Co-Infections and Their Prognostic Impact on Melioidosis Mortality: A Systematic Review and Individual Patient Data Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction Process and Outcome
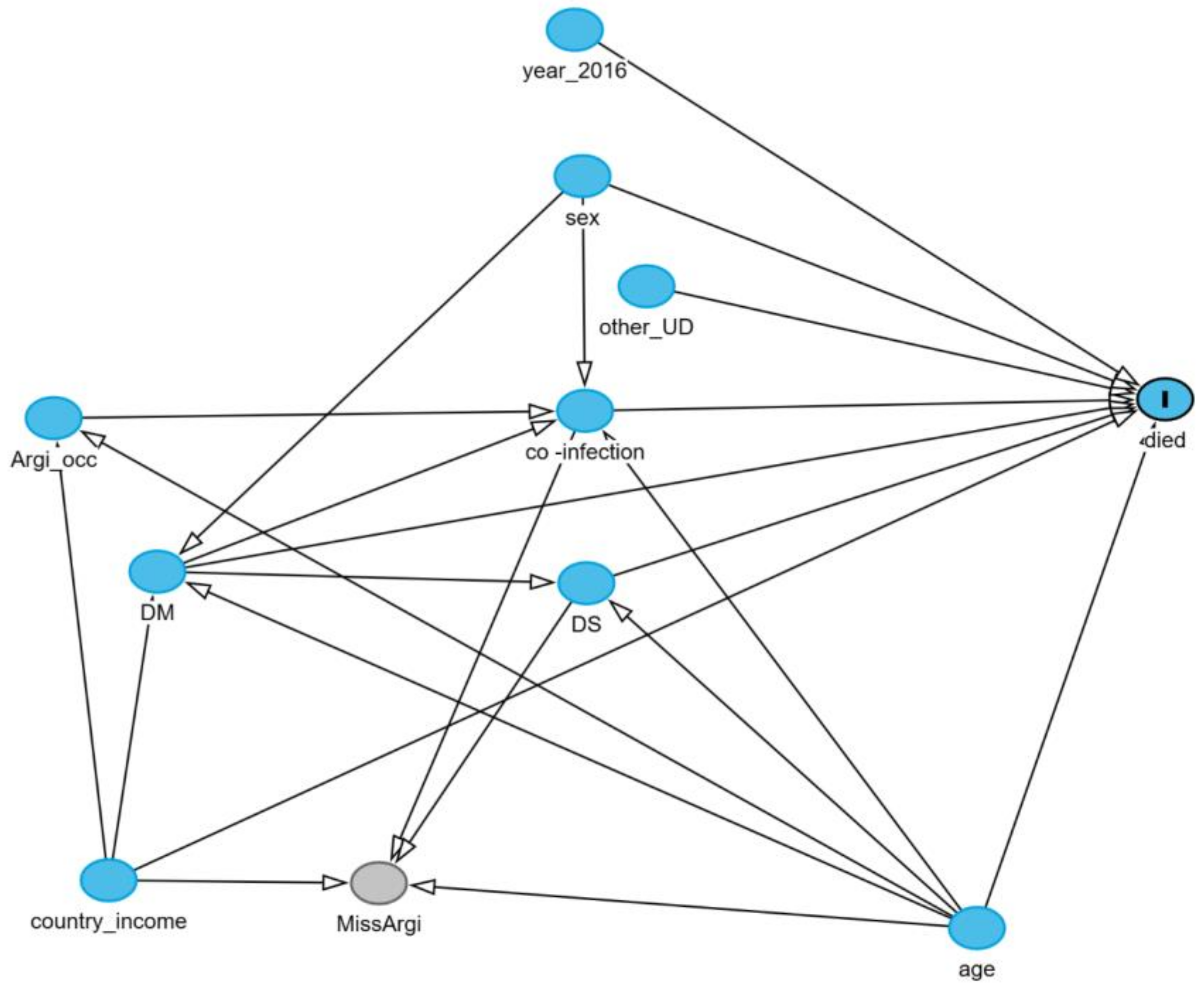
2.5. Conceptual Causal Diagram
2.6. Statistical Analysis
3. Results
3.1. Results of the Search
3.2. Study Characteristics
3.3. Patient Characteristics
3.4. Missing Data
3.5. Causal Associations Between Prognostic Factors and Death
3.5.1. Complete Case Analysis
3.5.2. Multiple Imputation Sensitivity Analysis
3.5.3. Post Hoc Sensitivity Analysis for Only Evidential Support Causal Diagram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, A.C.; Currie, B.J. Melioidosis: Epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 2005, 18, 383–416. [Google Scholar] [PubMed]
- Meumann, E.M.; Limmathurotsakul, D.; Dunachie, S.J.; Wiersinga, W.J.; Currie, B.J. Burkholderia pseudomallei and melioidosis. Nat. Rev. Microbiol. 2024, 22, 155–169. [Google Scholar] [CrossRef]
- Shetty, A.K.; Boloor, R.; Sharma, V.; Bhat, G.H. Melioidosis and pulmonary tuberculosis co-infection in a diabetic. Ann. Thorac. Med. 2010, 5, 113–115. [Google Scholar]
- Tan, S.Y. Tuberculosis and Melioidosis at Distinct Sites Occurring Simultaneously. Case Rep. Infect. Dis. 2020, 2020, 9818129. [Google Scholar]
- Gunasena, J.B.; De Silva, S.T. Double-trouble: A rare case of co-infection with melioidosis and leptospirosis from Sri Lanka. Trop. Dr. 2023, 53, 332–337. [Google Scholar]
- Mohd Ali, M.R.; Mohamad Safiee, A.W.; Thangarajah, P.; Fauzi, M.H.; Muhd Besari, A.; Ismail, N.; Yean Yean, C. Molecular detection of leptospirosis and melioidosis co-infection: A case report. J. Infect. Public Health 2017, 10, 894–896. [Google Scholar] [CrossRef]
- Chierakul, W.; Wuthiekanun, V.; Chaowagul, W.; Amornchai, P.; Cheng, A.C.; White, N.J.; Day, N.P.; Peacock, S.J. Short report: Disease severity and outcome of melioidosis in HIV coinfected individuals. Am. J. Trop. Med. Hyg. 2005, 73, 1165–1166. [Google Scholar]
- Koh, G.C.; Schreiber, M.F.; Bautista, R.; Maude, R.R.; Dunachie, S.; Limmathurotsakul, D.; Day, N.P.; Dougan, G.; Peacock, S.J. Host responses to melioidosis and tuberculosis are both dominated by interferon-mediated signaling. PLoS ONE 2013, 8, e54961. [Google Scholar]
- Limmathurotsakul, D.; Peacock, S.J. Melioidosis: A clinical overview. Br. Med. Bull. 2011, 99, 125–139. [Google Scholar]
- Hin, H.S.; Ramalingam, R.; Chunn, K.Y.; Ahmad, N.; Ab Rahman, J.; Mohamed, M.S. Fatal co-infection-melioidosis and leptospirosis. Am. J. Trop. Med. Hyg. 2012, 87, 737–740. [Google Scholar] [CrossRef]
- Suputtamongkol, Y.; Chaowagul, W.; Chetchotisakd, P.; Lertpatanasuwun, N.; Intaranongpai, S.; Ruchutrakool, T.; Budhsarawong, D.; Mootsikapun, P.; Wuthiekanun, V.; Teerawatasook, N.; et al. Risk factors for melioidosis and bacteremic melioidosis. Clin. Infect. Dis. 1999, 29, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Textor, J.; Hardt, J.; Knüppel, S. DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology 2011, 22, 745. [Google Scholar] [PubMed]
- Carpenter, J.R.; Smuk, M. Missing data: A statistical framework for practice. Biometrical journal. Biom. Z. 2021, 63, 915–947. [Google Scholar]
- Hughes, R.A.; Heron, J.; Sterne, J.A.C.; Tilling, K. Accounting for missing data in statistical analyses: Multiple imputation is not always the answer. Int. J. Epidemiol. 2019, 48, 1294–1304. [Google Scholar] [CrossRef]
- van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 2007, 16, 219–242. [Google Scholar]
- Azri, M.; Ismail, I.H. First documented co-infection case of cat-scratch disease and melioidosis in Malaysia: A cause of undifferentiated prolonged febrile illness. Med. J. Malays. 2022, 77, 255–257. [Google Scholar]
- Samad, I.; Wang, M.C.; Chong, V.H. Intracerebral coinfection with Burkholderia pseudomallei and Cryptococcus neoformans in a patient with systemic lupus erythematosus. Southeast Asian J. Trop. Med. Public Health 2014, 45, 352–356. [Google Scholar]
- Pongrithsukda, V.; Simakachorn, N.; Pimda, J. Childhood melioidosis in northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 1988, 19, 309–316. [Google Scholar]
- Macedo, R.N.; Rocha, F.A.; Rolim, D.B.; Vilar, D.C.; Araújo, F.M.; Vieira, N.N.; Teixeira, J.R.; Carvalho, M.C.; Oliveira, F.G.; Cavalcanti, L.P. Severe coinfection of melioidosis and dengue fever in Northeastern Brazil: First case report. Rev. Da Soc. Bras. De Med. Trop. 2012, 45, 132–133. [Google Scholar]
- Thongton, W.; Salee, P.; Nuntachit, N. Melioidosis in HIV and HCV co-infected patient: A case report. J. Infect. Dis. Antimicrob. Agents 2019, 36, 41–44. [Google Scholar]
- Pumpradit, W.; Ariyoshi, K.; Petkanchanapong, W.; Wichukchinda, N.; Chaiprasert, A.; Rojanawat, A.; Sawanpanyalert, P.; Pathipvanich, P. Mycobacterium avium and Burkholderia pseudomallei (Melioidosis) coinfection in an HIV-positive patient. Asian Pac. J. Allergy Immunol. 2006, 24, 239–243. [Google Scholar] [PubMed]
- Gulati, U.; Nanduri, A.C.; Juneja, P.; Kaufman, D.; Elrod, M.G.; Kolton, C.B.; Gee, J.E.; Garafalo, K.; Blaney, D.D. Case Report: A Fatal Case of Latent Melioidosis Activated by COVID-19. Am. J. Trop. Med. Hyg. 2022, 106, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Ke, B.X.; Chen, C.N.; Xiao, H.L.; Liu, M.Z.; Xiong, Y.C.; Bai, R.; Chen, J.D.; Ke, C.W. First co-infection case of melioidosis and Japanese encephalitis in China. BMC Infect. Dis. 2018, 18, 452. [Google Scholar] [CrossRef]
- Kahandawaarachchi, I.C.I.; Premawansa, G.S.; Warnasuriya, W.; Dassanayake, M.; Corea, E. A case report of co-infection of Melioidosis and cutaneous Leishmaniasis. BMC Infect. Dis. 2017, 17, 533. [Google Scholar] [CrossRef]
- Lim, K.; Shukeri, W.; Mazlan, M.; Rafiqi, M.; Abidin, H. Fulminant septic shock from melioidosis and leptospirosis co-infections. Anaesth. Pain Intensive Care 2022, 26, 257–259. [Google Scholar] [CrossRef]
- Sapian, M.; Khair, M.T.; How, S.H.; Rajalingam, R.; Sahhir, K.; Norazah, A.; Khebir, V.; Jamalludin, A.R. Outbreak of melioidosis and leptospirosis co-infection following a rescue operation. Med. J. Malays. 2012, 67, 293–297. [Google Scholar]
- Chetchotisakd, P.; Mootsikapun, P.; Anunnatsiri, S.; Jirarattanapochai, K.; Choonhakarn, C.; Chaiprasert, A.; Ubol, P.N.; Wheat, L.J.; Davis, T.E. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: A previously unrecognized clinical entity. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2000, 30, 29–34. [Google Scholar] [CrossRef]
- Azali, H.Y.; Norly, S.; Wong, L.M.; Tan, K.S.; Safian, N.M. Liver abscess caused by tuberculosis and melioidosis. Asian J. Surg. 2007, 30, 138–140. [Google Scholar] [CrossRef]
- Garg, R.; Shaw, T.; Vandana, K.E.; Magazine, R.; Mukhopadhyay, C. Melioidosis in Suspected Recurrent Tuberculosis: A disease in disguise. J. Infect. Dev. Ctries. 2020, 14, 312–316. [Google Scholar] [CrossRef]
- Kim, S.W.; Kwon, G.-Y.; Kim, B.; Kwon, D.; Shin, J.; Bae, G.-R. Imported Melioidosis in South Korea: A Case Series with a Literature Review. Osong Public Health Res. Perspect. 2015, 6, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Rubel, A.R.; Mani, B.I.; Kishore, P.V.; Chong, V.H. Pulmonary tuberculosis and melioidosis coinfection in Brunei Darussalam: The importance of awareness and screening. West. Pac. Surveill. Response J. WPSAR 2022, 13, 1–6. [Google Scholar] [PubMed]
- Shenoy, V.; Kamath, M.P.; Hegde, M.C.; D’Souza, T.; Mammen, S.S. Melioidosis and tuberculosis: Dual pathogens in a neck abscess. J. Laryngol. Otol. 2009, 123, 1285–1287. [Google Scholar] [PubMed]
- Sulaiman, H.; Ponnampalavanar, S.; Mun, K.S.; Italiano, C.M. Cervical abscesses due to co-infection with Burkholderia pseudomallei, Salmonella enterica serovar Stanley and Mycobacterium tuberculosis in a patient with diabetes mellitus. BMC Infect. Dis. 2013, 13, 527. [Google Scholar] [CrossRef]
- Chit Yee, D.; Aung, H.; Mg Mg, B.; Htun, W.; Janurian, N.; Bancone, G.; Watthanaworawit, W.; Proux, S.; Pyae Phyo, A.; Nosten, F. Case Report: A case report of multiple co-infections (melioidosis, paragonimiasis, COVID-19 and tuberculosis) in a patient with diabetes mellitus and thalassemia-trait in Myanmar [version 2; peer review: 1 approved, 1 approved with reservations]. Wellcome Open Res. 2022, 7, 160. [Google Scholar]
- Yuhana, M.Y.; Tanganuchitcharnchai, A.; Sujariyakul, P.; Sonthayanon, P.; Chotivanich, K.; Paris, D.H.; Pukrittayakamee, S.; Blacksell, S.D.; Hanboonkunupakarn, B. Melioidosis and scrub typhus co-infection in a patient presenting with acute undifferentiated febrile illness. JKKI J. Kedokt. Dan Kesehat. Indones. 2019, 10, 86–90. [Google Scholar] [CrossRef]
- Lu, P.L.; Tseng, S.H. Fatal septicemic melioidosis in a young military person possibly co-infected with Leptospira interrogans and Orientia tsutsugamushi. Kaohsiung J. Med. Sci. 2005, 21, 173–178. [Google Scholar]
- Pontes, D.G.; Ribeir, A.J.V.; Filho, J.H.M.; Freita, Â.E.d.H.A.; Gomes, V.C.C. An adolescent with type 1 diabetes and coinfection with melioidosis and COVID-19: A case report. Residência Pediátrica 2023, 13, 1296. [Google Scholar]
- Tong, T.K.; Kuang, C.Y.; Gani, Y.M.; Heng, B.S.L.; Zaini, A.B.B.; Abdullah, S.; Chidambaran, S.K. COVID-19 with melioidosis and cutaneous mucormycosis—A case report. Med. J. Malays. (MJM) Case Rep. 2022, 1, 24–27. [Google Scholar]
- Hemarajata, P.; Baghdadi, J.D.; Hoffman, R.; Humphries, R.M. Burkholderia pseudomallei: Challenges for the Clinical Microbiology Laboratory. J. Clin. Microbiol. 2016, 54, 2866–2873. [Google Scholar] [CrossRef]
- Jain, M.; Ratna, H.V.K.; Mohanty, S.; Padhi, S.; Tripathy, S. Coinfection of Melioidosis and Tuberculosis Causing Infective Lumbar Spondylodiscitis: A Rare Case Report. JBJS Case Connect. 2023, 13, e22.00770. [Google Scholar]
- Jin, J.L.; Ning, Y.X. Septicemic melioidosis: A case report and literature review. J. Thorac. Dis. 2014, 6, E1–E4. [Google Scholar] [PubMed]
- Leelarasamee, A. Burkholderia pseudomallei: The unbeatable foe? Southeast Asian J. Trop. Med. Public Health 1998, 29, 410–415. [Google Scholar] [PubMed]
- Cagliero, J.; Villanueva, S.Y.A.M.; Matsui, M. Leptospirosis Pathophysiology: Into the Storm of Cytokines. Front. Cell. Infect. Microbiol. 2018, 8, 204. [Google Scholar]
- Tang, X.D.; Ji, T.T.; Dong, J.R.; Feng, H.; Chen, F.Q.; Chen, X.; Zhao, H.Y.; Chen, D.K.; Ma, W.T. Pathogenesis and Treatment of Cytokine Storm Induced by Infectious Diseases. Int. J. Mol. Sci. 2021, 22, 13009. [Google Scholar] [CrossRef]
- Lebeau, G.; Vagner, D.; Frumence, É.; Ah-Pine, F.; Guillot, X.; Nobécourt, E.; Raffray, L.; Gasque, P. Deciphering SARS-CoV-2 Virologic and Immunologic Features. Int. J. Mol. Sci. 2020, 21, 5932. [Google Scholar] [CrossRef]
- Moghaddam, M.M.; Behzadi, E.; Sedighian, H.; Goleij, Z.; Kachuei, R.; Heiat, M.; Fooladi, A.A.I. Regulation of immune responses to infection through interaction between stem cell-derived exosomes and toll-like receptors mediated by microRNA cargoes. Front. Cell. Infect. Microbiol. 2024, 14, 1384420. [Google Scholar]
- Krishnananthasivam, S.; Sathkumara, H.D.; Corea, E.; Natesan, M.; De Silva, A.D. Gene Expression Profile of Human Cytokines in Response to Burkholderia pseudomallei Infection. mSphere 2017, 2, e00121-17. [Google Scholar]
- Pan, J.; Zhang, X.; Xu, J.; Chang, Z.; Xin, Z.; Wang, G. Landscape of Exhausted T Cells in Tuberculosis Revealed by Single-Cell Sequencing. Microbiol. Spectr. 2023, 11, e0283922. [Google Scholar]
- Yang, T.Y.; Lin, C.L.; Yao, W.C.; Lio, C.F.; Chiang, W.P.; Lin, K.; Kuo, C.F.; Tsai, S.Y. How mycobacterium tuberculosis infection could lead to the increasing risks of chronic fatigue syndrome and the potential immunological effects: A population-based retrospective cohort study. J. Transl. Med. 2022, 20, 99. [Google Scholar]
- Monack, D.M.; Mueller, A.; Falkow, S. Persistent bacterial infections: The interface of the pathogen and the host immune system. Nat. Rev. Microbiol. 2004, 2, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Dunachie, S.J.; Jenjaroen, K.; Reynolds, C.J.; Quigley, K.J.; Sergeant, R.; Sumonwiriya, M.; Chaichana, P.; Chumseng, S.; Ariyaprasert, P.; Lassaux, P.; et al. Infection with Burkholderia pseudomallei—Immune correlates of survival in acute melioidosis. Sci. Rep. 2017, 7, 12143. [Google Scholar] [CrossRef]
- Serdar, C.C.; Cihan, M.; Yücel, D.; Serdar, M.A. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Med. 2021, 31, 010502. [Google Scholar] [CrossRef] [PubMed]
- Silva Miranda, M.; Breiman, A.; Allain, S.; Deknuydt, F.; Altare, F. The tuberculous granuloma: An unsuccessful host defence mechanism providing a safety shelter for the bacteria? Clin. Dev. Immunol. 2012, 2012, 139127. [Google Scholar] [CrossRef]
- Ashenafi, S.; Muvva, J.R.; Mily, A.; Snäll, J.; Zewdie, M.; Chanyalew, M.; Rehn, A.; Rahman, S.; Aseffa, G.; Bekele, A.; et al. Immunosuppressive Features of the Microenvironment in Lymph Nodes Granulomas from Tuberculosis and HIV-Co-Infected Patients. Am. J. Pathol. 2022, 192, 653–670. [Google Scholar]
- Shanmuganathan, G.; Orujyan, D.; Narinyan, W.; Poladian, N.; Dhama, S.; Parthasarathy, A.; Ha, A.; Tran, D.; Velpuri, P.; Nguyen, K.H.; et al. Role of Interferons in Mycobacterium tuberculosis Infection. Clin. Pract. 2022, 12, 788–796. [Google Scholar] [CrossRef]
- Flynn, J.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar]
- Huaman, M.A.; Sterling, T.R. Treatment of Latent Tuberculosis Infection-An Update. Clin. Chest Med. 2019, 40, 839–848. [Google Scholar]
- Panigrahi, M.K.; Bal, S.K.; Tripathy, T.P.; Moorthy, A.; Mohanty, S.K.; Mahapatra, A.; Bhuniya, S. Leptospirosis and melioidosis coinfection presenting as acute respiratory distress syndrome and osteomyelitis: Case report and systematic review. J. Infect. Dev. Ctries. 2024, 18, 1301–1307. [Google Scholar]
- Sun, A.H.; Liu, X.X.; Yan, J. Leptospirosis is an invasive infectious and systemic inflammatory disease. Biomed. J. 2020, 43, 24–31. [Google Scholar] [CrossRef]
- Petakh, P.; Isevych, V.; Kamyshnyi, A.; Oksenych, V. Weil’s Disease—Immunopathogenesis, Multiple Organ Failure, and Potential Role of Gut Microbiota. Biomolecules 2022, 12, 1830. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Flórez, M.; Torres-Hoyos, D.; Miranda-Brand, Y.; Boudreau, R.L.; Gallego-Gómez, J.C.; Vicente-Manzanares, M. Dengue Virus Infection Alters Inter-Endothelial Junctions and Promotes Endothelial–Mesenchymal-Transition-like Changes in Human Microvascular Endothelial Cells. Viruses 2023, 15, 1437. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [PubMed]
- Cappellari, R.; D’Anna, M.; Menegazzo, L.; Bonora, B.M.; Albiero, M.; Avogaro, A.; Fadini, G.P. Diabetes mellitus impairs circulating proangiogenic granulocytes. Diabetologia 2020, 63, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Birnie, E.; Virk, H.S.; Savelkoel, J.; Spijker, R.; Bertherat, E.; Dance, D.A.B.; Limmathurotsakul, D.; Devleesschauwer, B.; Haagsma, J.A.; Wiersinga, W.J. Global burden of melioidosis in 2015: A systematic review and data synthesis. Lancet. Infect. Dis. 2019, 19, 892–902. [Google Scholar] [CrossRef]
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 2016, 1, 15008. [Google Scholar] [CrossRef]
- Hanson, J.; Smith, S.; Stewart, J.; Horne, P.; Ramsamy, N. Melioidosis-a disease of socioeconomic disadvantage. PLoS Neglected Trop. Dis. 2021, 15, e0009544. [Google Scholar] [CrossRef]
- Kwanhian, W.; Jiranantasak, T.; Kessler, A.T.; Tolchinsky, B.E.; Parker, S.; Songsri, J.; Wisessombat, S.; Pukanha, K.; Testamenti, V.A.; Khrongsee, P.; et al. Investigation of Melioidosis Outbreak in Pig Farms in Southern Thailand. Vet. Sci. 2020, 7, 9. [Google Scholar] [CrossRef]
- Songsri, J.; Chatatikun, M.; Wisessombat, S.; Mala, W.; Phothaworn, P.; Senghoi, W.; Palachum, W.; Chanmol, W.; Intakhan, N.; Chuaijit, S.; et al. Diagnostic accuracy of automation and non-automation techniques for identifying Burkholderia pseudomallei: A systematic review and meta-analysis. J. Infect. Public Health 2024, 17, 102438. [Google Scholar] [CrossRef]
- Lau, S.K.; Sridhar, S.; Ho, C.C.; Chow, W.N.; Lee, K.C.; Lam, C.W.; Yuen, K.Y.; Woo, P.C. Laboratory diagnosis of melioidosis: Past, present and future. Exp. Biol. Med. 2015, 240, 742–751. [Google Scholar] [CrossRef]
- Pitman, M.C.; Luck, T.; Marshall, C.S.; Anstey, N.M.; Ward, L.; Currie, B.J. Intravenous therapy duration and outcomes in melioidosis: A new treatment paradigm. PLoS Neglected Trop. Dis. 2015, 9, e0003586. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.P.; Marshall, C.S.; Anstey, N.M.; Ward, L.; Currie, B.J. 2020 Review and revision of the 2015 Darwin melioidosis treatment guideline; paradigm drift not shift. PLOS Neglected Trop. Dis. 2020, 14, e0008659. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M. The Health Gap: The Challenge of an Unequal World: The argument. Int. J. Epidemiol. 2017, 46, 1312–1318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richards, S.E.; Wijeweera, C.; Wijeweera, A. Lifestyle and socioeconomic determinants of diabetes: Evidence from country-level data. PLoS ONE 2022, 17, e0270476. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharma, S.; Yadav, P.K.; Dahal, R.; Shrestha, S.K.; Bhandari, S.; Thapaliya, K.P. Agriculture in relation to socioeconomic status of Tharu in Chitwan of Nepal. J. Agric. Food Res. 2021, 6, 100243. [Google Scholar]
- Fujishiro, K.; Xu, J.; Gong, F. What does “occupation” represent as an indicator of socioeconomic status?: Exploring occupational prestige and health. Soc. Sci. Med. 2010, 71, 2100–2107. [Google Scholar] [CrossRef]
- Hassan, M.R.; Pani, S.P.; Peng, N.P.; Voralu, K.; Vijayalakshmi, N.; Mehanderkar, R.; Aziz, N.A.; Michael, E. Incidence, risk factors and clinical epidemiology of melioidosis: A complex socio-ecological emerging infectious disease in the Alor Setar region of Kedah, Malaysia. BMC Infect. Dis. 2010, 10, 302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mardhiah, K.; Wan-Arfah, N.; Naing, N.N.; Hassan, M.R.A.; Chan, H.K. The Cox model of predicting mortality among melioidosis patients in Northern Malaysia: A retrospective study. Medicine 2021, 100, e26160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menon, R.; Baby, P.; Kumar, V.A.; Surendran, S.; Pradeep, M.; Rajendran, A.; Suju, G.; Ashok, A. Risk Factors for Mortality in Melioidosis: A Single-Centre, 10-Year Retrospective Cohort Study. Sci. World J. 2021, 2021, 8154810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michels, E.H.; Butler, J.M.; Reijnders, T.D.; Cremer, O.L.; Scicluna, B.P.; Uhel, F.; Peters-Sengers, H.; Schultz, M.J.; Knight, J.C.; van Vught, L.A.; et al. Association between age and the host response in critically ill patients with sepsis. Crit. Care 2022, 26, 385. [Google Scholar] [CrossRef]
- Dias, S.P.; Brouwer, M.C.; van de Beek, D. Sex and Gender Differences in Bacterial Infections. Infect. Immun. 2022, 90, e0028322. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Alrashed, F.A.; Iqbal, M.; Alsubiheen, A.M.; Ahmad, T. Exploring determinants of sex and family history-based disparity in type 2 diabetes mellitus prevalence among clinical patients. BMC Public Health 2024, 24, 682. [Google Scholar] [CrossRef]
- Kronsteiner, B.; Chaichana, P.; Sumonwiriya, M.; Jenjaroen, K.; Chowdhury, F.R.; Chumseng, S.; Teparrukkul, P.; Limmathurotsakul, D.; Day, N.P.J.; Klenerman, P.; et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur. J. Immunol. 2019, 49, 1092–1106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raj, S.; Sistla, S.; Sadanandan, D.M.; Kadhiravan, T.; Rameesh, B.M.S.; Amalnath, D. Clinical Profile and Predictors of Mortality among Patients with Melioidosis. J. Glob. Infect. Dis. 2023, 15, 72–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
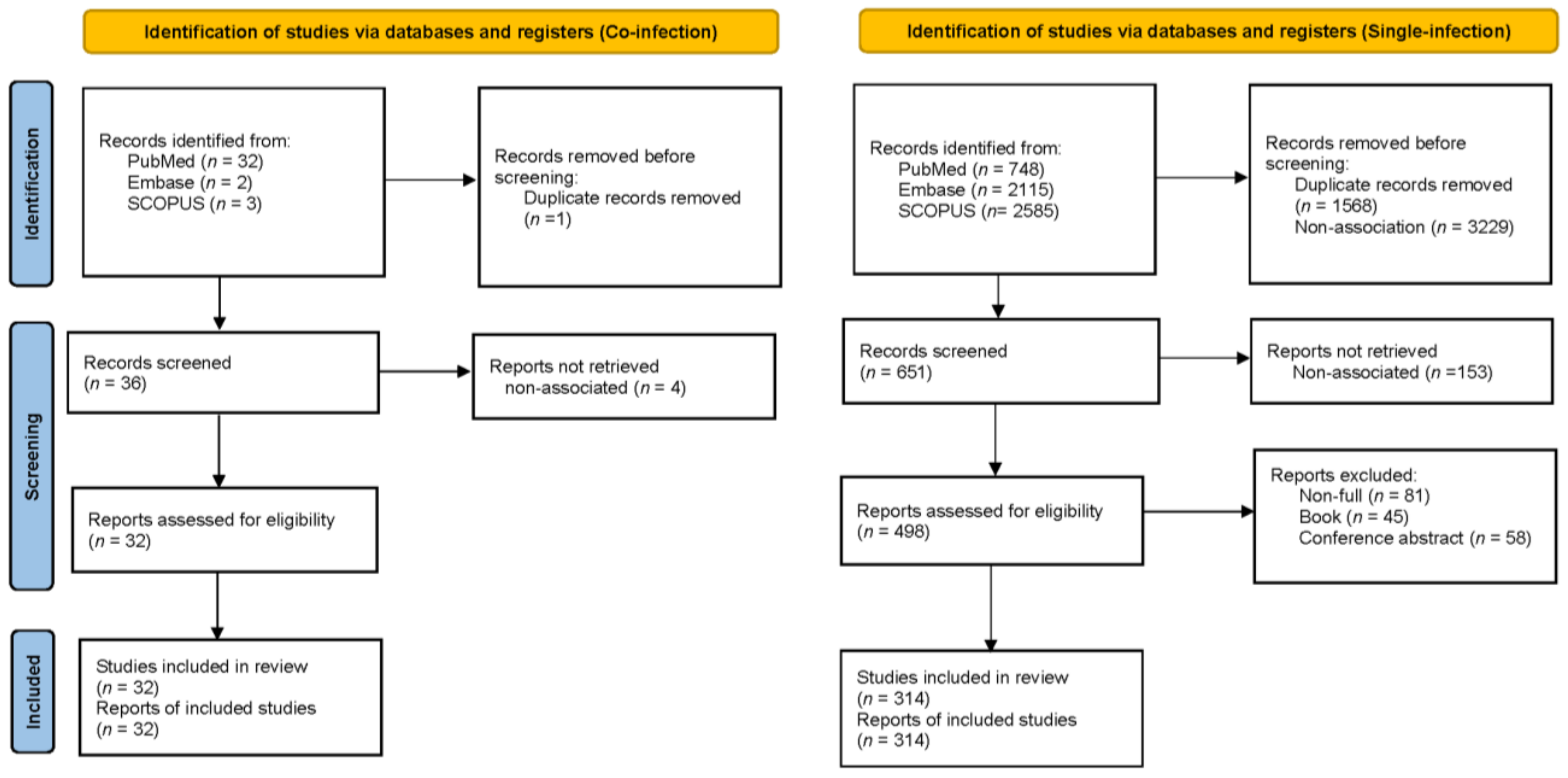
| Characteristics | Unreported Data n (%) | All Patients (n = 509) | Co-Infection (n = 55) | Single-Infection (n = 454) |
|---|---|---|---|---|
| Number of studies | 346 | 32 | 314 | |
| Study-level characteristics | ||||
| Year of publication | 0 (0.0) | |||
| 1998–2015 | 212 (41.7) | 32 (58.2) | 180 (39.6) | |
| 2016–2023 | 297 (58.4) | 23 (41.8) | 274 (60.4) | |
| Socio-economic country status | 0 (0.0) | |||
| High income | 167 (32.9) | 4 (7.2) | 163 (35.9) | |
| Upper-middle income | 166 (32.7) | 36 (35.5) | 130 (28.6) | |
| Lower-middle income | 175 (34.5) | 15 (27.3) | 160 (3.5) | |
| Patient-level characteristics | ||||
| Age (year) | 0 (0.0) | 45.4 ± 18.1 | 39.7 ± 18.4 | 46.1 ± 18.1 |
| ≤18 | 60 (11.9) | 7 (12.7) | 53 (11.7) | |
| 19–49 | 217 (43.0) | 26 (47.3) | 191 (42.1) | |
| ≥50 | 228 (45.1) | 22 (40.0) | 206 (45.4) | |
| Sex | 0 (0.0) | |||
| Male | 357 (70.1) | 45 (81.8) | 312 (68.7) | |
| Female | 152 (29.9) | 10 (18.2) | 142 (31.3) | |
| Agricultural occupation | 309 (60.7) | |||
| Related | 96 (18.9) | 19 (34.5) | 77 (17.0) | |
| Not related | 104 (20.4) | 12 (21.8) | 92 (2.0) | |
| Diabetes mellitus | 1 (0.2) | |||
| Presence | 262 (51.5) | 30 (54.5) | 232 (51.1) | |
| Absence | 246 (48.3) | 24 (43.6) | 222 (48.9) | |
| Other underlying diseasea | 1 (0.2) | |||
| Presence | 200 (39.3) | 16 (29.1) | 184 (40.5) | |
| Absence | 308 (60.5) | 38 (69.1) | 270 (5.9) | |
| Type of infection | 0 (0.0) | |||
| Single-infection | 454 (89.2) | 0 | 454 (100) | |
| Co-infection | 55 (10.8) | 55 (100) | 0 | |
| Dissemination of disease b | 3 (0.6) | |||
| Disseminated | 286 (56.2) | 34 (61.8) | 252 (55.5) | |
| Non-disseminated | 220 (43.2) | 18 (32.7) | 202 (44.5) |
| Co-Infection Organism | Alive n (%) | Death n (%) |
|---|---|---|
| TB | 14 (77.8) | 4 (22.2) |
| Leptospira | 3 (25.0) | 9 (75.0) |
| HIV | 10 (83.3) | 2 (16.7) |
| Other bacteria | 3 (75.0) | 1 (25.0) |
| Other viruses | 8 (66.7) | 4 (33.3) |
| Other mycobacteria | 2 (100.0) | 0 (0.0) |
| Fungus/yeast | 1 (50.0) | 1 (50.0) |
| Protozoa/Trematodes | 2 (100.0) | 0 (0.0) |
| Prognosis Factor | Alive | Death | Adjusted OR, CCA (95% CI) | p Value | Adjusted OR, MI (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Model 1 Publication year | (n = 509, 100%) | |||||
| 1998–2015 | 160 (75.5) | 52 (24.5) | Reference | NA | ||
| 2016–2023 | 233 (78.5) | 64 (21.5) | 0.88 (0.36–2.16) | 0.781 | NA | NA |
| Model 2 Socio-economic country status | (n = 508, 99.9%) | (n = 509, 100.0%) | ||||
| High income | 131 (78.4) | 36 (21.6) | Reference | Reference | ||
| High-middle income | 118 (71.1) | 48 (28.9) | 2.11 (0.73–6.05) | 0.166 | 2.11 (0.73–6.05) | 0.166 |
| Low-middle income | 143 (81.7) | 32 (18.3) | 1.03 (0.36–3.00) | 0.950 | 1.03 (0.36–3.00) | 0.950 |
| Model 3 Age groups | (n = 505, 98.8%) | (n = 509, 100.0%) | ||||
| Age ≤ 19 | 45 (75.0) | 15 (25.0) | Reference | Reference | ||
| Age 20–49 | 168 (77.4) | 49 (22.6) | 0.62 (0.17–2.34) | 0.483 | 0.62 (0.17–2.37) | 0.489 |
| Age ≥ 50 | 177 (77.6) | 51 (21.4) | 0.50 (0.13–1.94) | 0.314 | 0.50 (0.13–1.98) | 0.327 |
| Model 4 Sex | (n = 509, 100.0%) | |||||
| Female | 82 (76.6) | 25 (23.4) | Reference | NA | ||
| Male | 274 (79.0) | 73 (21.0) | 0.67 (0.30–1.48) | 0.319 | NA | NA |
| Model 5 Agricultural occupation | (n = 199, 39.0%) | (n = 509, 100.0%) | ||||
| Not related | 82 (78.9) | 22 (21.2) | Reference | Reference | ||
| Related | 76 (79.2) | 20 (20.8) | 0.82 (0.32–2.07) | 0.669 | 0.78 (0.29–2.16) | 0.632 |
| Model 6 Diabetes mellitus | (n = 503, 98.8%) | (n = 509, 100.0%) | ||||
| Absence | 194 (78.9) | 52 (21.1) | Reference | Reference | ||
| presence | 199 (76.0) | 63 (24.0) | 1.35 (0.60–3.05) | 0.471 | 1.36 (0.60–3.07) | 0.461 |
| Model 7 Other underlying | (n = 508, 99.9%) | (n = 509, 100.0%) | ||||
| Absence | 247 (80.2) | 61 (19.8) | Reference | Reference | ||
| presence | 146 (73.0) | 54 (27.0) | 1.57 (0.76–3.24) | 0.225 | 1.59 (0.77–3.27) | 0.212 |
| Model 8 Type of infection | (n = 503, 98.8%) | (n = 509, 100.0%) | ||||
| Single-infection | 356 (78.4) | 98 (21.6) | Reference | Reference | ||
| Co-infection | 37 (67.3) | 18 (32.7) | 2.70 (0.53–13.90) | 0.235 | 3.17 (0.60–16.62) | 0.172 |
| Model 9 Dissemination of disease | (n = 500, 98.2%) | (n = 509,100.0%) | ||||
| Non disseminated | 191 (86.8) | 29 (13.2) | Reference | Reference | ||
| Disseminated | 200 (69.9) | 86 (30.1) | 4.93 (2.14–11.37) | <0.001 | 4.70 (2.06–10.69) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongyikul, P.; Klangbud, W.K.; Chatatikun, M.; Phinyo, P. Co-Infections and Their Prognostic Impact on Melioidosis Mortality: A Systematic Review and Individual Patient Data Meta-Analysis. Epidemiologia 2025, 6, 17. https://doi.org/10.3390/epidemiologia6020017
Wongyikul P, Klangbud WK, Chatatikun M, Phinyo P. Co-Infections and Their Prognostic Impact on Melioidosis Mortality: A Systematic Review and Individual Patient Data Meta-Analysis. Epidemiologia. 2025; 6(2):17. https://doi.org/10.3390/epidemiologia6020017
Chicago/Turabian StyleWongyikul, Pakpoom, Wiyada Kwanhian Klangbud, Moragot Chatatikun, and Phichayut Phinyo. 2025. "Co-Infections and Their Prognostic Impact on Melioidosis Mortality: A Systematic Review and Individual Patient Data Meta-Analysis" Epidemiologia 6, no. 2: 17. https://doi.org/10.3390/epidemiologia6020017
APA StyleWongyikul, P., Klangbud, W. K., Chatatikun, M., & Phinyo, P. (2025). Co-Infections and Their Prognostic Impact on Melioidosis Mortality: A Systematic Review and Individual Patient Data Meta-Analysis. Epidemiologia, 6(2), 17. https://doi.org/10.3390/epidemiologia6020017







