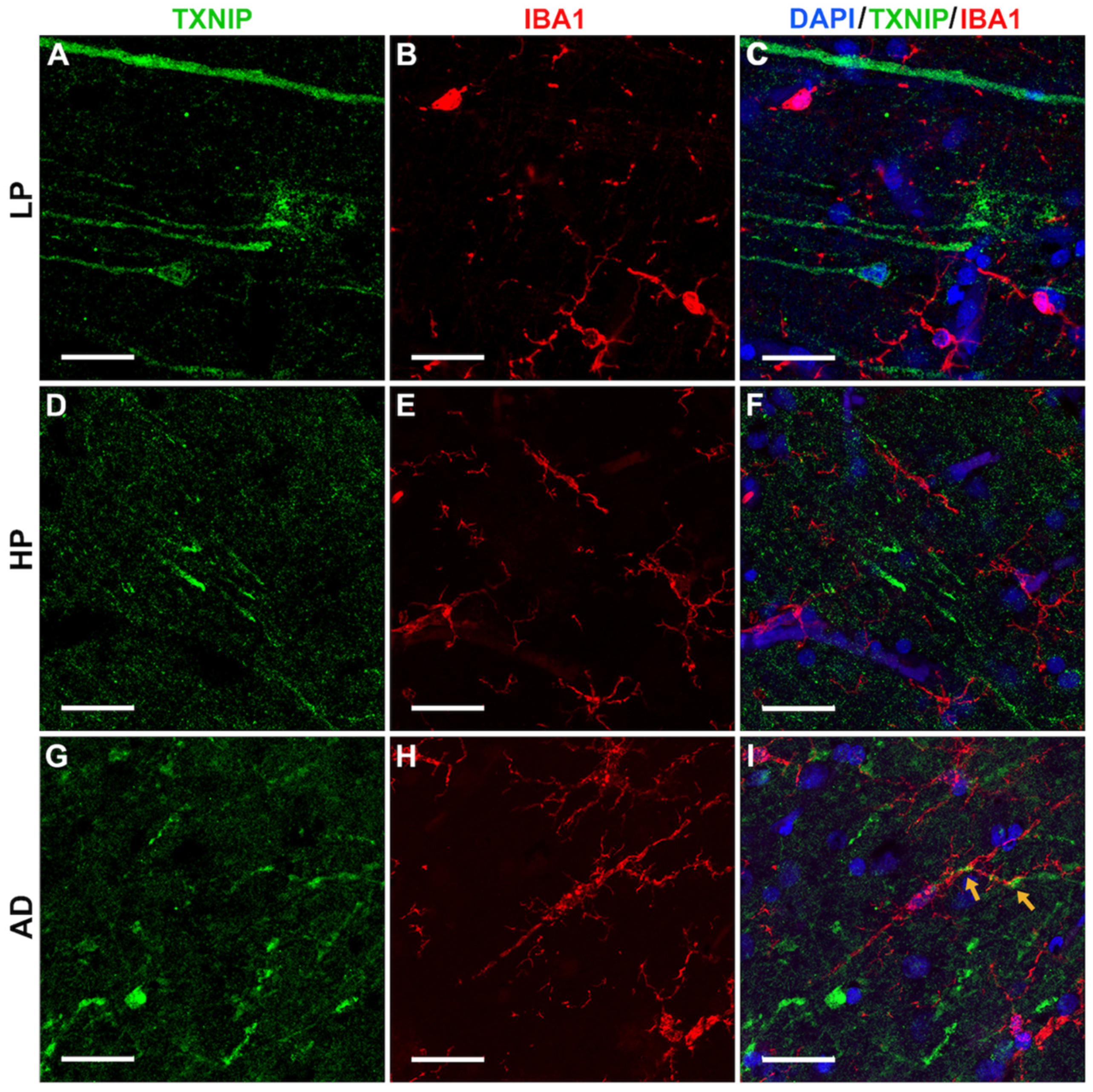
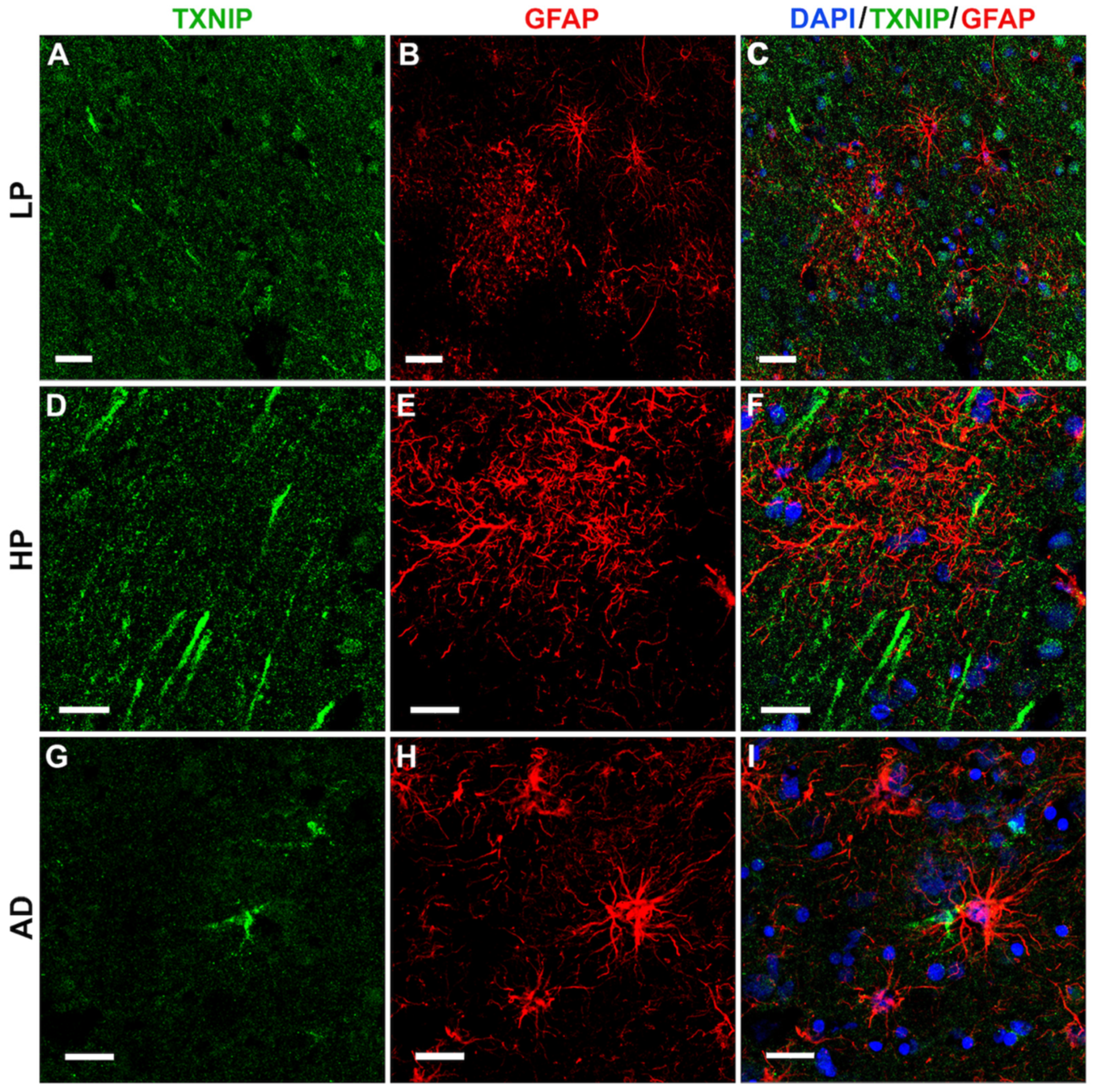
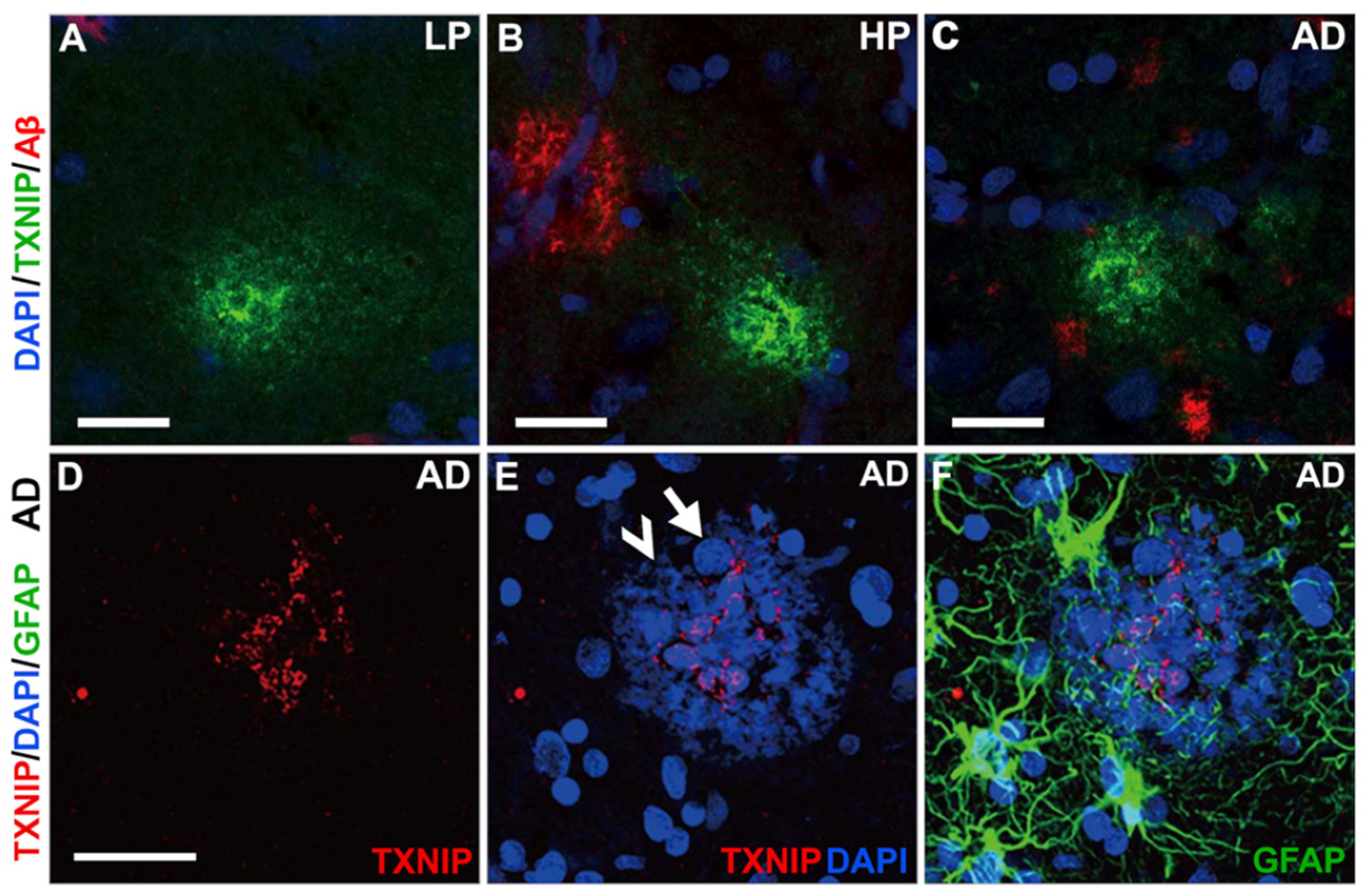
3.5. Additional Features of TXNIP Immunoreactive Structures (Confocal Microscopy)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Saleem, U.; Sabir, S.; Niazi, S.G.; Naeem, M.; Ahmad, B. Role of Oxidative Stress and Antioxidant Defense Biomarkers in Neurodegenerative Diseases. Crit. Rev. Eukaryot. Gene Expr. 2020, 30, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Han, S.H.; Im, J.Y.; Yang, Y.; Cho, E.W.; Um, H.D.; Kim, D.K.; Lee, K.W.; Han, P.L.; Rhee, S.G.; et al. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J. Immunol. 2000, 164, 6287–6295. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.C.; De Keulenaer, G.W.; Yoshioka, J.; Kassik, K.A.; Lee, R.T. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ. Res. 2002, 91, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Wondafrash, D.Z.; Nire’a, A.T.; Tafere, G.G.; Desta, D.M.; Berhe, D.A.; Zewdie, K.A. Thioredoxin-Interacting Protein as a Novel Potential Therapeutic Target in Diabetes Mellitus and Its Underlying Complications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 43–51. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ismael, S.; Nasoohi, S.; Sakata, K.; Liao, F.-F.; McDonald, M.P.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) Associated NLRP3 Inflammasome Activation in Human Alzheimer’s Disease Brain. J. Alzheimers. Dis. 2019, 68, 255–265. [Google Scholar] [CrossRef]
- Su, C.-J.; Feng, Y.; Liu, T.-T.; Liu, X.; Bao, J.-J.; Shi, A.-M.; Hu, D.-M.; Liu, T.; Yu, Y.-L. Thioredoxin-interacting protein induced alpha-synuclein accumulation via inhibition of autophagic flux: Implications for Parkinson’s disease. CNS Neurosci. Ther. 2017, 23, 717–723. [Google Scholar] [CrossRef]
- Ismael, S.; Wajidunnisa; Sakata, K.; McDonald, M.P.; Liao, F.-F.; Ishrat, T. ER stress associated TXNIP-NLRP3 inflammasome activation in hippocampus of human Alzheimer’s disease. Neurochem. Int. 2021, 148, 105104. [Google Scholar] [CrossRef]
- Schulze, P.C.; Yoshioka, J.; Takahashi, T.; He, Z.; King, G.L.; Lee, R.T. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J. Biol. Chem. 2004, 279, 30369–30374. [Google Scholar] [CrossRef]
- Zhao, Q.; Che, X.; Zhang, H.; Fan, P.; Tan, G.; Liu, L.; Jiang, D.; Zhao, J.; Xiang, X.; Liang, Y.; et al. Thioredoxin-interacting protein links endoplasmic reticulum stress to inflammatory brain injury and apoptosis after subarachnoid haemorrhage. J. Neuroinflamm. 2017, 14, 104. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ji, L.; Xing, W.; Zhang, W.; Zhou, H.; Qian, X.; Wang, X.; Gao, F.; Sun, X.; Zhang, H. Acute hyperglycaemia enhances oxidative stress and aggravates myocardial ischaemia/reperfusion injury: Role of thioredoxin-interacting protein. J. Cell. Mol. Med. 2013, 17, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Bharti, V.; Zhou, H.; Hoi, V.; Tan, H.; Wu, Z.; Nagakannan, P.; Eftekharpour, E.; Wang, J.-F. Upregulation of Thioredoxin-Interacting Protein in Brain of Amyloid-β Protein Precursor/Presenilin 1 Transgenic Mice and Amyloid-β Treated Neuronal Cells. J. Alzheimer’s Dis. 2019, 72, 139–150. [Google Scholar] [CrossRef]
- Fertan, E.; Rodrigues, G.J.; Wheeler, R.V.; Goguen, D.; Wong, A.A.; James, H.; Stadnyk, A.; Brown, R.E.; Weaver, I.C.G. Cognitive Decline, Cerebral-Spleen Tryptophan Metabolism, Oxidative Stress, Cytokine Production, and Regulation of the Txnip Gene in a Triple Transgenic Mouse Model of Alzheimer Disease. Am. J. Pathol. 2019, 189, 1435–1450. [Google Scholar] [CrossRef]
- Melone, M.A.B.; Dato, C.; Paladino, S.; Coppola, C.; Trebini, C.; Giordana, M.T.; Perrone, L. Verapamil Inhibits Ser202/Thr205 Phosphorylation of Tau by Blocking TXNIP/ROS/p38 MAPK Pathway. Pharm. Res. 2018, 35, 44. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xie, C.; Gabbita, S.P.; Markesbery, W.R. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Radic. Biol. Med. 2000, 28, 418–427. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Serrano, G.; Shill, H.A.; Walker, D.G.; Lue, L.; Roher, A.E.; Dugger, B.N.; Maarouf, C.; et al. Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program. Neuropathology 2015, 35, 354–389. [Google Scholar] [CrossRef]
- Beach, T.G.; Sue, L.I.; Walker, D.G.; Sabbagh, M.N.; Serrano, G.; Dugger, B.N.; Mariner, M.; Yantos, K.; Henry-Watson, J.; Chiarolanza, G.; et al. Striatal amyloid plaque density predicts Braak neurofibrillary stage and clinicopathological Alzheimer’s disease: Implications for amyloid imaging. J. Alzheimers. Dis. 2012, 28, 869–876. [Google Scholar] [CrossRef]
- Buyandelger, U.; Walker, D.G.; Taguchi, H.; Yanagisawa, D.; Tooyama, I. Novel fluorinated derivative of curcumin negatively regulates thioredoxin-interacting protein expression in retinal pigment epithelial and macrophage cells. Biochem. Biophys. Res. Commun. 2020, 532, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Tang, T.M.; Lue, L.-F. Increased expression of toll-like receptor 3, an anti-viral signaling molecule, and related genes in Alzheimer’s disease brains. Exp. Neurol. 2018, 309, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.G.; Lue, L.-F.; Beach, T.G.; Tooyama, I. Microglial Phenotyping in Neurodegenerative Disease Brains: Identification of Reactive Microglia with an Antibody to Variant of CD105/Endoglin. Cells 2019, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Weksler, B.B.; Subileau, E.A.; Perrière, N.; Charneau, P.; Holloway, K.; Leveque, M.; Tricoire-Leignel, H.; Nicotra, A.; Bourdoulous, S.; Turowski, P.; et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2005, 19, 1872–1874. [Google Scholar] [CrossRef]
- Lanciotti, M.; Montaldo, P.G.; Folghera, S.; Lucarelli, E.; Cornaglia-Ferraris, P.; Ponzoni, M. A combined evaluation of biochemical and morphological changes during human neuroblastoma cell differentiation. Cell. Mol. Neurobiol. 1992, 12, 225–240. [Google Scholar] [CrossRef]
- Mendsaikhan, A.; Tooyama, I.; Bellier, J.-P.; Serrano, G.E.; Sue, L.I.; Lue, L.-F.; Beach, T.G.; Walker, D.G. Characterization of lysosomal proteins Progranulin and Prosaposin and their interactions in Alzheimer’s disease and aged brains: Increased levels correlate with neuropathology. Acta Neuropathol. Commun. 2019, 7, 215. [Google Scholar] [CrossRef]
- Levendusky, M.C.; Basle, J.; Chang, S.; Mandalaywala, N.V.; Voigt, J.M.; Dearborn, R.E.J. Expression and regulation of vitamin D3 upregulated protein 1 (VDUP1) is conserved in mammalian and insect brain. J. Comp. Neurol. 2009, 517, 581–600. [Google Scholar] [CrossRef]
- Matsuura, M.; Abe, H.; Tominaga, T.; Sakurai, A.; Murakami, T.; Kishi, S.; Bando, Y.; Minakuchi, J.; Nagai, K.; Doi, T. A Novel Method of DAPI Staining for Differential Diagnosis of Renal Amyloidosis. J. Med. Investig. 2017, 64, 217–221. [Google Scholar] [CrossRef][Green Version]
- Tsubaki, H.; Tooyama, I.; Walker, D.G. Thioredoxin-Interacting Protein (TXNIP) with Focus on Brain and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 9357. [Google Scholar] [CrossRef]
- Nasoohi, S.; Ismael, S.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and Neurodegenerative Diseases: Regulation and Implication. Mol. Neurobiol. 2018, 55, 7900–7920. [Google Scholar] [CrossRef]
- Ding, R.; Ou, W.; Chen, C.; Liu, Y.; Li, H.; Zhang, X.; Chai, H.; Ding, X.; Wang, Q. Endoplasmic reticulum stress and oxidative stress contribute to neuronal pyroptosis caused by cerebral venous sinus thrombosis in rats: Involvement of TXNIP/peroxynitrite-NLRP3 inflammasome activation. Neurochem. Int. 2020, 141, 104856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Che, X.; Zhang, H.; Tan, G.; Liu, L.; Jiang, D.; Zhao, J.; Xiang, X.; Sun, X.; He, Z. Thioredoxin-Interacting Protein Mediates Apoptosis in Early Brain Injury after Subarachnoid Haemorrhage. Int. J. Mol. Sci. 2017, 18, 854. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Robinson, K.A.; Brock, J.W.; Buse, M.G. Posttranslational regulation of thioredoxin-interacting protein. J. Mol. Endocrinol. 2013, 50, 59–71. [Google Scholar] [CrossRef]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef]
- Malek-Ahmadi, M.; Beach, T.; Obradov, A.; Sue, L.; Belden, C.; Davis, K.; Walker, D.G.; Lue, L.; Adem, A.; Sabbagh, M.N. Increased Alzheimer’s disease neuropathology is associated with type 2 diabetes and ApoE ε.4 carrier status. Curr. Alzheimer Res. 2013, 10, 654–659. [Google Scholar] [CrossRef]
- Bendlin, B.B. Antidiabetic therapies and Alzheimer disease. Dialogues Clin. Neurosci. 2019, 21, 83–91. [Google Scholar]
- de la Monte, S.M. The Full Spectrum of Alzheimer’s Disease Is Rooted in Metabolic Derangements That Drive Type 3 Diabetes. Adv. Exp. Med. Biol. 2019, 1128, 45–83. [Google Scholar] [CrossRef]
- Kimura, N. Diabetes Mellitus Induces Alzheimer’s Disease Pathology: Histopathological Evidence from Animal Models. Int. J. Mol. Sci. 2016, 17, 503. [Google Scholar] [CrossRef]
- Lanzillotta, C.; Tramutola, A.; Di Giacomo, G.; Marini, F.; Butterfield, D.A.; Di Domenico, F.; Perluigi, M.; Barone, E. Insulin resistance, oxidative stress and mitochondrial defects in Ts65dn mice brain: A harmful synergistic path in down syndrome. Free Radic. Biol. Med. 2021, 165, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Yamaguchi, K.; Matsui, K.; Sano, T.; Kubota, T.; Hashimoto, T.; Mano, A.; Yamada, K.; Matsuo, Y.; Kubota, N.; et al. Differential effects of diet- and genetically-induced brain insulin resistance on amyloid pathology in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Chornenkyy, Y.; Wang, W.-X.; Wei, A.; Nelson, P.T. Alzheimer’s disease and type 2 diabetes mellitus are distinct diseases with potential overlapping metabolic dysfunction upstream of observed cognitive decline. Brain Pathol. 2019, 29, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Smith, C.D.; Abner, E.A.; Schmitt, F.A.; Scheff, S.W.; Davis, G.J.; Keller, J.N.; Jicha, G.A.; Davis, D.; Wang-Xia, W.; et al. Human cerebral neuropathology of Type 2 diabetes mellitus. Biochim. Biophys. Acta 2009, 1792, 454–469. [Google Scholar] [CrossRef]
- Dos Santos Matioli, M.N.P.; Suemoto, C.K.; Rodriguez, R.D.; Farias, D.S.; da Silva, M.M.; Leite, R.E.P.; Ferretti-Rebustini, R.E.L.; Farfel, J.M.; Pasqualucci, C.A.; Jacob Filho, W.; et al. Diabetes is Not Associated with Alzheimer’s Disease Neuropathology. J. Alzheimer’s Dis. 2017, 60, 1035–1043. [Google Scholar] [CrossRef]
- Abner, E.L.; Nelson, P.T.; Kryscio, R.J.; Schmitt, F.A.; Fardo, D.W.; Woltjer, R.L.; Cairns, N.J.; Yu, L.; Dodge, H.H.; Xiong, C.; et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimer’s Dement. 2016, 12, 882–889. [Google Scholar] [CrossRef]
- Shinohara, M.; Tashiro, Y.; Suzuki, K.; Fukumori, A.; Bu, G.; Sato, N. Interaction between APOE genotype and diabetes in cognitive decline. Alzheimer’s Dement. 2020, 12, e12006. [Google Scholar] [CrossRef]



| Gender (M:F) | Mean Age ± SD/y | PMI/h | APOE4 | Plaque Score ± SEM | Tangle Score ± SEM | Braak Score | |
|---|---|---|---|---|---|---|---|
| LP (n = 14) | 8/6 | 85.7 ± 8.1 | 3.2 ± 1.0 | 3.8% (1/26) | 2.1 ± 0.6 | 4.8 ± 0.7 | I–IV |
| HP (n = 11) | 4/7 | 83.6 ± 6.9 | 3.0 ± 0.7 | 20% (4/20) | 10.6 ± 0.8 | 4.5 ± 0.7 | III–IV |
| AD (n = 13) | 9/4 | 80.2 ± 6.2 | 3.5 ± 0.8 | 38.5% (10/26) | 14.3 ± 0.2 | 13.9 ± 0.5 | V–VI |
| Gender (M:F) | Mean Age ± SD/y | PMI/h | APOE4 | Plaque Score ± SEM | Tangle Score ± SEM | Braak Score | |
|---|---|---|---|---|---|---|---|
| LP (n = 12) | 7/5 | 86.8 ± 7.2 | 2.6 ± 0.6 | 8.3% (2/24) | 1.7 ± 0.62 | 4.1 ± 0.6 | I–IV |
| HP (n = 14) | 9/5 | 83.8 ± 5.5 | 3.1 ± 0.7 | 21.4% (6/28) | 9.0 ± 0.5 | 3.5 ± 0.5 | I–IV |
| AD (n = 13) | 5/8 | 78.0 ± 9.4 | 3.0 ± 0.9 | 38.5% (10/26) | 14.3 ± 0.2 | 13.2 ± 0.8 | IV–VI |
| Antigen | Antibody | Supplier | Host Species | Catalog No. | Application | Dilution |
|---|---|---|---|---|---|---|
| TXNIP | TXNIP (JY2) | Novus | Mouse/Mono | NBP1-54578 | IHC/WB | 1:200–500 |
| TXNIP | TXNIP | Bethyl | Rabbit/Poly | A303-229A | WB | 1:10,000 |
| TXNIP | TXNIP | Abcam | Rabbit/Mono | ab188865 | IHC/WB | 1:2000 |
| TXNIP | TXNIP | Proteintech | Rabbit/Poly | 18243-1-AP | WB | 1:2000 |
| TRX | TRX | Abcam | Rabbit/Mono | ab133524 | IHC/WB | 1:1000–5000 |
| β-actin | HRP-β-actin | Abcam | Mouse/Mono | ab49900 | WB | 1:30,000 |
| Aβ1-42 | Amyloid β (mOC64) | Abcam | Rabbit/Mono | ab201060 | IHC | 1:1000 |
| P-tau | P-tau (S202) | Abcam | Rabbit/Mono | ab108387 | IHC/WB | 1:3000–5000 |
| P-tau | P-tau (S396) | Abcam | Rabbit/Mono | ab109390 | IHC/WB | 1:3000–5000 |
| MAP2 | MAP2 | Abcam | Chicken/Poly | ab92434 | IHC | 1:1000 |
| GFAP | GFAP | Dako | Rabbit/Poly | Z0334 | IHC | 1:2000 |
| IBA1 | IBA1 | Abcam | Goat/Poly | ab5076 | IHC | 1:1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsubaki, H.; Mendsaikhan, A.; Buyandelger, U.; Tooyama, I.; Walker, D.G. Localization of Thioredoxin-Interacting Protein in Aging and Alzheimer’s Disease Brains. NeuroSci 2022, 3, 166-185. https://doi.org/10.3390/neurosci3020013
Tsubaki H, Mendsaikhan A, Buyandelger U, Tooyama I, Walker DG. Localization of Thioredoxin-Interacting Protein in Aging and Alzheimer’s Disease Brains. NeuroSci. 2022; 3(2):166-185. https://doi.org/10.3390/neurosci3020013
Chicago/Turabian StyleTsubaki, Haruka, Anarmaa Mendsaikhan, Undral Buyandelger, Ikuo Tooyama, and Douglas G. Walker. 2022. "Localization of Thioredoxin-Interacting Protein in Aging and Alzheimer’s Disease Brains" NeuroSci 3, no. 2: 166-185. https://doi.org/10.3390/neurosci3020013
APA StyleTsubaki, H., Mendsaikhan, A., Buyandelger, U., Tooyama, I., & Walker, D. G. (2022). Localization of Thioredoxin-Interacting Protein in Aging and Alzheimer’s Disease Brains. NeuroSci, 3(2), 166-185. https://doi.org/10.3390/neurosci3020013






