1. Introduction
Globally, no less than 4.4 billion (56%) of the world’s population resides in cities, and this proportion is anticipated to reach 68% by the year 2050 [
1]. By 2045, the global urban population will approach 6 billion, and this implies that city authorities ought to plan for such a growth as well as provide the required basic services, infrastructure, and housing [
1]. One of the greatest challenges in cities is ensuring a reliable supply of food [
2]. City inhabitants subsist on either imported food originating from complex supply chains or urban horticulture, which may lead to food insecurity, as witnessed in the crucible of the COVID-19 pandemic [
3,
4].
In recent times, urban horticulture has remained pivotal to the food, social, economic, and ecological sustainability of cities [
5]. Like elsewhere in Africa [
6], urban vegetable farming in wetlands and riverbanks is a common feature of Ugandan cities [
7]. However, urbanization has led to various anthropogenic activities that can lead to the pollution of water resources, enrichment of pollutants in soils and, consequently, pollutant bioaccumulation in edible tissues of plants cultivated on such soils [
8]. Uganda, an East African country, is a late urbanizer characterized by speculative urbanism. In the absence of lucrative industrial opportunities, investments in land and real estate as well as agriculture as a capital gains strategy are the last resort [
9,
10]. As of May 2023, the country had more than 16 cities, most of which were municipalities in 2019 [
9,
11].
Mbarara City is among the top five rapidly growing cities in Uganda. It is expanding at a rate that is believed to be faster than that of Kampala, the country’s central business district and capital city [
9]. In the suburban areas of Mbarara city, urban agriculture is practiced, especially on the banks of River Rwizi [
12]. Some of the crops grown include bananas, coffee, tea, sugarcane and vegetables (cabbages, spinach, amaranth, pumpkin) [
13,
14]. Such urban agricultural activities coupled with indiscriminate disposal of municipal wastes and sewage, brick laying and sand mining along the river bank [
15] have been speculated to impact the quality of water in Lake Victoria, a lentic East African water resource. Reports on the pollution of Lake Victoria are widely documented, but studies investigating the potential sources of its water contaminants, especially from the transboundary, in-country and influent rivers such as Nzoia, Rwizi and Kagera, are still limited [
16,
17].
For River Rwizi (the focus of this study), there have been reports on its water quality and concentration of trace metals (TMTs), nutrients and polycyclic aromatic compounds in water, sediments, damselfly larvae (
Ceriagrion glabrum) and selected fish species (
Brycinus sadleri and
Barbus altianalis) [
18,
19,
20,
21,
22]. Heavy metals, sometimes called trace metals or potentially toxic elements, are naturally present in the earth’s crust, but they are enriched in the environment following their release from various anthropogenic activities. In the case of water, TMTs such as manganese (Mn), zinc (Zn), cadmium (Cd), lead (Pb), chromium (Cr), copper (Cu) and nickel (Ni) have been reported to induce toxic physiological effects when they are ingested at higher concentrations than their thresholds in animals. For example, environmental exposure to Pb contributes to at least 500,000 new cases of children with intellectual disabilities every year [
23]. Lead poisoning following long-term exposure has been associated with hypertension [
24], and several inorganic Pb compounds are probable human carcinogens [
25]. The environmental and toxic effects of TMTs largely depend on the metal in question, its exposure concentration, the oxidation state, the chemical form, and the exposure route, among other extrinsic factors [
26].
To our knowledge, no previous efforts have been devoted to the examination of the transferability and health risks of regulatory TMTs in soils and leafy vegetables cultivated on the banks of River Rwizi. We herein report on the trace metal contamination of soils as well as their accumulation in edible leafy vegetables grown on the banks of River Rwizi in Mbarara City, Western Uganda. Ecological and human health risks were assessed, and, in all cases, the results were compared with findings of previous studies conducted around the world in a similar setting to assess the relative pollution levels of River Rwizi’s urban vegetable gardens in a global context.
2. Materials and Methods
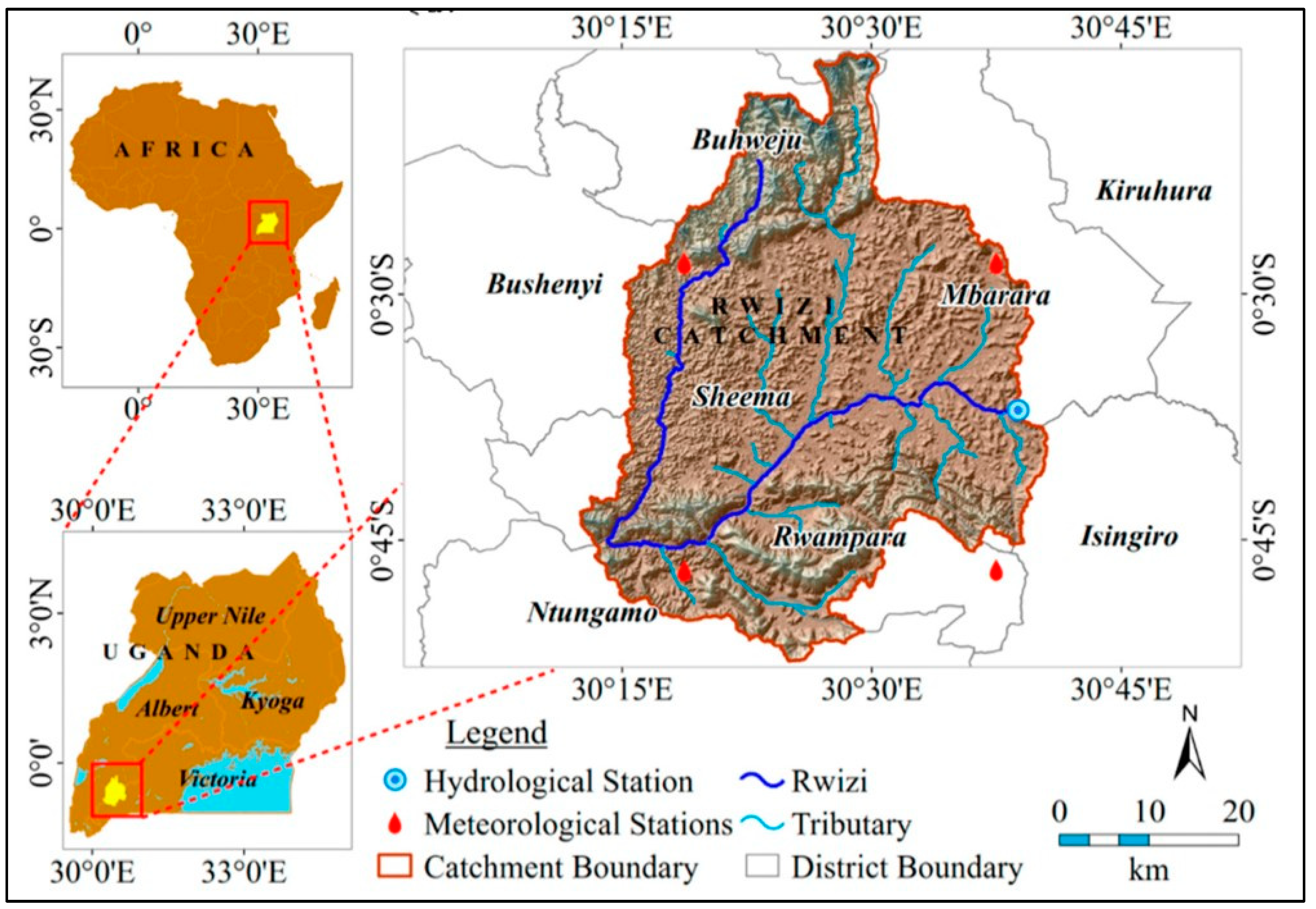
2.1. Description of the Targeted River and Its Stretch in Mbarara City
River Rwizi in the upstream areas of Ankole (or otherwise the Bukola River in the downstream areas of Buganda kingdom) is an 8200 km river (
Figure 1). It is an influent river that discharges its water into the Ugandan portion of L. Victoria through interconnected wetlands of the Kooki lakes (i.e., Lake Mburo, Lake Kachera, Lake Nakivale and Lake Kijanebarola) system and the Sango Bay wetland forest [
27,
28,
29]. The river is traced to originate from the mountainous parts of Buhweju district. It has various tributaries emanating from the hilly regions of the Nkore area, covering a total distance of approximately 55 km with a catchment area of 2521 km
2 [
22]. The river flows eastward for about 57 km until the gauge at Mbarara water works before it joins the Kagera transboundary river, which then pours into L. Victoria and progresses through the White Nile until the Mediterranean Sea in Egypt [
30].
Also known as the “Life-line River” [
20,
30], River Rwizi is the main river that traverses Mbarara District and is thus the principal source of water for millions of people and livestock in Mbarara City boroughs. It is the biggest and longest river in the Ankole sub-region, serving 14 districts (Buhweju, Bushenyi, Isingiro, Kiruhura, Kyotera, Lyantonde, Rakai, Sheema, Mbarara, Rubirizi, Rwampara, Sembabule, and Ntungamo). Various anthropogenic activities are evident on River Rwizi. For example, there is sand mining, washing, brick laying, agriculture and dumping of medical and industrial wastes as well as treated water from Kakoba and Taso wastewater treatment plants [
31]. More than 200 farmers illegally have hectares of land on the banks of River Rwizi, which have accelerated the degradation of its water quality, biodiversity, colonization by the invasive water hyacinth especially at the Taso village bridge, and reduction in its water levels such that it is traversable on foot [
18,
19,
27]. Coupled with the looming impacts of the decadal climate change, there are worries that River Rwizi may be at the brink of drying up [
30,
32].
2.2. Collection of Soil and Vegetable Samples
Sampling of both vegetables and soils was performed from January 2023 to April 2023. Five sampling sites were selected in the cultivated areas along the river at least 500 m apart, and these were based on the intensity of cultivation or potential anthropogenic activities (
Table 1). Five samples of each vegetable were collected in triplicate per site, giving a total of 75 samples. These included cabbages (
Brassica oleracea var. capitata), pumpkin (
Cucurbita pepo L.), spinach (
Spinacia oleracea L.), black nightshade (
Solanum nigrum L.), and green amaranth (
Amaranthus hybridus L.) (
Figure 2). The corresponding soil samples (from depths of 0 to 15 cm) were obtained using a plastic scoop [
33] from the same positions where the vegetables were uprooted (n = 75). All the samples were packed in labelled paper bags, sealed and transported to the laboratory.
2.3. Sample Preparation Procedures
The vegetable samples were washed under running tap water and then rinsed with distilled water to preclude contamination by pesticides, fertilizers and dust. Thereafter, they were blotted dry with tissue paper, put on labelled stainless-steel pans and dried in a hot air oven for 24–72 h at 70 °C [
34].
The dried samples were cooled and then pulverized using a non-metallic motor and pestle to reduce the particle size. Each vegetable sample (2.0 g) was weighed and transferred into a 250 mL Pyrex glass beaker. Perchloric acid (70%, 5 mL) was added followed by concentrated nitric acid (15 mL). The sample was kept at room temperature for 1 h to allow for cold digestion, after which the sample was heated in a sand bath to achieve heat digestion. Digestion went on until no more brown fumes were present, and then the sides of the beaker were washed down with double distilled water to ensure all the sample was in solution.
Soil samples, on the other hand, were dried at 110 °C in an oven for 24 h. The dried samples were cooled and pulverized using a porcelain motor and pestle to reduce the particle size. For each soil sample, 1.0 g was weighed and transferred into a 250 mL beaker. Hydrochloric acid (37%, 5 mL) was added followed by concentrated nitric acid (15 mL). Cold digestion and heat digestion were then carried out for the vegetable samples above.
2.4. Trace Metal Analysis
Each digested sample was cooled and then filtered into 100 mL volumetric flasks. The filtrate was carefully washed down the flask and topped up to the mark with double distilled water. The flask was stoppered and the contents were shaken well. The diluent used was 1% nitric acid prepared using double distilled water. The samples were thereafter analyzed on a flame atomic absorption spectrometer (Analyst 300, Perkin Elmer, Waltham, MA, USA).
We ensured the quality of our data through the use of reagents of analytical grade, analysis of procedural blanks and spiked samples. The analytical recoveries obtained in the analyzed blanks and spikes ranged from 97.9% to 99.5%. The relative standard deviations were found to range from 4.4% to 5.1%.
2.5. Assessment of TMTs Bioconcentration in Leafy Vegetables
To establish whether or not the leafy vegetables grown on contaminated soils on the river bank were bioaccumulating the TMTs, the bioconcentration factor (BCF) was calculated using Equation (1) [
35].
Conventionally, BCF values higher than 1 confirm that a plant is a hyperaccumulator of the metal in question, whereas values less than 1 are indicative of an excluder [
36].
2.6. Human Health Risk Assessment Due to Exposure to the TMTs
Human health risk assessments were used to examine the relationship between the environment and human health. The model proposed by United States Environmental Protection Agency [
37], and used by previous studies [
35,
38] was adopted in this study. According to the US EPA carcinogenic chemical classification standards, the trace metal(loid)s investigated in this study can pose chronic non-cancer risks, but among which Cd and Pb can also pose cancer health risks [
39]. From a sensitivity perspective, we further delineated such exposures by children (as a sensitive group) and adults (as representative of the general population), given that the toxicity of trace metalloids are expected to be influenced by the age of the intoxicated organism [
26].
2.6.1. Non-Cancer Health Risks
The chronic daily intake (mg kg
−1day
−1) through inhalation (CDI
INH), incidental ingestion (CDI
ING), and dermal contact (CDI
DC) with soils was calculated using Equations (2)–(4) [
40]. Similarly, the estimated daily intake (EDI) for direct ingestion of the contaminated leafy vegetables (mg kg
−1 day
−1) was calculated using Equation (5) [
38,
41].
Supplementary Materials (Table S1) describe the factors used, their units and values [
34,
40,
42,
43,
44,
45].
Target Hazard Quotient (THQ) was calculated to establish non-cancer risks from the TMTs through the three different exposure routes (Equations (6)–(9)). Due to the well-established additive effects of TMTs, the hazard index (HI) was established as the sum of the THQ of the individual element through a given exposure pathway (Equation (10)) [
40].
where
RfDING,
RfDINH and
RfDDC are the oral (direct ingestion), inhalation and dermal reference doses of the specific element.
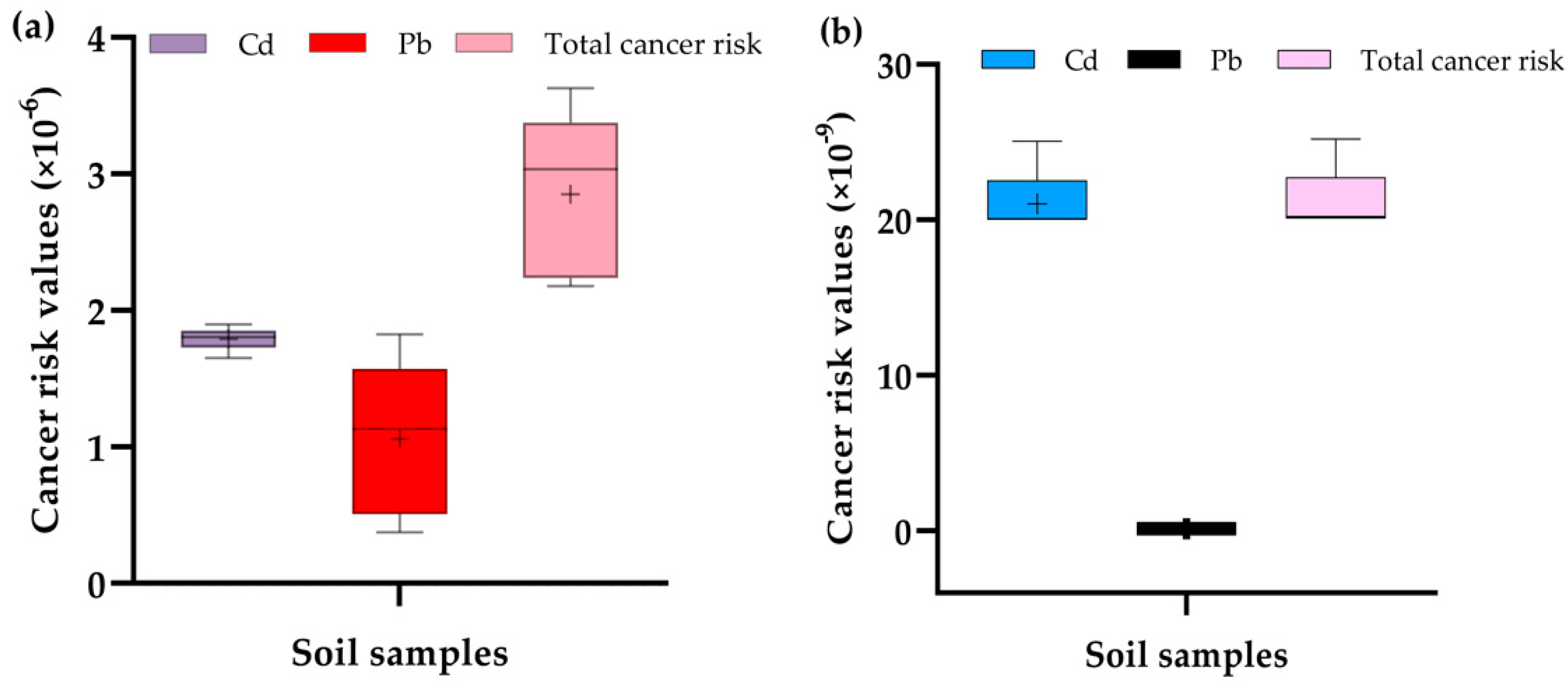
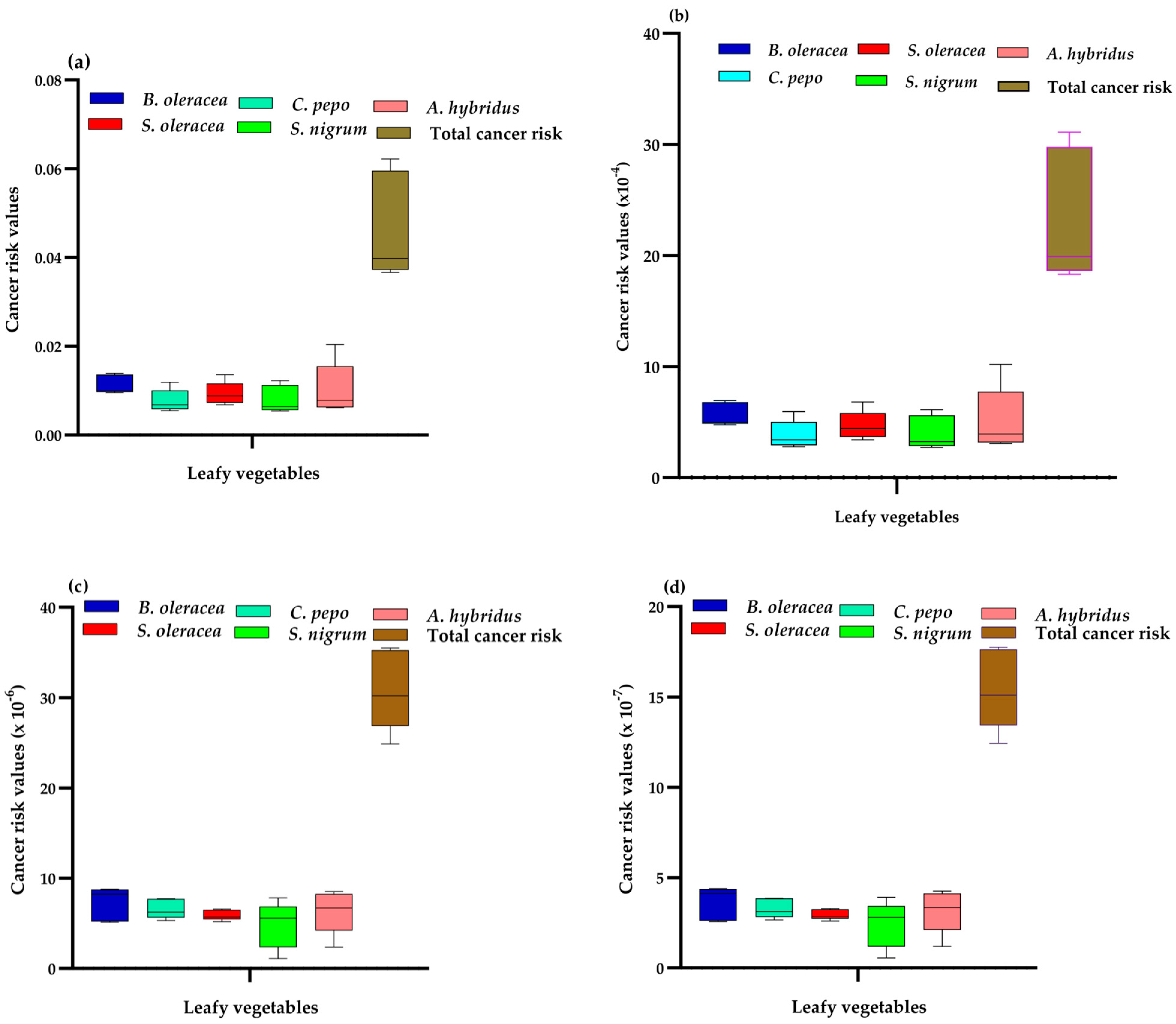
2.6.2. Cancer Health Risks Evaluation
To assess the probable cancer risks (CR), the incremental lifetime cancer risk for ingestion of the carcinogenic elements (Cd and Pb) was estimated for direct ingestion of soils or vegetables using Equations (11) and (12) [
40].
where IGCSF is the ingestion cancer slope factor = 5.01 × 10
−1 and 8.5 × 10
−3 mg kg
−1 day
−1, respectively, for Cd and Pb [
40].
2.7. Soil Pollution Extents and Ecological Health Risks Posed by the TMTs
To decipher soil pollution levels of the TMTs along the banks of River Rwizi, geostatistical indices, i.e., the contamination factor [
46] and geo-accumulation index [
47], were calculated (Equations (13) and (14)). This was performed with respect to the concentrations of TMTs in the soils to the concentration of the metals in uncontaminated soils sampled from Mbarara City in our previous study [
33] for Mn (695.3 mg kg
−1), Cd (0.13 mg kg
−1), Pb (19.88 mg kg
−1), and Cr (40.69 mg kg
−1). The background concentrations for Cu (38.9 mg kg
−1) and Zn (64.00 mg kg
−1) used are the global averages of the upper continental crust [
48].
where C
TMT is the concentration of the TMT in the soil sample, C
bg is the background concentration of the same TMT in the background soil sample, and 1.5 is the background matrix correction factor [
33]. The classification of CF
s and I
geo values is described in
Table S2.
To discern the cumulative pollution load in a particular soil sample, the pollution load index (PLDI) was calculated using Equation (15).
wherein CF
S1 to CF
S6 are the calculated contamination factors for the six TMTs.
The potential ecological risk index technique based on the work of Hakanson [
46] was used to assess the sensitivity of the biotic community to the TMTs. The potential ecological risk coefficient (
) and potential ecological risk index (PERI) were calculated (Equations (16) and (17)).
From which
= biological toxic factor of the TMTs: Mn = Zn = 1, Cd = 30, Pb = Cu = 5, and Cr = 2 [
49]. The risk characterization criterion is provided in
Table S2.
2.8. Statistical Analysis
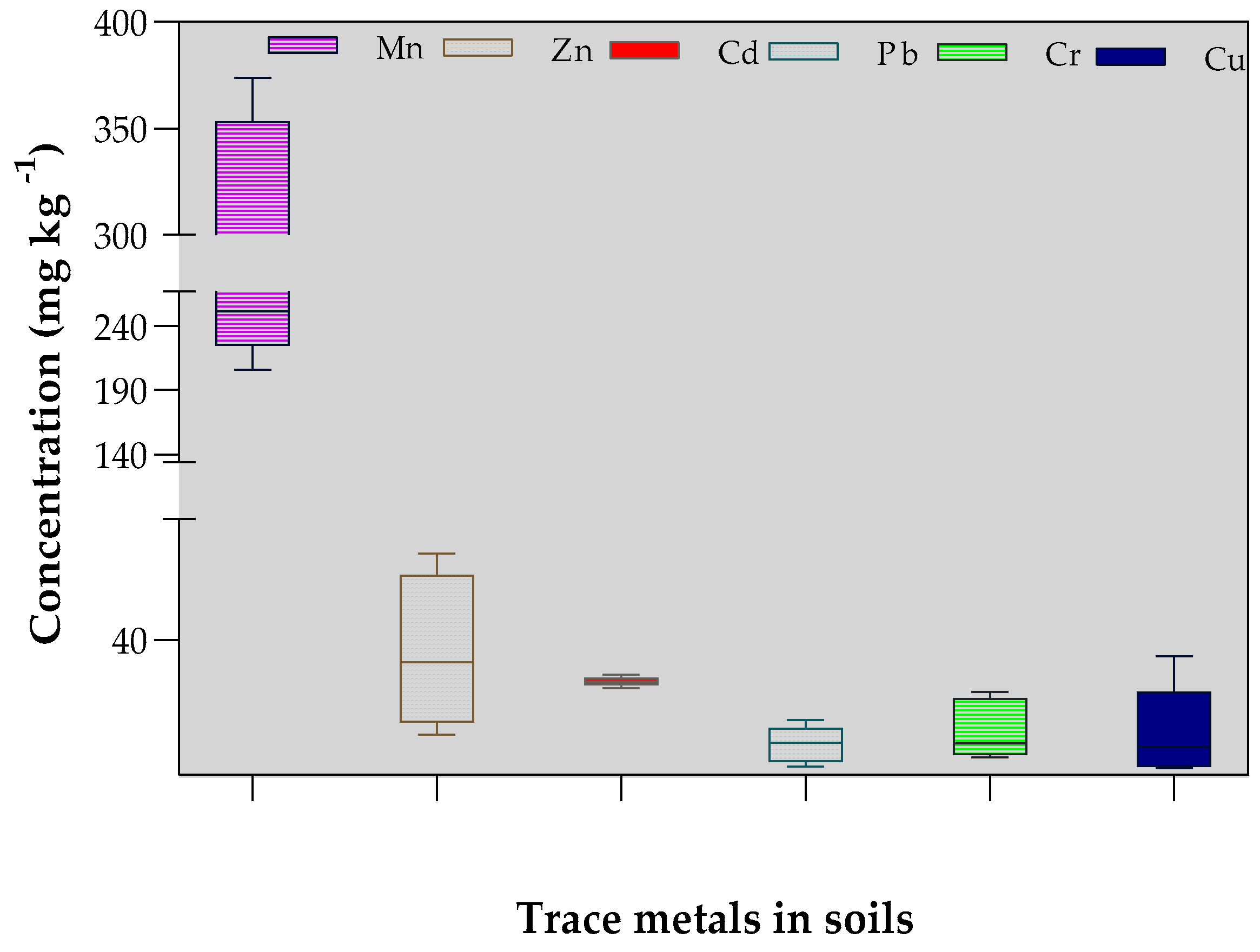
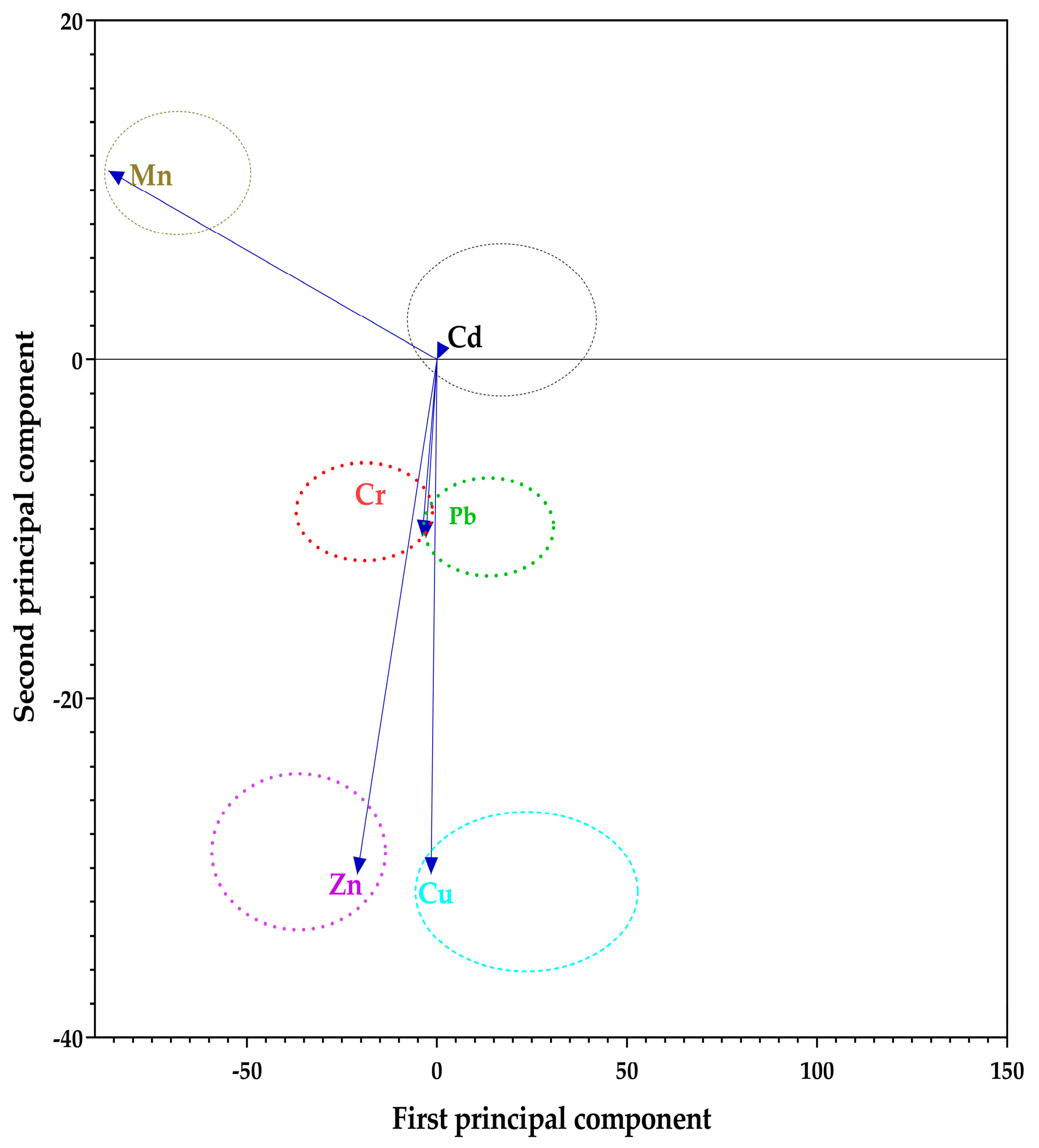
All the numerical data were captured in Microsoft Excel where descriptive statistics were used to summarize them as means with standard deviations. Pearson’s correlation analysis was used to establish potential pairwise relationships between the concentration of TMTs in the soils and the leafy vegetable tissues. To establish the hypothetical sources of the TMTs, Principal Component Analysis (PCA) was used. Further, PCA components were transformed using a varimax rotation with Kaiser normalization post analysis. One-way analysis of variance (ANOVA) at p < 0.05 was employed to identify potential significant differences among the means for the TMTs at the different sites. All statistical analyses and data visualizations proceeded in GraphPad Prism (v9.3.1 for Windows, GraphPad Software, San Diego, CA, USA).