Impact of Membrane Thickness on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr/Ru Catalyst
Abstract
1. Introduction
2. Experiment
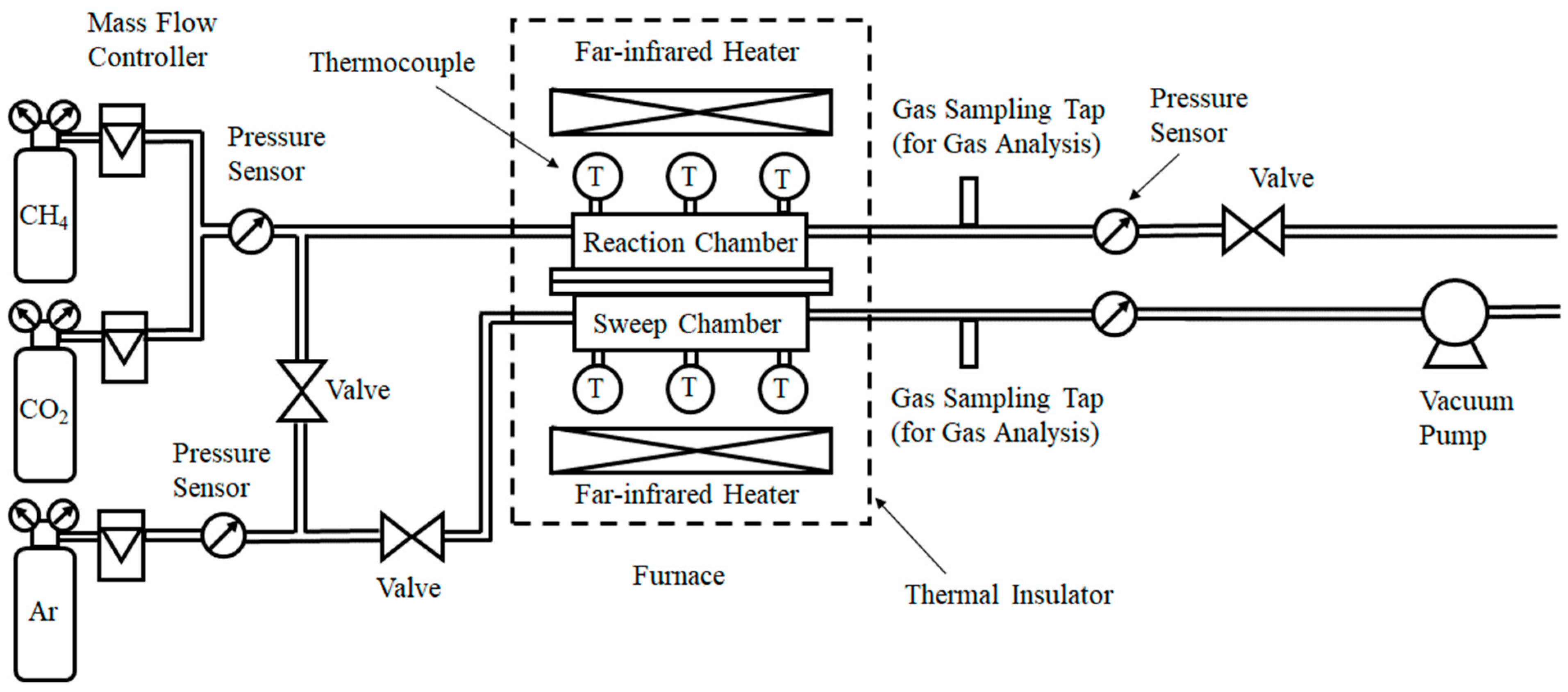
2.1. Experimental Set-Up
2.2. Assessment Factor to Evaluate the Performance of BDR Mmbrane Ractor in This Study
3. Results and Discussion
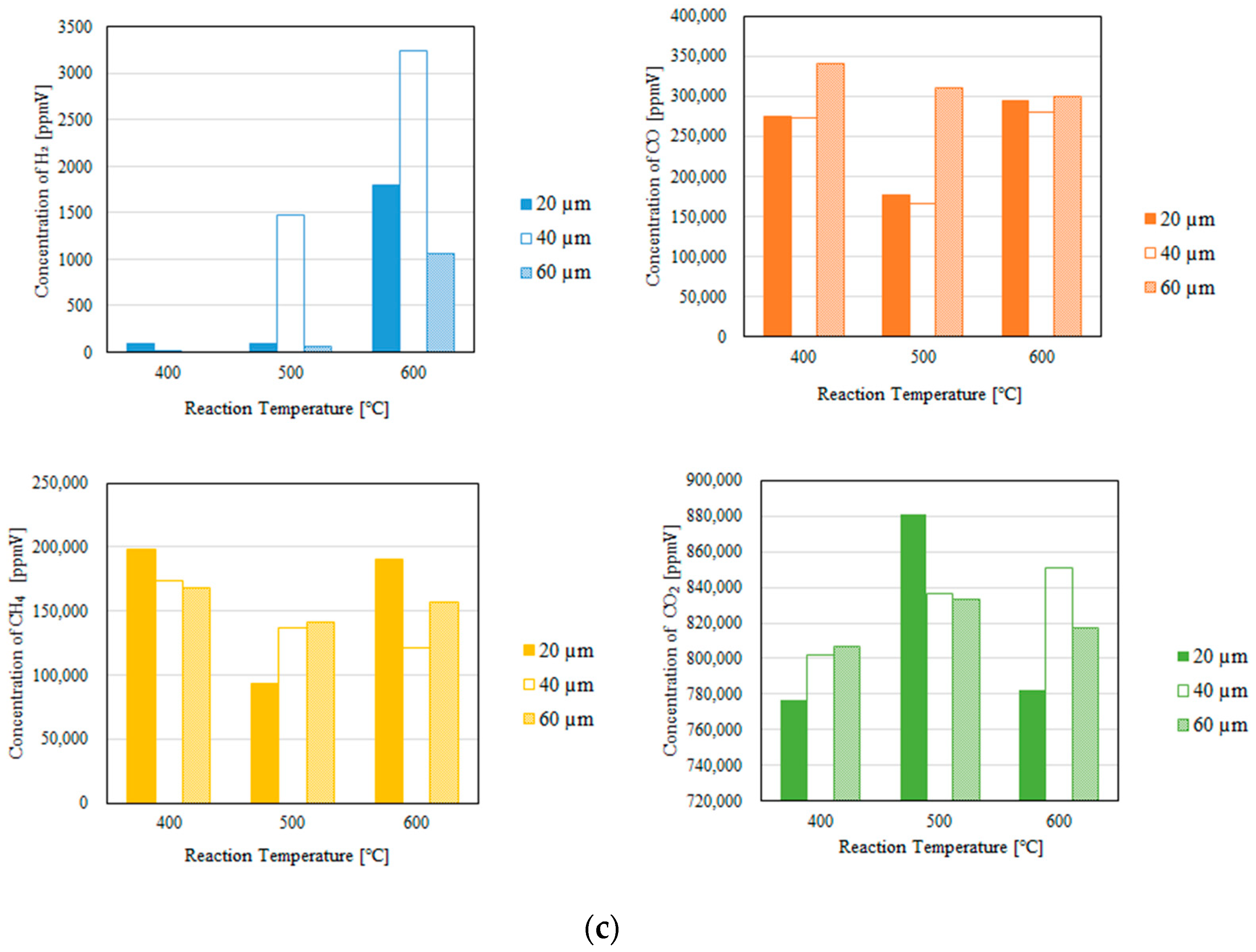
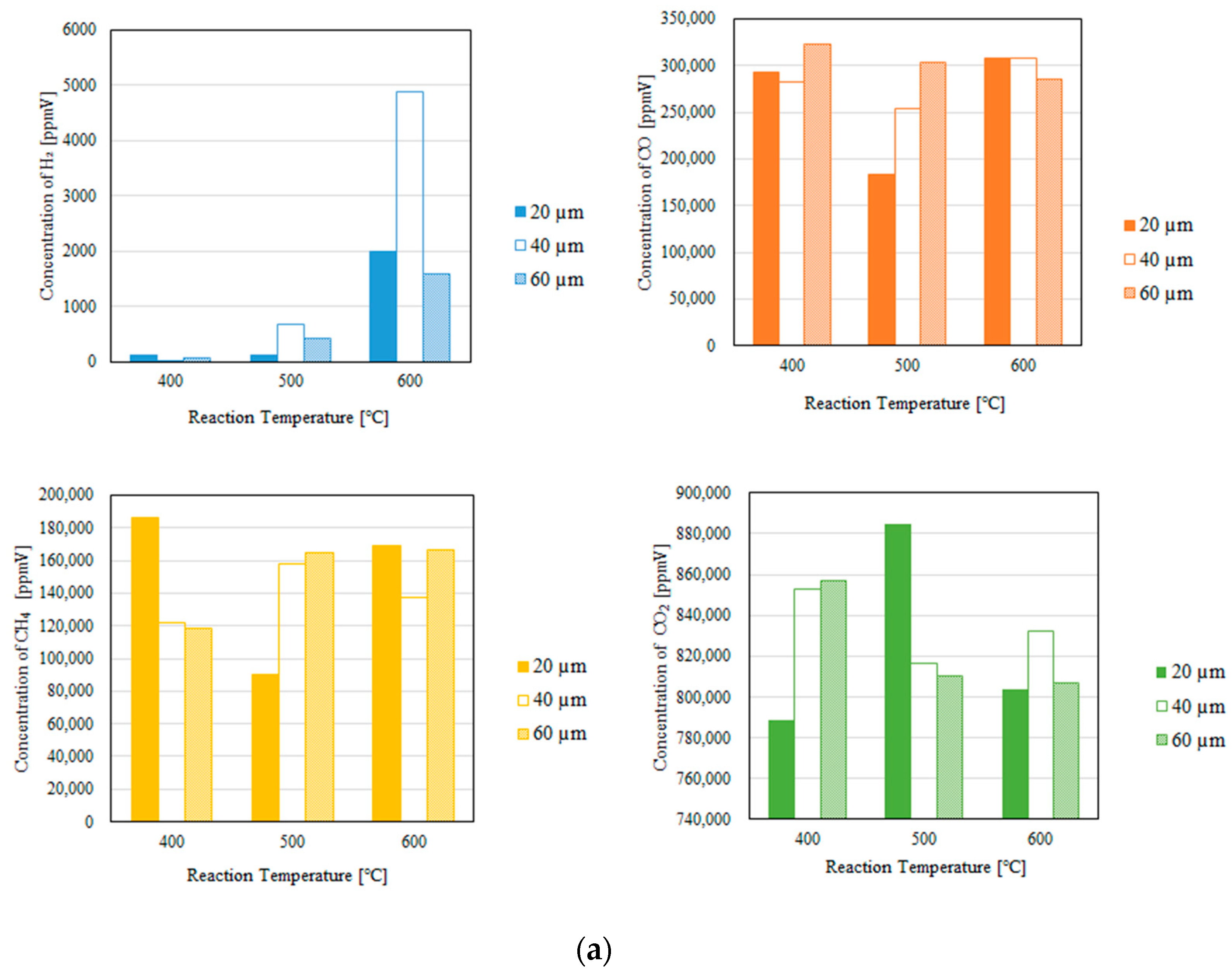
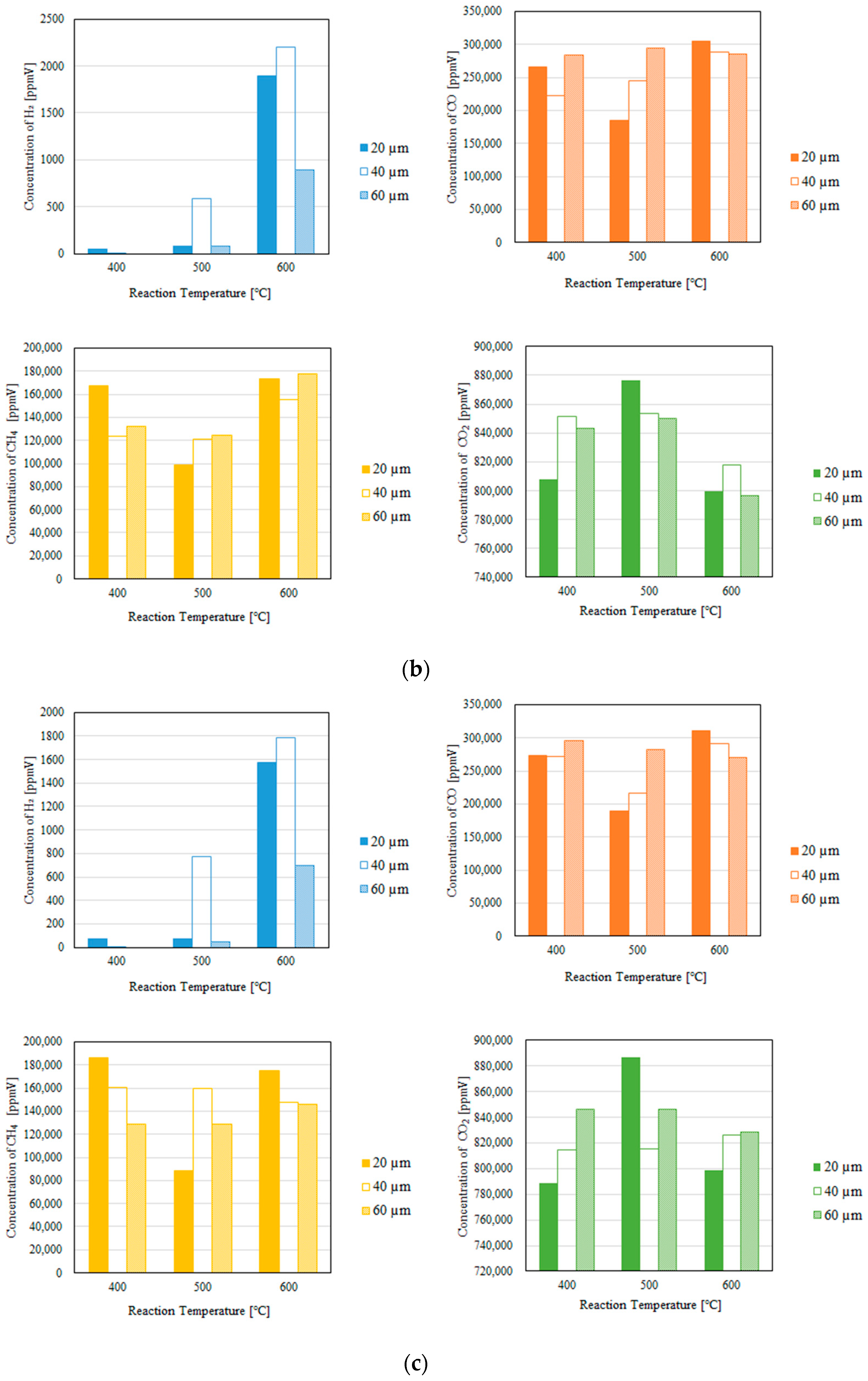
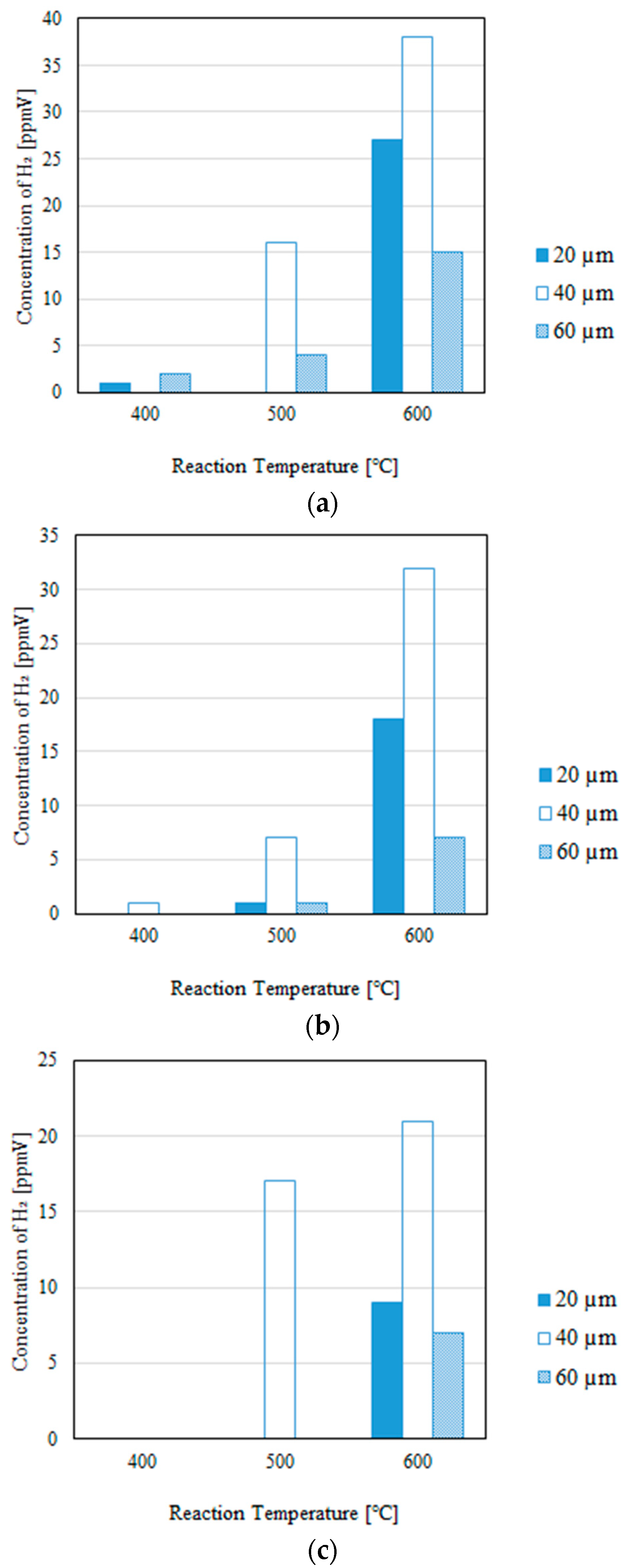
3.1. Impact of the Thickness of the Pd/Cu Membrane on Each Gas Concentration in the Reaction Chamber and the Concentration of H2 in the Sweep Chamber, Changing the Initial Reaction Temperature and the Differential Pressure Between the Reaction Chamber and the Sweep Chamber with and Without a Sweep Gas
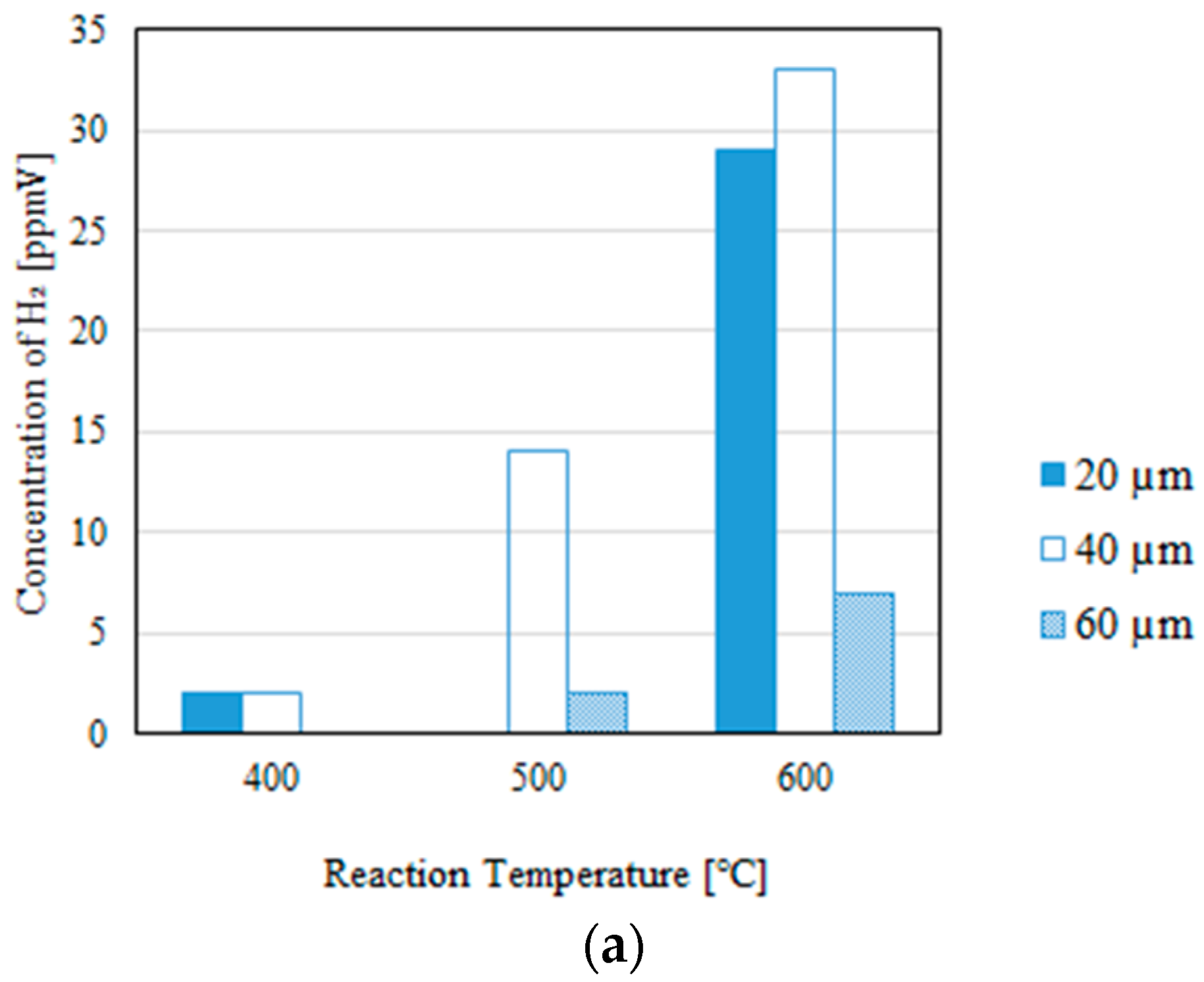
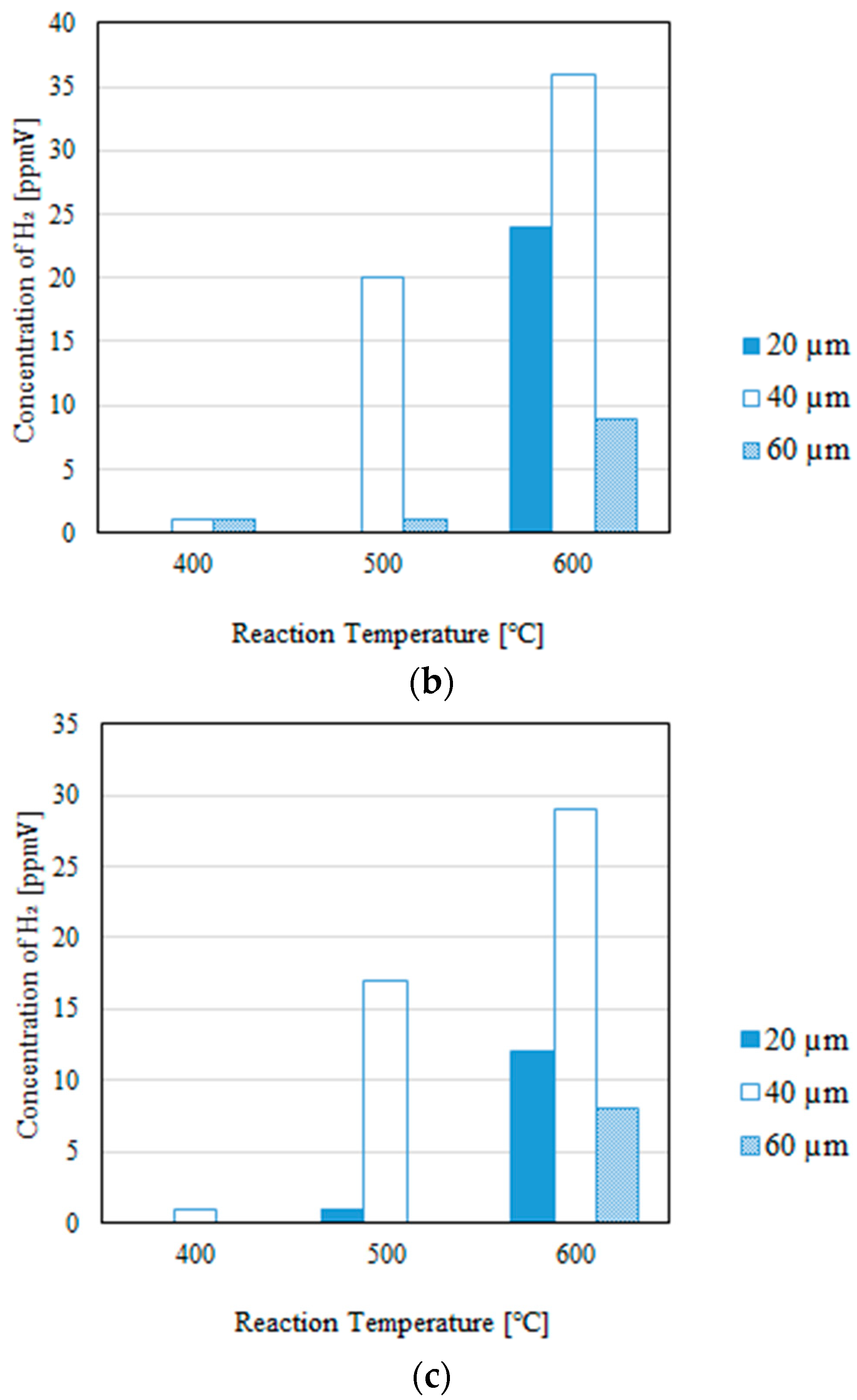
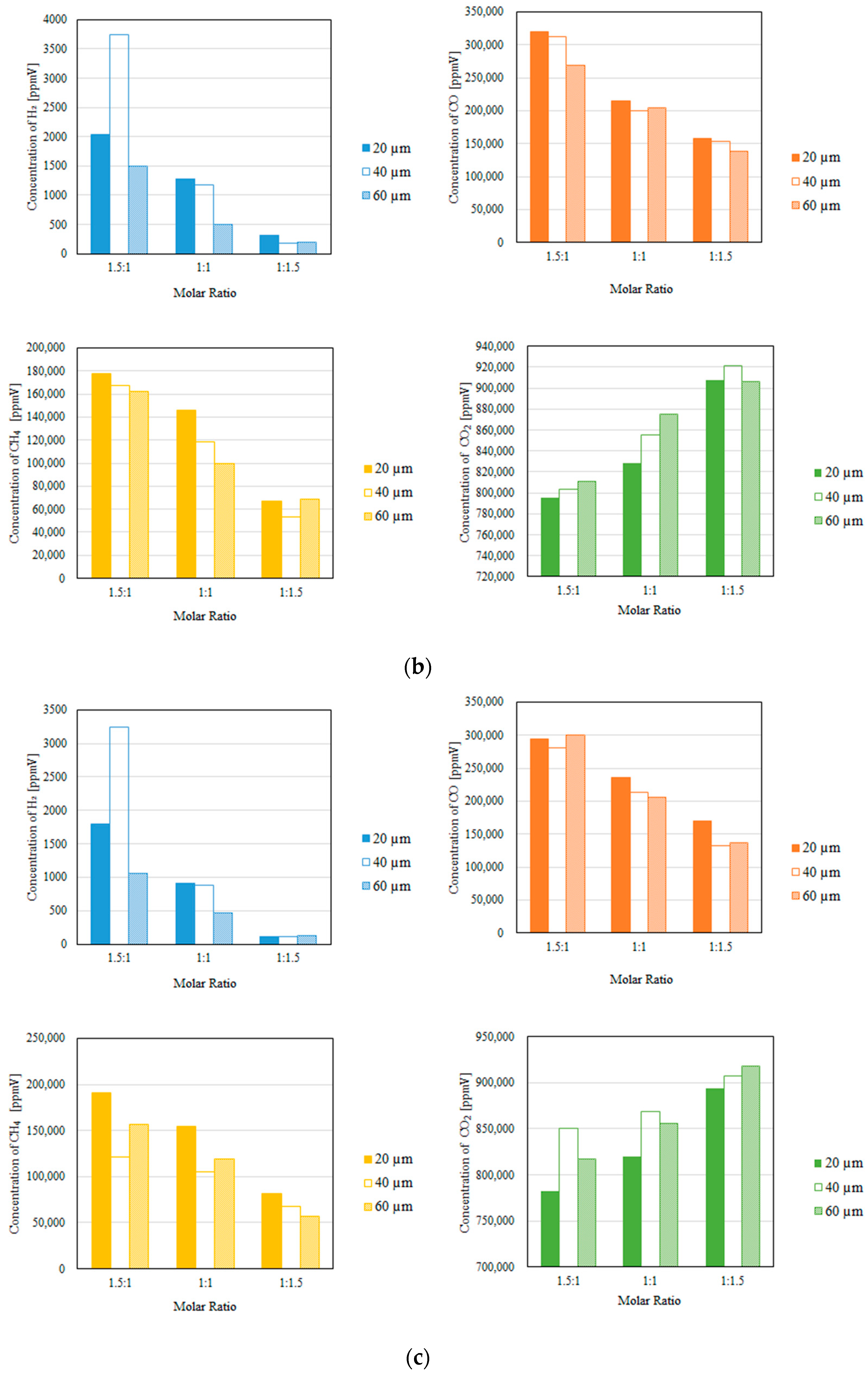
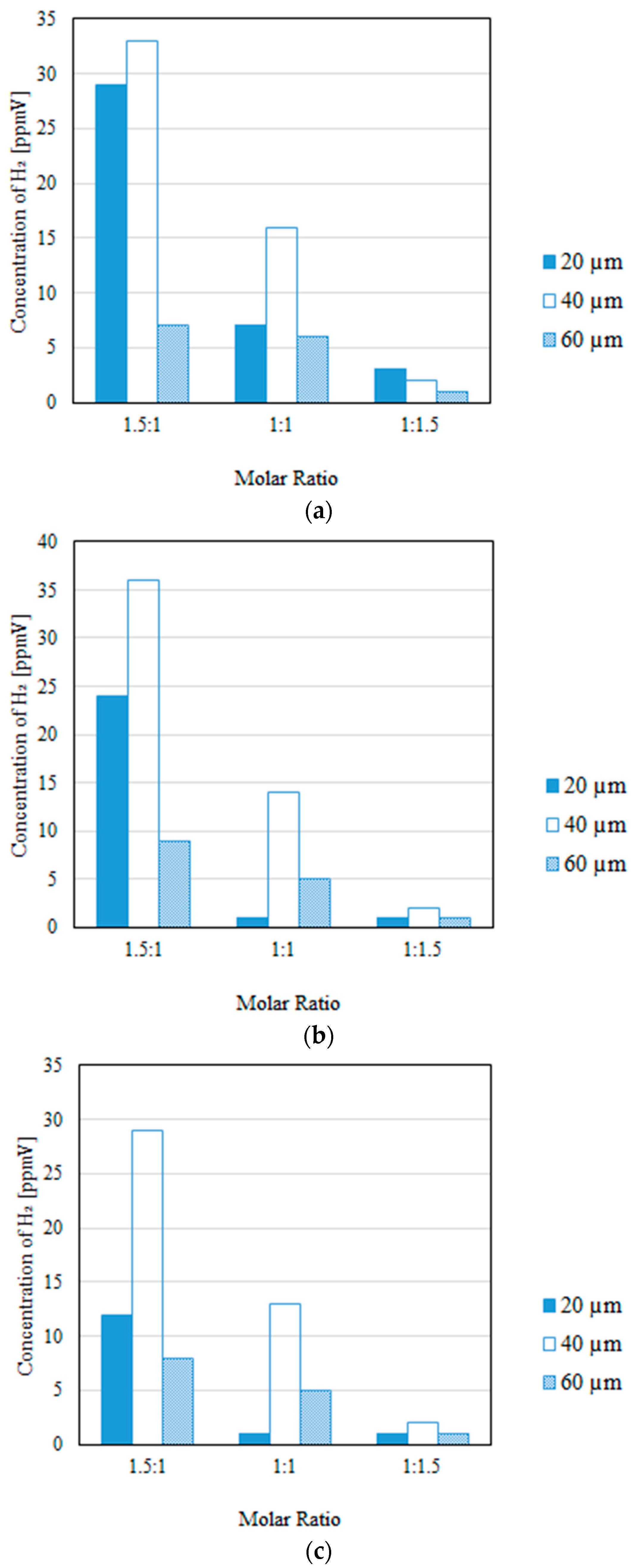
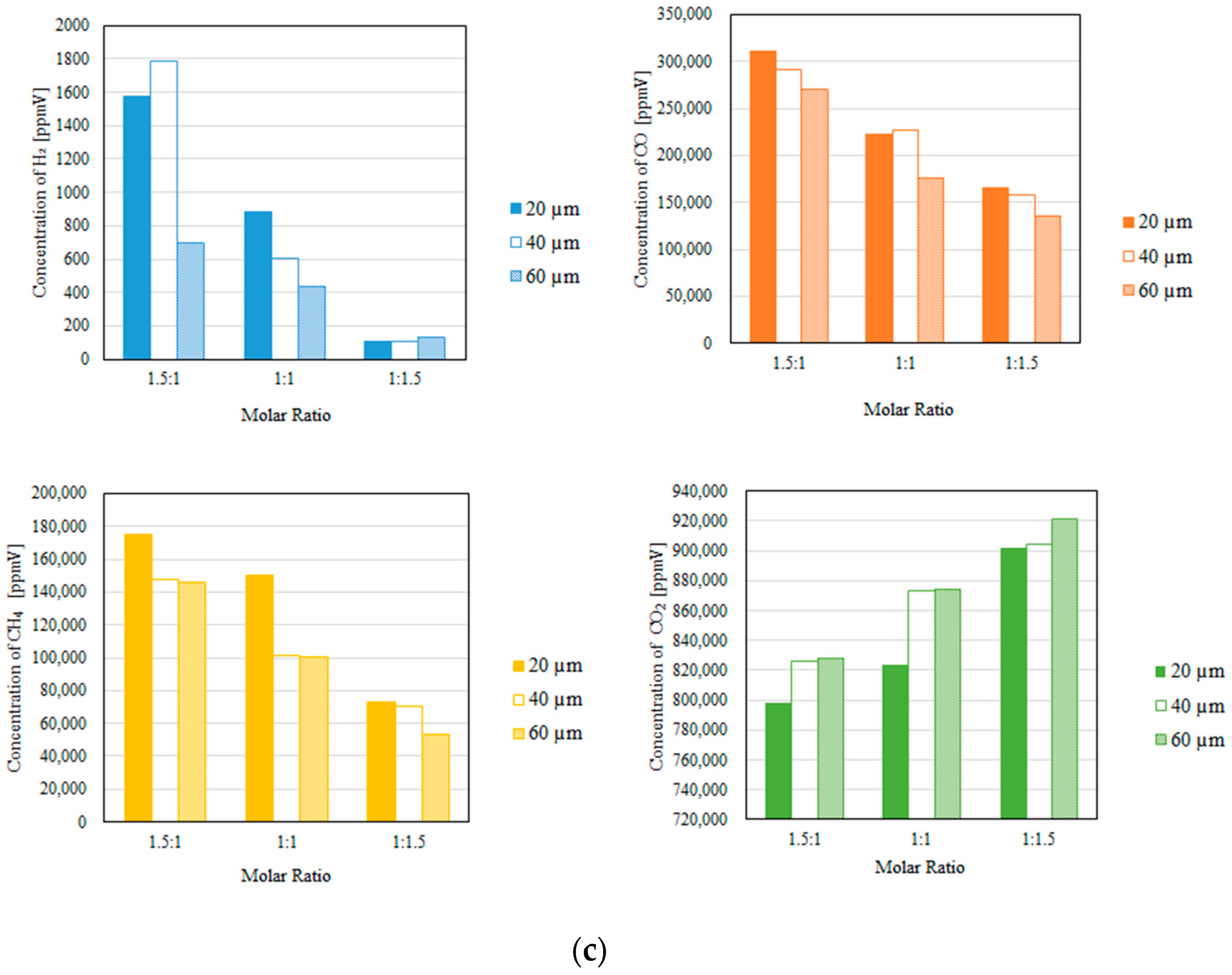
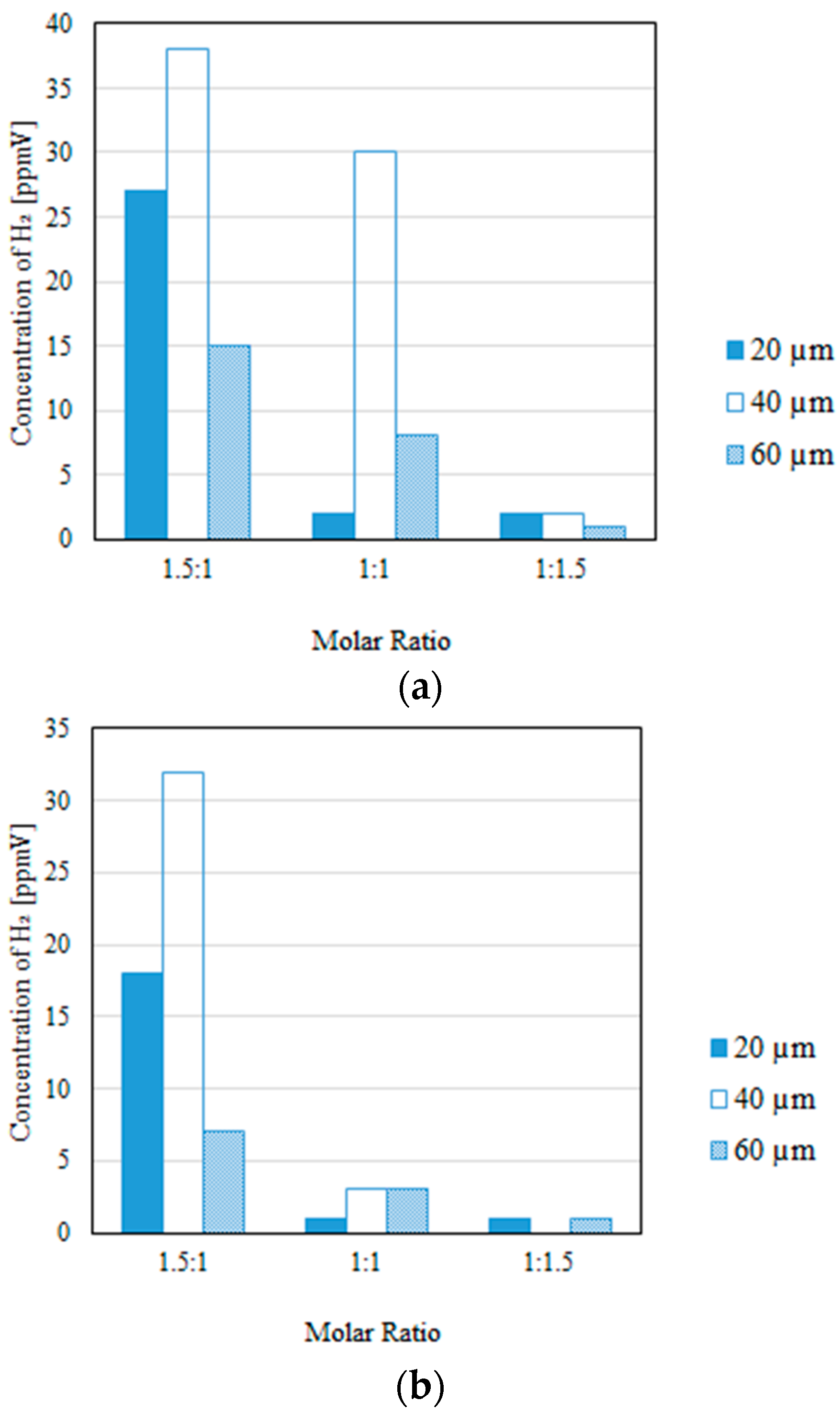
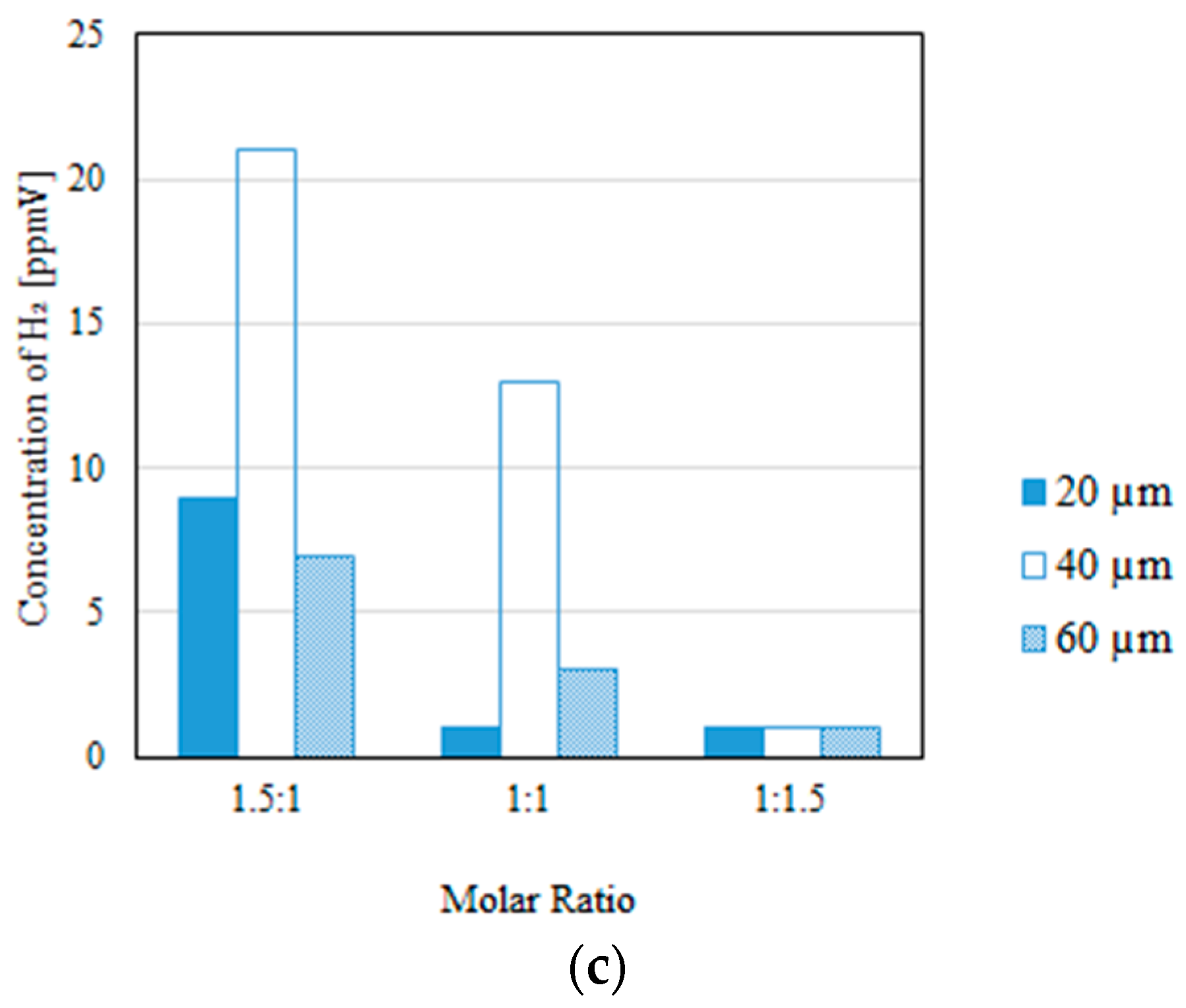
3.2. Impact of the Thickness of the Pd/Cu Membrane on Each Gas Concentration in the Reaction Chamber and the Concentration of H2 in the Sweep Chamber, Changing the Molar Ratio and the Differential Pressure Between the Reaction Chamber and the Sweep Chamber with and Without a Sweep Gas
- (i)
- H2 is produced via Equations (9) and (13).
- (ii)
- The produced H2 is consumed via Equations (10) and (11), resulting in the production of CO, CH4, and H2O.
- (iii)
- The produced CO is consumed via Equations (14) and (16), resulting in the production of C, CO2, and H2O.
3.3. Comparison of Assessment Factors for the Investigated Experimental Conditions
4. Conclusions
- (i)
- We reveal that the concentration of H2 in the reaction chamber increases with an increase in the reaction temperature irrespective of the thickness of the Pd/Cu membrane, the differential pressure between the reaction chamber and the sweep chamber, and the existence of a sweep gas. The reaction progresses well with a rise in the reaction temperature since DR, RWGS, and SR are endothermic reactions.
- (ii)
- We reveal that the concentration in the sweep chamber increases with an increase in the reaction temperature irrespective of the thickness of Pd/Cu membrane, the differential pressure between the reaction chamber and the sweep chamber, and the existence of a sweep gas. When the concentration of H2 in the reaction chamber is larger at higher reaction temperature, the driving force to penetrate through the Pd/Cu membrane is larger because of the high H2 partial differential pressure between the reaction chamber and the sweep chamber.
- (iii)
- It is revealed that the largest concentration of H2 in the reaction chamber and that in the sweep chamber are obtained for a molar ratio of CH4:CO2 = 1.5:1, irrespective of the thickness of Pd/Cu membrane, the differential pressure between the reaction chamber and the sweep chamber, and the existence of a sweep gas.
- (iv)
- The highest concentration of H2 is mainly obtained for the thickness of 40 μm among the investigated conditions, i.e., the reaction temperature, the molar ratio of CH4:CO2, the differential pressure between the reaction chamber and the sweep chamber, and the existence of a sweep gas. This study claims that the optimum thickness is 40 μm.
- (v)
- It is clarified that the highest concentration of H2 is obtained for the thickness of 40 μm, the molar ratio of CH4:CO2 = 1.5:1, and the differential pressure between the reaction chamber and the sweep chamber of 0 MPa without a sweep gas, which is 4890 ppmV in the reaction chamber and 38 ppmV in the sweep chamber, respectively. Under these conditions, the CH4 conversion, H2 yield, and thermal efficiency are 75.0%, 0.214%, and 2.92%, respectively.
- (vi)
- In the near future, the following objectives can be considered: (i) to optimize the catalyst shape and composition (i.e., the pore size and the weight ratio of Ni, Cr, and Ru); (ii) to optimize the thickness and the composition of the Pd/Cu membrane; and (iii) to match the H2 separation rate of the Pd/Cu membrane and the H2 production rate of the Ni/Cr/Ru catalyst, determining the optimum operation condition.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Sugiarto, J.R.; Yoon, W.; Irshad, M.; Jo, H.; Bibi, S.S.; Kim, S.K.; Khan, M.K.; Kim, J. High-yield penetranes-plus production via hydrogenation of carbon dioxide: Revealing new roles of zirconia as promoter of iron catalyst with long-term stability. J. Energy Chem. 2025, 102, 431–442. [Google Scholar] [CrossRef]
- Kalai, D.Y.; Stangeland, K.; Jin, R.; Tucho, W.M.; Yu, Z. Biogas dry reforming for syngas production on La promoted hydrotalcite-derived Ni Catalyst. Int. J. Hydrogen Energy 2018, 43, 19438–19450. [Google Scholar] [CrossRef]
- World Bioenergy Association. Available online: https://worldbioenergy.org/global-bioenergy-statistics (accessed on 7 November 2024).
- The Japan Gas Association. Available online: https://www.gas.or.jp/gas-life/biogas/ (accessed on 7 November 2024).
- Nishimura, A.; Takada, T.; Ohata, S.; Kolhe, M.L. Biogas dry reforming for hydrogen through membrane reactor utilizing negative pressure. Fuels 2021, 2, 194–209. [Google Scholar] [CrossRef]
- Nishimura, A.; Ichikawa, M.; Yamada, S.; Ichii, R. The characteristics of a Ni/Cr/Ru catalyst for a biogas dry reforming membrane reactor using a Pd/Cu membrane and a comparison of it with a Ni/Cr catalyst. Hydrogen 2024, 5, 414–435. [Google Scholar] [CrossRef]
- Nishimura, A.; Sato, R.; Hu, E. An energy production system powered by solar heat with biogas dry reforming reactor and solar heat with biogas dry reforming reactor and solid oxide fuel cell. Smart Grid Renew. Energy 2023, 14, 85–106. [Google Scholar] [CrossRef]
- Yoo, E.; Choi, D.S.; Kim, J.; Kim, Y.H.; Kim, N.Y.; Joo, B. Effects of operating parameters and feed gas compositions on the dry reforming of methane over the Ni/Al2O3 catalyst. Catalysts 2023, 13, 602. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Deo, G. Process and catalyst improvements for the dry reforming of methane. Chem. Eng. Sci. 2023, 276, 118767. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Rabelo-Neto, R.C.; Bion, N.; Toniolo, F.S.; Noronha, B. Dry reforming of methane over embedded Ni nanoparticles in CeZrO2: Effect of Ce/Zr ratio and H2O addition. Int. J. Hydrogen Energy 2024, 71, 1151–1163. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Ju, T.; Hao, S. Influence of H2S and NH3 on biogas dry reforming using Ni catalyst: A study on single and synergetic effect. Front. Environ. Sci. Eng. 2023, 17, 32. [Google Scholar] [CrossRef]
- Kiani, P.; Meshksar, M.; Rahimpour, M.R. Biogas reforming over La-promoted Ni/SBA-16 catalyst for syngas production: Catalytic structure and process activity investigation. Int. J. Hydrogen Energy 2023, 48, 6262–6274. [Google Scholar] [CrossRef]
- Miao, C.; Chen, S.; Shang, K.; Liang, L.; Ouyang, J. Highly active Ni-Ru bimetallic catalyst integrated with MFI zeolite loaded cerium zirconium oxide for dry reforming of methane. ACS Appl. Mater. Interfaces 2022, 14, 47616–47632. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.D.; Faroldi, B.; Cornaglia, L.M.; Tarditi, A.M. Development of catalytic membranes over PdAu selective films for hydrogen production through the dry reforming of methane. Mol. Catal. 2020, 481, 100643. [Google Scholar] [CrossRef]
- Andraos, S.; Abbas-Ghaleb, R.; Chlala, D.; Vita, A.; Italiano, C.; Lagana, M.; Pino, L.; Nakhl, M.; Specchia, S. Production of hydrogen by methane dry reforming over ruthenium-nickel based catalysts deposited on Al2O3, MgAl2O4, and YSZ. Int. J. Hydrogen Energy 2019, 44, 25706–25716. [Google Scholar] [CrossRef]
- Nishimura, A.; Ohata, S.; Okukura, K.; Hu, E. The impact of operating conditions on the performance of CH4 dry reforming membrane reactor for H2 production. J. Energy Power Technol. 2020, 2, 1–19. [Google Scholar] [CrossRef]
- Cherbanski, R.; Kotkowski, T.; Molga, E. Thermogravimetiric analysis of coking during dry reforming methane. Int. J. Hydrogen Energy 2023, 48, 7346–7360. [Google Scholar] [CrossRef]
- Nishimura, A.; Yamada, S.; Ichii, R.; Ichikawa, M.; Hayakawa, T.; Kolhe, M.L. Hydrogen yield enhancement in biogas dry reforming with a Ni/Cr catalyst: A numerical study. Energies 2024, 17, 5421. [Google Scholar] [CrossRef]
- Nishimura, A.; Ito, S.; Ichikawa, M.; Kolhe, M.L. Impact of thickness of Pd/Cu membrane on performance of biogas dry reforming membrane reactor utilizing Ni/Cr catalyst. Fuels 2024, 5, 439–457. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Ghahremani, M.; Amiri, T.Y.; Basile, A. Performance evaluation of Pd-Ag membrane reactor in glycerol steam reforming process: Development of the CFD model. Int. J. Hydrogen Energy 2019, 44, 1000–1009. [Google Scholar] [CrossRef]
- Bosko, M.L.; Munera, J.F.; Lombardo, E.A.; Cornaglia, L.M. Dry reforming of methane in membrane reactors using Pd and Pd-Ag composite membranes on a NaA zeolite modified porous stainless steel support. J. Membr. Sci. 2010, 364, 17–26. [Google Scholar] [CrossRef]
- Sumrunronnasak, S.; Tantayanon, S.; Kiatgamolchai, S.; Sukonket, T. Improved hydrogen production from dry reforming reaction using a catalytic packed-bed membrane reactor with Ni-based catalyst and dense PdAgCu alloy membrane. Int. J. Hydrogen Energy 2016, 41, 2621–2630. [Google Scholar] [CrossRef]





| Parameters | Values |
|---|---|
| Initial reaction temperature [°C] | 400, 500, and 600 |
| Pressure of supply gas [MPa] | 0.10 |
| Differential pressure between the reaction chamber and the sweep chamber [MPa] | 0, 0.010, and 0.020 |
| Molar ratio of provided CH4:CO2 (flow rate of provided CH4:CO2 [NL/min]) | 1.5:1, 1:1, and 1:1.5 (1.088:0.725, 0.725:0.725, 0.725:1.088) |
| Feed ratio of sweep gas to supply gas [−] | 0 (W/O), 1.0 (W) |
| Thickness of Pd/Cu Membrane [μm] | Sweep Gas | CH4 Conversion [%] | CO2 Conversion [%] | H2 Yield [%] | H2 Selectivity [%] | CO Selectivity [%] | H2 Recovery [%] | Permeation Flux [mol/(m2·s)] | Thermal Efficiency [%] |
|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||
| 20 | W/O | 67.1 | −93.6 | 0.259 | 0.933 | 99.1 | 0.933 | 0 | 3.55 |
| W | 71.8 | −101 | 0.167 | 0.649 | 99.4 | 1.34 | 0 | 1.46 | |
| 40 | W/O | 75.0 | −106 | 0.214 | 0.766 | 99.2 | 1.29 | 0 | 2.92 |
| W | 77.1 | −108 | 0.411 | 1.57 | 98.4 | 0.771 | 0 | 3.59 | |
| 60 | W/O | 69.1 | −97.2 | 5.72 × 10−2 | 0.238 | 99.8 | 1.02 | 0 | 0.783 |
| W | 72.3 | −102 | 0.132 | 0.552 | 99.4 | 0.944 | 0 | 1.16 | |
| (b) | |||||||||
| 20 | W/O | 64.9 | −59.4 | 0.241 | 0.812 | 99.2 | 0.290 | 0 | 2.76 |
| W | 69.8 | −64.5 | 0.167 | 0.557 | 99.4 | 0.120 | 0 | 1.22 | |
| 40 | W/O | 79.9 | −74.6 | 0.148 | 0.585 | 99.4 | 1.08 | 0 | 1.69 |
| W | 79.7 | −74.2 | 0.236 | 1.030 | 99.0 | 1.27 | 0 | 1.71 | |
| 60 | W/O | 81.2 | −76.0 | 8.80 × 10−2 | 0.425 | 99.6 | 0.682 | 0 | 1.00 |
| W | 76.2 | −71.0 | 0.103 | 0.483 | 99.5 | 0.776 | 0 | 0.750 | |
| (c) | |||||||||
| 20 | W/O | 80.0 | −49.0 | 0.129 | 0.660 | 99.3 | 0.291 | 0 | 1.18 |
| W | 75.5 | −46.0 | 0.116 | 0.561 | 99.4 | 0.216 | 0 | 0.677 | |
| 40 | W/O | 88.2 | −54.6 | 2.21 × 10−2 | 0.102 | 99.9 | 1.13 | 0 | 0.200 |
| W | 79.3 | −48.6 | 4.49 × 10−2 | 0.239 | 99.8 | 0.557 | 0 | 0.261 | |
| 60 | W/O | 80.4 | −49.4 | 1.39 × 10−2 | 7.41 × 10−2 | 99.9 | 0.900 | 0 | 0.126 |
| W | 84.2 | −51.9 | 2.71 × 10−2 | 0.151 | 99.8 | 0.462 | 0 | 0.158 | |
| Thickness of Pd/Cu Membrane [μm] | Sweep Gas | CH4 Conversion [%] | CO2 Conversion [%] | H2 Yield [%] | H2 Selectivity [%] | CO Selectivity [%] | H2 Recovery [%] | Permeation Flux [mol/(m2·s)] | Thermal Efficiency [%] |
|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||
| 20 | W/O | 70.4 | −98.9 | 0.171 | 0.638 | 99.4 | 1.17 | 5.00 × 10−4 | 2.34 |
| W | 71.0 | −99.8 | 0.160 | 0.624 | 99.4 | 0.938 | 5.00 × 10−4 | 1.40 | |
| 40 | W/O | 72.1 | −101 | 0.315 | 1.16 | 98.8 | 0.953 | 2.50 × 10−4 | 4.31 |
| W | 74.2 | −104 | 0.186 | 0.751 | 99.2 | 1.43 | 2.50 × 10−4 | 1.62 | |
| 60 | W/O | 72.9 | −103 | 0.126 | 0.549 | 99.5 | 0.595 | 1.67 × 10−4 | 1.73 |
| W | 70.5 | −99.2 | 7.47 × 10−2 | 0.311 | 99.7 | 0.781 | 1.67 × 10−4 | 0.653 | |
| (b) | |||||||||
| 20 | W/O | 70.8 | −65.6 | 0.128 | 0.595 | 99.4 | 7.80 × 10−2 | 5.00 × 10−4 | 1.47 |
| W | 68.9 | −63.7 | 0.115 | 0.509 | 99.5 | 8.70 × 10−2 | 5.00 × 10−4 | 0.841 | |
| 40 | W/O | 76.3 | −71.1 | 0.118 | 0.570 | 99.4 | 1.19 | 2.50 × 10−4 | 1.34 |
| W | 76.1 | −70.9 | 7.82 × 10−2 | 0.330 | 99.7 | 0.384 | 2.50 × 10−4 | 0.571 | |
| 60 | W/O | 80.1 | −75.0 | 4.97 × 10−2 | 0.240 | 99.8 | 1.01 | 1.67 × 10−4 | 0.565 |
| W | 80.1 | −75.0 | 4.40 × 10−2 | 0.200 | 99.8 | 0.682 | 1.67 × 10−4 | 0.320 | |
| (c) | |||||||||
| 20 | W/O | 83.2 | −51.2 | 4.01 × 10−2 | 0.202 | 99.8 | 0.312 | 5.00 × 10−4 | 0.366 |
| W | 86.7 | −53.6 | 2.34 × 10−2 | 0.117 | 99.9 | 0.535 | 5.00 × 10−4 | 0.136 | |
| 40 | W/O | 86.6 | −53.5 | 2.32 × 10−2 | 0.115 | 99.9 | 1.08 | 2.50 × 10−4 | 0.210 |
| W | 86.3 | −53.4 | 1.59 × 10−2 | 8.36 × 10−2 | 99.9 | 0 | 2.50 × 10−4 | 9.32 × 10−2 | |
| 60 | W/O | 82.9 | −51.1 | 2.47 × 10−2 | 0.140 | 99.9 | 0.506 | 1.67 × 10−4 | 0.225 |
| W | 83.0 | −51.2 | 1.68 × 10−2 | 9.51 × 10−2 | 99.9 | 0.743 | 1.67 × 10−4 | 9.77 × 10−2 | |
| Thickness of Pd/Cu Membrane [μm] | Sweep Gas | CH4 Conversion [%] | CO2 Conversion [%] | H2 Yield [%] | H2 Selectivity [%] | CO Selectivity [%] | H2 Recovery [%] | Permeation Flux [mol/(m2·s)] | Thermal Efficiency [%] |
|---|---|---|---|---|---|---|---|---|---|
| (a) | |||||||||
| 20 | W/O | 68.2 | −95.6 | 0.151 | 0.611 | 99.4 | 0.661 | 7.07 × 10−4 | 2.08 |
| W | 70.8 | −99.6 | 0.132 | 0.508 | 99.5 | 0.567 | 7.07 × 10−4 | 1.16 | |
| 40 | W/O | 79.8 | −113 | 0.273 | 1.12 | 98.9 | 0.885 | 3.54 × 10−4 | 3.74 |
| W | 75.4 | −106 | 0.151 | 0.601 | 99.4 | 1.16 | 3.54 × 10−4 | 1.31 | |
| 60 | W/O | 73.9 | −104 | 8.90 × 10−2 | 0.349 | 99.7 | 0.749 | 2.36 × 10−4 | 1.22 |
| W | 75.6 | −107 | 5.85 × 10−2 | 0.257 | 99.7 | 0.997 | 2.36 × 10−4 | 0.510 | |
| (b) | |||||||||
| 20 | W/O | 69.1 | −63.9 | 9.12 × 10−2 | 0.384 | 99.6 | 0.110 | 7.07 × 10−4 | 1.05 |
| W | 69.9 | −64.7 | 8.87 × 10−2 | 0.396 | 99.6 | 0.113 | 7.07 × 10−4 | 0.649 | |
| 40 | W/O | 78.9 | −73.8 | 8.91 × 10−2 | 0.405 | 99.6 | 1.46 | 3.54 × 10−4 | 1.01 |
| W | 79.8 | −74.6 | 6.17 × 10−2 | 0.265 | 99.7 | 2.11 | 3.54 × 10−4 | 0.442 | |
| 60 | W/O | 76.2 | −71.1 | 4.75 × 10−2 | 0.228 | 99.8 | 1.05 | 2.36 × 10−4 | 0.539 |
| W | 80.0 | −74.9 | 4.40 × 10−2 | 0.248 | 99.8 | 0.682 | 2.36 × 10−4 | 0.320 | |
| (c) | |||||||||
| 20 | W/O | 79.7 | −48.9 | 1.41 × 10−2 | 6.62 × 10−2 | 99.9 | 0.885 | 7.07 × 10−4 | 0.128 |
| W | 81.8 | −50.4 | 1.35 × 10−2 | 6.51 × 10−2 | 99.9 | 0.926 | 7.07 × 10−4 | 7.82 × 10−2 | |
| 40 | W/O | 83.0 | −51.2 | 1.50 × 10−2 | 8.68 × 10−2 | 99.9 | 1.67 | 3.54 × 10−4 | 0.135 |
| W | 82.5 | −50.8 | 1.35 × 10−2 | 6.64 × 10−2 | 99.9 | 0.923 | 3.54 × 10−4 | 7.85 × 10−2 | |
| 60 | W/O | 85.8 | −53.0 | 1.67 × 10−2 | 9.63 × 10−2 | 99.9 | 0.748 | 2.36 × 10−4 | 0.152 |
| W | 86.6 | −53.6 | 1.64 × 10−2 | 9.60 × 10−2 | 99.9 | 0.762 | 2.36 × 10−4 | 9.52 × 10−2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Ichikawa, M.; Hayakawa, T.; Yamada, S.; Ichii, R.; Kolhe, M.L. Impact of Membrane Thickness on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr/Ru Catalyst. Fuels 2025, 6, 18. https://doi.org/10.3390/fuels6010018
Nishimura A, Ichikawa M, Hayakawa T, Yamada S, Ichii R, Kolhe ML. Impact of Membrane Thickness on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr/Ru Catalyst. Fuels. 2025; 6(1):18. https://doi.org/10.3390/fuels6010018
Chicago/Turabian StyleNishimura, Akira, Mizuki Ichikawa, Taisei Hayakawa, Souta Yamada, Ryoma Ichii, and Mohan Lal Kolhe. 2025. "Impact of Membrane Thickness on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr/Ru Catalyst" Fuels 6, no. 1: 18. https://doi.org/10.3390/fuels6010018
APA StyleNishimura, A., Ichikawa, M., Hayakawa, T., Yamada, S., Ichii, R., & Kolhe, M. L. (2025). Impact of Membrane Thickness on Characteristics of Biogas Dry Reforming Membrane Reactor Using Pd/Cu Membrane and Ni/Cr/Ru Catalyst. Fuels, 6(1), 18. https://doi.org/10.3390/fuels6010018







