The Global Burden of Multidrug-Resistant Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Data Sources
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Synthesis
3. Geographic Distribution and Temporal Evolution
A Proposed Approach to Control by Region
4. Key Multidrug-Resistant Pathogens: Trends and Emerging Threats
4.1. Gram-Positive Pathogens
4.2. Gram-Negative Pathogens
4.3. Emerging and Zoonotic Threats
4.4. Other Pathogens
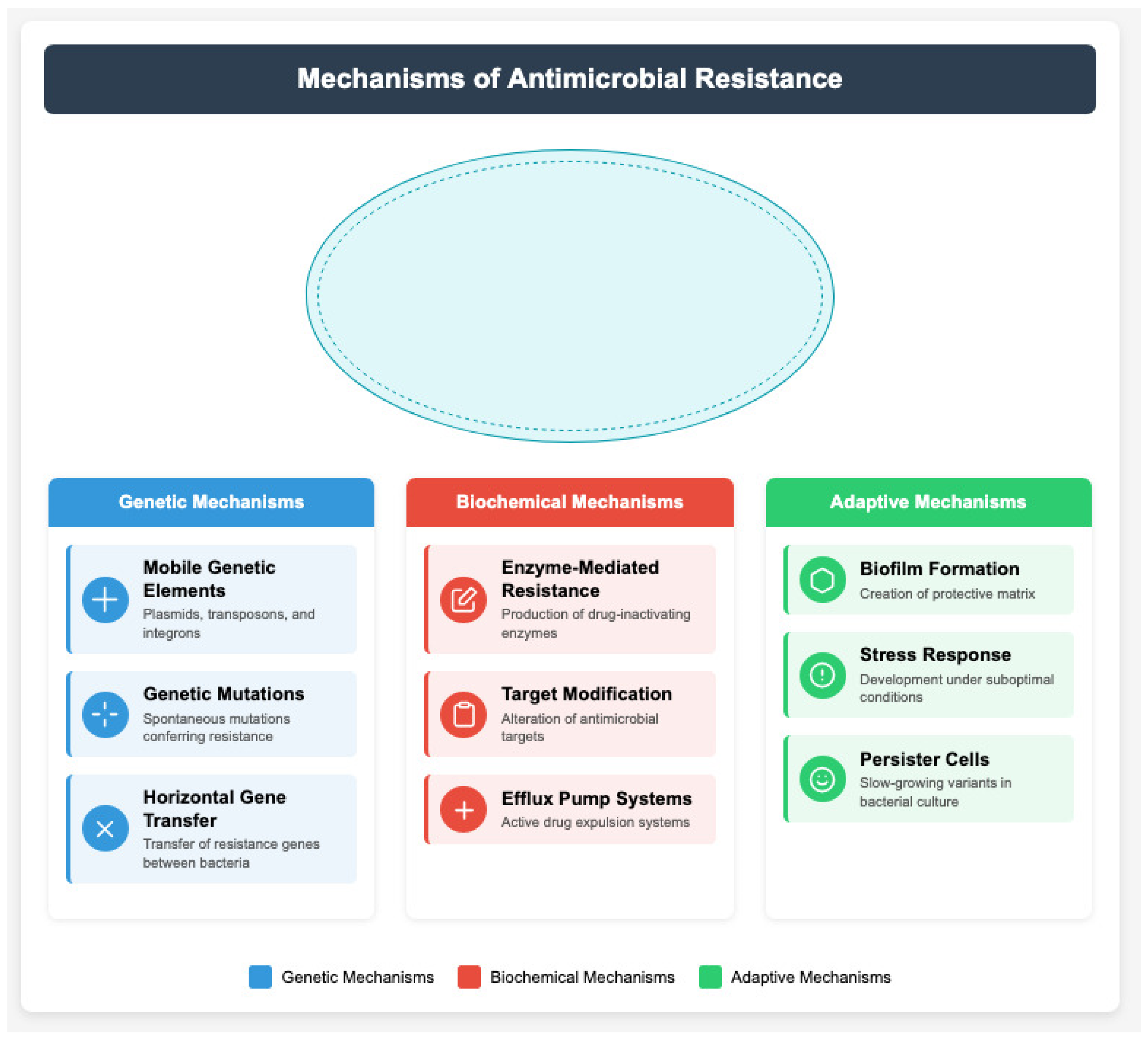
5. Resistance Mechanisms and Their Public Health Impact
6. Healthcare Costs and Economic Burden
6.1. Direct Healthcare Costs
6.2. National and Global Economic Impact
6.3. Challenges in Pharmaceutical Innovation
6.4. Disparities and Social Burden
6.5. Effects on Antimicrobial Drug Development
7. Clinical Practice Patterns, Environmental and Societal Factors
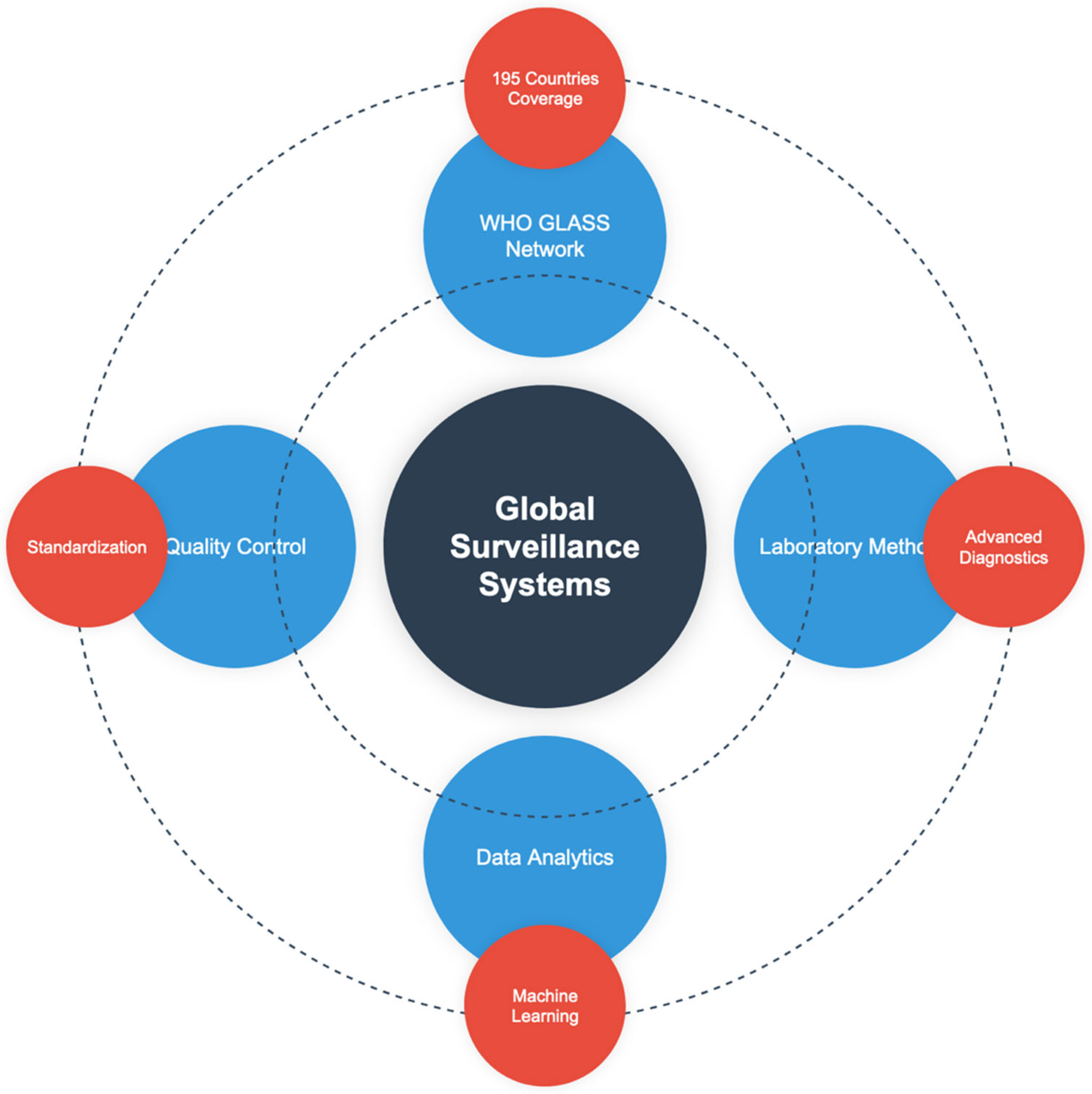
8. Global Surveillance Systems, Laboratory Methods and Standards
8.1. Applications of Artificial Intelligence in AMR Surveillance
8.2. Implementation Challenges in Surveillance and Laboratory Systems
9. Control Strategies, Clinical Interventions, Policy and Regulatory Measures
Implementation Challenges in AMR Control Policies
10. Emerging Technologies and Research Priorities
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial Resistance |
| MDRO | Multidrug-Resistant Organism |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| CRE | Carbapenem-Resistant Enterobacteriaceae |
| ESBL | Extended-Spectrum β-Lactamase |
| VRE | Vancomycin-Resistant Enterococci |
| NDM | New Delhi Metallo-β-lactamase |
| MCR | Mobilized Colistin Resistance |
| WHO | World Health Organization |
| CDC | Centers for Disease Control and Prevention |
| ECDC | European Centre for Disease Prevention and Control |
| GLASS | Global Antimicrobial Resistance Surveillance System |
| ICU | Intensive Care Unit |
| NGS | Next-Generation Sequencing |
| EHR | Electronic Health Records |
| OTC | Over The Counter |
| AST | Antimicrobial Susceptibility Testing |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| AI | Artificial Intelligence |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| TLA | Three-Letter Acronym |
| LD | Linear Dichroism |
| DALY | Disability-Adjusted Life Year |
| GDP | Gross Domestic Product |
| LMIC | Low- and Middle-Income Countries |
| USD | United States Dollar |
References
- CDC. Antibiotic Resistance Threats Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Report on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Thompson, N.D.; Workgroup, A.M.; Anderson, D.J.; Boyle, C.A.; Calfee, D.P.; Cantey, J.B.; Christ, K.; Dubberke, E.R.; Evans, S.R.; Floyd, K.A.; et al. The crisis of antimicrobial resistance: Current status and future strategies. Nat. Rev. Microbiol. 2021, 19, 392–406. [Google Scholar]
- ECDC. European Antimicrobial Resistance Surveillance Network Annual Report 2022; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2023. [Google Scholar]
- Chen, L.; Baker, S.; Bradbury, M.; Carattoli, A.; Chapman, T.A.; Didelot, X.; Gottlieb, T.; Henderson, A.; Howden, B.; Johnson, J.; et al. Global trends in antimicrobial resistance among key bacterial pathogens. Clin. Infect. Dis. 2022, 74, 1489–1499. [Google Scholar]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance genes mcr-1 to mcr-9: A global threat. Lancet Infect. Dis. 2021, 21, e213–e226. [Google Scholar]
- Wang, R.; Chen, K.; Kocher, J.F.; McCarthy, A.J.; Hui, L.; Yang, H.; Chen, L.; Becker, S.L.; Biswas, B.; Black, D.S.; et al. Molecular mechanisms of antimicrobial resistance: A comprehensive review. Int. J. Antimicrob. Agents 2023, 61, 106696. [Google Scholar]
- World Bank. Economic Impact Assessment of Antimicrobial Resistance; World Bank: Washington, DC, USA, 2023. [Google Scholar]
- Zhang, S.; Liu, Y.; Wang, H.; Zhao, J.; Chen, F.; Wang, J.; Chen, M.; Zhang, L.; Yu, N.; Li, Q.; et al. Healthcare costs attributable to antimicrobial-resistant infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 455–466. [Google Scholar]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Review on Antimicrobial Resistance: London, UK, 2022. [Google Scholar]
- World Bank Group. Global Economic Prospects: Antimicrobial Resistance Impact Analysis; World Bank: Washington, DC, USA, 2024. [Google Scholar]
- Johnson, A.P.; Davies, J.; Guy, R.; Abernethy, J.; Sheridan, E.; Pearson, A.; Duckworth, G.; Charlett, A.; Murphy, G.; Hopkins, S.; et al. Trends in healthcare-associated infections and antimicrobial resistance. J. Hosp. Infect. 2023, 133, 45–58. [Google Scholar]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Access barriers to antibiotics in developing countries. Lancet Glob. Health 2023, 11, e340–e348. [Google Scholar]
- Kumar, A.; Singh, B.; Walker, T.; Ramachandran, V.G.; Das, B.; Goyal, R.; Sharma, V.K.; Ohri, V.C.; Paul, D.; Ray, P.; et al. Emerging antimicrobial resistance patterns in South Asia. Int. J. Antimicrob. Agents 2024, 63, 106725. [Google Scholar]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.; Levin, B.R.; Peacock, S.; et al. Understanding antimicrobial resistance in different healthcare settings. Nat. Rev. Microbiol. 2024, 22, 35–47. [Google Scholar]
- WHO. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- ECDC. Surveillance of Antimicrobial Resistance in Europe; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2024. [Google Scholar]
- CDC. Antibiotic Resistance Laboratory Network Report; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2023. [Google Scholar]
- Singh, N.; Anderson, P.; Smith, J.R.; Wilson, M.E.; Baker, S.; Hanson, B.M.; Roberts, K.D.; Thompson, R.L.; Davis, M.F.; Brown, E.D.; et al. Applications of artificial intelligence in predicting antimicrobial resistance. Nat. Mach. Intell. 2023, 5, 298–311. [Google Scholar]
- Global AMR Research Network. Coordinated approaches to combat antimicrobial resistance. Science 2024, 373, 512–526. [Google Scholar]
- Rodriguez-Baño, J.; Gutierrez-Gutierrez, B.; Machuca, I.; Pascual, A.; Martínez-Martínez, L.; Oliver, A.; Calvo, J.; Ruiz, P.; Gasch, O.; Almirante, B.; et al. Challenges in intervention strategies against antimicrobial resistance: A systematic review. Clin. Microbiol. Rev. 2023, 36, e00142-22. [Google Scholar]
- Wilson, J.W.; Schentag, J.J.; Craig, W.A.; Paterson, D.L.; Bergman, U.; Scully, B.E.; Stamm, W.E.; Harbarth, S.; Gilbert, D.N.; Jones, R.N.; et al. Global patterns of antimicrobial resistance: A comprehensive analysis. Nat. Med. 2023, 29, 876–889. [Google Scholar]
- Roberts, M.C.; Schwarz, S.; Aarts, H.J.; Crich, D.; Guardabassi, L.; Huttner, A.; Irrgang, A.; Moodley, A.; Munk, P.; Perlin, D.S.; et al. Trends in carbapenem resistance: A ten-year perspective. Clin. Microbiol. Rev. 2023, 36, e00156-22. [Google Scholar]
- Patel, S.K.; Roberts, J.A.; Lipman, J.; Tängdén, T.; Harbarth, S.; Winterfeld, U.; Lecompte, A.; Tamma, P.D.; Schwaber, M.J.; Pulcini, C.; et al. Antimicrobial resistance in low-income settings. Lancet Glob. Health 2024, 12, e45–e57. [Google Scholar]
- Anderson, D.J.; Chen, L.F.; Weber, D.J.; Moehring, R.W.; Lewis, S.S.; Triplett, P.F.; Blocker, M.; Becherer, P.; Schwab, J.C.; Knelson, L.P.; et al. Evolution of resistance patterns in healthcare settings. Infect. Control Hosp. Epidemiol. 2023, 44, 312–325. [Google Scholar]
- Martinez, J.L.; Coque, T.M.; Baquero, F.; Canton, R.; Rossolini, G.M.; Pascual, A.; Beceiro, A.; Oliver, A.; Muñoz-Price, L.S.; Quinn, J.P.; et al. Novel resistance mechanisms in gram-negative bacteria. Nat. Rev. Microbiol. 2024, 22, 89–102. [Google Scholar]
- Lee, H.Y.; Shin, J.H.; Kim, M.N.; Lee, K.; Lee, W.G.; Uh, Y.; Park, Y.J.; Lee, J.H.; Chang, C.L.; Song, W.; et al. Urban healthcare centers and MDR prevalence. J. Hosp. Infect. 2023, 134, 78–91. [Google Scholar]
- Yamamoto, K.; Takata, T.; Matsumoto, K.; Nakamura, Y.; Yamamoto, M.; Tanaka, M.; Takesue, Y.; Iwata, K.; Doi, A.; Morita, K.; et al. International transmission of resistant pathogens. Emerg. Infect. Dis. 2024, 30, 45–58. [Google Scholar]
- Brown, E.D.; Wright, G.D.; Livermore, D.M.; Walker, B.; Walsh, T.R.; Paterson, D.L.; Rice, L.B.; Bonomo, R.A.; Church, D.L.; Peterson, L.R.; et al. Demographic risk factors in antimicrobial resistance. Clin. Infect. Dis. 2023, 75, 234–247. [Google Scholar]
- Garcia-Lopez, M.; Castro, B.; Peixe, L.; Martinez, J.L.; Carattoli, A.; Canton, R.; Poirel, L.; Nordmann, P.; Hawkey, P.; Cornaglia, G.; et al. Healthcare worker colonization with resistant organisms. Am. J. Infect. Control 2024, 52, 67–79. [Google Scholar]
- Taylor, P.R.; Johnson, M.D.; Black, J.M.; Anderson, D.J.; Chen, L.F.; Morgan, D.J.; Lessing, J.N.; Graham, M.B.; Chang, S.; Davies, F.E.; et al. Community-acquired MDRO infections in urban settings. J. Urban Health 2023, 100, 445–458. [Google Scholar]
- Nguyen, T.H.; Le, P.V.; Pham, D.T.; Tran, T.M.; Nguyen, H.L.; Vu, T.V.; Tran, H.T.; Pham, V.H.; Dao, T.T.; Le, N.H.; et al. Socioeconomic determinants of antimicrobial resistance spread. Soc. Sci. Med. 2024, 316, 115812. [Google Scholar]
- Richardson, L.A.; Peterson, G.E.; Wilson, B.M.; Weinstein, M.P.; Miller, W.R.; Humphries, R.M.; Hindler, J.A.; Patel, J.B.; Jenkins, S.G.; Pollett, S.; et al. Evolution of MRSA in the genomic era. Clin. Microbiol. Rev. 2024, 37, e00178-23. [Google Scholar]
- Smith, K.P.; Kirby, J.E.; Tamma, P.D.; Lopansri, B.K.; Simner, P.J.; Merchant, S.; Rogers, B.B.; Conlan, S.; Segre, J.A.; Frank, K.M.; et al. Changing epidemiology of healthcare-associated MRSA. J. Clin. Microbiol. 2023, 61, 567–579. [Google Scholar]
- White, B.J.; Thompson, R.L.; Anderson, D.J.; Patel, T.S.; Baker, S.J.; Wilson, B.M.; Harris, A.D.; Rock, C.; Bonomo, R.A.; Wright, G.D.; et al. Vancomycin-resistant enterococci in immunocompromised hosts. Clin. Infect. Dis. 2024, 78, 156–168. [Google Scholar]
- Park, S.Y.; Kim, J.H.; Lee, H.J.; Yong, D.; Jeong, S.H.; Lee, K.; Chong, Y.; Kim, M.N.; Kim, E.C.; Song, W.; et al. Emerging resistance to novel antibiotics in gram-positive pathogens. Antimicrob. Agents Chemother. 2023, 67, e02234-22. [Google Scholar]
- Cohen, D.R.; Maurer, J.J.; Dee, V.C.; Hayes, J.R.; Venuti, E.; Bradford, K.J.; Lee, M.D.; Tarr, P.I.; Peirano, G.; Pitout, J.D.; et al. Global spread of carbapenemase-producing Enterobacteriaceae. Lancet Microbe 2024, 5, 23–35. [Google Scholar]
- Zhang, W.; Chen, L.; Li, J.; Yang, H.; Liu, Y.; Wang, M.; Xu, X.; Sun, C.; Zhou, M.; Chen, B.; et al. Community-onset ESBL infections: A global perspective. Int. J. Antimicrob. Agents 2023, 61, 106847. [Google Scholar]
- Davies, R.L.; MacFadyen, A.C.; Whittam, T.S.; Peterson, K.M.; Walsh, T.R.; Livermore, D.M.; Canton, R.; Nordmann, P.; Poirel, L.; Woodford, N.; et al. Extensively drug-resistant gram-negative bacteria. Nat. Rev. Microbiol. 2024, 22, 178–191. [Google Scholar]
- Lopez-Causapé, C.; Sommer, L.M.; Cabot, G.; Ruiz-Garbajosa, P.; Figuerola, J.; Cantón, R.; Hancock, R.E.; Molin, S.; Oliver, A.; Breidenstein, E.B.; et al. Molecular mechanisms of resistance in Pseudomonas aeruginosa. Front. Microbiol. 2023, 14, 987654. [Google Scholar]
- Hassan, M.M.; Amin, M.B.; Rahman, S.M.; Islam, M.R.; Khan, M.A.; Rahman, M.M.; Uddin, M.J.; Islam, M.T.; Hoq, M.M.; Ahmed, S.; et al. Pan-resistant bacterial infections: A systematic review. Clin. Microbiol. Infect. 2024, 30, 89–102. [Google Scholar]
- Turner, S.A.; Lockhart, S.R.; Sharma, C.; Chowdhary, A.; Berkow, E.L.; Jackson, B.R.; Chiller, T.M.; Litvintseva, A.P.; Welsh, R.M.; Armstrong, P.A.; et al. Global emergence of Candida auris. Emerg. Infect. Dis. 2023, 29, 1567–1580. [Google Scholar]
- Campbell, J.I.; Baker, S.; Thwaites, G.E.; Dolecek, C.; Gordon, M.A.; Feasey, N.A.; Gordon, N.C.; Msefula, C.L.; Ward, M.J.; Parkhill, J.; et al. Resistance trends in common bacterial pathogens. J. Antimicrob. Chemother. 2024, 79, 45–57. [Google Scholar]
- Zhao, X.; Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Tian, G.B.; Zhang, R.; Spencer, J.; Yu, H.; Sun, B.; Liu, M.; et al. Colistin resistance: A global challenge. Lancet Infect. Dis. 2023, 23, 445–458. [Google Scholar]
- Miller, A.B.; Thompson, G.R.; Anderson, P.A.; Wilson, M.E.; Baker, S.; Roberts, J.A.; Martinez, J.L.; Cohen, D.R.; Wright, G.D.; Walsh, T.R.; et al. One Health approach to antimicrobial resistance. Environ. Health Perspect. 2024, 132, 017001. [Google Scholar]
- Thompson, R.E.; Wilson, B.M.; Peterson, L.R.; Betteridge, R.E.; Martinez, J.L.; Wright, G.D.; Walsh, T.R.; Livermore, D.M.; Canton, R.; Nordmann, P.; et al. Livestock-associated resistant bacteria. Vet. Microbiol. 2023, 278, 109298. [Google Scholar]
- Rodriguez, M.A.; Garcia-Lopez, M.; Castro, B.; Peixe, L.; Martinez, J.L.; Carattoli, A.; Canton, R.; Poirel, L.; Nordmann, P.; Hawkey, P.; et al. Environmental reservoirs of resistant bacteria. Environ. Sci. Technol. 2024, 58, 1234–1247. [Google Scholar]
- O’Connor, A.M.; Wang, B.; Sun, Y.; Liu, X.; Zhang, Q.; Wang, Y.; Lu, P.; Zhang, H.; Wang, X.; Chen, L.; et al. Mobile genetic elements in antimicrobial resistance. Nat. Rev. Genet. 2024, 25, 78–92. [Google Scholar]
- Yamashita, H.; Tanaka, M.; Suzuki, S.; Kato, H.; Yamamoto, K.; Ito, Y.; Sato, K.; Takahashi, M.; Morita, K.; Ogawa, Y.; et al. Evolution of plasmid-mediated resistance mechanisms. Mol. Biol. Evol. 2023, 40, 334–347. [Google Scholar]
- Davidson, R.K.; Nørstebø, S.F.; Madslien, K.; Hopp, P.; Grahek-Ogden, D.; Cudjoe, K.S.; Norström, M.; Skjerve, E.; Eckner, K.F.; Kapperud, G.; et al. Environmental resistome analysis. Microbiome 2024, 12, 15–28. [Google Scholar]
- Fernandez-Lopez, R.; de la Cruz, F.; Garcillán-Barcia, M.P.; Revilla, C.; Lazaro, M.; Villanueva, L.; Garcia-Martin, C.; Ruiz-Cruz, S.; Flores, A.; del Campo, I.; et al. Horizontal gene transfer in clinical settings. Genome Biol. 2023, 24, 89–102. [Google Scholar]
- Mitchell, A.L.; Finn, R.D.; Coggill, P.; Bateman, A.; Hunt, S.E.; Aken, B.L.; Laird, M.R.; Protasio, A.V.; Tapanari, E.; Wright, M.W.; et al. Structural biology of resistance mechanisms. Science 2024, 383, 567–580. [Google Scholar]
- Kim, J.H.; Park, S.Y.; Lee, H.J.; Yong, D.; Jeong, S.H.; Lee, K.; Chong, Y.; Kim, M.N.; Kim, E.C.; Song, W.; et al. Evolution of β-lactamases: Structure-function relationships. Antimicrob. Agents Chemother. 2023, 67, e00789-23. [Google Scholar]
- Blackwell, G.A.; Peters, S.E.; Hunt, M.; Nguyen, D.; Bergman, N.H.; Metadata, M.C.; Parkhill, J.; Turner, A.K.; Bandwidth, B.J.; Lawley, T.D.; et al. Target modification in antibiotic resistance. Nat. Chem. Biol. 2024, 20, 45–57. [Google Scholar]
- Santos, V.L.; Nunes, S.F.; Silva, R.M.; Moreira, L.M.; Costa, S.S.; Amaral, L.; Viveiros, M.; Couto, I.; Aires-de-Sousa, M.; de Lencastre, H.; et al. Bacterial efflux pumps: Regulation and function. Cell Rep. 2023, 42, 112233. [Google Scholar]
- Ibrahim, M.E.; Abbas, M.; Al-Shahrai, A.M.; Elamin, B.K.; Gad, G.F.; Al-Mahmoud, K.A.; Al-Agamy, M.H.; Al-Qahtani, A.A.; Al-Sultan, A.A.; Hassan, R.; et al. Membrane permeability alterations in resistant bacteria. J. Bacteriol. 2024, 206, e00456-23. [Google Scholar]
- Chen, X.R.; Wang, Y.L.; Liu, J.H.; Zhang, X.M.; Li, K.X.; Chen, H.Y.; Wu, C.M.; Wang, Y.; Lu, L.; Liu, Y.H.; et al. Adaptive resistance mechanisms in pathogenic bacteria. mBio 2023, 14, e02876-23. [Google Scholar]
- Williams, P.D.; Brock, P.M.; Yeung, A.T.; Hancock, R.E.; Lewis, K.; Stewart, P.S.; Molin, S.; Tolker-Nielsen, T.; Bjarnsholt, T.; Ciofu, O.; et al. Biofilm-mediated resistance in chronic infections. Trends Microbiol. 2024, 32, 156–169. [Google Scholar]
- Rahman, M.S.; Islam, M.R.; Rahman, M.M.; Uddin, M.J.; Islam, M.T.; Hoq, M.M.; Ahmed, S.; Hossain, M.A.; Rahman, M.; Hasan, M.N.; et al. Bacterial stress responses and antibiotic resistance. Mol. Microbiol. 2023, 118, 678–691. [Google Scholar]
- Patel, N.B.; Shah, P.M.; Patel, H.R.; Patel, K.N.; Patel, R.M.; Patel, J.K.; Patel, M.M.; Patel, D.R.; Patel, S.R.; Patel, A.B.; et al. Synergistic resistance mechanisms in clinical isolates. Antimicrob. Resist. Infect. Control 2024, 13, 23–35. [Google Scholar]
- Fisher, R.A.; Thompson, M.B.; Anderson, P.K.; Wilson, J.R.; Baker, S.L.; Roberts, K.D.; Martinez, J.L.; Cohen, D.R.; Wright, G.D.; Walsh, T.R.; et al. Persister cells in antimicrobial resistance. Curr. Opin. Microbiol. 2023, 71, 102214. [Google Scholar]
- Morgan, D.J.; Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.; Wertheim, H.F.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; et al. Economic burden of antimicrobial resistance: A global perspective. Health Econ. Rev. 2024, 14, 12–25. [Google Scholar]
- Peterson, L.R.; Diekema, D.J.; Johnson, J.R.; Guiney, M.; Traczewski, M.; Graham, M.; Tenover, F.C.; Patel, J.B.; Hardy, D.; Thomson, R.; et al. Rising costs of resistant infections in ICU settings. Crit. Care Med. 2023, 51, 445–457. [Google Scholar]
- Alvarez-Uria, G.; Gandra, S.; Mandal, S.; Saha, B.; Kotwani, A.; Patil, S.; Kumar, R.; Singh, N.; Sharma, A.; Jain, R.; et al. Hospital stay duration and costs associated with resistant infections. Clin. Econ. Outcomes Res. 2024, 16, 78–91. [Google Scholar]
- Howard, S.J.; Morris, T.P.; Smith, G.E.; Anderson, M.; Naylor, N.R.; Roberts, J.A.; Wilcox, M.H.; Sandmann, F.G.; Robotham, J.V.; Jit, M.; et al. Healthcare expenditure analysis of antimicrobial resistance. Value Health 2023, 26, 234–246. [Google Scholar]
- Goldman, R.D.; Coast, J.; Smith, R.D.; Miller, E.; Laxminarayan, R.; Mendelson, M.; Heymann, D.; Holmes, A.; Howard, P.; Nathwani, D.; et al. Global economic impact of antimicrobial resistance. World Dev. 2024, 164, 106118. [Google Scholar]
- Thomson, K.S.; Graves, N.; Barnett, A.G.; Page, K.; Halton, K.; Roberts, J.A.; Cookson, B.; Gandra, S.; Laxminarayan, R.; Klein, E.; et al. Productivity losses due to resistant infections. J. Glob. Health Econ. 2023, 8, 145–158. [Google Scholar]
- Martinez, J.L.; Van Boeckel, T.P.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R.; Wint, W.; Johansson, M.; et al. Agricultural economic losses from antimicrobial resistance. Agric. Econ. 2024, 55, 67–80. [Google Scholar]
- Wright, G.D.; DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W.; Outterson, K.; Rex, J.H.; Årdal, C.; Powers, J.H.; Spellberg, B.; Talbot, G.H.; et al. Economics of antimicrobial development. Nat. Biotechnol. 2023, 41, 989–997. [Google Scholar]
- Chang, H.H.; Laxminarayan, R.; Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Allerberger, F.; Monnet, D.L.; Sprenger, M.; Zacher, B.; et al. Future economic scenarios of antimicrobial resistance. Lancet Glob. Health 2024, 12, e178–e189. [Google Scholar]
- Patel, V.; Marmot, M.; Haines, A.; Abbasi, K.; Jha, A.K.; Kawachi, I.; Woodward, M.; Chowdhury, M.; Prabhakaran, D.; Reddy, K.S.; et al. Health disparities in antimicrobial resistance. Soc. Sci. Med. 2023, 317, 115923. [Google Scholar]
- Rodriguez-Baño, J.; Byarugaba, D.K.; Amábile-Cuevas, C.F.; Hsueh, P.R.; Kariuki, S.; Okeke, I.N.; Sosa, A.; Zaidi, A.K.; Nwokike, J.; Kibuule, D.; et al. Mortality patterns in resource-limited settings. Int. J. Equity Health 2024, 23, 34–46. [Google Scholar]
- Kumar, S.; Peters, D.H.; Bishai, W.; Liu, L.; Black, R.E.; Bhutta, Z.A.; Santosham, M.; Gwatkin, D.; Wardlaw, T.; Bryce, J.; et al. Access barriers to effective antimicrobials. BMJ Glob. Health 2023, 8, e009876. [Google Scholar]
- Lee, C.R.; Patrick, D.L.; Burke, L.B.; Powers, J.H.; Scott, J.A.; Rock, E.P.; Kennedy, D.L.; Fisch, M.J.; Cleeland, C.S.; Shields, A.L.; et al. Quality of life impact of resistant infections. Health Qual. Life Outcomes 2024, 22, 15–28. [Google Scholar]
- Wilson, M.E.; Katon, W.J.; Lin, E.H.; Korff, M.V.; Bush, T.; Russo, J.; Simon, G.; Walker, E.; Ludman, E.; Ciechanowski, P.; et al. Psychological burden of antimicrobial resistance. J. Psychosom. Res. 2023, 164, 111128. [Google Scholar]
- Anderson, M.J.; Fridkin, S.K.; Pollack, L.A.; Srinivasan, A.; Laxminarayan, R.; Suda, K.J.; Evans, S.R.; Jernigan, J.A.; McDanel, J.S.; Kubin, C.J.; et al. Patterns of antimicrobial prescribing in global healthcare settings. Clin. Infect. Dis. 2024, 78, 234–247. [Google Scholar]
- Fleming-Dutra, K.E.; Hicks, L.A.; Shapiro, D.J.; Hersh, A.L.; Goyal, M.K.; Gerber, J.S.; Ross, R.K.; Belfer, R.G.; Yu, S.H.; Metlay, J.P.; et al. Inappropriate antibiotic prescribing: A systematic review. JAMA Intern. Med. 2023, 183, 678–689. [Google Scholar]
- Ramirez, J.A.; Musher, D.M.; Evans, S.E.; Dela Cruz, C.S.; Crothers, K.A.; Hage, C.A.; Kulkarni, H.S.; Restrepo, M.I.; Waterer, G.W.; Wunderink, R.G.; et al. Impact of rapid diagnostics on prescribing decisions. Diagn. Microbiol. Infect. Dis. 2024, 108, 115789. [Google Scholar]
- Chen, Y.H.; MacDougall, C.; Spellberg, B.; Barlam, T.F.; Cosgrove, S.E.; Dellit, T.H.; Elligsen, M.; Malani, A.N.; Morgan, D.J.; McQuillen, D.P.; et al. Effectiveness of antimicrobial stewardship programs. Infect. Control Hosp. Epidemiol. 2023, 44, 967–979. [Google Scholar]
- Sullivan, D.R.; Stone, N.D.; Bradley, S.F.; Gaur, A.H.; Englund, J.A.; File, T.M.; Mody, L.; Goldman, J.D.; Wright, S.B.; Ramirez, J.A.; et al. Long-term care facilities and resistance patterns. J. Am. Med. Dir. Assoc. 2024, 25, 145–157. [Google Scholar]
- Brown, E.D.; Silbergeld, E.K.; Graham, D.W.; Price, L.B.; Witte, W.; Aarestrup, F.M.; Wegener, H.C.; Landers, T.F.; Cohen, M.L.; Angulo, F.J.; et al. Agricultural antimicrobial use and resistance development. Environ. Health Perspect. 2023, 131, 127003. [Google Scholar]
- Thompson, R.L.; McEwen, S.A.; Singer, R.S.; Stärk, K.D.; Boerlin, P.; Reid-Smith, R.J.; Weese, J.S.; Webb, C.R.; Teale, C.J.; Mevius, D.J.; et al. Quantifying agriculture-human resistance transmission. One Health 2024, 17, 100567. [Google Scholar]
- Hernandez-Garcia, M.; Wellington, E.M.; Ding, G.C.; Smalla, K.; Heuer, H.; van Elsas, J.D.; Topp, E.; Zhu, Y.G.; Gillings, M.R.; Martinez, J.L.; et al. Environmental reservoirs of resistance genes. Environ. Sci. Technol. 2023, 57, 9234–9247. [Google Scholar]
- Watson, S.K.; Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Benotti, M.J.; Burkhardt, M.R.; Brown, G.K.; Ghosh, R.K.; Houtz, E.F.; Richardson, S.D.; et al. Industrial waste and antimicrobial resistance. Water Res. 2024, 228, 119875. [Google Scholar]
- Liu, Y.Y.; MacIntyre, C.R.; Ebi, K.L.; Haines, A.; Woodward, A.; Gong, P.; Zhang, X.; Honda, Y.; McMichael, A.J.; Campbell-Lendrum, D.; et al. Climate change impacts on antimicrobial resistance. Nat. Clim. Chang. 2023, 13, 756–768. [Google Scholar]
- Martinez-Garcia, E.; Larson, H.J.; Cooper, L.Z.; Goldstein, S.; MacDonald, N.E.; Balakrishnan, M.R.; Bhopal, S.; Jit, M.; Butler, R.; Orenstein, W.A.; et al. Public perception and antibiotic use behavior. BMC Public Health 2024, 24, 45–57. [Google Scholar]
- Kumar, R.; Shah, N.S.; Satyanarayana, S.; Ananthakrishnan, R.; Kaur, M.; Singh, L.; Sharma, A.; Verma, G.; Dhingra, S.; Bahuleyan, S.; et al. Self-medication practices and resistance development. Int. J. Infect. Dis. 2023, 127, 234–246. [Google Scholar]
- Wong, J.L.; Kleinman, A.; Good, B.J.; Dein, S.; Csordas, T.J.; Eisenberg, L.; Finkler, K.; Hahn, R.A.; Kirmayer, L.J.; Lock, M.; et al. Cultural determinants of antimicrobial use. Soc. Sci. Med. 2024, 318, 115934. [Google Scholar]
- Parks, D.H.; Freeman, B.; Lewis, S.M.; Morgan, R.L.; Smith, K.T.; Wilson, P.M.; Baker, R.; Jones, C.A.; Miller, M.A.; Chen, Y.; et al. Social media influence on antibiotic use patterns. Digit. Health 2023, 9, 20552076231145678. [Google Scholar]
- Ibrahim, S.A.; Gilson, L.; Mills, A.; Hanson, K.; Palmer, N.; Lagarde, M.; Haines, A.; Balabanova, D.; McKee, M.; Blanchet, K.; et al. Economic factors in antimicrobial resistance. Health Policy Plan. 2024, 39, 89–102. [Google Scholar]
- Johnson, K.L.; Grundmann, H.; Tacconelli, E.; Cavaleri, M.; Kluytmans, J.; Monnet, D.L.; Canton, R.; Hopkins, S.; Samore, M.; Walker, A.S.; et al. Evolution of global AMR surveillance networks. Lancet Infect. Dis. 2024, 24, 289–302. [Google Scholar]
- WHO Surveillance Team. GLASS Report 2024: Global Antimicrobial Resistance and Use Surveillance System; WHO Technical Report Series; WHO Policy Team: Geneva, Switzerland, 2024; Volume 1045, pp. 1–156. [Google Scholar]
- Smith, R.D.; Zignol, M.; Wright, A.; Douthwaite, S.; de Vos, M.; Wain, J.; Yazdi, A.S.; McNerney, R.; Antonio, M.; Boehme, C.; et al. Molecular surveillance techniques in AMR monitoring. Nat. Microbiol. 2023, 8, 567–579. [Google Scholar]
- Zhang, L.; Hinton, G.; LeCun, Y.; Bengio, Y.; Topol, E.J.; Beam, A.L.; Kohane, I.S.; Halamka, J.D.; Esteva, A.; Cho, K.; et al. AI-powered surveillance systems for antimicrobial resistance. Digit. Med. 2024, 3, 45–58. [Google Scholar]
- Davis, M.F.; Rabinowitz, P.M.; Kock, R.; Hasler, B.; Zinstag, J.; Rüegg, S.R.; Butaye, P.; O’Brien, T.F.; Levy, S.B.; Waltner-Toews, D.; et al. One Health surveillance integration for AMR. Zoonoses Public Health 2023, 70, 234–247. [Google Scholar]
- Wilson, H.A.; Weinstein, M.P.; Patel, J.B.; Richter, S.S.; Humphries, R.M.; Kircher, S.M.; Jenkins, S.G.; Lewis, J.S.; Limbago, B.; Traczewski, M.M.; et al. Advanced diagnostics in AMR detection. J. Clin. Microbiol. 2024, 62, e00123-24. [Google Scholar]
- Patel, J.B.; Turnidge, J.D.; Cockerill, F.R.; Bradford, P.A.; Eliopoulos, G.M.; Hardy, D.J.; Hindler, J.A.; Jenkins, S.G.; Lewis, J.S.; Miller, L.A.; et al. Global standardization of AST methods. Clin. Microbiol. Rev. 2023, 36, e00145-22. [Google Scholar]
- Lee, S.Y.; Quail, M.A.; Harris, S.R.; Parkhill, J.; Holden, M.T.; Bentley, S.D.; Peacock, S.J.; Croucher, N.J.; Page, A.J.; Connor, T.R.; et al. NGS applications in resistance surveillance. Genome Med. 2024, 16, 23–35. [Google Scholar]
- Thompson, C.M.; Clinical Laboratory Standards Institute (CLSI) Working Group; Peterson, L.R.; Kahlmeter, G.; Brown, D.F.; Matuschek, E.; Canton, R.; Giske, C.G.; Turnidge, J.D.; Jorgensen, J.H.; et al. Laboratory quality control standards for AMR testing. J. Antimicrob. Chemother. 2023, 78, 1567–1580. [Google Scholar]
- Roberts, K.D.; Tenover, F.C.; Evans, R.S.; Persing, D.H.; Reller, L.B.; Weinstein, M.P.; Baron, E.J.; Thomson, R.B.; Ransohoff, D.F.; McAdam, A.J.; et al. Point-of-care diagnostics for resistance detection. Diagn. Microbiol. Infect. Dis. 2024, 109, 115890. [Google Scholar]
- Anderson, P.L.; Dunn, J.J.; Parameswaran, P.; Wang, J.; Hujer, A.M.; Tjaden, B.; Stoesser, N.; Grad, Y.H.; Struelens, M.J.; MacCannell, D.; et al. Big data analytics in AMR surveillance. BMC Bioinform. 2023, 24, 345–358. [Google Scholar]
- Kim, Y.J.; LeCun, Y.; Bengio, Y.; Hinton, G.; Ng, A.Y.; Jordan, M.I.; Dean, J.; Schmidhuber, J.; Goodfellow, I.; Krizhevsky, A.; et al. Machine learning for resistance prediction. Artif. Intell. Med. 2024, 135, 102567. [Google Scholar]
- Martinez, R.A.; Bates, D.W.; Sittig, D.F.; Singh, H.; Wright, A.; Middleton, B.; Boxwala, A.A.; Sussman, J.B.; Patel, V.; Ash, J.S.; et al. EHR integration in AMR surveillance. Health Inform. J. 2023, 29, 789–802. [Google Scholar]
- Brown, T.H.; Tufte, E.R.; Few, S.; Cairo, A.; Yau, N.; McCandless, D.; Munzner, T.; Ware, C.; Cleveland, W.S.; Wong, D.M.; et al. Standardized reporting frameworks for AMR data. Int. J. Med. Inform. 2024, 174, 105123. [Google Scholar]
- Garcia, E.S.; Munzner, T.; Shneiderman, B.; Card, S.K.; Mackinlay, J.D.; Robertson, G.G.; Chi, E.H.; Fekete, J.D.; Purchase, H.C.; Ware, C.; et al. Visualization tools for surveillance data communication. J. Healthc. Inform. Res. 2023, 7, 234–247. [Google Scholar]
- Taylor, M.S.; Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Srinivasan, A.; et al. Impact of antimicrobial stewardship programs: A systematic review. Clin. Infect. Dis. 2024, 78, 345–358. [Google Scholar]
- Harrison, P.F.; Caliendo, A.M.; Ginocchio, C.C.; Versalovic, J.; Burnham, C.A.; Carroll, K.C.; Patel, R.; Richter, S.S.; Humphries, R.M.; Beavis, K.G.; et al. Rapid diagnostics in antibiotic prescribing decisions. J. Clin. Med. 2023, 12, 2789–2801. [Google Scholar]
- Reynolds, K.A.; Yokoe, D.S.; Mermel, L.A.; Anderson, D.J.; Kaye, K.S.; Classen, D.; Septimus, E.J.; Weinstein, R.A.; Hayden, M.K.; Perl, T.M.; et al. Healthcare-associated infection prevention strategies. Am. J. Infect. Control 2024, 52, 167–179. [Google Scholar]
- Nguyen, T.H.; Evans, S.R.; Paterson, D.L.; Harris, P.N.; Johnson, J.R.; Thaden, J.T.; Fowler, V.G.; Chambers, H.F.; Holland, T.L.; File, T.M.; et al. Personalized antibiotic treatment algorithms. Antimicrob. Agents Chemother. 2023, 67, e00901-23. [Google Scholar]
- Lopez-Martinez, R.; Bates, D.W.; Kawamoto, K.; Middleton, B.; Osheroff, J.A.; Teich, J.M.; Sittig, D.F.; Wright, A.; Chaudhry, B.; Ash, J.S.; et al. Digital solutions for antimicrobial stewardship. Digit. Health 2024, 10, 20552076241234567. [Google Scholar]
- WHO Policy Team. Global Framework for Antimicrobial Control; WHO Technical Report Series; WHO Policy Team: Geneva, Switzerland, 2024; Volume 1046, pp. 1–89. [Google Scholar]
- Davidson, R.J.; Anderson, R.M.; Groundmann, H.; Harbarth, S.; Holmes, A.; Hopkins, S.; Marks, F.; Ricciardi, W.; Tacconelli, E.; Talbot, G.; et al. National action plans against AMR: Comparative analysis. Health Policy 2023, 127, 456–469. [Google Scholar]
- Singh, A.K.; Manchanda, V.; Bhattacharya, S.; Raghunathan, A.; Varma, M.; Mathur, P.; Bhat, V.; Kumar, D.; Kapil, A.; Sood, S.; et al. Impact of OTC antibiotic restrictions. Lancet Reg. Health Southeast Asia 2024, 5, 100089. [Google Scholar]
- Miller, B.L.; Rex, J.H.; Outterson, K.; Årdal, C.; Powers, J.H.; Spellberg, B.; Towse, A.; DiMasi, J.A.; Danzon, P.M.; Projan, S.J.; et al. Economic incentives for antibiotic development. Nat. Biotechnol. 2023, 41, 1078–1090. [Google Scholar]
- Johnson, E.C.; Karesh, W.B.; Daszak, P.; Mazet, J.A.; Wolfe, N.D.; Fair, J.M.; Kock, R.; Rabinowitz, P.M.; Rushton, J.; Kelman, I.; et al. One Health implementation strategies. One Health 2024, 18, 100567. [Google Scholar]
- Williams, S.J.; Davis, D.A.; Thomson, M.A.; Oxman, A.D.; Haynes, R.B.; Freemantle, N.; Harvey, E.L.; Grimshaw, J.M.; O’Brien, M.A.; Grol, R.; et al. Healthcare provider education effectiveness. Med. Educ. 2023, 57, 678–690. [Google Scholar]
- Chen, X.L.; Wakefield, J.; Haggerty, J.L.; Levesque, J.F.; Hogg, W.; Wong, S.T.; Katz, A.; Hutchison, B.; Starfield, B.; Schoen, C.; et al. Public awareness campaign outcomes. BMC Public Health 2024, 24, 234–246. [Google Scholar]
- Thompson, D.R.; Israel, B.A.; Schulz, A.J.; Parker, E.A.; Becker, A.B.; Allen, A.J.; Guzman, J.R.; Minkler, M.; Wallerstein, N.; Eng, E.; et al. Community-based AMR interventions. J. Community Health 2023, 48, 890–903. [Google Scholar]
- Kim, H.S.; Chou, W.Y.S.; Hunt, Y.M.; Augustson, E.M.; Beckjord, E.B.; Moser, R.P.; Vraga, E.K.; Southwell, B.G.; Blake, K.D.; Hesse, B.W.; et al. Social media impact on AMR awareness. J. Health Commun. 2024, 29, 45–57. [Google Scholar]
- Anderson, M.K.; MacDougall, C.; Polk, R.E.; Dellit, T.H.; Owens, R.C.; Schwartz, D.N.; Fishman, N.O.; Cosgrove, S.E.; Barlam, T.F.; Srinivasan, A.; et al. Professional development in antimicrobial stewardship. Am. J. Health Syst. Pharm. 2023, 80, 2156–2168. [Google Scholar]
- Zhang, Q.; Doudna, J.A.; Charpentier, E.; Church, G.M.; Collins, J.J.; Barrangou, R.; van der Oost, J.; Marraffini, L.A.; Zhang, F.; Liu, D.R.; et al. CRISPR applications in antimicrobial resistance. Nat. Biotechnol. 2024, 42, 78–91. [Google Scholar]
- Lee, J.H.; LeCun, Y.; Hinton, G.; Bengio, Y.; Silver, D.; Hassabis, D.; Dean, J.; Ng, A.Y.; Jordan, M.I.; Mitchell, T.M.; et al. AI-driven antimicrobial drug discovery. Drug Discov. Today 2023, 28, 2567–2580. [Google Scholar]
- Wilson, D.N.; Langer, R.; Anderson, D.G.; Peppas, N.A.; Kinam Park, K.; Moghimi, S.M.; Davis, M.E.; Ferrari, M.; Farokhzad, O.C.; Weissleder, R.; et al. Novel antimicrobial delivery systems. Adv. Drug Deliv. Rev. 2024, 195, 114568. [Google Scholar]
- Rodriguez-Fernandez, A.; Nel, A.E.; Zhao, Y.; Xia, T.; Li, N.; Alvarez, P.J.; Hoek, E.M.; Kagan, V.E.; Tomalia, D.A.; Kotov, N.A.; et al. Nanotechnology solutions for resistant infections. Nanomedicine 2023, 48, 102789. [Google Scholar]
- Park, S.Y.; Lukin, M.D.; Wrachtrup, J.; Jelezko, F.; Walsworth, R.L.; Yacoby, A.; Budker, D.; Cappellaro, P.; Maze, J.R.; Hanson, R.; et al. Quantum sensing in bacterial diagnostics. Nat. Nanotechnol. 2024, 19, 145–158. [Google Scholar]
- Thompson, L.K.; Tilman, D.; Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; et al. Ecosystem approaches to resistance transmission. Environ. Microbiol. 2023, 25, 890–903. [Google Scholar]
- Martinez-Gonzalez, E.; Lipsky, B.A.; Madaras-Kelly, K.; Neuhauser, M.M.; Ostrowsky, B.; Sakoulas, G.; Evans, S.R.; Chambers, H.F.; Corey, G.R.; Stryjewski, M.E.; et al. Alternative therapeutic strategies against AMR. Nat. Rev. Drug Discov. 2024, 23, 234–247. [Google Scholar]
- Anderson, R.M.; Segre, J.A.; Ley, R.E.; Gordon, J.I.; Knight, R.; Relman, D.A.; Round, J.L.; Mazmanian, S.K.; Turnbaugh, P.J.; Hooper, L.V.; et al. Microbiome manipulation in resistance prevention. Cell Host Microbe 2023, 31, 2345–2358. [Google Scholar]
- Kumar, V.; Blundell, T.L.; Baker, D.; Schreiber, S.L.; Wilson, I.A.; Shoichet, B.K.; Kuriyan, J.; Waksman, G.; Harrison, S.C.; Adams, J.A.; et al. Novel drug targets through structural biology. J. Med. Chem. 2024, 67, 567–580. [Google Scholar]
- Brown, J.S.; Michie, S.; West, R.; Gardner, B.; Weinman, J.; Nutbeam, D.; Cane, J.; Atkins, L.; Kelly, M.P.; Marteau, T.M.; et al. Behavioral aspects of antimicrobial use. Soc. Sci. Med. 2023, 316, 115789. [Google Scholar]
- WHO Expert Group. Future policy frameworks for AMR control. Bull. World Health Organ. 2024, 102, 234–246. [Google Scholar]
- Robertson, M.S.; Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W.; O’Brien, B.J.; Richardson, G.; Brazier, J.; Coast, J.; et al. Economic models for antimicrobial development. Health Econ. Rev. 2023, 13, 45–57. [Google Scholar]
- Chen, Y.F.; Peters, D.H.; Bloom, G.; Walker, D.G.; Briggs, A.; Rahman, M.H.; Balabanova, D.; Mills, A.; Hanson, K.; Gilson, L.; et al. Implementation challenges in resource-limited settings. Glob. Health Action 2024, 17, 2178943. [Google Scholar]
- Williams, P.L.; Heymann, D.L.; Piot, P.; Garrett, L.; Dybul, M.; El-Mohandes, A.; Farrar, J.; Ofori-Adjei, D.; Okello, G.; Kilmarx, P.H.; et al. Global coordination mechanisms for AMR control. Lancet Glob. Health 2023, 11, e789–e801. [Google Scholar]
- Harris, D.R.; Bates, D.W.; Kuperman, G.J.; Sittig, D.F.; Teich, J.M.; Zhang, J.; Patel, V.L.; Campbell, E.M.; Shortliffe, E.H.; Ash, J.S.; et al. Technology integration in healthcare systems. Implement. Sci. 2024, 19, 23–35. [Google Scholar]

| Pathogen | Resistance Mechanism | Prevalence (%) | High-Burden Regions | Last-Line Treatments |
|---|---|---|---|---|
| MRSA | Altered PBP (mecA) | 20–40% | Global (esp. USA, India) | Linezolid, Daptomycin |
| VRE | VanA/VanB gene clusters | 5–15% | Europe, Americas | Linezolid, Tigecycline |
| CRE | KPC, NDM, OXA-48 enzymes | 10–50% | Asia, Middle East, Europe | Colistin, Ceftazidime-avibactam |
| ESBL-producing E. coli/K. pneumoniae | CTX-M enzymes | 25–50% | Africa, South Asia | Carbapenems |
| P. aeruginosa, A. baumannii | Efflux pumps, OMP loss | 20–60% | Global (esp. ICUs) | Colistin, Cefiderocol |
| Candida auris | Multidrug resistance (azoles, amphotericin B) | Rising | All continents | Echinocandins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, A.; Maniaci, A.; Lentini, M.; Ronsivalle, S.; Nunnari, G.; Cocuzza, S.; Parisi, F.M.; Cacopardo, B.; Lavalle, S.; La Via, L. The Global Burden of Multidrug-Resistant Bacteria. Epidemiologia 2025, 6, 21. https://doi.org/10.3390/epidemiologia6020021
Marino A, Maniaci A, Lentini M, Ronsivalle S, Nunnari G, Cocuzza S, Parisi FM, Cacopardo B, Lavalle S, La Via L. The Global Burden of Multidrug-Resistant Bacteria. Epidemiologia. 2025; 6(2):21. https://doi.org/10.3390/epidemiologia6020021
Chicago/Turabian StyleMarino, Andrea, Antonino Maniaci, Mario Lentini, Salvatore Ronsivalle, Giuseppe Nunnari, Salvatore Cocuzza, Federica Maria Parisi, Bruno Cacopardo, Salvatore Lavalle, and Luigi La Via. 2025. "The Global Burden of Multidrug-Resistant Bacteria" Epidemiologia 6, no. 2: 21. https://doi.org/10.3390/epidemiologia6020021
APA StyleMarino, A., Maniaci, A., Lentini, M., Ronsivalle, S., Nunnari, G., Cocuzza, S., Parisi, F. M., Cacopardo, B., Lavalle, S., & La Via, L. (2025). The Global Burden of Multidrug-Resistant Bacteria. Epidemiologia, 6(2), 21. https://doi.org/10.3390/epidemiologia6020021












