Access to Blood Glucose Testing in Peru: Who Is Getting Tested?
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Data
2.2. Study Population
2.3. Outcome
2.4. Exposure
2.5. Covariates
2.6. Data Analysis
2.7. Ethical Considerations
2.8. Reporting Standards
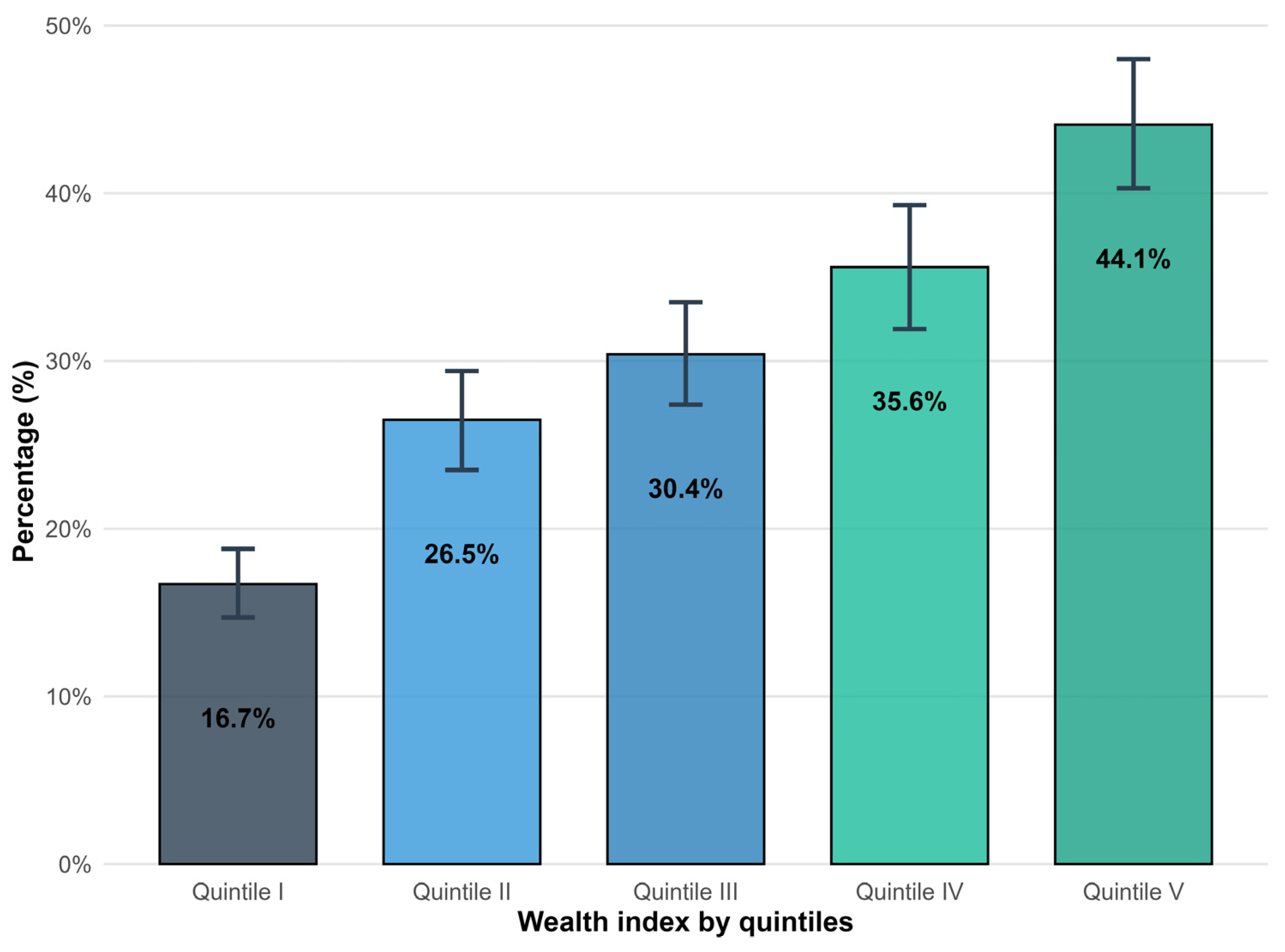
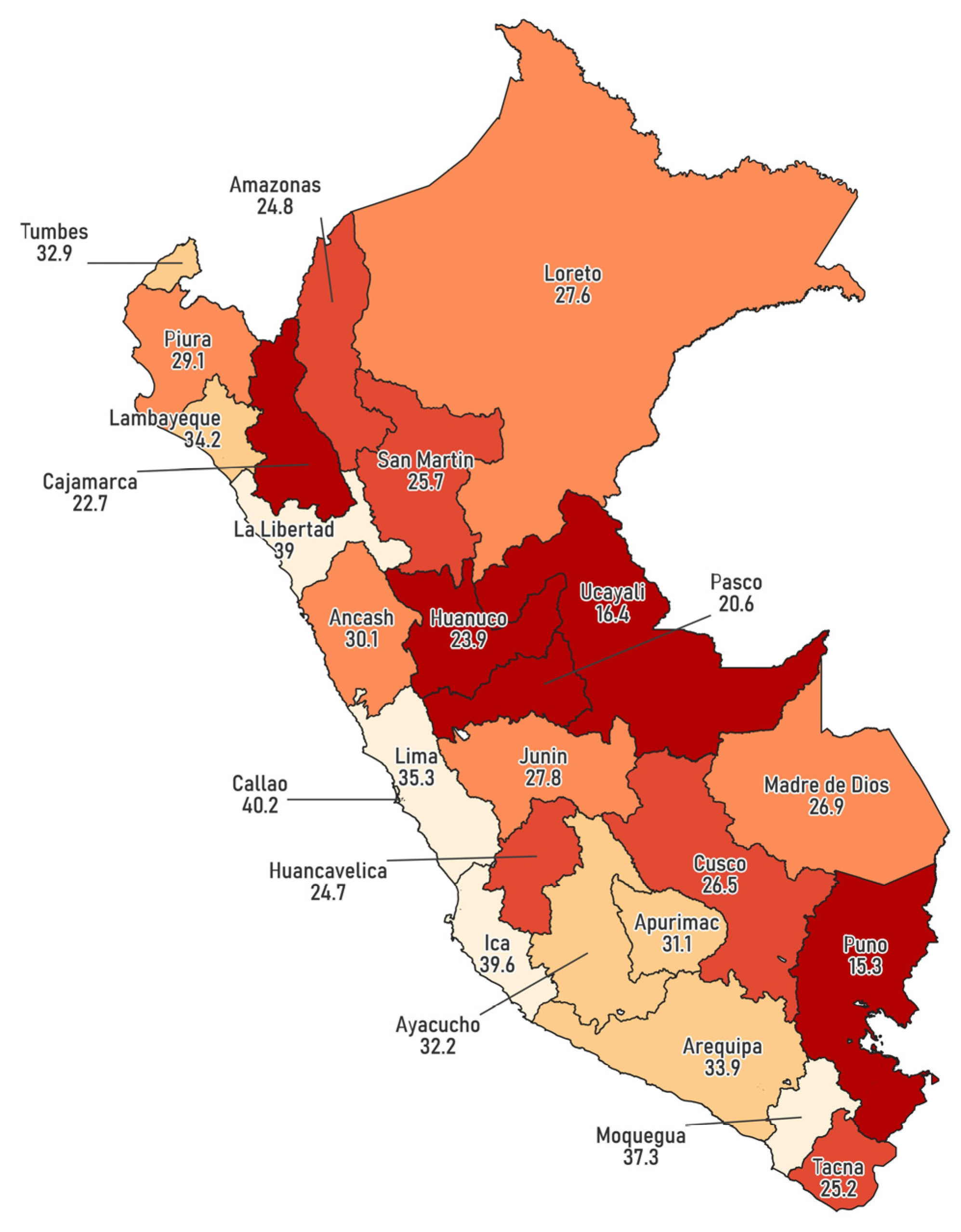
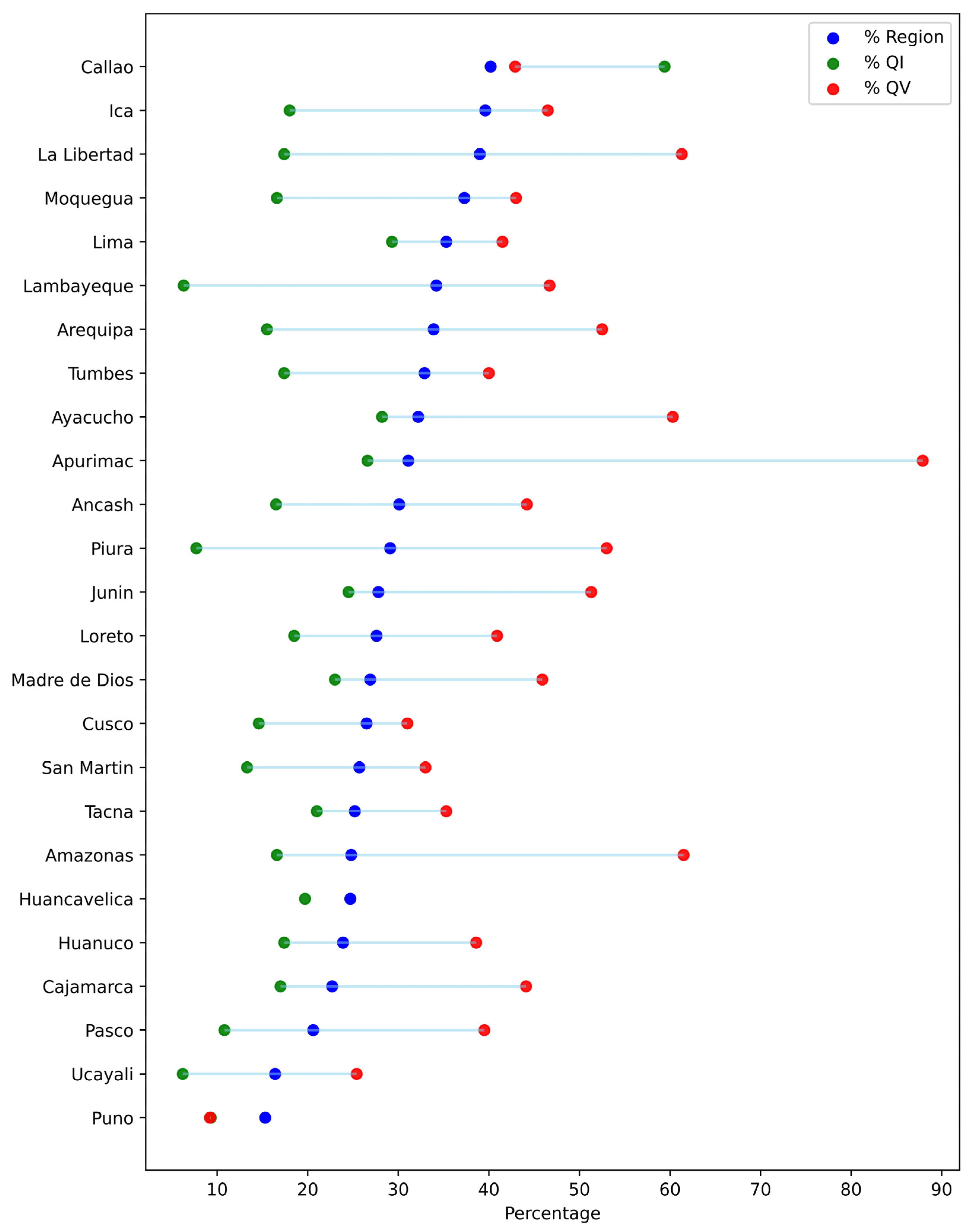
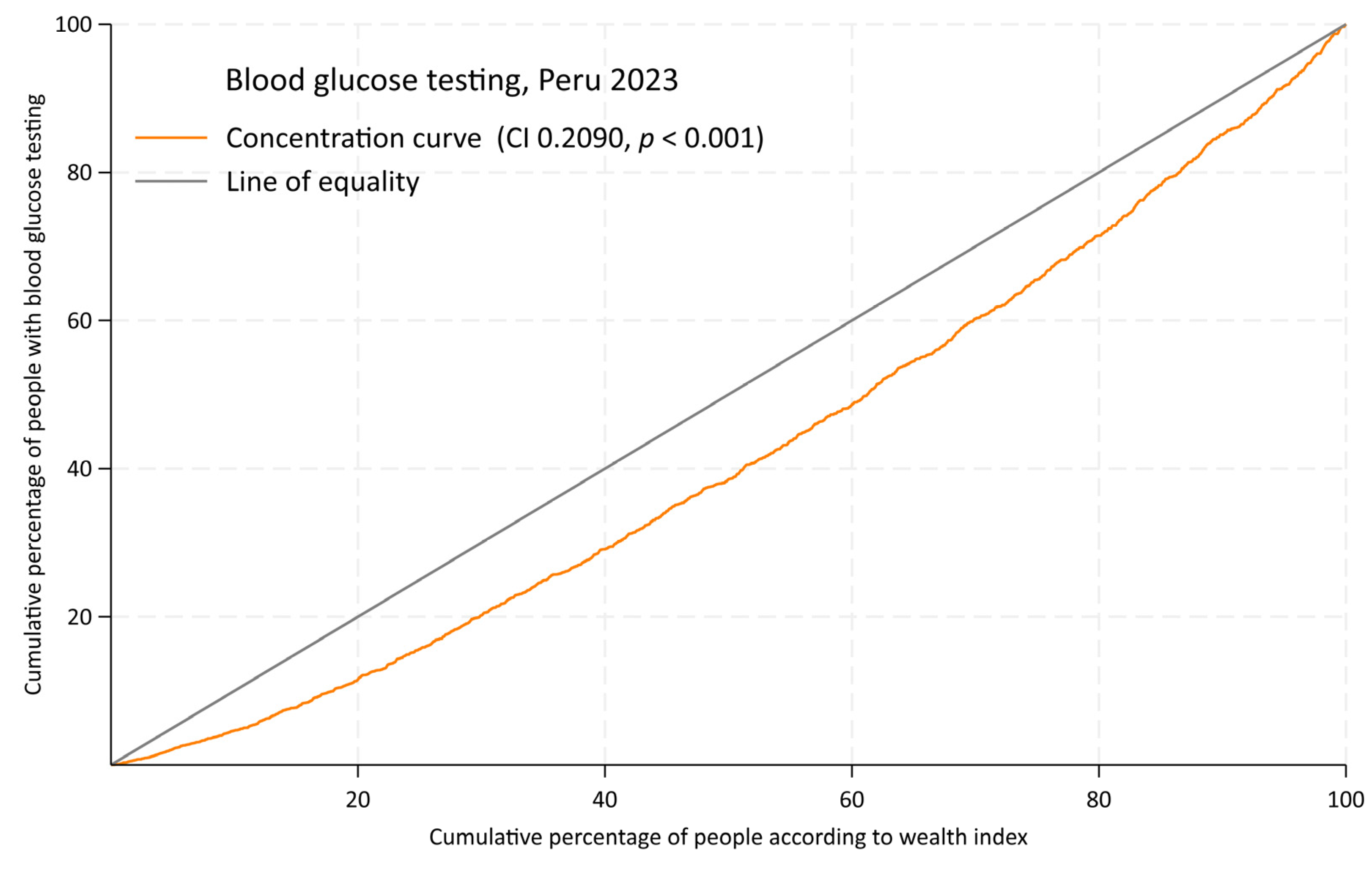
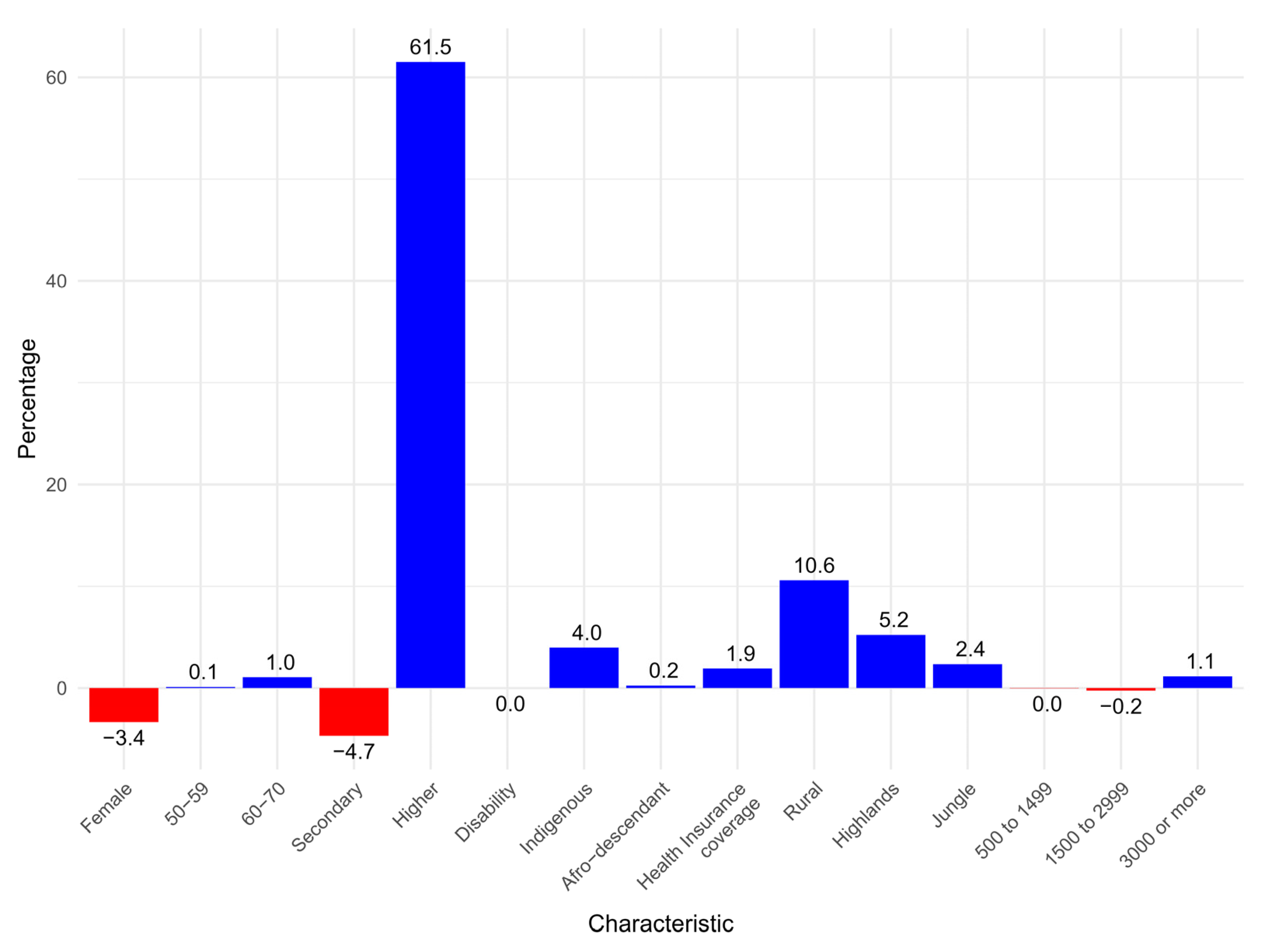
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhou, X.D.; Shapiro, M.D.; Lip, G.Y.H.; Tilg, H.; Valenti, L.; Somers, V.K.; Byrne, C.D.; Targher, G.; Yang, W.; et al. Global burden of metabolic diseases, 1990–2021. Metabolism 2024, 160, 155999. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022, 23, e13366. [Google Scholar] [CrossRef]
- Chande, A.T.; Rishishwar, L.; Conley, A.B.; Valderrama-Aguirre, A.; Medina-Rivas, M.A.; Jordan, I.K. Ancestry effects on type 2 diabetes genetic risk inference in Hispanic/Latino populations. BMC Med. Genet. 2020, 21, 132. [Google Scholar] [CrossRef]
- Ogurtsova, K.; Guariguata, L.; Barengo, N.C.; Ruiz, P.L.-D.; Sacre, J.W.; Karuranga, S.; Sun, H.; Boyko, E.J.; Magliano, D.J. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022, 183, 109118. [Google Scholar] [CrossRef]
- The Lancet Diabetes & Endocrinology. Undiagnosed type 2 diabetes: An invisible risk factor. Lancet Diabetes Endocrinol. 2024, 12, 215. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Davidson, K.W.; Barry, M.J.; Mangione, C.M.; Cabana, M.; Caughey, A.B.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Krist, A.H.; et al. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 326, 736. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, A. Type 2 diabetes and health care costs in Latin America: Exploring the need for greater preventive medicine. BMC Med. 2014, 12, 136. [Google Scholar] [CrossRef]
- Kaul, P.; Chu, L.M.; Dover, D.C.; Yeung, R.O.; Eurich, D.T.; Butalia, S. Disparities in adherence to diabetes screening guidelines among males and females in a universal care setting: A population-based study of 1,380,697 adults. Lancet Reg. Health-Am. 2022, 14, 100320. [Google Scholar] [CrossRef]
- Ricci-Cabello, I.; Ruiz-Pérez, I.; De Labry-Lima, A.O.; Márquez-Calderón, S. Do social inequalities exist in terms of the prevention, diagnosis, treatment, control and monitoring of diabetes? A systematic review: Social inequalities in health-care. Health Soc. Care Community 2010, 18, 572–587. [Google Scholar] [CrossRef]
- Ares-Blanco, S.; López-Rodríguez, J.A.; Vela, M.F.; Polentinos-Castro, E.; del Cura-González, I. Sex and income inequalities in preventive services in diabetes. Eur. J. Gen. Pract. 2023, 29, 2159941. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Esenarro, L.; Contreras Rojas, M.; Del Canto y Dorador, J.; Vílchez Dávila, W. Guía Técnica Para la Valoración Nutricional Antropométrica de la Persona Adulta Mayor; Ministerio de Salud, Instituto Nacional de Salud: Lima, Peru, 2013; pp. 1–50. Available online: http://bvs.minsa.gob.pe/local/MINSA/2858.pdf (accessed on 16 January 2025).
- World Health Organization. Manual de Vigilancia STEPS de la OMS: El Método STEPwise de la OMS Para la Vigilancia de los Factores de Riesgo de las Enfermedades Crónicas; World Health Organization: Geneva, Switzerland, 2006; Available online: https://iris.who.int/handle/10665/43580 (accessed on 25 January 2025).
- Rutstein, S.O.; Johnson, K. The DHS Wealth Index; ORC Macro: Calverton, MD, USA, 2004; Available online: http://www.measuredhs.com/pubs/pdf/CR6/CR6.pdf (accessed on 25 January 2025).
- O'Neill, J.; Tabish, H.; Welch, V.; Petticrew, M.; Pottie, K.; Clarke, M.; Evans, T.; Pardo, J.P.; Waters, E.; White, H.; et al. Applying an equity lens to interventions: Using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J. Clin. Epidemiol. 2014, 67, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Madans, J.H.; Loeb, M.E.; Altman, B.M. Measuring disability and monitoring the UN Convention on the Rights of Persons with Disabilities: Ehe work of the Washington Group on Disability Statistics. BMC Public Health 2011, 11, S4. [Google Scholar] [CrossRef]
- Rao, J.N.K.; Scott, A.J. The Analysis of Categorical Data from Complex Sample Surveys: Chi-Squared Tests for Goodness of Fit and Independence in Two-Way Tables. J. Am. Stat. Assoc. 1981, 76, 221. [Google Scholar] [CrossRef]
- Wagstaff, A.; Van Doorslaer, E. Measuring inequalities in health in the presence of multiple-category morbidity indicators. Health Econ. 1994, 3, 281–289. [Google Scholar] [CrossRef]
- O’Donnell, O.; Van Doorslaer, E.; Wagstaff, A.; Lindelow, M. Analyzing Health Equity Using Household Survey Data: A Guide to Techniques and Their Implementation; The World Bank: Washington, DC, USA, 2007; Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/633931468139502235/analyzing-health-equity-using-household-survey-data-a-guide-to-techniques-and-their-implementation (accessed on 15 January 2025).
- Erreygers, G. Correcting the Concentration Index. J. Health Econ. 2009, 28, 504–515. [Google Scholar] [CrossRef]
- Koolman, X.; van Doorslaer, E. On the interpretation of a concentration index of inequality. Health Econ. 2004, 13, 649–656. [Google Scholar] [CrossRef]
- Yiengprugsawan, V.; Lim, L.L.; Carmichael, G.A.; Dear, K.B.; Sleigh, A.C. Decomposing socioeconomic inequality for binary health outcomes: An improved estimation that does not vary by choice of reference group. BMC Res. Notes 2010, 3, 57. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Ngene, N.C.; Khaliq, O.P.; Moodley, J. Inequality in health care services in urban and rural settings in South Africa. Afr. J. Reprod. Health 2023, 27, 87–95. [Google Scholar]
- Dawkins, B.; Renwick, C.; Ensor, T.; Shinkins, B.; Jayne, D.; Meads, D. What factors affect patients’ ability to access healthcare? An overview of systematic reviews. Trop. Med. Int. Health 2021, 26, 1177–1188. [Google Scholar] [CrossRef]
- Calderón, M.; Alvarado-Villacorta, R.; Barrios, M.; Quiroz-Robladillo, D.; Naupay, D.R.G.; Obregon, A.; Chávez, S.C.; Glaser, L.; Carnero, A.M.; Cortez-Vergara, C.; et al. Health need assessment in an indigenous high-altitude population living on an island in Lake Titicaca, Perú. Int. J. Equity Health 2019, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Perry, B.; Gesler, W. Physical access to primary health care in Andean Bolivia. Soc. Sci. Med. 2000, 50, 1177–1188. [Google Scholar] [CrossRef]
- Ministerio de Salud. Análisis de Situación de Salud del Perú, 2021; Centro Nacional de Epidemiología, Prevención y Control de Enfermedades: Lima, Peru, March 2023; Available online: https://bvs.minsa.gob.pe/local/MINSA/6279.pdf (accessed on 16 January 2025).
- Carrillo-Larco, R.M.; Guzman-Vilca, W.C.; Leon-Velarde, F.; Bernabe-Ortiz, A.; Jimenez, M.M.; Penny, M.E.; Gianella, C.; Leguía, M.; Tsukayama, P.; Hartinger, S.M.; et al. Peru—Progress in health and sciences in 200 years of independence. Lancet Reg. Health-Am. 2022, 7, 100148. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.K.; Lim, H.K. Socioeconomic and lifestyle determinants of blood glucose screening in Malaysia. Asian Biomed. 2017, 9, 683–690. [Google Scholar] [CrossRef]
- Sharma, S.K.; Nambiar, D.; Sankar, H.; Joseph, J.; Surendran, S.; Benny, G. Decomposing socioeconomic inequality in blood pressure and blood glucose testing: Evidence from four districts in Kerala, India. Int. J. Equity Health 2022, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Abuduxike, G.; Aşut, Ö.; Vaizoğlu, S.A.; Cali, S. Health-Seeking Behaviors and its Determinants: A Facility-Based Cross-Sectional Study in the Turkish Republic of Northern Cyprus. Int. J. Health Policy Manag. 2020, 9, 240–249. [Google Scholar] [CrossRef]
- Pinkhasov, R.M.; Wong, J.; Kashanian, J.; Lee, M.; Samadi, D.B.; Pinkhasov, M.M.; Shabsigh, R. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int. J. Clin. Pract. 2010, 64, 475–487. [Google Scholar] [CrossRef]
- Anagaw, T.F.; Mazengia, E.M.; Bogale, E.K.; Fenta, E.T.; Eshetu, H.B.; Kebede, N.; Gelaw, S.S.; Zewdie, A.; Kassie, T.D. Health-seeking behavior among non-communicable disease patients globally, systematic review and meta-analysis. Sage Open Med. 2023, 11, 20503121231215236. [Google Scholar] [CrossRef]
- Viera, A.J.; Thorpe, J.M.; Garrett, J.M. Effects of sex, age, and visits on receipt of preventive healthcare services: A secondary analysis of national data. BMC Health Serv. Res. 2006, 6, 15. [Google Scholar] [CrossRef]
- Vaidya, V.; Partha, G.; Karmakar, M. Gender Differences in Utilization of Preventive Care Services in the United States. J. Women's Health 2012, 21, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Longarzo, M.; Mele, G.; Alfano, V.; Salvatore, M.; Cavaliere, C. Gender Brain Structural Differences and Interoception. Front. Neurosci. 2021, 14, 586860. [Google Scholar] [CrossRef] [PubMed]
- Grabauskaitė, A.; Baranauskas, M.; Griškova-Bulanova, I. Interoception and gender: What aspects should we pay attention to? Conscious Cogn. 2017, 48, 129–137. [Google Scholar] [CrossRef]
- Addis, M.E.; Mahalik, J.R. Men, masculinity, and the contexts of help seeking. Am. Psychol. 2003, 58, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.; Guthold, R.; Cowan, M.; Savin, S.; Bhatti, L.; Armstrong, T.; Bonita, R. The World Health Organization STEPwise Approach to Noncommunicable Disease Risk-Factor Surveillance: Methods, Challenges, and Opportunities. Am. J. Public Health 2016, 106, 74–78. [Google Scholar] [CrossRef]
- Campos Briceño, G.R.; Condor Iturrizaga, R.M. La etnicidad en el Perú y su naturaleza multidimensional: Una propuesta de medición. Desde El Sur. 2022, 14, e0012. [Google Scholar] [CrossRef]
- Schneider, A.L.C.; Pankow, J.S.; Heiss, G.; Selvin, E. Validity and Reliability of Self-reported Diabetes in the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2012, 176, 738–743. [Google Scholar] [CrossRef]
- Guo, H.; Yu, Y.; Ye, Y.; Zhou, S. Accuracy of Self-Reported Hypertension, Diabetes, and Hyperlipidemia among Adults of Liwan, Guangzhou, China. Iran. J. Public Health 2020, 49, 1622–1630. [Google Scholar] [CrossRef]
| Characteristic | n | % Weighted |
|---|---|---|
| Gender | ||
| Male | 4007 | 46.4 |
| Female | 5492 | 53.6 |
| Age groups (years) | ||
| 35–49 | 6472 | 59.4 |
| 50–59 | 2116 | 29.0 |
| 60–70 | 911 | 11.6 |
| Education level | ||
| Up to primary | 2773 | 22.5 |
| Secondary | 3882 | 42.2 |
| Higher | 2844 | 35.3 |
| Disability | ||
| No | 9395 | 98.7 |
| Yes | 104 | 1.3 |
| Self-identified ethnicity | ||
| Others | 4962 | 60.6 |
| Indigenous | 3551 | 28.1 |
| Afro-descendant | 986 | 11.3 |
| Health insurance coverage | ||
| No | 1201 | 15.3 |
| Yes | 8298 | 84.7 |
| Area of residence | ||
| Urban | 6483 | 85.1 |
| Rural | 3016 | 14.9 |
| Geographic region | ||
| Coast | 4063 | 65.8 |
| Highlands | 3240 | 22.5 |
| Jungle | 2196 | 11.7 |
| Residence altitude (m.a.s.l.) | ||
| 0 to 499 | 4903 | 68.6 |
| 500 to 1499 | 1135 | 7.9 |
| 1500 to 2999 | 1494 | 10.7 |
| 3000 or more | 1967 | 12.8 |
| Blood Glucose Testing | |||||
|---|---|---|---|---|---|
| No | Yes | ||||
| Characteristic | % Weighted | (95% CI) | % Weighted | (95% CI) | p-Value |
| Gender | |||||
| Male | 70.4 | (68.2–72.5) | 29.6 | (27.5–31.8) | 0.005 |
| Female | 65.9 | (63.7–68.1) | 34.1 | (31.9–36.3) | |
| Age groups (years) | |||||
| 35–49 | 69.9 | (68.0–71.6) | 30.1 | (28.4–32.0) | 0.001 |
| 50–59 | 67.4 | (64.0–70.7) | 32.6 | (29.3–36.0) | |
| 60–70 | 59.6 | (54.6–64.5) | 40.4 | (35.5–45.4) | |
| Education level | |||||
| Up to primary | 77.4 | (74.8–79.9) | 22.6 | (20.1–25.2) | <0.001 |
| Secondary | 71.7 | (69.3–74.0) | 28.3 | (26.0–30.7) | |
| Higher | 57.5 | (54.7–60.2) | 42.5 | (39.8–45.3) | |
| Disability | |||||
| No | 68.0 | (66.4–69.5) | 32.0 | (30.5–33.6) | 0.910 |
| Yes | 68.7 | (54.2–80.4) | 31.3 | (19.6–45.8) | |
| Self-identified ethnicity | |||||
| Others | 64.8 | (62.7–66.9) | 35.2 | (33.1–37.3) | <0.001 |
| Indigenous | 73.3 | (70.7–75.7) | 26.7 | (24.3–29.3) | |
| Afro-descendant | 71.9 | (67.8–75.6) | 28.1 | (24.4–32.2) | |
| Health insurance coverage | |||||
| No | 83.0 | (79.1–86.3) | 17.0 | (13.7–20.9) | <0.001 |
| Yes | 65.3 | (63.6–66.9) | 34.7 | (33.1–36.4) | |
| Area of residence | |||||
| Urban | 65.7 | (63.9–67.4) | 34.3 | (32.6–36.1) | <0.001 |
| Rural | 80.9 | (79.1–82.6) | 19.1 | (17.4–20.9) | |
| Geographic region | |||||
| Coast | 64.7 | (62.5–66.8) | 35.3 | (33.2–37.5) | <0.001 |
| Highlands | 74.4 | (72.2–76.5) | 25.6 | (23.5–27.8) | |
| Jungle | 74.2 | (71.9–76.3) | 25.8 | (23.7–28.1) | |
| Residence altitude (m.a.s.l.) | |||||
| 0 to 499 | 65.3 | (63.2–67.3) | 34.7 | (32.7–36.8) | <0.001 |
| 500 to 1499 | 73.2 | (69.2–76.8) | 26.8 | (23.2–30.8) | |
| 1500 to 2999 | 71.1 | (67.8–74.2) | 28.9 | (25.8–32.2) | |
| 3000 or more | 76.8 | (73.9–79.4) | 23.2 | (20.6–26.1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra Valencia, J.; Hernández-Vásquez, A.; Rojas-Roque, C.; Vargas-Fernández, R. Access to Blood Glucose Testing in Peru: Who Is Getting Tested? Epidemiologia 2025, 6, 20. https://doi.org/10.3390/epidemiologia6020020
Guerra Valencia J, Hernández-Vásquez A, Rojas-Roque C, Vargas-Fernández R. Access to Blood Glucose Testing in Peru: Who Is Getting Tested? Epidemiologia. 2025; 6(2):20. https://doi.org/10.3390/epidemiologia6020020
Chicago/Turabian StyleGuerra Valencia, Jamee, Akram Hernández-Vásquez, Carlos Rojas-Roque, and Rodrigo Vargas-Fernández. 2025. "Access to Blood Glucose Testing in Peru: Who Is Getting Tested?" Epidemiologia 6, no. 2: 20. https://doi.org/10.3390/epidemiologia6020020
APA StyleGuerra Valencia, J., Hernández-Vásquez, A., Rojas-Roque, C., & Vargas-Fernández, R. (2025). Access to Blood Glucose Testing in Peru: Who Is Getting Tested? Epidemiologia, 6(2), 20. https://doi.org/10.3390/epidemiologia6020020







