Thoracic outlet syndrome (TOS) represents a complex entity characterized by different neurovascular signs and symptoms involving the upper limb and one of the most controversial diagnoses in clinical medicine [
1,
9]. As noted above, bone abnormalities (cervical ribs, exostosis of the first rib or clavicle, elongated transverse process of C7, excessive callus of the clavicle or first rib) and congenital or acquired soft-tissue abnormalities (fibrous bands and ligaments, muscular hypertrophy or fibrosis, post-traumatic changes) represent the most frequent causes of TOS [
2,
4]. True neurogenic TOS with characteristic clinical-instrumental findings in the C8/T1 nerve root distribution and confirmed neurophysiological abnormality is rare. Typically, clinical manifestations include intrinsic hand muscles hypotrophy (especially thenar weakness and atrophy) and sensory symptoms involving the T1 dermatome more than the C8 dermatome [
10]. Conversely, nonspecific TOS is usually characterized by the presence of pain accompanied by paresthesias, weakness, and dysfunction of the hand, again most frequently in the ulnar nerve distribution [
5]. Thus, the presence of objective clinical signs, such as intrinsic hand muscle atrophy, could suggest a clinical picture of true neurologic TOS due to the presence of a cervical rib rather than nonspecific TOS [
3]. In addition to the congenital or acquired soft-tissue abnormalities associated with TOS, anterior or middle scalene hypertrophy is responsible for the reduction of the interscalene triangle space. Instead, isolated SM hypertrophy represents a rare condition that could lead to a reduction in the costoclavicular space and brachial plexus compression [
8]. The utility of electrodiagnostic studies in TOS is still controversial [
4]. Tsao et al. reviewed the electrodiagnostic findings of true neurogenic TOS, which reflects an axon loss lower plexopathy with a predominant T1 sensory and motor nerve fibers involvement. The contemporary implication of medial antebrachial cutaneous (MABC) SNAP and median CMAP associated with a neurogenic pattern on needle EMG in abductor pollicis brevis (APB) indeed represents the most common neurophysiological pattern in true neurogenic TOS [
10]. Those findings were in accordance with the anatomic relationship between the lower brachial plexus and the fibrous band, which extends from the first thoracic rib to any C7 bone abnormalities and compresses the plexus from below, thus affecting primarily T1 sensory and motor fibers. Nevertheless, combined abnormalities in ulnar SNAP and MAP were present in about the 40-percent of cases, as in our patient [
10]. In the setting of clavicular trauma, the most susceptible brachial plexus branch involved was the medial cord that crosses the first rib and the underlying SM directly posterior to the middle segment of the clavicle. This produces sensory abnormalities in the medial aspect of the arm, forearm, or hand and the medial 1.5 digits (ulnar nerve) [
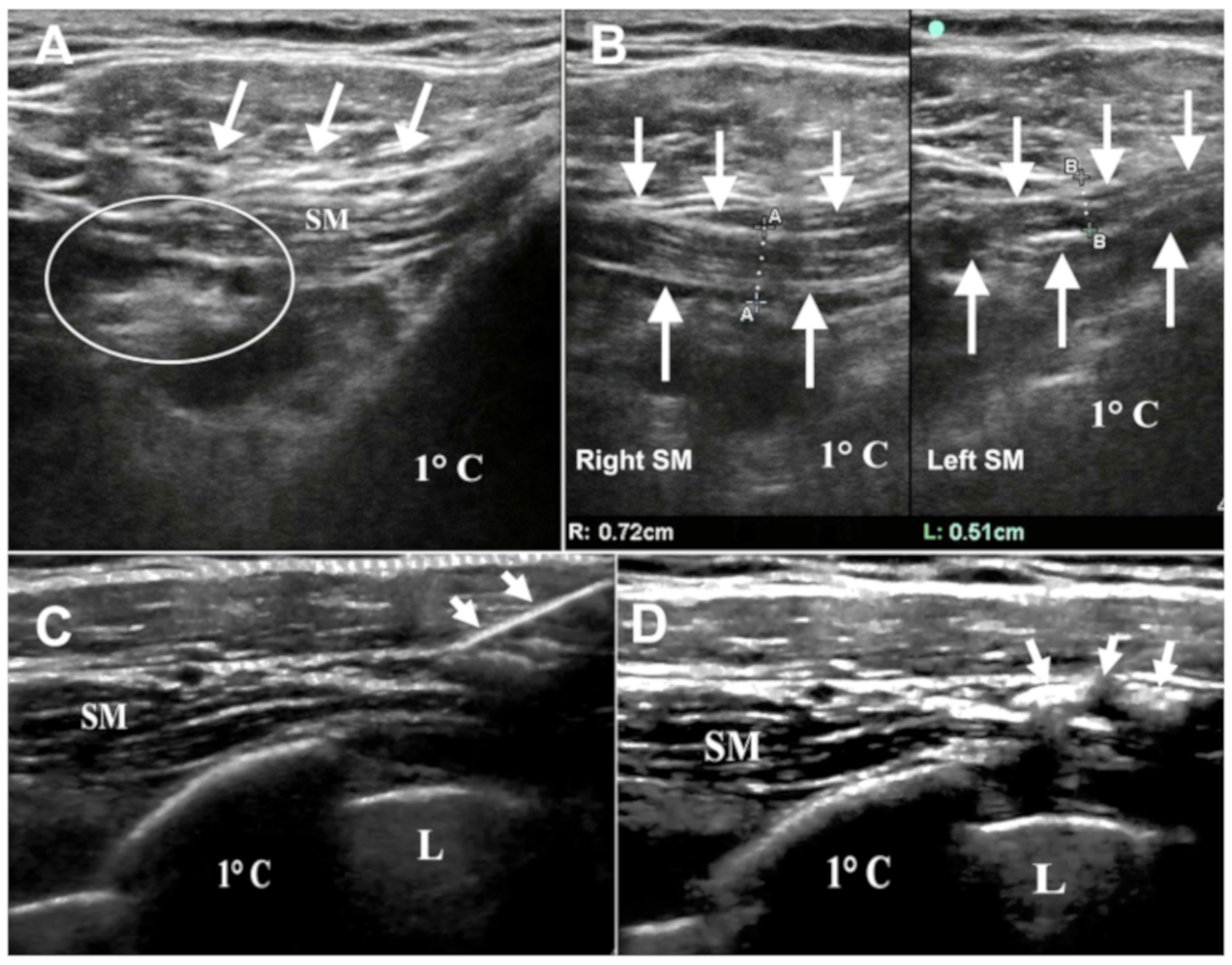
3]. A similar condition could be seen in our patient: the prominent C8 motor and sensory fibers involvement may be due to the anatomical relationship between the lower brachial plexus and SM that could compress the nerve trunk from the above. Thus, these considerations, associated with the presence of clinical objective motor signs (i.e., atrophy and weakness of right intrinsic hand muscles) and electrodiagnostic findings, might explain the different lower brachial plexus involvement between our case and the classic true neurogenic TOS. Moreover, in our case, the EMG profile before treatment was indicative of partial axonal damage, with a CMAP amplitude of 40% compared to the contralateral limb. At the 6-month follow-up, the amplitude of the CMAP was increased up to 70% of the healthy side, confirming an initial reinnervation process. SM is a small muscle located in the costoclavicular space; its tendon originates from the junction of the first rib with costal cartilage and its fleshy fibers joint laterally to the inferior surface of the clavicle [
8]. Its posterior surface is separated from the first rib by the subclavian vessels and brachial plexus. Indeed, changes in this muscle can cause compression of the brachial neurovascular bundle, as previously described [
8]. We know that SM pulls the point of the shoulder downwards and forwards and steadies the clavicle during movements of the shoulder [
8]. In our patient repetitive gun recoil during hunting and skeet shooting may have led to abnormal muscle activation, correlated to its physiological function, resulting in hypertrophy. This hypothesis was confirmed by brachial plexus echography that revealed the rare condition of SM hypertrophy, which has led to the compression of the lower part of the brachial plexus. MRI represents an efficient technique to detect muscle hypertrophy, abnormal muscles or fibrous bands; however, it is characterized by the presence of some instrumental practical limitations, such as the supine position of the patient with restriction of arm elevation during the examination [
1]. Another limitation is the difficulty to delineate and identify the anatomic structures in thin individuals with little adipose tissue [
1]. On the other hand, ultrasonography may be suitable to the position of the patient so that it could be performed with a raised arm. Even if the technique is operator-dependent, it represents a supplementary evaluation in patients with clinical and neurophysiological features suggestive of TOS and negative MRI studies [
1]. Thus, a clinical and instrumental approach, including neuroimaging and ultrasound techniques, could be useful for a correct diagnosis of brachial plexus compressions, including hypertrophy of muscles of the costoclavicular space. Physiotherapy with stretching exercises for the neck and shoulder, postural training, anti-inflammatories, and analgesic agent injections represent the most common conservative options in the treatment of TOS [
4]. There are few clinical experiences in the literature reporting the efficacy of botulinum chemodenervation in Neurogenic TOS [
6,
7]. Jordan et al. performed a retrospective clinical analysis of patients treated with botulinum toxin using ultrasonography and electromyography as targeting techniques [
6]. Simultaneous Botulinum toxin-A injections of the anterior and middle scalene, subclavius, and pectoralis minor muscles were performed, leading to a significant reduction in pain in the majority of cases. In these patients, SM was injected just to provide additional decompression of the costoclavicular space, even if the hypertrophy was not radiologically detected [
6]. Torriani et al. reported their experience of ultrasound-guided BTX-A treatment in patients with symptoms and signs suggestive of TOS; the target of injection was different in every patient, according to clinical examination [
7]. An enlarged SM with secondary constriction of the costoclavicular space was detected in two patients, and just one of them received the injections, in combination with the treatment of anterior scalene and pectoralis minor, with subsequent clinical benefit [
7]. Furthermore, a Cochrane review on therapy of TOS concluded that there was no significant effect of treatment with the BTX injection into the scalene muscles over placebo in terms of pain relief or improvements in disability, improving only paresthesias at six months follow-up [
9]. However, as highlighted in the review, BTX injections cannot be judged to have no effect in the treatment of TOS until other potential anatomical locations are trialed, such as the pectoralis minor and subclavius muscles [
9]. In clinical practice, botulinum toxin is mainly dedicated to dystonia and spasticity treatment, situations in which the symptoms are related to the muscle itself, in terms of pain-rigidity, postural alterations, or loss of performance. In other conditions, such as piriform syndrome or neurogenic TOS secondary to muscle hypertrophy (as in our case), a hypertrophic muscle exerts compression on a nerve structure, leading to the development of symptoms [
4,
6,
7,
9]. Once the causal relationship has been established, in case of failure of physical therapy and before a possible surgical approach, treatment with botulinum toxin should be considered [
4,
6,
7,
9].