Analyzing Differences in Viral Dynamics Between Vaccinated and Unvaccinated RSV Patients
Abstract
1. Introduction
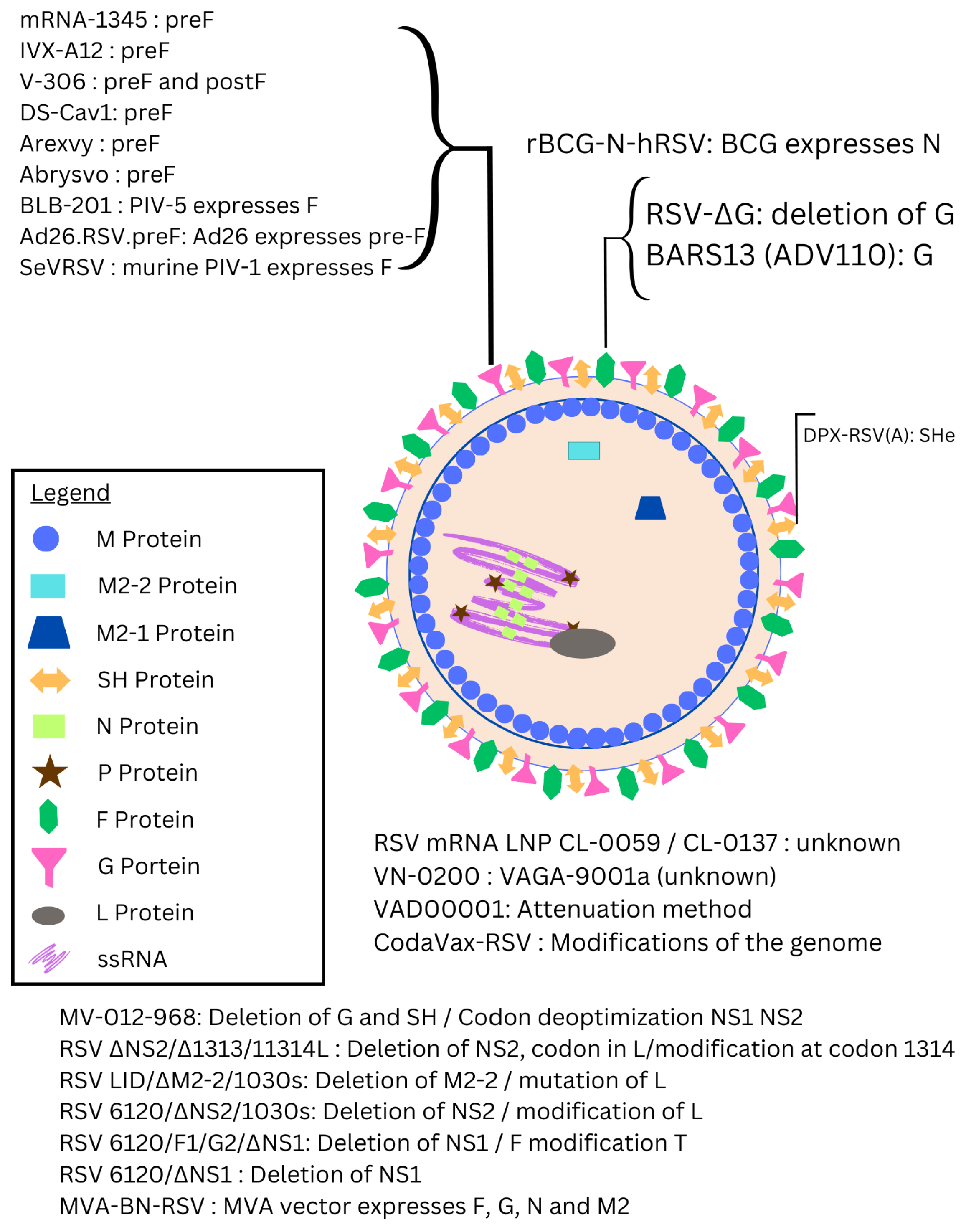
- mRNA Vaccines: Although mRNA vaccines rose to prominence with COVID-19, their application in RSV has been more recent, largely inspired by the success seen during the pandemic [20]. Efforts to develop mRNA vaccines for RSV are underway [21,22], with one mRNA vaccine already having received FDA approval [23]. Other mRNA vaccines are still being investigated, with the aim of achieving rapid, scalable production and targeted immune responses for populations at high risk, such as infants and older adults.
- Live-attenuated RSV vaccines have been in development for decades. However, early versions had setbacks, such as enhanced respiratory disease (ERD) in some young children, prompting significant adjustments [24]. Today, improved live-attenuated RSV vaccines are being tested, particularly those that are safer for RSV-naïve infants [25,26].
- Subunit/Virus-Like Particle (VLP)-Based Vaccines: Subunit vaccines, which consist of purified pathogen fragments, gained traction after the approval of the hepatitis B vaccine in 1986 [27]. Subunit vaccines targeting the RSV F protein have long been a focus due to the protein’s role in viral fusion [28,29]. Virus-like particles represent a recent advancement, resembling the virus structure but lacking the ability to replicate, making them safer for vulnerable populations [30].
- Recombinant Viral-Vector-Based Vaccines: The use of viral vectors in vaccines began in 1972 [31], with recent developments harnessing modified viruses to deliver genetic information and produce target proteins. This strategy provides flexibility in targeting the RSV proteins most critical for immunity, like F and G, and has shown promise for long-term efficacy in clinical studies [32].
2. Materials and Methods
2.1. Experimental Data
2.2. Mathematical Model
2.3. Fitting the Model to Data
3. Results
3.1. Model Fit to Data
3.2. Assessment of Identifiability
3.3. Parameter Comparison
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vekemans, J.; Moorthy, V.; Giersing, B.; Friede, M.; Hombach, J.; Arora, N.; Modjarrad, K.; Smith, P.G.; Karron, R.; Graham, B.; et al. Respiratory syncytial virus vaccine research and development: World Health Organization technological roadmap and preferred product characteristics. Vaccine 2019, 37, 7394–7395. [Google Scholar] [CrossRef]
- Sevendal, A.T.K.; Hurley, S.; Bartlett, A.W.; Rawlinson, W.; Walker, G.J. Systematic Review of the Efficacy and Safety of RSV-Specific Monoclonal Antibodies and Antivirals in Development. Rev. Med. Virol. 2024, 34, e2576. [Google Scholar] [CrossRef]
- Topalidou, X.; Kalergis, A.M.; Papazisis, G. Respiratory Syncytial Virus Vaccines: A Review of the Candidates and the Approved Vaccines. Pathogens 2023, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.; Hennessey, P.; Formica, M.; Cox, C.; Walsh, E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Ruckwardt, T.J.; Morabito, K.M.; Graham, B.S. Determinants of early life immune responses to RSV infection. Curr. Opin. Virol. 2016, 16, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simoes, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory Syncytial Virus—A Comprehensive Review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef]
- Panatto, D.; Domnich, A.; Lai, P.L.; Ogliastro, M.; Bruzzone, B.; Galli, C.; Stefanelli, F.; Pariani, E.; Orsi, A.; Icardi, G. Epidemiology and molecular characteristics of respiratory syncytial virus (RSV) among italian community-dwelling adults, 2021/22 season. BMC Infect. Dis. 2023, 23, 134. [Google Scholar] [CrossRef]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of severe RSV disease among immunocompromised children and adults: A 10 year retrospective study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef]
- Walsh, E.; Lee, N.; Sander, I.; Stolper, R.; Zakar, J.; Wyffels, V.; Myers, D.; Fleischhackl, R. RSV-associated hospitalization in adults in the USA: A retrospective chart review investigating burden, management strategies, and outcomes. Health Sci. Rep. 2022, 5, e556. [Google Scholar] [CrossRef]
- Wilkinson, T.; Beaver, S.; Macartney, M.; Mcarthur, E.; Yadav, V.; Lied-Lied, A. Burden of respiratory syncytial virus in adults in the United Kingdom: A systematic literature review and gap analysis. Influenza Other Respir. Viruses 2023, 17, e13188. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Williams, T.C.; Viboud, C.; Campbell, H.; Chen, J.; Spiro, D.J. RSV genomic diversity and the development of a globally effective RSV intervention. Vaccine 2021, 39, 2811–2820. [Google Scholar] [CrossRef]
- Rezende, W.; Neal, H.E.; Dutch, R.E.; Piedra, P.A. The RSV F p27 peptide: Current knowledge, important questions. Front. Microbiol. 2023, 14, 1219846. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Tripp, R.A. RSV Replication, Transmission, and Disease Are Influenced by the RSV G Protein. Viruses 2022, 14, 2396. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Tran, K.C.; Luthra, P.; Teng, M.N.; He, B. Function of the respiratory syncytial virus small hydrophobic protein. J. Virol. 2007, 81, 8361–8366. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.C.; Boukhvalova, M.S.; Morrison, T.G.; Vogel, S.N. A multifaceted approach to RSV vaccination. Hum. Vaccines Immunother. 2018, 14, 1734–1745. [Google Scholar]
- Wildenbeest, J.G.; Lowe, D.M.; Standing, J.F.; Butler, C.C. Respiratory syncytial virus infections in adults: A narrative review. Lancet Respir. Med. 2024, 12, 822–836. [Google Scholar] [CrossRef]
- Ohmit, S.; Moler, F.; Monto, A.; Khan, A. Ribavirin utilization and clinical effectiveness in children hospitalized with respiratory syncytial virus infection. J. Clin. Epidemiol. 1996, 49, 963–967. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Anderson, E.J.; Goldstein, M. The Future of Respiratory Syncytial Virus Disease Prevention and Treatment. Infect. Dis. Ther. 2021, 10, 47–60. [Google Scholar] [CrossRef]
- Schlake, T.; Thess, A.; Fotin-Mleczek, M.; Kallen, K.J. Developing mRNA-vaccine technologies. RNA Biol. 2012, 9, 1319–1330. [Google Scholar] [CrossRef]
- Fitz-Patrick, D.; Mihara, H.; Mills, A.; Mithani, R.; Kapoor, A.; Dhar, R.; Wilson, L.; Guo, R.; Simorellis, A.K.; Panozzo, C.A.; et al. Safety and immunogenicity of an mRNA-based RSV vaccine in Japanese older adults aged ≥ 60 years: A phase 1, randomized, observer-blind, placebo-controlled trial. Respir. Investig. 2024, 62, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Goswami, J.; Baqui, A.H.; Doreski, P.A.; Marc, G.P.; Jimenez, G.; Ahmed, S.; Zaman, K.; Duncan, C.J.A.; Ujiie, M.; Ramet, M.; et al. Humoral Immunogenicity of mRNA-1345 RSV Vaccine in Older Adults. J. Infect. Dis. 2024, 230, e996–e1006. [Google Scholar] [CrossRef]
- Anfaal, Z.; Khan, Z.A.; Aslam, M.A. FDA Approves mRESVIA: Embracing the New Era of RSV Prevention With Advanced mRNA Technology. Ann. Pharmacother. 2024. [Google Scholar] [CrossRef]
- Schickli, J.H.; Dubovsky, F.; Tang, R.S. Challenges in developing a pediatric RSV vaccine. Hum. Vaccines 2009, 5, 582–591. [Google Scholar] [CrossRef]
- Levy, M.; Chen, J.W.; Kaiser, J.A.; Park, H.S.; Liu, X.; Yang, L.; Santos, C.; Buchholz, U.J.; Le Nouen, C. Intranasal respiratory syncytial virus vaccine attenuated by codon-pair deoptimization of seven open reading frames is genetically stable and elicits mucosal and systemic immunity and protection against challenge virus replication in hamsters. PLoS Pathog. 2024, 20, e1012198. [Google Scholar] [CrossRef]
- Alamares-Sapuay, J.; Kishko, M.; Lai, C.; Parrington, M.; Delagrave, S.; Herbert, R.; Castens, A.; Swerczek, J.; Luongo, C.; Yang, L.; et al. Mutations in the F protein of the live-attenuated respiratory syncytial virus vaccine candidate ΔNS2/Δ1313/I1314L increase the stability of infectivity and content of prefusion F protein. PLoS ONE 2024, 19, e0301773. [Google Scholar] [CrossRef]
- Cid, R.; Bolivar, J. Platforms for Production of Protein-Based Vaccines: From Classical to Next-Generation Strategies. Biomolecules 2021, 11, 1072. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, M.J.G.; Cox, F.; Koornneef, A.; Yu, X.; van Overveld, D.; Le, L.; van den Hoogen, W.; Vaneman, J.; Thoma, A.; Voorzaat, R.; et al. A foldon-free prefusion F trimer vaccine for respiratory syncytial virus to reduce off-target immune responses. Nat. Microbiol. 2024, 9, 3254–3267. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Y.; Bai, Y.; Zhu, B.; Chang, F.; Lu, Y. Efficacy, Safety, and Immunogenicity of Subunit Respiratory Syncytial Virus Vaccines: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Vaccines 2024, 12, 879. [Google Scholar] [CrossRef]
- Mandviwala, A.S.; Huckriede, A.L.W.; Arankalle, V.A.; Patil, H.P. Mucosal delivery of a prefusogenic-F, glycoprotein, and matrix proteins-based virus-like particle respiratory syncytial virus vaccine induces protective immunity as evidenced by challenge studies in mice. Virology 2024, 598, 110194. [Google Scholar] [CrossRef]
- Jackson, D.; Berg, P.; Symons, R. Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40—Circular SV40 DNA Molecules Containing Lambda Phage Genes and Galactose Operon of Escherchia coli. Proc. Natl. Acad. Sci. USA 1972, 69, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- van Heesbeen, R.; Bastian, A.R.; Omoruyi, E.; Rosen, J.; Comeaux, C.A.; Callendret, B.; Heijnen, E. Immunogenicity and safety of different dose levels of Ad26.RSV.preF/RSV preF protein vaccine in adults aged 60 years and older: A randomized, double-blind, placebo-controlled, phase 2a study. Vaccine 2024, 42, 126273. [Google Scholar] [CrossRef]
- Kingwell, K. RSV vaccines score landmark FDA approvals. Nat. Rev. Drug Discov. 2023, 22, 523–525. [Google Scholar] [CrossRef]
- Venkatesan, P. First RSV vaccine approvals. Lancet Microbe 2023, 4, E577. [Google Scholar] [CrossRef]
- Syed, Y.Y. Respiratory Syncytial Virus Prefusion F Subunit Vaccine: First Approval of a Maternal Vaccine to Protect Infants. Pediatr. Drugs 2023, 25, 729–734. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Aliabadi, N.; Goodwin, G.; Slezak, J.; Hong, V.; Ackerson, B.; Liu, Q.; Shaw, S.; Welsh, S.; Stern, J.A.; et al. Estimated Vaccine Effectiveness for Respiratory Syncytial Virus-Related Lower Respiratory Tract Disease. JAMA Netw. Open 2024, 7, e2450832. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Kabir, G.; Schultz, S.; Silbernagl, G.; Schmidt, D.; Jenkins, V.A.; Weidenthaler, H.; Stroukova, D.; Martin, B.K.; De Moerlooze, L. Reduced Respiratory Syncytial Virus Load, Symptoms, and Infections: A Human Challenge Trial of MVA-BN-RSV Vaccine. J. Infect. Dis. 2023, 228, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Jenkins, V.; Silbernagl, G.; Chavez, M.P.V.; Schmidt, D.; Schnorfeil, F.; Schultz, S.; Chen, L.; Salgado, F.; Jacquet, J.M.; et al. A multivalent RSV vaccine based on the modified vaccinia Ankara vector shows moderate protection against disease caused by RSV in older adults in a phase 3 clinical study. Vaccine 2024, 42, 126427. [Google Scholar] [CrossRef]
- Boianelli, A.; Nguyen, V.K.; Ebensen, T.; Schulze, K.; Wilk, E.; Sharma, N.; Bruder, S.S.K.D.; Toapanta, F.R.; Guzman, C.A.; Meyer-Hermann, M.; et al. Modeling Influenza Virus Infection: A Roadmap for Influenza Research. Viruses 2015, 7, 5274–5304. [Google Scholar] [CrossRef]
- Gallagher, M.E.; Brooke, C.B.; Ke, R.; Koelle, K. Causes and Consequences of Spatial Within-Host Viral Spread. Viruses 2018, 10, 627. [Google Scholar] [CrossRef]
- Zitzmann, C.; Ke, R.; Ribeiro, R.M.; Perelson, A.S. How robust are estimates of key parameters in standard viral dynamic models? PLoS Comput. Biol. 2024, 20, e1011437. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.K.; Klawonn, F.; Mikolajczyk, R.; Hernandez-Vargas, E.A. Analysis of Practical Identifiability of a Viral Infection Model. PLoS ONE 2016, 11, e0167568. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Hernandez-Vargas, E.A. Parameter Estimation in Mathematical Models of Viral Infections Using R. In Influenza Virus: Virus Methods and Protocols; Yamauchi, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1836, pp. 531–549. [Google Scholar] [CrossRef]
- González-Parra, G.; Dobrovolny, H.M. Assessing Uncertainty in A2 Respiratory Syncytial Virus Viral Dynamics. Comput. Math. Meth. Med. 2015, 2015, 567589. [Google Scholar] [CrossRef][Green Version]
- González-Parra, G.; De Ridder, F.; Huntjens, D.; Roymans, D.; Ispas, G.; Dobrovolny, H.M. A comparison of RSV and influenza in vitro kinetic parameters reveals differences in infecting time. PLoS ONE 2018, 13, e0192645. [Google Scholar] [CrossRef]
- González-Parra, G.; Dobrovolny, H.M. A quantitative assessment of dynamical differences of RSV infections in vitro and in vivo. Virology 2018, 523, 129–139. [Google Scholar] [CrossRef]
- Beauchemin, C.A.; Kim, Y.I.; Yu, Q.; Ciaramella, G.; DeVincenzo, J.P. Uncovering critical properties of the human respiratory syncytial virus by combining in vitro assays and in silico analyses. PLoS ONE 2019, 14, e0214708. [Google Scholar] [CrossRef]
- Wethington, D.; Harder, O.; Uppulury, K.; Stewart, W.C.; Chen, P.; King, T.; Reynolds, S.D.; Perelson, A.S.; Peeples, M.E.; Niewiesk, S.; et al. Mathematical modelling identifies the role of adaptive immunity as a key controller of respiratory syncytial virus in cotton rats. J. Roy. Soc. Interface 2019, 16, 20190389. [Google Scholar] [CrossRef]
- Heumann, R.; Duncan, C.; Stobart, C.C.; Kaschner, S. Dynamical Differences in Respiratory Syncytial Virus. Bull. Math. Biol. 2022, 84, 11. [Google Scholar] [CrossRef]
- González-Parra, G.; Dobrovolny, H.M. Modeling of fusion inhibitor treatment of RSV in African green monkeys. J. Theor. Biol. 2018, 456, 62–73. [Google Scholar] [CrossRef]
- Patel, K.; Kirkpatrick, C.M.; Nieforth, K.A.; Chanda, S.; Zhang, Q.; McClure, M.; Fry, J.; Symons, J.A.; Blatt, L.M.; Beigelman, L.; et al. Respiratory syncytial virus-A dynamics and the effects of lumicitabine, a nucleoside viral replication inhibitor, in experimentally infected humans. J. Antimicrob. Chemother. 2019, 74, 442–452. [Google Scholar] [CrossRef]
- Jessie, B.; Dobrovolny, H. The role of syncytia during viral infections. J. Theor. Biol. 2021, 525, 110749. [Google Scholar] [CrossRef]
- Noffel, Z.; Dobrovolny, H.M. Quantifying the effect of defective viral genomes in respiratory syncytial virus infections. Math. Biosci. Eng. 2023, 20, 12666–12681. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Dobrovolny, H.M. A study of the effects of age on the dynamics of RSV in animal models. Virus Res. 2021, 304, 198524. [Google Scholar] [CrossRef]
- González-Parra, G.; Dobrovolny, H.M. The rate of viral transfer between upper and lower respiratory tracts determines RSV illness duration. J. Math. Biol. 2019, 79, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Petrie, S.M.; Guarnaccia, T.; Laurie, K.L.; Hurt, A.C.; McVernon, J.; McCaw, J.M. Reducing Uncertainty in Within-Host Parameter Estimates of Influenza Infection by Measuring Both Infectious and Total Viral Load. PLoS ONE 2013, 8, e64098. [Google Scholar] [CrossRef]
- Lagarias, J.; Reeds, J.; Wright, M.; Wright, P. Convergence properties of the Nelder-Mead simplex method in low dimensions. SIAM J. Optim. 1998, 9, 112–147. [Google Scholar] [CrossRef]
- Stoffer, D.; Wall, K. Bootstrapping State Space Models—Gaussian Maximum Likelihood Estimation and the Kalman Filter. J. Am. Stat. Assoc. 1991, 86, 1024–1033. [Google Scholar] [CrossRef]
- Hooker, K.L.; Ganusov, V.V. Impact of Oseltamivir Treatment on Influenza A and B Virus Dynamics in Human Volunteers. Front. Microbiol. 2021, 12, 631211. [Google Scholar] [CrossRef]
- Malinczak, C.A.; Fonseca, W.; Mire, M.M.; Parolia, A.; Chinnaiyan, A.; Rasky, A.J.; Morris, S.; Yagi, K.; Bermick, J.R.; Lukacs, N.W. Sex-associated early-life viral innate immune response is transcriptionally associated with chromatin remodeling of type-I IFN-inducible genes. Mucosal Immunol. 2023, 16, 578–592. [Google Scholar] [CrossRef]
- Jastorff, A.; Gymnopoulou, E.; Salas, J.; Merrall, E.; Buntinx, E.; Martin, C.; Askling, H.H.; Schenkenberger, I.; Yuste, A.C.; Smith, W.; et al. Safety and immunogenicity of the Ad26/protein preF RSV vaccine in adults aged 18 to 59 years with and without at-risk comorbidities for severe respiratory syncytial virus disease: A phase 3, randomized, controlled, immunobridging trial. Vaccine 2025, 43, 126514. [Google Scholar] [CrossRef]
- Cayatte, C.; Bennett, A.S.; Rajani, G.M.; Hostetler, L.; Maynard, S.K.; Lazzaro, M.; McTamney, P.; Ren, K.; O’Day, T.; McCarthy, M.P.; et al. Inferior immunogenicity and efficacy of respiratory syncytial virus fusion proteinbased subunit vaccine candidates in aged versus young mice. PLoS ONE 2017, 12, e0188708. [Google Scholar] [CrossRef] [PubMed]
- Cherukuri, A.; Stokes, K.L.; Patton, K.; Kuo, H.; Sakamoto, K.; Lambert, S.; Stillman, E.; Moore, M.L.; Lee, S. An adjuvanted respiratory syncytial virus fusion protein induces protection in aged BALB/c mice. Immun. Ageing 2012, 9, 21. [Google Scholar] [CrossRef]
- Liyanage, Y.R.; Heitzman-Breen, N.; Tuncer, N.; Ciupe, S.M. Identifiability investigation of within-host models of acute virus infection. BiorXiv 2024. [Google Scholar] [CrossRef]
- Miao, H.; Xia, X.; Perelson, A.S.; Wu, H. On Identifiability of Nonlinear ODE Models and Applications in Viral Dynamics. SIAM Rev. 2011, 53, 3–39. [Google Scholar] [CrossRef]
- Beauchemin, C.A.; McSharry, J.J.; Drusano, G.L.; Nguyen, J.T.; Went, G.T.; Ribeiro, R.M.; Perelson, A.S. Modeling amantadine treatment of influenza A virus in vitro. J. Theor. Biol. 2008, 254, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Peterson, D.R.; Falsey, A.R. Viral Shedding and Immune Responses to Respiratory Syncytial Virus Infection in Older Adults. J. Infect. Dis. 2013, 207, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.T.; Bont, L.J.; Zar, H.; Polack, F.P.; Park, C.; Claxton, A.; Borok, G.; Butylkova, Y.; Wegzyn, C. Respiratory syncytial virus hospitalization and mortality: Systematic review and meta-analysis. Pediatr. Pulmonol. 2017, 52, 556–569. [Google Scholar] [CrossRef]
- Elena Cordova-Davalos, L.; Hernandez-Mercado, A.; Berenice Barron-Garcia, C.; Rojas-Martinez, A.; Jimenez, M.; Salinas, E.; Cervantes-Garcia, D. Impact of genetic polymorphisms related to innate immune response on respiratory syncytial virus infection in children. Virus Genes 2022, 58, 501–514. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Zhang, H.; Wei, Z.; Wei, D.; Yang, G.; Demongeot, J.; Zeng, Q. A mathematical model simulating the adaptive immune response in various vaccines and vaccination strategies. Sci. Rep. 2024, 14, 23995. [Google Scholar] [CrossRef]
- Handel, A.; Li, Y.; McKay, B.; Pawelek, K.A.; Zarnitsyna, V.; Antia, R. Exploring the impact of inoculum dose on host immunity and morbidity to inform model-based vaccine design. PLoS Comput. Biol. 2018, 14, e1006505. [Google Scholar] [CrossRef]
- Smith, R.J.; Schwartz, E.J. Predicting the potential impact of a cytotoxic T-lymphocyte HIV vaccine: How often should you vaccinate and how strong should the vaccine be? Math. Biosci. 2008, 212, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Nokes, D. Do we need 3 doses of hepatitis B vaccine? Vaccine 1999, 17, 2667–2673. [Google Scholar] [CrossRef] [PubMed]


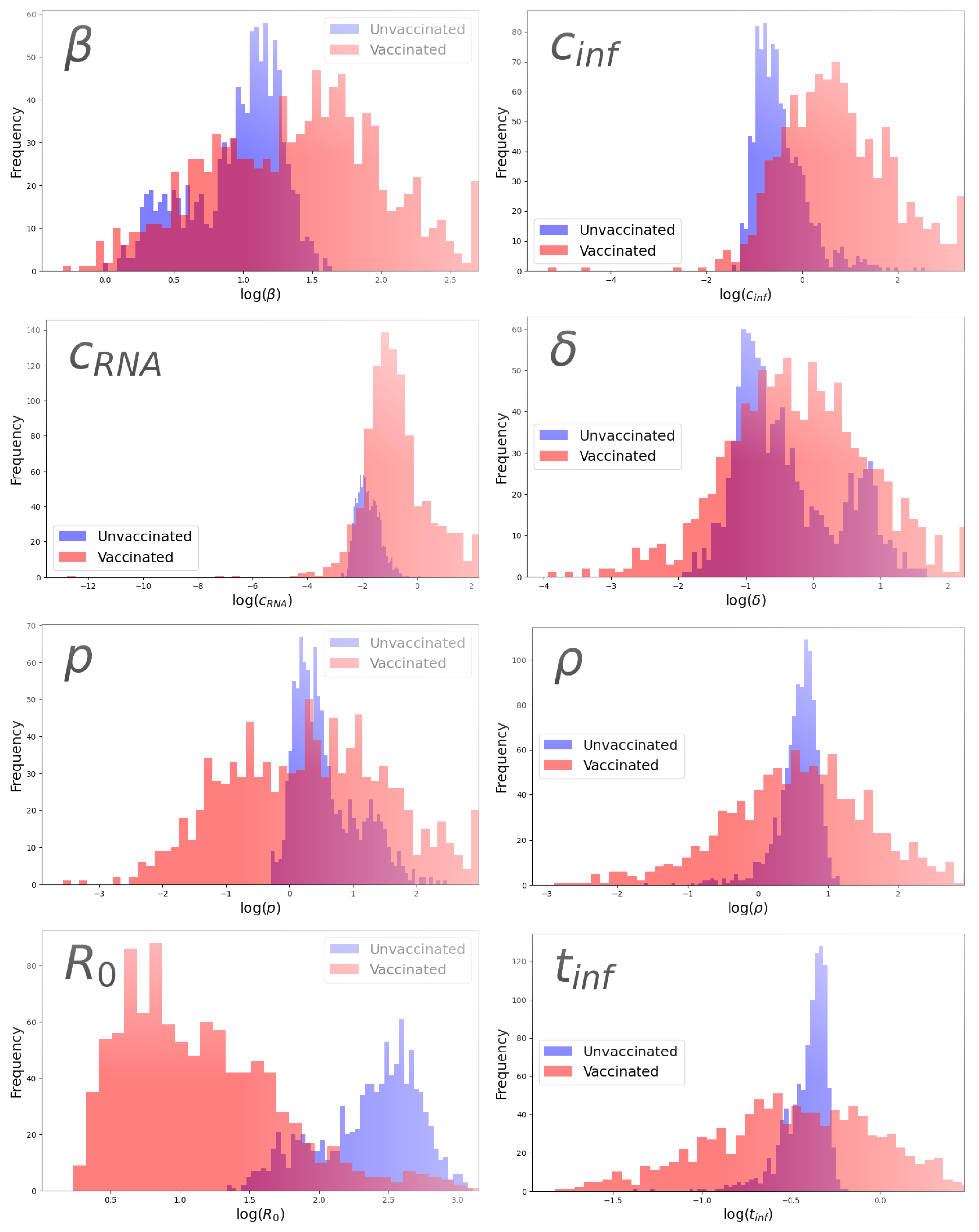
| Parameter | Group | Parameter Value | 95% CI |
|---|---|---|---|
| β (pfu/mL·d)−1 | Unvaccinated | 2.80 | [1.29, 4.17] |
| Vaccinated | 4.18 | [1.14, 12.7] | |
| (/d) | Unvaccinated | 0.554 | [0.228, 3.24] |
| Vaccinated | 0.748 | [0.0956, 5.59] | |
| p (pfu/mL·d−1) | Unvaccinated | 1.54 | [0.899, 5.61] |
| Vaccinated | 1.39 | [0.133, 15.2] | |
| (/d) | Unvaccinated | 0.565 | [0.313, 2.64] |
| Vaccinated | 1.92 | [0.307, 24.9] | |
| (/d) | Unvaccinated | 0.160 | [0.0881, 0.432] |
| Vaccinated | 0.398 | [0.0635, 7.20] | |
| (copies/pfu) | Unvaccinated | 1.88 | [0.791, 2.65] |
| Vaccinated | 1.80 | [0.175, 13.3] | |
| Unvaccinated | 13.8 | [4.95, 18.3] | |
| Vaccinated | 4.05 | [1.45, 15.6] | |
| (d) | Unvaccinated | 0.681 | [0.470, 0.764] |
| Vaccinated | 0.587 | [0.229, 1.41] |
| Parameter | Avg. p-Value (Wilcoxon) | Avg. p-Value (Levene) |
|---|---|---|
| 0.059 | 0.0091 | |
| 0.40 | 0.36 | |
| p | 0.56 | 0.15 |
| 0.0039 | 0.030 | |
| l0.0087 | 0.047 | |
| 0.44 | 0.028 | |
| 0.0015 | 0.304 | |
| 0.49 | 0.0035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, A.; Satani, S.; Dobrovolny, H.M. Analyzing Differences in Viral Dynamics Between Vaccinated and Unvaccinated RSV Patients. Epidemiologia 2025, 6, 16. https://doi.org/10.3390/epidemiologia6020016
Suri A, Satani S, Dobrovolny HM. Analyzing Differences in Viral Dynamics Between Vaccinated and Unvaccinated RSV Patients. Epidemiologia. 2025; 6(2):16. https://doi.org/10.3390/epidemiologia6020016
Chicago/Turabian StyleSuri, Arjan, Sahaj Satani, and Hana M. Dobrovolny. 2025. "Analyzing Differences in Viral Dynamics Between Vaccinated and Unvaccinated RSV Patients" Epidemiologia 6, no. 2: 16. https://doi.org/10.3390/epidemiologia6020016
APA StyleSuri, A., Satani, S., & Dobrovolny, H. M. (2025). Analyzing Differences in Viral Dynamics Between Vaccinated and Unvaccinated RSV Patients. Epidemiologia, 6(2), 16. https://doi.org/10.3390/epidemiologia6020016







