Exploring the Potentials of Halophytes in Addressing Climate Change-Related Issues: A Synthesis of Their Biological, Environmental, and Socioeconomic Aspects
Abstract
1. Introduction
2. Methodology for Literature Search
3. Biological Aspects of Halophytes
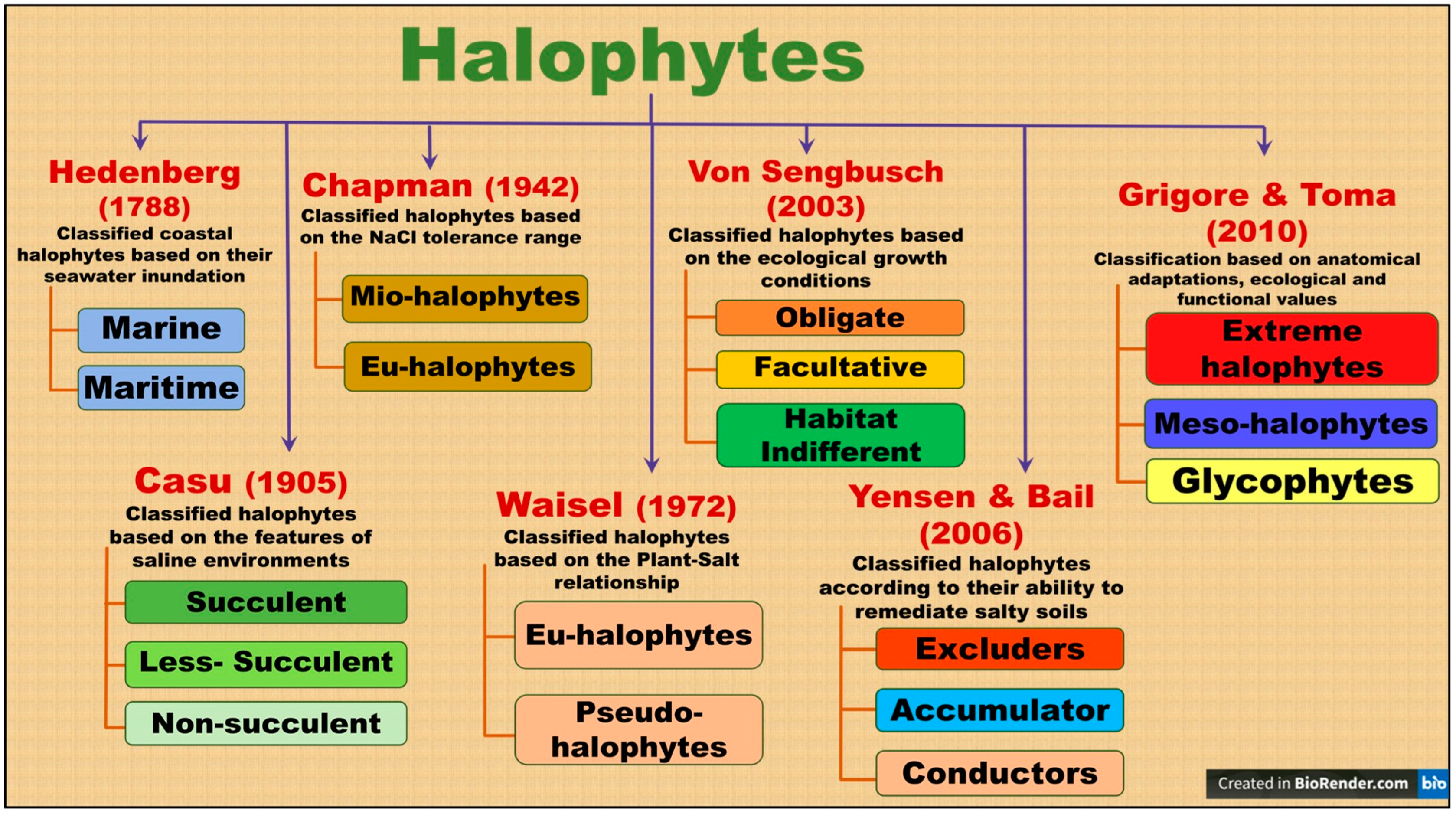
3.1. Distribution and Classification
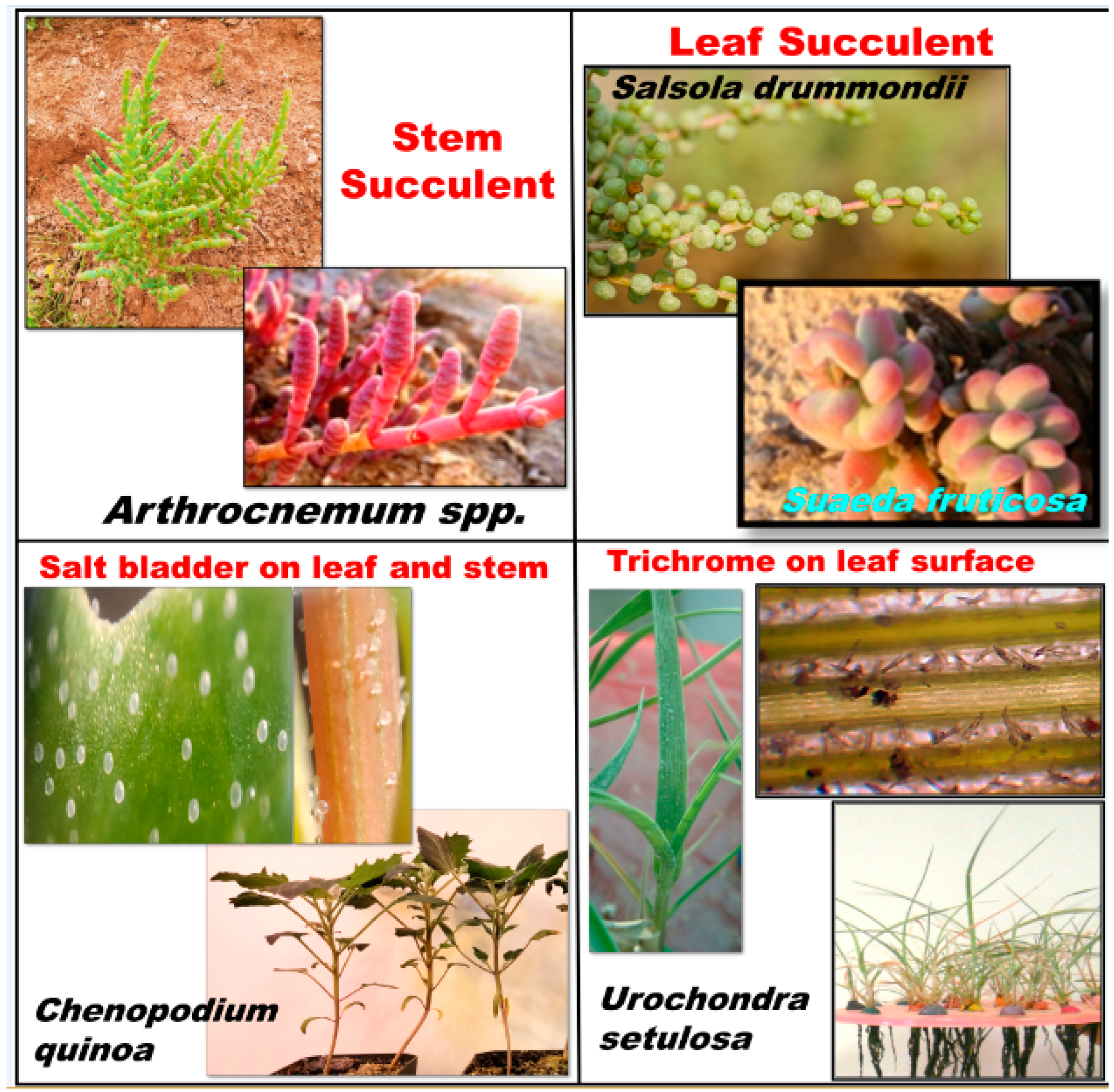
3.2. Morphological Features of Halophytes to Cope with Salt Stress?
3.3. Physio-Chemical and Molecular Mechanisms of Halophytes to Cope with Salinity
3.4. Biological Diversity of Halophytes
4. Environmental Aspects of Halophytes
4.1. Ecological Roles and Functions of Halophytes in Different Saline Habitats
- Mangrove mangles: Mangroves are woody halophytes found in intertidal zones of tropical and subtropical regions [84,85]. They have pneumatophores (aerial roots), which help them to cope with anoxic waterlogging conditions and also have salt glands to excrete excess salt through their leaves [86]. Mangroves are highly productive ecosystems capable of sequestering large amounts of carbon in their biomass and sediments [87], which is termed blue carbon. Mangroves can store approximately 694 Mg C ha−1 blue carbon [88]. They also guard the coastline from erosion, storm surges, and tsunamis. Furthermore, they also provide a habitat for a variety of fishes, crabs, and birds [87].
- Salt marshes: Salt marsh halophytes possess succulent leaves, which store water to dilute salts. Salt marshes are also among the highly productive ecosystems with large quantities of blue carbon sequestered in their below-ground biomass and sediments [89]. For instance, salt marshes along Tampa bay, Florida, USA contained as much as 66.4 Mg C ha−1 blue carbon [90]. They also buffer the effects of tides, waves, or floods. They are home to a variety of insects, mollusks, and birds [91].
- Coastal Sand dunes: Plants growing on the coastal sand dunes are adapted to harsh environments, characterized by frequent sand and wind blasting, low nutrient and water availability, high temperature, lack of shade, salt spray, and high soil salinity [92]. Their adaptations include deep root systems, vegetative growth, high nutrient use efficiency, thick outer layers, trichomes over leaves, and succulent leaves that protect them from sand scour, water loss, and nutrient limitation [92]. Dune halophytes help in increasing the diversity of organisms at both above and below the soil surface due to their roles as sources of carbon and role as hosts for insects, bacteria, fungi, birds, and mammals of various kinds [92]. Halophyte vegetation also covers the sand dunes to reduce the sand erosion/dunes movement to support recreational activities [27,92].
- Sabkhat or salt flats: Sabkhat (Singular: Sabkha) are characterized by sparse halophytic vegetation with specialized adaptations [93]. Salt flats are generally low-productivity ecosystems, where halophytes fix soil and provide habitats for some species of insects, reptiles, or birds [93]. Loughland and Cunnigham [94] reported 10 mammal and 21 reptile species from the sabkhat of Arabian Peninsula and 17 mammals and 9 reptiles from Central Asia. Similarly, Hogarth and Tigar [95] reported the occurrence of insects belonging to Hemiptera, Homoptera, Lepidoptera, and Coleoptera from the sabkhat.
- Playa: The playas are often considered synonymous to sabkhat, but differ mainly in geographical and hydrological characteristics [93,96]. Playas are often defined as inland permanent or occasionally inundated saline flats in proximity of a water body such as mountainous lakes [93]. Playa vegetation is halophytic in nature and has adaptations to cope with wet–dry cycles, and thereby, the rapid fluctuations of the environmental variables in these habitats [97]. A large number of playa halophytes are annuals, which endure periods of harsh conditions in the form of seed banks and actively grow when the conditions of the playa are conducive for plant growth such as after sufficient rainfall. Playa halophytes help in the formation of substrates by increasing the soil thickness and enhance the organic content of soil to support the next stage of vegetation succession [97].
| Habitat | Species (Examples) | Reference |
|---|---|---|
| Mangroves/mangles | Avicennia marina | [98] |
| Rhizophora mucronata | ||
| Ceriops tagal | ||
| Aegiceras corniculatum | ||
| Avicennia macrostachyum | [99] | |
| Avicennia germinans | ||
| Laguncularia racemosa | ||
| Salt marshes | Arthrocnemum macrostachyum | [27] |
| Arthrocnemum indicum | ||
| Aeluropus lagopoides | ||
| Sprolobolus tremulus | ||
| Cressa cretica | ||
| Spartina alterniflora | [100] | |
| Zostera japonica | ||
| Sarcocornia quinqueflora | [101] | |
| Salicornia spp. | [102] | |
| Coastal dunes | Cyperus conglomeratus | [27] |
| Heliotropium bacciferum | [103] | |
| Halopyrum mucronatum, | ||
| Ipomoea pes-caprae | ||
| Salsola imbricata | ||
| Sabkha/salt flats | Suaeda fruticosa | [104] |
| Limonium stocksii | ||
| Aeluropus lagooides | ||
| Urochondra setulosa | ||
| Arthrocnemum macrostachyum | [105] | |
| Cyperus aucheri | ||
| Halocnemum strobilaceum | ||
| Halopeplis perfoliata | ||
| Limonium spp. | ||
| Salicornia perennans | ||
| Seidlitzia rosmarinus | ||
| Tetraena spp. | ||
| Juncus rigidus, | ||
| Odyssea mucronata | ||
| Sporobolus spicatus | ||
| S. consimilis | ||
| Salsola drummondii | ||
| Suaeda vermiculata | ||
| Suaeda aegyptiaca | ||
| Anabasis setifera | ||
| Tetraena qatarense | ||
| Playa | Halogeton glomeratus | [106] |
| Lepidium latifolium | ||
| Peganum harmala | ||
| Suaeda heterophylla | [107] | |
| Salicornia rubra | [108] | |
| S. utahensis | ||
| Distichlis spicata | ||
| Allenrolfea occidentalis |
4.2. Effects of Climate Change Factors on Distribution, Diversity, and Productivity of Halophytes
4.3. Influence of Halophytes on the Soil Quality, Water Balance, Carbon Sequestration, Nutrient Cycling, and Biodiversity Conservation of Saline Ecosystems
4.4. Potential Threats and Challenges for Halophyte Conservation and Management
5. Socioeconomic Aspects of Halophytes
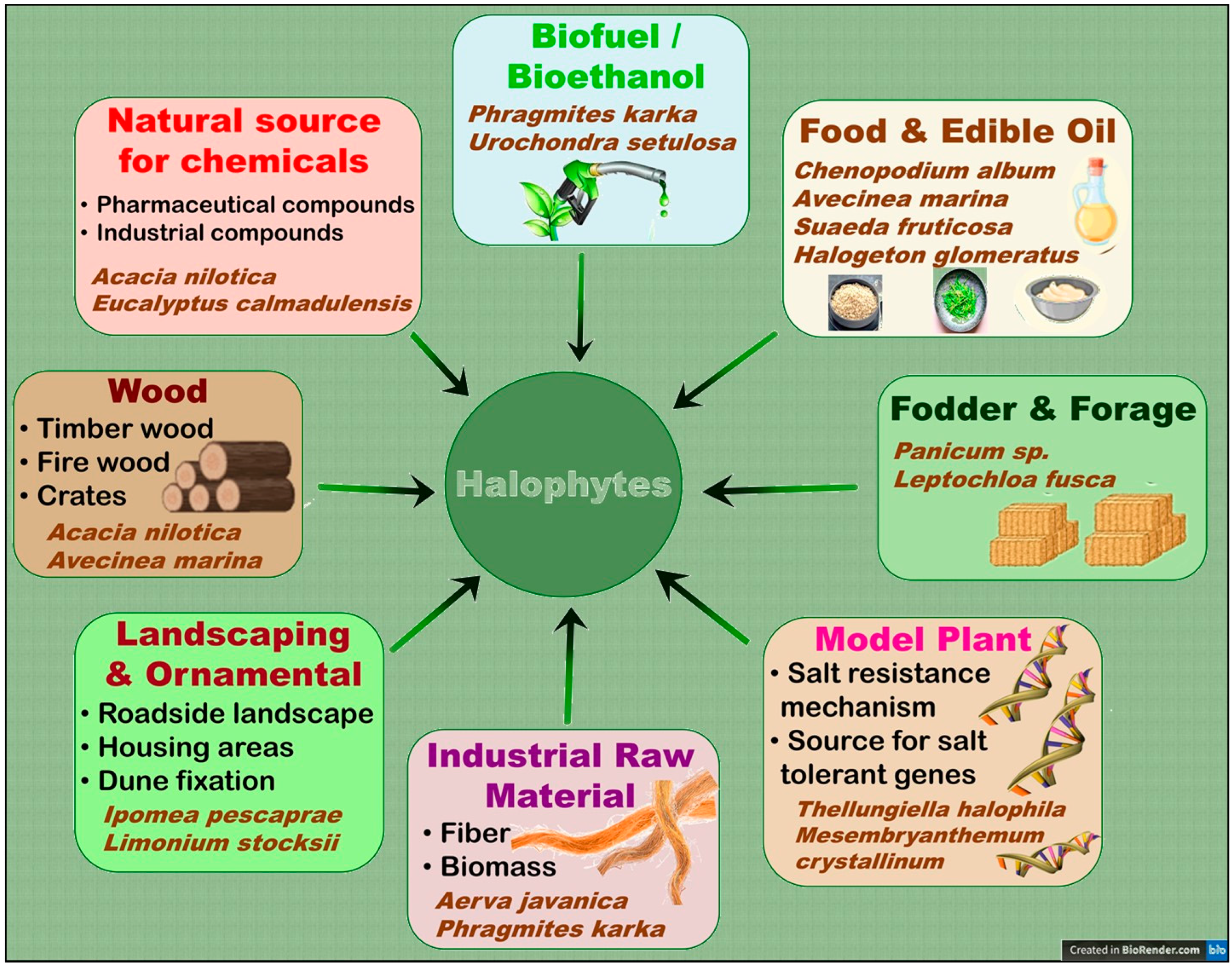
5.1. Potential Uses and Importance of Halophytes for Human Welfare
- Food security in arid/saline regions: Many halophytes can be a source of food for humans and livestock in arid and semi-arid regions, where soil salinity and fresh water scarcity are major constraints to crop production [3,7,128]. Khan et al. [79] reported that the halophyte grass Panicum turgidum can produce up to 60,000 kg year−1 ha−1 fresh biomass on saline lands and is comparable to maize in its fodder properties. Besides their use as fodder [79], halophytes can also be used to produce a variety of food products, including vegetables and grains [7]. Many halophytes such as quinoa, Purslane, and Salicornia have already gained popularity as alternative food crops [7,63,147,148]. In fact, quinoa grains are now widely sold and considered a super-food with multiple benefits [149,150]. In addition, Barreira et al. [148] evaluated the nutritional characteristics of the halophytes Sarcocornia perennis subsp. perennis, Sarcocornia perennis subsp. alpini and Salicornia ramosissima and Arthrocnemum macrostachyum and found them suitable for human consumption.
- Phytoremediation of saline/polluted soils: Halophytes have the remarkable ability to remove excess salts from soil and water, thereby improving the soil [122,151]. Farzi et al. [152] found that the halophytes Salicornia europaea, Salsola crassa, and Bienertia cycloptera had very good ability to reduce the water salinity of the constructed wetlands. Halophytes can also be used to treat wastewater from industries such as oil and gas [153,154]. In addition, many halophytes are hyper-accumulators of heavy metals and therefore can be used for the phytoremediation of heavy-metal-polluted soils [155]. In this context, Arthrocnemum macrostachyum [156], Halogeton glomeratus [157], Suaeda fruticosa, Atriplex lentiformis [158], Salicornia fruticosa [159], Tamarix africana [160], Sesuvium portulacastrum [161], Spartina alterniflora [162], Suaeda glauca, and Kochia scoparia [163] are some examples of halophytes with the ability to phytoremediate heavy-metal-polluted soils.
- Medicinal compounds and essential oil production: Halophytes have been used in traditional medicine to treat various ailments such as inflammation, pain, and infections [164,165]. For instance, Qasim et al. [164] reported that 45 halophytes of the coastal and near-coastal areas of Pakistan are being used to treat seven different disease conditions by locals, whereas Garcia-Caparros et al. [9] recently reported a total of 258 halophytes from different parts of the world with medicinal properties. Halophytes contain bioactive compounds that have antimicrobial, antioxidant, and anti-inflammatory properties [166,167]. For instance, the halophytes Sonchus brachyotus and Limonium tetragonum had high phenolic content, antioxidant and anti-inflammatory activities [168]. Similarly, another halophyte, Suaeda fruticosa, also had high anticancer, antioxidant, antidiabetic, and antimicrobial potential [169,170,171]. Halophytes are also source essential oils [124,125]. Two edible halophytes Crithmum maritimum and Inula crithmoïdes reportedly contain essential oil with good antimicrobial activity [172], whereas essential oil from a Tunisian halophyte Lobularia maritima has good potential to be used as a preservative in the meat industry.
- Biofuel and renewable energy production: Halophytes may also be used as a renewable source of bioenergy, such as biofuel, thus helping to reduce reliance on fossil fuels and fight climate change [173,174,175]. They can be grown on marginal lands, which are not suitable for conventional crops, thereby would reduce the food versus fuel dilemma [176]. Interestingly, many halophytes have a similar or even better lignocellulose composition compared to conventional biofuel feedstock, which makes the halophytic biomass highly suitable for the production of bio-ethanol, a common type of biofuel [175]. Similarly, the seeds of a number of halophytes are rich in oil, which can be converted into bio-diesel, another form of biofuel [176]. Many halophytes such as Alhagi maurorum, Atriplex rosea, Arthrocnemum macrostachyum, Cressa cretica, Halogeton glomeratus, Salicornia fruticosa, and Kosteletzkya virginica are reportedly promising candidates for the bio-diesel production [177]. In addition, many halophytes such as Salicornia spp. can also be a source of biogas/bio-methane production [178].
- Environmental conservation and eco-tourism: Halophytes play an important role in environmental conservation through various ecosystem services such as coast protection, soil stabilization, and land reclamation and carbon sequestration [124,127]. Halophytes such as mangrove mangles can also be utilized for eco-tourism sites [6,179,180] and fish/shrimp farming [181,182,183]. In this context, Özcan et al. [184] reported that owing to its diverse topography and rich halophyte diversity, Kavak Delta of Northwest Turkey has high potential for eco-tourism. Likewise, artificial floating-mangrove jetties can help not only in coastal protection but also in game-fishing and carbon sequestration purposes [185]. Mangrove forests are also a popular tourist destination in Bali, Indonesia, for scientists and environmentalists, where they can experience natural scenic view, explore diverse plants and animals, and understand the ecological and cultural significance of mangroves [186]. Sundarban mangrove forest, which is recognized as a UNESCO World Heritage Site, is another example of a popular tourist attraction site [187].
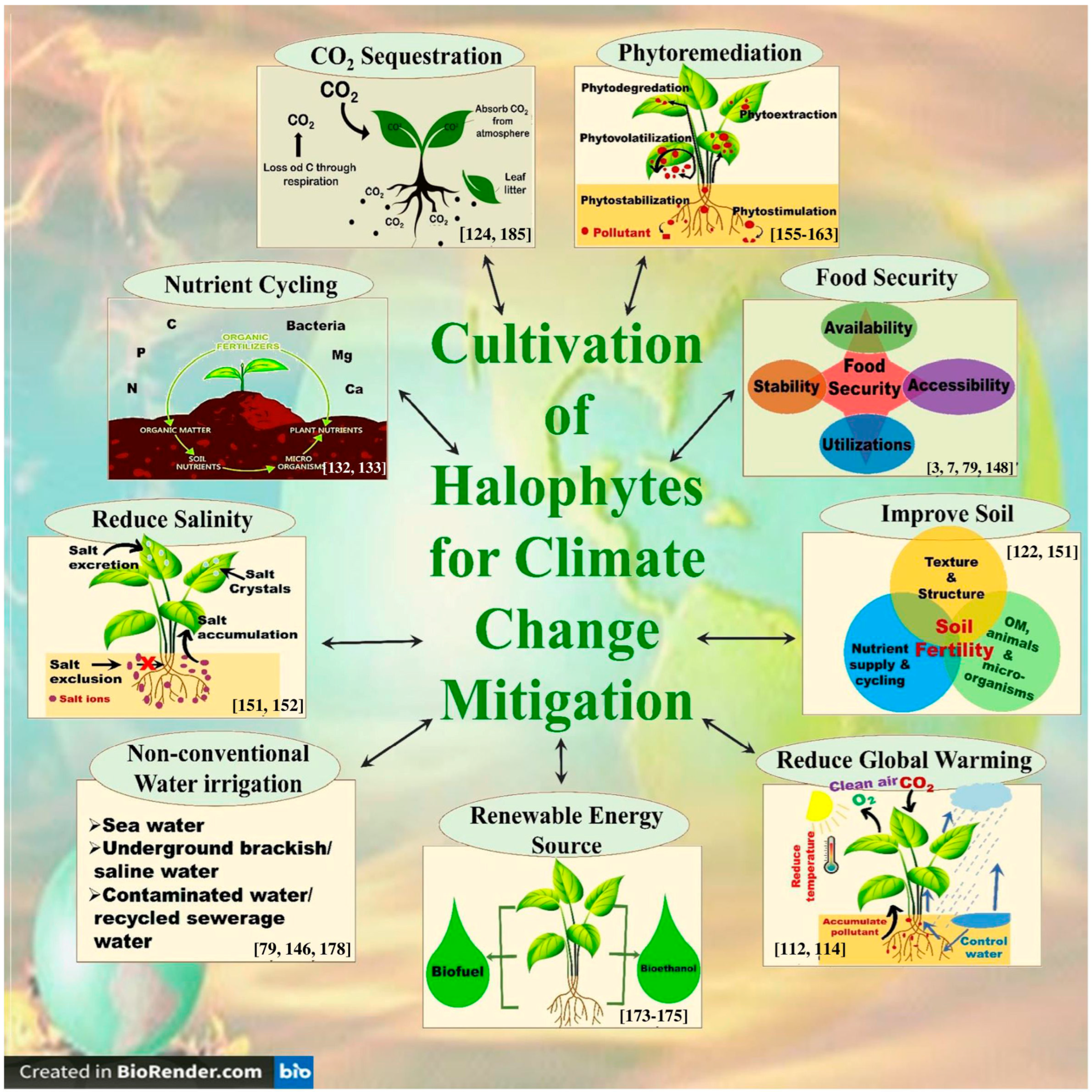
5.2. Challenges for Large Scale Cultivation of Halophytes
6. Discussion
- Collaborative research projects involving academic institutions, research centers, governmental agencies, and the corporate sector to hasten the development of halophyte-based solutions and close the gap between knowledge and application.
- Develop extension services and training programs to educate farmers and agricultural communities about halophyte cultivation techniques.
- The development of markets and marketing campaigns for halophyte products.
- Ensuring and safeguarding the intellectual property rights for halophyte varieties developed through local breeding initiatives, which can also promote private sector investment.
- Establishing regulatory frameworks or organizations for land rights, water use, harvesting, farming, and environmental impact. However, rules and regulations should be flexible enough to accommodate the developing halophyte utilization field.
- Governmental and international organizations’ research funding and programs might encourage academic institutions and research organizations to investigate the potentials of halophytes.
- Offering subsidies, tax exemptions, or preferential access to resources like saline water or land for halophyte production to farmers as incentives to adopt halophyte cultivation.
- International collaboration, conferences, and seminars to exchange information on halophyte research and development.
- Public education and awareness initiatives on halophytes’ benefits and their involvement in tackling issues like food security and climate change.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as new model plant species for salt tolerance strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef]
- Hameed, A.; Khan, M.A. Halophytes: Biology and economic potentials. Karachi Univ. J. Sci. 2011, 39, 40–44. [Google Scholar]
- Flowers, T.J.; Troke, P.F.; Yeo, A.R. The mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1977, 28, 89–121. [Google Scholar] [CrossRef]
- Grigore, M.N. Definition and Classification of Halophytes as an Ecological Group of Plants. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 3–50. [Google Scholar]
- Luković, M.; Aćić, S.; Šoštarić, I.; Pećinar, I.; Dajić Stevanović, Z. Management and Ecosystem Services of Halophytic Vegetation. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–31. [Google Scholar]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Garcia-Caparrós, P.; Nogales, A.; Abreu, M.M.; Santos, E.; Cortinhas, A.L.; Caperta, A.D. Sustainable agricultural management of saline soils in arid and semi-arid Mediterranean regions through halophytes, microbial and soil-based technologies. Environ. Exp. Bot. 2023, 212, 105397. [Google Scholar] [CrossRef]
- Garcia-Caparros, P.; Al-Azzawi, M.J.; Flowers, T.J. Economic Uses of Salt-Tolerant Plants. Plants 2023, 12, 2669. [Google Scholar] [CrossRef]
- Flowers, T.J.; Muscolo, A. Introduction to the special issue: Halophytes in a changing world. AoB Plants 2015, 7, plv020. [Google Scholar] [CrossRef]
- Grigore, M.N.; Vicente, O. Wild Halophytes: Tools for Understanding Salt Tolerance Mechanisms of Plants and for Adapting Agriculture to Climate Change. Plants 2023, 12, 221. [Google Scholar] [CrossRef]
- Koricheva, J.; Gurevitch, J. Uses and misuses of meta-analysis in plant ecology. J. Ecol. 2014, 102, 828–844. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A systematic review of plants with antibacterial activities: A taxonomic and phylogenetic perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Al-Ansari, N.; Hu, Y.; Acharki, S.; Vishwakarma, D.K.; Aghelpour, P.; Zubair, M.; Wandolo, C.A.; Elbeltagi, A. Misconceptions of reference and potential evapotranspiration: A PRISMA-guided comprehensive review. Hydrology 2022, 9, 153. [Google Scholar] [CrossRef]
- Angon, P.B.; Tahjib-Ul-Arif, M.; Samin, S.I.; Habiba, U.; Hossain, M.A.; Brestic, M. How do plants respond to combined drought and salinity stress?—A systematic review. Plants 2022, 11, 2884. [Google Scholar] [CrossRef] [PubMed]
- O’leary, J.W.; Glenn, E.P. Global distribution and potential for halophytes. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Tasks for Vegetation Science; Squires, V.R., Ayoub, A.T., Eds.; Springer: Dordrecht, The Netherlands, 1994; Volume 32, pp. 7–17. [Google Scholar]
- Shamsutdinov, N.Z.; Shamsutdinova, E.Z.; Orlovsky, N.S.; Shamsutdinov, Z.S. Halophytes: Ecological features, global resources, and outlook for multipurpose use. Her. Russ. Acad. Sci. 2017, 87, 1–11. [Google Scholar] [CrossRef]
- Chapman, V.J. The new perspective in the halophytes. Q. Rev. Biol. 1942, 17, 291–311. [Google Scholar] [CrossRef]
- Waisel, Y. The Biology of Halophytes; Elsevier: London, UK; Academic: Cambridge, MA, USA, 1972. [Google Scholar]
- Von Sengbusch, P. Halophytes Botanik Online; University of Hamburg: Hamburg, Germany, 2003. [Google Scholar]
- Yensen, N.P.; Biel, K.Y. Soil remediation via salt-conduction and the hypotheses of halosynthesis and photoprotection. In Ecophysiology of High Salinity Tolerant Plants; Khan, M.A., Weber, D.J., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 40, pp. 313–344. [Google Scholar]
- Grigore, M.N.; Toma, C. A proposal for a new halophytes classification, based on integrative anatomy observations. Oltenia J. Stud. Nat. Sci. 2010, 26, 45–50. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Flowers, T.J.; Yeo, A.R. Breeding for salinity resistance in crop plants: Where next? Funct. Plant Biol. 1995, 22, 875–884. [Google Scholar] [CrossRef]
- Pérez Cuadra, V.; Verolo, M.; Cambi, V. Morphological and anatomical traits of halophytes. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–20. [Google Scholar]
- Hameed, M.; Ashraf, M.; Ahmad, M.S.A.; Naz, N. Structural and functional adaptations in plants for salinity tolerance. In Plant Adaptation and Phytoremediation; Ashraf, M., Ozturk, M., Ahmad, M., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 151–170. [Google Scholar] [CrossRef]
- Khan, M.A.; Qaiser, M. Halophytes of Pakistan: Characteristics, distribution and potential economic usages. In Sabkha Ecosystems; Khan, M.A., Böer, B., Kust, G.M., Barth, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 129–153. [Google Scholar]
- Yun, P.; Shabala, S. Ion transport in salt glands and bladders in halophyte species. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 1859–1876. [Google Scholar]
- Gul, B.; Khan, M.A. Effect of growth regulators and osmotica in alleviating salinity effects on the germination of Salicornia utahensis. Pak. J. Bot. 2004, 35, 885–894. [Google Scholar]
- Gulzar, S.; Khan, M.A. Comparative salt tolerance of perennial grasses. In Ecophysiology of High Salinity Tolerant Plants. Tasks for Vegetation Science; Khan, M.A., Weber, D.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 40, pp. 239–253. [Google Scholar]
- Ak, P. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar]
- Ahmed, M.Z.; Shimazaki, T.; Gulzar, S.; Kikuchi, A.; Gul, B.; Khan, M.A.; Koyro, H.W.; Huchzermeyer, B.; Watanabe, K.N. The influence of genes regulating transmembrane transport of Na+ on the salt resistance of Aeluropus lagopoides. Funct. Plant Biol. 2013, 40, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Golldack, D.; Zhao, C.; Bohnert, H.J. The expression of HAK-type K+ transporters is regulated in response to salinity stress in common ice plant. Plant Physiol. 2002, 29, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Hussain, T.; Gulzar, S.; Aziz, I.; Gul, B.; Khan, M.A. Salt tolerance of a cash crop halophyte Suaeda fruticosa: Biochemical responses to salt and exogenous chemical treatments. Acta Physiol. Plant 2012, 34, 2331–2340. [Google Scholar] [CrossRef]
- Llanes, A.; Bertazza, G.; Palacio, G.; Luna, V. Different sodium salts cause different solute accumulation in the halophyte Prosopis strombulifera. Plant Biol. 2013, 15, 118–125. [Google Scholar] [CrossRef]
- Hassine, A.B.; Ghanem, M.E.; Bouzid, S.; Lutts, S. An inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus L. differ in their ability to accumulate proline and glycinebetaine in response to salinity and water stress. J. Exp. Bot. 2008, 59, 1315–1326. [Google Scholar] [CrossRef]
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Hedrich, R.; Shabala, S. Stomata in a saline world. Curr. Opin. Plant Biol. 2018, 46, 87–95. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Prashanth, S.R.; Sivaprakash, K.R.; Parida, A.K. Antioxidative response mechanisms in halophytes: Their role in stress defence. J. Genet. 2006, 85, 237–254. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Hussain, T.; Aziz, I.; Ahmad, N.; Gul, B.; Nielsen, B.L. Effects of salinity stress on chloroplast structure and function. Cells 2021, 10, 2023. [Google Scholar] [CrossRef]
- Hameed, A.; Gulzar, S.; Aziz, I.; Hussain, T.; Gul, B.; Khan, M.A. Effects of salinity and ascorbic acid on growth, water status and antioxidant system in a perennial halophyte. AoB Plants 2015, 7, plv004. [Google Scholar] [CrossRef]
- Benzarti, M.; Ben Rejeb, K.; Debez, A.; Messedi, D.; Abdelly, C. Photosynthetic activity and leaf antioxidative responses of Atriplex portulacoides subjected to extreme salinity. Acta Physiol. Plant 2012, 34, 1679–1688. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Srivastava, A.K.; Pandey, G.K.; Suprasanna, P. Halophytes in biosaline agriculture: Mechanism, utilization, and value addition. Land Degrad. Dev. 2018, 29, 1081–1095. [Google Scholar] [CrossRef]
- Diray-Arce, J.; Clement, M.; Gul, B.; Khan, M.A.; Nielsen, B.L. Transcriptome assembly, profiling and differential gene expression analysis of the halophyte Suaeda fruticosa provides insights into salt tolerance. BMC Genomics 2015, 16, 353. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Kurusu, T.; Kuchitsu, K.; Tada, Y. Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Front. Plant Sci. 2015, 6, 427. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Manuka, R.; Nikalje, G.C.; Penna, S. Halophytes as a potential resource for phytodesalination. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–21. [Google Scholar]
- Zhu, J.K. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol. 2000, 124, 941–948. [Google Scholar] [CrossRef]
- Denby, K.; Gehring, C. Engineering drought and salinity tolerance in plants: Lessons from genome-wide expression profiling in Arabidopsis. Trends Biotechnol. 2005, 23, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Bartels, D.; Dinakar, C. Balancing salinity stress responses in halophytes and non-halophytes: A comparison between Thellungiella and Arabidopsis thaliana. Funct. Plant Biol. 2013, 40, 819–831. [Google Scholar] [CrossRef]
- Kazachkova, Y.; Eshel, G.; Pantha, P.; Cheeseman, J.M.; Dassanayake, M.; Barak, S. Halophytism: What have we learnt from Arabidopsis thaliana relative model systems? Plant Physiol. 2018, 178, 972–988. [Google Scholar] [CrossRef]
- Koch, M.A.; German, D.A. Taxonomy and systematics are key to biological information: Arabidopsis, Eutrema (Thellungiella), Noccaea and Schrenkiella (Brassicaceae) as examples. Front. Plant Sci. 2013, 4, 267. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Raddatz, N.; Pardo, J.M.; Yun, D.J. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol. Plant 2021, 171, 546–558. [Google Scholar] [CrossRef]
- Akyol, T.Y.; Yilmaz, O.; Uzilday, B.; Uzilday, R.Ö.; Türkan, İ. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13. [Google Scholar]
- Li, H.; Wang, H.; Wen, W.; Yang, G. The antioxidant system in Suaeda salsa under salt stress. Plant Signal. Behav. 2020, 15, 1771939. [Google Scholar] [CrossRef] [PubMed]
- Moinoddini, F.; Mirshamsi, K.A.; Bagheri, A.; Jalilian, A. Genome-wide analysis of annexin gene family in Schrenkiella parvula and Eutrema salsugineum suggests their roles in salt stress response. PLoS ONE 2023, 18, e0280246. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An overview of the emerging trends of the Salicornia L. genus as a sustainable crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Katel, S.; Yadav, S.P.S.Y.; Turyasingura, B.; Mehta, A. Salicornia as a salt-tolerant crop: Potential for addressing climate change challenges and sustainable agriculture development. Turk. J. Food Agric. Sci. 2023, 5, 55–67. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa–a model crop for understanding salt-tolerance mechanisms in halophytes. Plant Biosyst. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Jaikishun, S.; Li, W.; Yang, Z.; Song, S. Quinoa: In perspective of global challenges. Agronomy 2019, 9, 176. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.E.; Shabala, S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Jarvis, D.E.; Ho, Y.S.; Lightfoot, D.J.; Schmöckel, S.M.; Li, B.; Borm, T.J.; Ohyanagi, H.; Mineta, K.; Michell, C.T.; Saber, N.; et al. The genome of Chenopodium quinoa. Nature 2017, 542, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Matanguihan, J.B.; Fuentes, F.F.; Gómez-Pando, L.R.; Jellen, E.N.; Maughan, P.J.; Jarvis, D.E. Quinoa breeding and genomics. Plant Breed. Rev. 2018, 42, 257–320. [Google Scholar]
- Guo, S.; Yin, H.; Zhang, X.; Zhao, F.; Li, P.; Chen, S.; Zhao, Y.; Zhang, H. Molecular cloning and characterization of a vacuolar H+-pyrophos-phatase gene, SsVP, from the halophyte Suaeda salsa and its overexpression increases salt and drought tolerance of Arabidopsis. Plant Mol. Biol. 2006, 60, 41–50. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Prinz, K.; Weising, K.; Hensen, I. Genetic structure of coastal and inland populations of the annual halophyte Suaeda maritima (L.) dumort. in Central Europe, inferred from amplified fragment length polymorphism markers. Plant Biol. 2009, 11, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.Z.; Gilani, S.A.; Kikuchi, A.K.I.R.A.; Gulzar, S.; Khan, M.A.; Watanabe, K.N. Population diversity of Aeluropus lagopoides: A potential cash crop for saline land. Pak. J. Bot. 2011, 43, 595–605. [Google Scholar]
- Xu, W.; Wang, J.; Tian, C.; Shi, W.; Wang, L. Genome-Wide Development of Polymorphic Microsatellite Markers and Genetic Diversity Analysis for the Halophyte Suaeda aralocaspica (Amaranthaceae). Plants 2023, 12, 1865. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.H.; Dassanayake, M.; Haas, J.S.; Kropornika, A.; Wright, C.; d’Urzo, M.P.; Hong, H.; Ali, S.; Hernandez, A.; Lambert, G.M.; et al. Genome structures and halophyte-specific gene expression of the extremophile Thellungiella parvula in comparison with Thellungiella salsuginea (Thellungiella halophila) and Arabidopsis. Plant Physiol. 2010, 154, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, M.; Gucel, S.; Guvensen, A.; Kadis, C.; Kounnamas, C. Halophyte plant diversity, coastal habitat types and their conservation status in Cyprus. In Sabkha Ecosystems. Tasks for Vegetation Science; Öztürk, M., Böer, B., Barth, H.J., Clüsener-Godt, M., Khan, M., Breckle, S.W., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 46, pp. 99–111. [Google Scholar]
- United Nations. Biodiversity—Our Strongest Natural Defense against Climate Change. 2022. Available online: https://www.un.org/en/climatechange/science/climate-issues/biodiversity (accessed on 3 September 2023).
- Khan, M.A.; Ansari, R.; Ali, H.; Gul, B.; Nielsen, B.L. Panicum turgidum, a potentially sustainable cattle feed alternative to maize for saline areas. Agric. Ecosyst. Environ. 2009, 129, 542–546. [Google Scholar] [CrossRef]
- Debez, A.; Huchzermeyer, B.; Abdelly, C.; Koyro, H.W. Current challenges and future opportunities for a sustainable utilization of halophytes. In Sabkha Ecosystems. Tasks for Vegetation Science; Öztürk, M., Böer, B., Barth, H.J., Clüsener-Godt, M., Khan, M., Breckle, S.W., Eds.; Springer: Dordrecht, The Netherlands, 2010; Volume 3, pp. 59–77. [Google Scholar]
- Bueno, M.; Cordovilla, M.P. Ecophysiology and Uses of Halophytes in Diverse Habitats. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–25. [Google Scholar]
- Singh, J.P.; Rathore, V.S.; Mangalassery, S.; Dayal, D. Diversity and Utilization of Halophytes of Hot Arid Rangelands: A Review. Ann. Arid Zone 2019, 58, 65–77. [Google Scholar]
- Milchakova, N. Ecosystem Services of Seagrasses. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–21. [Google Scholar]
- Walsh, G.E. Mangroves: A review. In Ecology of Halophytes; Robert, J.M., Ed.; Elsevier: London, UK, 1974; pp. 51–174. [Google Scholar]
- Krauss, K.W.; Ball, M.C. On the halophytic nature of mangroves. Trees 2013, 27, 7–11. [Google Scholar] [CrossRef]
- Parida, A.K.; Jha, B. Salt tolerance mechanisms in mangroves: A review. Trees 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Alongi, D.M. Impacts of climate change on blue carbon stocks and fluxes in mangrove forests. Forests 2022, 13, 149. [Google Scholar] [CrossRef]
- Alongi, D.M. Carbon balance in salt marsh and mangrove ecosystems: A global synthesis. J. Mar. Sci. Eng. 2020, 8, 767. [Google Scholar] [CrossRef]
- Radabaugh, K.R.; Moyer, R.P.; Chappel, A.R.; Powell, C.E.; Bociu, I.; Clark, B.C.; Smoak, J.M. Coastal blue carbon assessment of mangroves, salt marshes, and salt barrens in Tampa Bay, Florida, USA. Estuaries Coasts 2018, 41, 1496–1510. [Google Scholar] [CrossRef]
- Friess, D.A.; Yando, E.S.; Alemu, J.B.; Wong, L.W.; Soto, S.D.; Bhatia, N. Ecosystem services and disservices of mangrove forests and salt marshes. In Oceanography and Marine Biology; Hawkins, S.J., Allcock, A.L.A., Bates, E., Evans, A.J., Firth, L.B., McQuaid, C.D., Russell, B.D., Smith, I.P., Swearer, S.E., Todd, P.A., Eds.; Taylor & Francis: Oxford, UK, 2020; pp. 107–142. [Google Scholar]
- Maun, M.A. The Biology of Coastal Sand Dunes; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Barth, H.J.; Böer, B. Sabkha Ecosystems, The Arabian Peninsula and Adjacent Countries; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002; Volume 1. [Google Scholar]
- Loughland, R.A.; Cunningham, P.L. Vertebrate fauna of Sabkhat from the Arabian Peninsula: A review of Mammalia, Reptilia and Amphibia. In Sabkha Ecosystems; Barth, H.J., Böer, B., Eds.; Springer Kluwer Academic: Dordrecht, The Netherland, 2002; pp. 255–266. [Google Scholar]
- Hogarth, P.J.; Tigar, B.J. Ecology of sabkha arthropods. In Sabkha Ecosystems; Barth, H.J., Böer, B., Eds.; Springer Kluwer Academic: Dordrecht, The Netherland, 2002; pp. 267–282. [Google Scholar]
- Briere, P.R. Playa, playa lake, sabkha: Proposed definitions for old terms. J. Arid Environ. 2000, 45, 1–7. [Google Scholar] [CrossRef]
- Haukos, D.A.; Smith, L.M. Ecology of playa lakes. In Waterfowl Management Handbook; U.S. Fish and Wildlife Service: Fort Collins, CO, USA, 1992. Available online: https://digitalcommons.unl.edu/icwdmwfm/19 (accessed on 3 September 2023).
- Aziz, I.; Khan, F. Distribution, Ecology and Ecophysiology of Mangroves in Pakistan. In Sabkha Ecosystems. Tasks for Vegetation Science; Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B., Eds.; Springer: Dordrecht, The Netherland, 2014; pp. 55–66. [Google Scholar]
- Adame, M.F.; Reef, R.; Santini, N.S.; Najera, E.; Turschwell, M.P.; Hayes, M.A.; Masque, P.; Lovelock, C.E. Mangroves in arid regions: Ecology, threats, and opportunities. Estuar. Coast. Shelf Sci. 2021, 248, 106796. [Google Scholar] [CrossRef]
- Yue, S.; Zhou, Y.; Xu, S.; Zhang, X.; Liu, M.; Qiao, Y.; Gu, R.; Xu, S.; Zhang, Y. Can the non-native salt marsh halophyte Spartina alterniflora threaten native seagrass (Zostera japonica) habitats? A case study in the Yellow River Delta, China. Front. Plant Sci. 2021, 12, 643425. [Google Scholar] [CrossRef]
- Vårhammar, A.; McLean, C.M.; Yu, R.M.K.; MacFarlane, G.R. Uptake and partitioning of metals in the Australian saltmarsh halophyte, samphire (Sarcocornia quinqueflora). Aquat. Bot. 2019, 156, 25–37. [Google Scholar] [CrossRef]
- Alfheeaid, H.A.; Raheem, D.; Ahmed, F.; Alhodieb, F.S.; Alsharari, Z.D.; Alhaji, J.H.; BinMowyna, M.N.; Saraiva, A.; Raposo, A. Salicornia bigelovii, S. brachiata and S. herbacea: Their Nutritional Characteristics and an Evaluation of Their Potential as Salt Substitutes. Foods 2022, 11, 3402. [Google Scholar] [CrossRef] [PubMed]
- Mujeeb, A.; Aziz, I.; Ahmed, M.Z.; Alvi, S.K.; Shafiq, S. Comparative assessment of heavy metal accumulation and bio-indication in coastal dune halophytes. Ecotoxicol. Environ. Saf. 2020, 195, 110486. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B. Salt tolerant plants of coastal sabkhas of Pakistan. In Sabkha Ecosystems; Barth, H.J., Böer, B., Eds.; Springer Kluwer Academic: Dordrecht, The Netherland, 2002; pp. 123–140. [Google Scholar]
- Ghazanfar, S.A.; Böer, B.; Al Khulaidi, A.W.; El-Keblawy, A.; Alateeqi, S. Plants of Sabkha ecosystems of the Arabian Peninsula. In Sabkha Ecosystems; Gul, B., Böer, B., Khan, M., Clüsener-Godt, M., Hameed, A., Eds.; Springer: Dordrecht, The Netherland, 2019; Asia/Pacific 2019; Volume VI, pp. 55–80. [Google Scholar]
- Ahmed, M.Z.; Khan, M.A. Tolerance and recovery responses of playa halophytes to light, salinity and temperature stresses during seed germination. Flora 2010, 205, 764–771. [Google Scholar] [CrossRef]
- Hameed, A.; Ahmed, M.Z.; Gulzar, S.; Gul, B.; Alam, J.; Hegazy, A.K.; Alatar, A.R.A.; Khan, M.A. Seed germination and recovery responses of Suaeda heterophylla to abiotic stresses. Pak. J. Bot. 2013, 45, 1649–1656. [Google Scholar] [CrossRef]
- Harris, L.C.; Gul, B.; Khan, M.A.; Hansen, L.D.; Smith, B.N. Seasonal changes in respiration of halophytes in salt playas in the Great Basin, USA. Wetl. Ecol. Manag. 2001, 9, 463–468. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Walters, D.C.; Reay, W.G.; Carr, J.A. Sea level driven marsh expansion in a coupled model of marsh erosion and migration. Geophys. Res. Lett. 2016, 43, 4366–4373. [Google Scholar] [CrossRef]
- Friess, D.A.; Krauss, K.W.; Taillardat, P.; Adame, M.F.; Yando, E.S.; Cameron, C.; Sasmito, S.D.; Sillanpää, M. Mangrove blue carbon in the face of deforestation, climate change, and restoration. Annu. Plant Rev. 2020, 3, 427–456. [Google Scholar]
- Asadullah; Bano, A. Climate Change Modulates Halophyte Secondary Metabolites to Reshape Rhizosphere Halobacteria for Biosaline Agriculture. Appl. Sci. 2023, 13, 1299. [Google Scholar] [CrossRef]
- Geissler, N.; Lieth, H.; Koyro, H.W. The Ecologically and Economically Sustainable Use of Naturally Salt-Resistant Plants in the Context of Global Changes. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Ahmad, P., Wani, M., Eds.; Springer: New York, NY, USA, 2013; Volume 1, pp. 145–162. [Google Scholar]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Jiang, J.; DeAngelis, D.L.; Teh, S.Y.; Krauss, K.W.; Wang, H.; Li, H.; Smith, I.T.J.; Koh, H.L. Defining the next generation modeling of coastal ecotone dynamics in response to global change. Ecol. Model. 2016, 326, 168–176. [Google Scholar] [CrossRef]
- Mahdavi, P.; Bergmeier, E. Distribution of C4 plants in sand habitats of different climatic regions. Folia Geobot. 2018, 53, 201–211. [Google Scholar] [CrossRef]
- Borges, F.O.; Santos, C.P.; Paula, J.R.; Mateos-Naranjo, E.; Redondo-Gomez, S.; Adams, J.B.; Caçador, I.; Fonseca, V.F.; Reis-Santos, P.; Duarte, B.; et al. Invasion and extirpation potential of native and invasive Spartina species under climate change. Front. Mar. Sci. 2021, 8, 696333. [Google Scholar] [CrossRef]
- Estrelles, E.; Biondi, E.; Galiè, M.; Mainardi, F.; Hurtado, A.; Soriano, P. Aridity level, rainfall pattern and soil features as key factors in germination strategies in salt-affected plant communities. J. Arid Environ. 2015, 117, 1–9. [Google Scholar] [CrossRef]
- Moreno, J.; Terrones, A.; Juan, A.; Alonso, M.Á. Halophytic plant community patterns in Mediterranean saltmarshes: Shedding light on the connection between abiotic factors and the distribution of halophytes. Plant Soil 2018, 430, 185–204. [Google Scholar] [CrossRef]
- Martinez, J.P.; Ledent, J.F.; Bajji, M.; Kinet, J.M.; Lutts, S. Effect of water stress on growth, Na+ and K+ accumulation and water use efficiency in relation to osmotic adjustment in two populations of Atriplex halimus L. Plant Growth Regul. 2003, 41, 63–73. [Google Scholar] [CrossRef]
- Williams, K.; Ewel, K.C.; Stumpf, R.P.; Putz, F.E.; Workman, T.W. Sea-level rise and coastal forest retreat on the west coast of Florida, USA. Ecology 1999, 80, 2045–2063. [Google Scholar] [CrossRef]
- Xue, L.; Li, X.; Yan, Z.; Zhang, Q.; Ding, W.; Huang, X.; Tian, B.; Ge, Z.; Yin, Q. Native and non-native halophytes resiliency against sea-level rise and saltwater intrusion. Hydrobiologia 2018, 806, 47–65. [Google Scholar] [CrossRef]
- Karakas, S.; Dikilitas, M.; Tıpırdamaz, R. Phytoremediation of Salt-Affected Soils Using Halophytes. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–18. [Google Scholar]
- Sarath, N.G.; Sruthi, P.; Shackira, A.M.; Puthur, J.T. Halophytes as effective tool for phytodesalination and land reclamation. In Frontiers in Plant-Soil Interaction; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 459–494. [Google Scholar]
- Shaygan, M.; Baumgartl, T. Reclamation of salt-affected land: A review. Soil Syst. 2022, 6, 61. [Google Scholar] [CrossRef]
- Turcios, A.E.; Miglio, R.; Vela, R.; Sánchez, G.; Bergier, T.; Włodyka-Bergier, A.; Cifuentes, J.I.; Pignataro, G.; Avellan, T.; Papenbrock, J. From natural habitats to successful application-Role of halophytes in the treatment of saline wastewater in constructed wetlands with a focus on Latin America. Environ. Exp. Bot. 2021, 190, 104583. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Zhang, B.; Xu, X. Halophyte planting improves saline-alkali soil and brings changes in physical and chemical properties and soil microbial communities. Pol. J. Environ. Stud. 2021, 30, 4767. [Google Scholar] [CrossRef] [PubMed]
- Waris, M.; Baig, J.A.; Talpur, F.N.; Kazi, T.G.; Afridi, H.I. An environmental field assessment of soil quality and phytoremediation of toxic metals from saline soil by selected halophytes. J. Environ. Health Sci. Eng. 2022, 20, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Khan, M.A.; Ansari, R.; Gul, B.; Qadir, M. Crop diversification through halophyte production on salt-prone land resources. CABI Rev. 2007, 2007, 8. [Google Scholar] [CrossRef]
- Ezcurra, P.; Ezcurra, E.; Garcillán, P.P.; Costa, M.T.; Aburto-Oropeza, O. Coastal landforms and accumulation of mangrove peat increase carbon sequestration and storage. Proc. Natl. Acad. Sci. USA 2016, 113, 4404–4409. [Google Scholar] [CrossRef]
- Owers, C.J.; Rogers, K.; Mazumder, D.; Woodroffe, C.D. Temperate coastal wetland near-surface carbon storage: Spatial patterns and variability. Estuar. Coast. Shelf Sci. 2020, 235, 106584. [Google Scholar] [CrossRef]
- Sousa, A.I.; Lillebø, A.I.; Pardal, M.A.; Caçador, I. Productivity and nutrient cycling in salt marshes: Contribution to ecosystem health. Estuar. Coast. Shelf Sci. 2010, 87, 640–646. [Google Scholar] [CrossRef]
- Rathore, A.P.; Chaudhary, D.R.; Jha, B. Biomass production, nutrient cycling, and carbon fixation by Salicornia brachiata Roxb: A promising halophyte for coastal saline soil rehabilitation. Int. J. Phytoremed. 2016, 18, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Shi, J.; Rui, J.; Yu, Y.; Huang, W.; Lu, Z.; Chen, Y.; Chen, X.; Dong, S.; Hu, Z.; et al. Halophyte vegetation influences soil microbial community of coastal salt marsh. J. Ocean Univ. China 2022, 21, 1549–1556. [Google Scholar] [CrossRef]
- Pham, H.T.; Ngô, V.T.; Pham, T.T.; Bùi, T.T. The Role of Mangroves in Supporting Ports and the Shipping Industry to Reduce Emissions and Water Pollution; CIFOR: Bogor, Indonesia, 2022; Volume 231. [Google Scholar]
- Thuy, P.T.; Van Anh, N.T.; Anh, N.T.T.; Hong, T.T.K.; Nuong, N.T.K.; Le Hoa, D.; Thuyen, P.T.; Long, H.T. The role of mangroves in supporting shipping industry commitments to environmental protection and sustainable development. In CIFOR Occasional Paper; CIFOR: Bogor, Indonesia, 2021. [Google Scholar]
- Gibson, R.; Atkinson, R.; Gordon, J. Loss, status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol. Annu. Rev. 2007, 45, 345–405. [Google Scholar]
- Costa, C.S.B.; Herrera, O.B. Halophytic Life in Brazilian Salt Flats: Biodiversity, Uses and Threats. In Sabkha Ecosystems. Tasks for Vegetation Science; Khan, M., Boër, B., Ȫzturk, M., Clüsener-Godt, M., Gul, B., Breckle, S.W., Eds.; Springer: Cham, Switzerland, 2016; Volume 48, pp. 11–27. [Google Scholar]
- Eliáš, P.; Dítě, D.; Dítě, Z. Halophytic Vegetation in the Pannonian Basin: Origin, Syntaxonomy, Threat, and Conservation. In Handbook of Halophyte; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 287–324. [Google Scholar]
- Brown, J.J.; Das, P.; Al-Saidi, M. Sustainable agriculture in the Arabian/Persian Gulf region utilizing marginal water resources: Making the best of a bad situation. Sustainability 2018, 10, 1364. [Google Scholar] [CrossRef]
- Öztürk, M.; Altay, V.; Güvensen, A. Sustainable Use of Halophytic Taxa as Food and Fodder: An Important Genetic Resource in Southwest Asia. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 235–257. [Google Scholar]
- Zhang, Y.; Tariq, A.; Hughes, A.C.; Hong, D.; Wei, F.; Sun, H.; Sardans, J.; Peñuelas, J.; Perry, G.; Qiao, J.; et al. Challenges and solutions to biodiversity conservation in arid lands. Sci. Total Environ. 2023, 857, 159695. [Google Scholar] [CrossRef]
- Koyro, H.W.; Huchzermeyer, B.; Harrouni, M.C. Comparison of strategies of halophytes from different plant families to avoid salt injury. In Plant Nutrition. Developments in Plant and Soil Sciences; Horst, W.J., Schenk, M.K., Bürkert, A., Claassen, N., Flessa, H., Frommer, W.B., Olfa, H.-W., Römheld, V., Sattelmacher, B., Schmidhalter, U., et al., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 92, pp. 414–415. [Google Scholar]
- Radulovich, R.; Umanzor, S. Halophyte use and cultivation. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 2517–2535. [Google Scholar]
- Centofanti, T.; Bañuelos, G. Practical uses of halophytic plants as sources of food and fodder. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Wallingford, UK, 2019; pp. 324–342. [Google Scholar] [CrossRef]
- Robertson, S.M.; Lyra, D.A.; Mateo-Sagasta, J.; Ismail, S.; Akhtar, M.J.U. Financial analysis of halophyte cultivation in a desert environment using different saline water resources for irrigation. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 347–364. [Google Scholar]
- Devi, S.; Kumar, A.; Arya, S.S.; Kumari, A.; Kumar, N.; Chand, G.; Mann, A.; Goyal, V.; Pooja. Economic Utilization and Potential of Halophytes. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 195–220. [Google Scholar]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Singh, K.V.; Singh, R. Quinoa (Chenopodium quinoa Willd), functional superfood for today’s world: A Review. World Sci. News 2016, 58, 84–96. Available online: https://www.infona.pl/resource/bwmeta1.element.psjd-e765ee65-4f8c-413f-9b30-e02c2b6c9172 (accessed on 1 September 2023).
- Salvador-Reyes, R.; Furlan, L.; Martínez-Villaluenga, C.; Dala-Paula, B.M.; Clerici, M.T.P.S. From ancient crop to modern superfood: Exploring the history, diversity, characteristics, technological applications, and culinary uses of Peruvian fava beans. Food Int. Res. 2023, 173, 113394. [Google Scholar] [CrossRef]
- Mushtaq, W.; Bedair, H.; Shakeel, A. Halophytes: A phytoremediation tool for salt-affected soils with special reference to indian subcontinent. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar]
- Farzi, A.; Borghei, S.M.; Vossoughi, M. The use of halophytic plants for salt phytoremediation in constructed wetlands. Int. J. Phytoremed. 2017, 19, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Yasseen, B.T.; Al-Thani, R.F. Endophytes and halophytes to remediate industrial wastewater and saline soils: Perspectives from Qatar. Plants 2022, 11, 1497. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Mudgal, A.; Mudgal, V.; Sagi, M.; Standing, D.; Davies, P.A. Desalination, Water Re-use, and Halophyte Cultivation in Salinized Regions: A Highly Productive Groundwater Treatment System. Environ. Sci. Technol. 2023, 57, 11863–11875. [Google Scholar] [CrossRef] [PubMed]
- Aziz, I.; Mujeeb, A. Halophytes for phytoremediation of hazardous metal (loid) s: A terse review on metal tolerance, bio-indication and hyperaccumulation. J. Hazard. Mater. 2022, 424, 127309. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Andrades-Moreno, L. Accumulation and tolerance characteristics of cadmium in a halophytic Cd-hyperaccumulator, Arthrocnemum macrostachyum. J. Hazard. Mater. 2010, 184, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.; Yao, L.; Meng, Y.; Ma, X.; Si, E.; Ren, P.; Yang, K.; Shang, X.; Wang, H. Halophyte Halogeton glomeratus, a promising candidate for phytoremediation of heavy metal-contaminated saline soils. Plant Soil 2019, 442, 323–331. [Google Scholar] [CrossRef]
- Devi, S.; Nandwal, A.S.; Angrish, R.; Arya, S.S.; Kumar, N.; Sharma, S.K. Phytoremediation potential of some halophytic species for soil salinity. Int. J. Phytoremed. 2016, 18, 693–696. [Google Scholar] [CrossRef]
- Salama, F.M.; Al-Huqail, A.A.; Ali, M.; Abeed, A.H. Cd Phytoextraction potential in halophyte Salicornia fruticosa: Salinity impact. Plants 2022, 11, 2556. [Google Scholar] [CrossRef]
- Santos, E.S.; Abreu, M.M.; Peres, S.; Magalhães, M.C.F.; Leitão, S.; Pereira, A.S.; Cerejeira, M.J. Potential of Tamarix africana and other halophyte species for phytostabilisation of contaminated salt marsh soils. J. Soils Sedim. 2017, 17, 1459–1473. [Google Scholar] [CrossRef]
- Ayyappan, D.; Sathiyaraj, G.; Ravindran, K.C. Phytoextraction of heavy metals by Sesuvium portulacastrum L. a salt marsh halophyte from tannery effluent. Int. J. Phytoremed. 2016, 18, 453–459. [Google Scholar] [CrossRef]
- Nalla, S.; Hardaway, C.J.; Sneddon, J. Phytoextraction of selected metals by the first and second growth seasons of Spartina alterniflora. Instrum. Sci. Technol. 2012, 40, 17–28. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, X.; Arif, M.; Chen, S.; Ma, M.; Zhu, K.; Chen, Q.; Wu, S.; Li, C. Strategy matters: Phytoremediation potential of native halophytes is jointly associated with their distinct salt tolerances. J. Clean. Prod. 2023, 425, 139060. [Google Scholar] [CrossRef]
- Qasim, M.; Gulzar, S.; Khan, M.A. Halophytes as medicinal plants. In Urbanisation, Land Use, Land Degradation and Environment; Ozturk, M., Mermut, A.R., Celik, A., Eds.; Daya Publishing House: Delhi, India, 2011; pp. 330–343. [Google Scholar]
- Ferreira, M.J.; Pinto, D.C.; Cunha, Â.; Silva, H. Halophytes as medicinal plants against human infectious diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 2, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Devi, S.; Ram, K.; Kumar, S.; Kumar, N.; Mann, A.; Kumar, A.; Chand, G. The Plants of Therapeutic Medicine. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 271–287. [Google Scholar]
- Lee, J.M.; Yim, M.J.; Choi, G.; Lee, M.S.; Park, Y.G.; Lee, D.S. Antioxidant and anti-inflammatory activity of six halophytes in Korea. Nat. Prod. Sci. 2018, 24, 40–46. [Google Scholar] [CrossRef]
- Oueslati, S.; Ksouri, R.; Falleh, H.; Pichette, A.; Abdelly, C.; Legault, J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012, 132, 943–947. [Google Scholar] [CrossRef]
- Ullah, S.; Bano, A.; Girmay, S.; Tan, G. Anticancer, antioxidant and antimicrobial activities of Suaeda fruticosa related to its phytochemical screening. Int. J. Phytoremed. 2012, 4, 284. [Google Scholar]
- Ahmad, I.; Gul, H.; Noureen, A.; Ujjan, J.A.; Manzoor, S.; Muhammad, W. Antimicrobial, Antioxidant and Antidiabetic Potential of Suaeda fruticosa L. Int. J. Emerg. Technol. 2021, 12, 155–160. [Google Scholar]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Debez, A.; Belghith, I.; Friesen, J.; Montzka, C.; Elleuche, S. Facing the challenge of sustainable bioenergy production: Could halophytes be part of the solution? J. Biol. Eng. 2017, 11, 27. [Google Scholar] [CrossRef]
- Behera, S.S.; Ramachandran, S. Potential uses of halophytes for biofuel production: Opportunities and challenges. Sustain. Biofuels 2021, 14, 425–448. [Google Scholar]
- Abideen, Z.; Ansari, R.; Hasnain, M.; Flowers, T.J.; Koyro, H.W.; El-Keblawy, A.; Abouleish, M.; Khan, M.A. Potential use of saline resources for biofuel production using halophytes and marine algae: Prospects and pitfalls. Front. Plant Sci. 2023, 14, 1026063. [Google Scholar] [CrossRef] [PubMed]
- Abideen, Z.; Hameed, A.; Koyro, H.W.; Gul, B.; Ansari, R.; Khan, M.A. Sustainable biofuel production from non-food sources-An overview. Emir. J. Food Agric. 2014, 26, 1057–1066. [Google Scholar] [CrossRef]
- Abideen, Z.; Qasim, M.; Rizvi, R.F.; Gul, B.; Ansari, R.; Khan, M.A. Oilseed halophytes: A potential source of biodiesel using saline degraded lands. Biofuels 2015, 6, 241–248. [Google Scholar] [CrossRef]
- Cayenne, A.; Turcios, A.E.; Thomsen, M.H.; Rocha, R.M.; Papenbrock, J.; Uellendahl, H. Halophytes as Feedstock for Biogas Production: Composition Analysis and Biomethane Potential of Salicornia spp. Plant Material from Hydroponic and Seawater Irrigation Systems. Fermentation 2022, 8, 189. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Sánchez-Arias, L.E. Creation of Mangrove “Productive Oases”: Community Participation for the Sustainable Utilization of Halophytes. In Mangroves and Halophytes: Restoration and Utilisation. Tasks for Vegetation Sciences; Lieth, H., Sucre, M.G., Herzog, B., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 43, pp. 85–96. [Google Scholar]
- Breckle, S.W. Halophytes and saline vegetation of Afghanistan, a potential rich source for people. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 49–66. [Google Scholar]
- Pinheiro, I.; Arantes, R.; do Espírito Santo, C.M.; do Nascimento Vieira, F.; Lapa, K.R.; Gonzaga, L.V.; Fett, R.; Barcelos-Oliveira, J.L.; Seiffert, W.Q. Production of the halophyte Sarcocornia ambigua and Pacific white shrimp in an aquaponic system with biofloc technology. Ecol. Eng. 2017, 100, 261–267. [Google Scholar] [CrossRef]
- Chu, Y.T.; Brown, P.B. Evaluation of Pacific whiteleg shrimp and three halophytic plants in marine aquaponic systems under three salinities. Sustainability 2020, 13, 269. [Google Scholar] [CrossRef]
- Colette, M.; Guentas, L.; Gunkel-Grillon, P.; Callac, N.; Della Patrona, L. Is halophyte species growing in the vicinity of the shrimp ponds a promising agri-aquaculture system for shrimp ponds remediation in New Caledonia? Mar. Pollut. Bull. 2022, 177, 113563. [Google Scholar] [CrossRef]
- Özcan, H.; Akbulak, C.; Kelkit, A.; Tosunoğlu, M.; İsmet, U. Ecotourism potential and management of kavak delta (northwest turkey). J. Coast. Res. 2009, 25, 781–787. [Google Scholar] [CrossRef]
- Böer, B.; Huot, C.; Sutcliffe, M. Floating Mangroves: The Solution to Reduce Atmospheric Carbon Levels and Land-Based Marine Pollution? In Sabkha Ecosystems: Cash Crop Halophyte and Biodiversity Conservation; Khan, M.A., Böer, B., Öztürk, M., Al Abdessalaam, T.Z., Clüsener-Godt, M., Gul, B., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume IV, pp. 327–333. [Google Scholar]
- Ginantra, I.K. Mangrove Conservation: An Ecotourism Approach. In Mangrove Biology, Ecosystem, and Conservation, Yllano, O.B., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Salam, M.A.; Lindsay, G.R.; Beveridge, M.C. Eco-tourism to protect the reserve mangrove forest the Sundarbans and its flora and fauna. Anatolia 2000, 11, 56–66. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Custódio, M.; Lillebø, A.I.; Calado, R.; Villasante, S. Halophytes as novel marine products–A consumers’ perspective in Portugal and policy implications. Mar. Policy 2021, 133, 104731. [Google Scholar] [CrossRef]
- Abdal, M.S. Salicornia production in Kuwait. World Appl. Sci. J. 2009, 6, 1033–1038. [Google Scholar]
- Ashour, N.; Arafat, S.M.; El-Haleem, A.A.; Serag, M.; Mandour, S.; Makki, B. Growing halophytes in Egypt for forage production and desertification control. Bull. Natl. Res. Cent. 1999, 4, 349–360. [Google Scholar]
- Jiang, D.; Huang, L.; Lin, S.; Li, Y. Allelopathic effects of euhalophyte Salicornia bigelovii on marine alga Skeletonema costatum. Allelopath. J. 2010, 25, 163–172. [Google Scholar]
- Jiang, D.; Huang, L.; Lin, Y.; Nie, L.; Lv, S.; Kuang, T.; Li, Y. Inhibitory effect of Salicornia europaea on the marine alga Skeletonema costatum. Sci. China Life Sci. 2012, 55, 551–558. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, L.; Zhang, Z.; Zhang, K.; Lv, S.; Li, Y. Inhibitory effects of halophyte Sesuvium portulacastrum on the marine diatom Skeletonema costatum. Allelopath. J. 2012, 29, 137–150. [Google Scholar]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Bibi, S.; Bibi, A.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H. Allelopathic Effects of the Invasive Prosopis juliflora (Sw.) DC. on Native Plants: Perspectives toward Agrosystems. Agronomy 2023, 13, 590. [Google Scholar] [CrossRef]
- Tahar, M.; Labani, A.; Rechache, M.; Terras, M. Assessment of the Allelopathic Effect of (Atriplex Canescens)” Fourwing Saltbush” on Germination of Seeds and Growth Parameters of (Artemisia Herba-Alba Asso). World J. Environ. Biosci. 2019, 8, 61–68. [Google Scholar]
- Casolo, V.; Tomasella, M.; De Col, V.; Braidot, E.; Savi, T.; Nardini, A. Water relations of an invasive halophyte (Spartina patens): Osmoregulation and ionic effects on xylem hydraulics. Funct. Plant Biol. 2014, 42, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, E.A.; Glenn, E.P.; Guntenspergen, G.R.; Brown, J.J.; Nelson, S.G. Salt tolerance and osmotic adjustment of Spartina alterniflora (Poaceae) and the invasive M haplotype of Phragmites australis (Poaceae) along a salinity gradient. Am. J. Bot. 2006, 93, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Barnard, P.; Moomaw, W.R. World scientists’ warning of a climate emergency. BioScience 2020, 70, 8–100. [Google Scholar] [CrossRef]
- Feizizadeh, B.; Alajujeh, K.M.; Makki, M. A scenario-based food security analysis and halophyte crop suitability assessment in dying lake environments impacted by climate change. Int. J. Appl. Earth Obs. Geoinf. 2023, 122, 103425. [Google Scholar] [CrossRef]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Ansari, R.; Gul, B.; Khan, M.A. Traditional ethnobotanical uses of medicinal plants from coastal areas. J. Coast. Life Med. 2014, 2, 22–30. [Google Scholar]
- Renna, M.; Gonnella, M. Ethnobotany, nutritional traits, and healthy properties of some halophytes used as greens in the Mediterranean basin. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–19. [Google Scholar]
- Öztürk, M.A.; Altay, V.; Nazish, M.; Ahmad, M.; Zafar, M. Ethnic Aspects of Halophytes and Importance in the Economy. In Halophyte Plant Diversity and Public Health, 1st ed.; Öztürk, M.A., Altay, V., Nazish, M., Ahmad, M., Zafar, M., Eds.; Springer: Cham, Switzerland, 2023; Volume 1, pp. 173–197. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, A.; Hussain, S.; Rasheed, A.; Ahmed, M.Z.; Abbas, S. Exploring the Potentials of Halophytes in Addressing Climate Change-Related Issues: A Synthesis of Their Biological, Environmental, and Socioeconomic Aspects. World 2024, 5, 36-57. https://doi.org/10.3390/world5010003
Hameed A, Hussain S, Rasheed A, Ahmed MZ, Abbas S. Exploring the Potentials of Halophytes in Addressing Climate Change-Related Issues: A Synthesis of Their Biological, Environmental, and Socioeconomic Aspects. World. 2024; 5(1):36-57. https://doi.org/10.3390/world5010003
Chicago/Turabian StyleHameed, Abdul, Sadiq Hussain, Aysha Rasheed, Muhammad Zaheer Ahmed, and Sahar Abbas. 2024. "Exploring the Potentials of Halophytes in Addressing Climate Change-Related Issues: A Synthesis of Their Biological, Environmental, and Socioeconomic Aspects" World 5, no. 1: 36-57. https://doi.org/10.3390/world5010003
APA StyleHameed, A., Hussain, S., Rasheed, A., Ahmed, M. Z., & Abbas, S. (2024). Exploring the Potentials of Halophytes in Addressing Climate Change-Related Issues: A Synthesis of Their Biological, Environmental, and Socioeconomic Aspects. World, 5(1), 36-57. https://doi.org/10.3390/world5010003







