The Efficacy of Cotrimoxazole for the Prevention of Pneumocystis jirovecii Pneumonia Among HIV-Exposed and Infected Children: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Outcome Measures
2.3. Inclusion and Exclusion Criteria
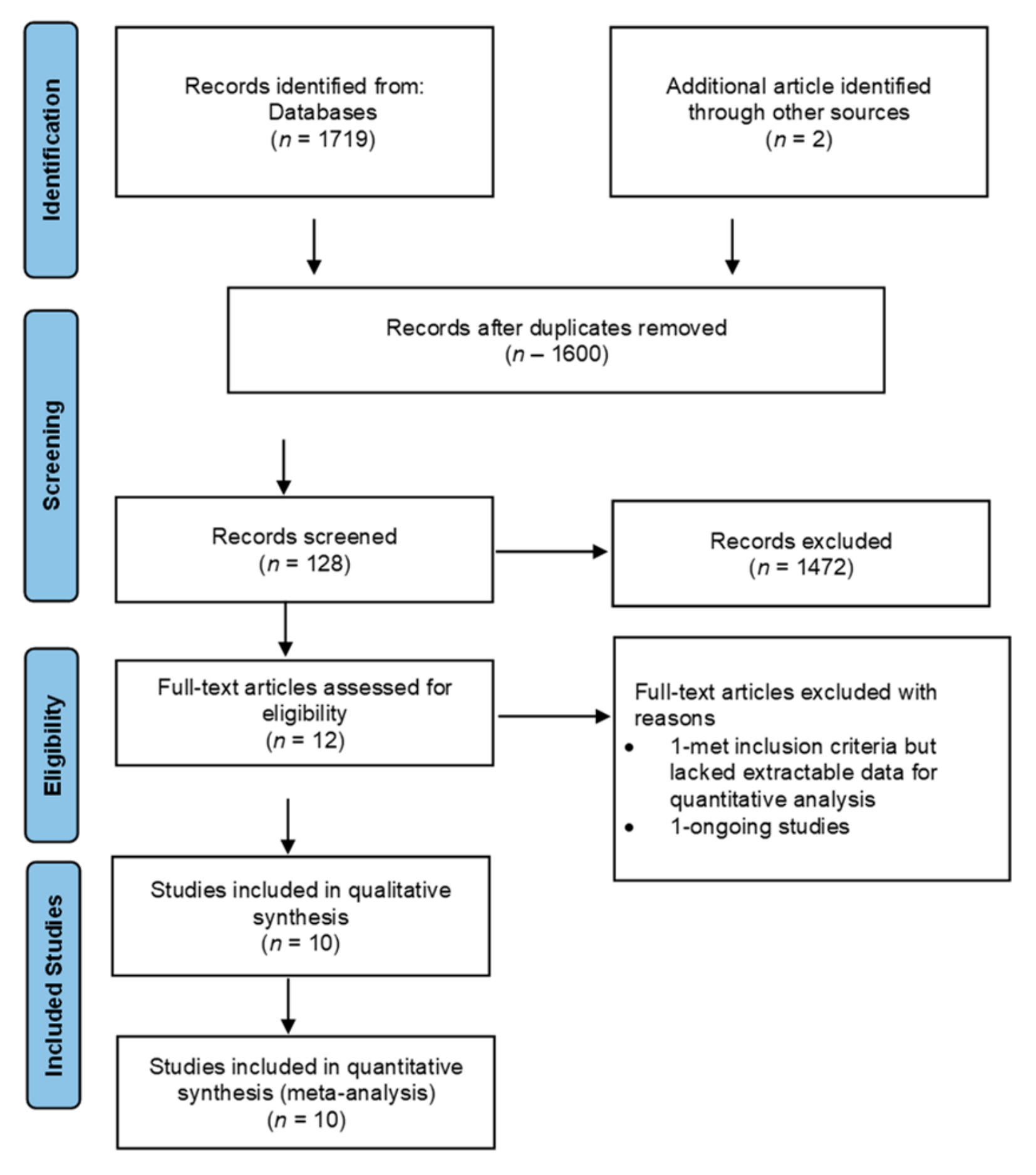
2.4. Selection Process of Studies
2.5. Qualitative Evaluation of Studies
2.6. Data Extraction
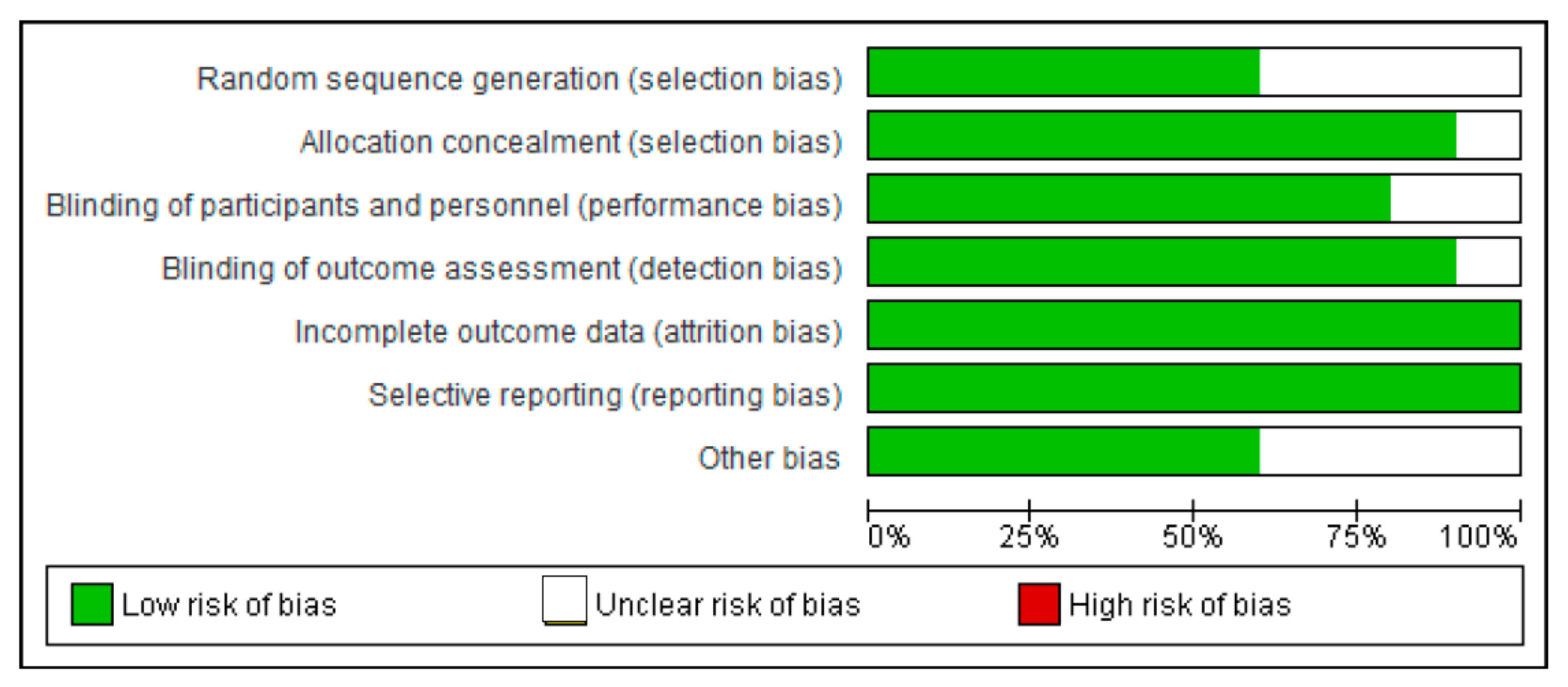
2.7. Risk of Bias Assessment
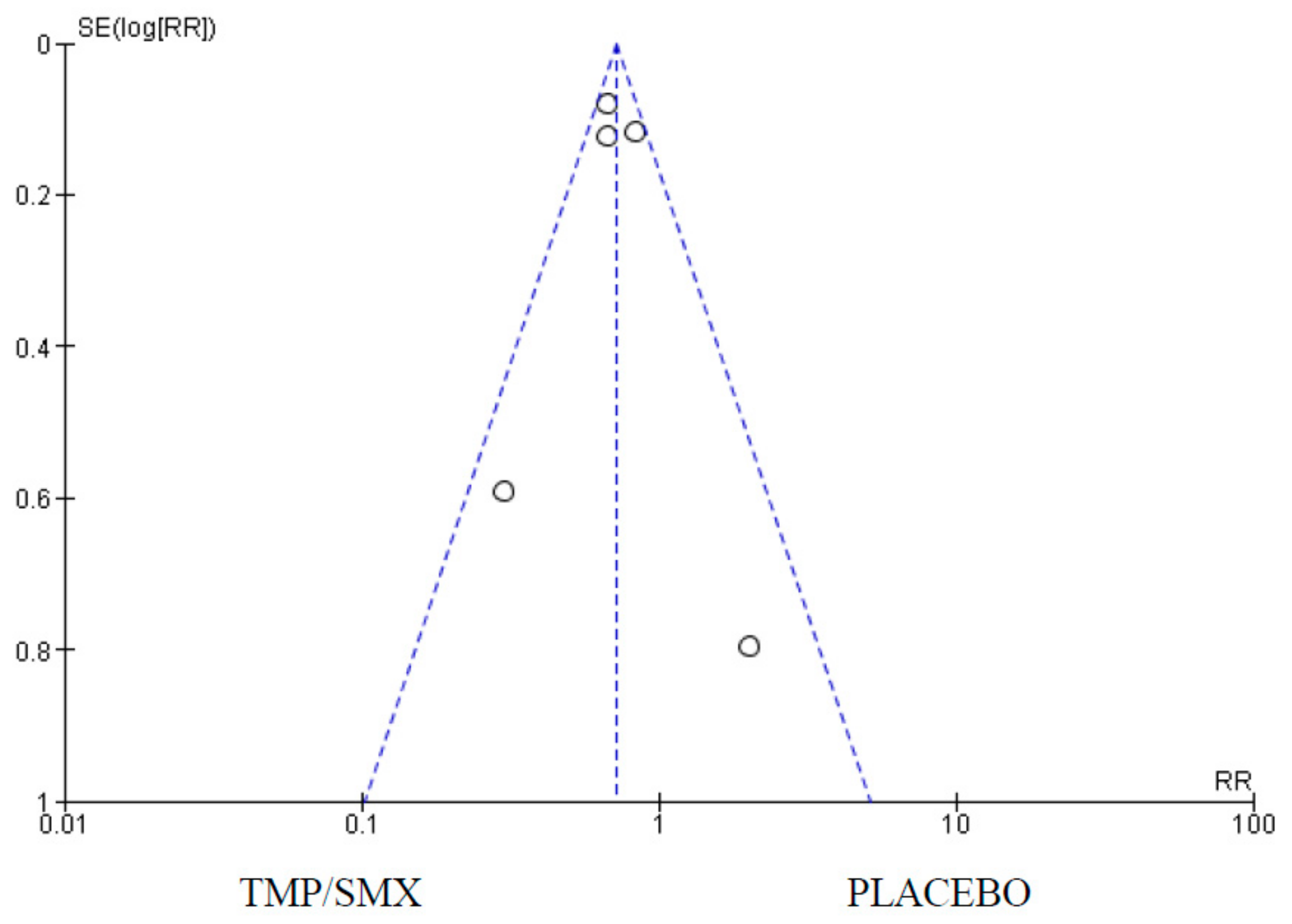
2.8. Data Analysis
3. Results
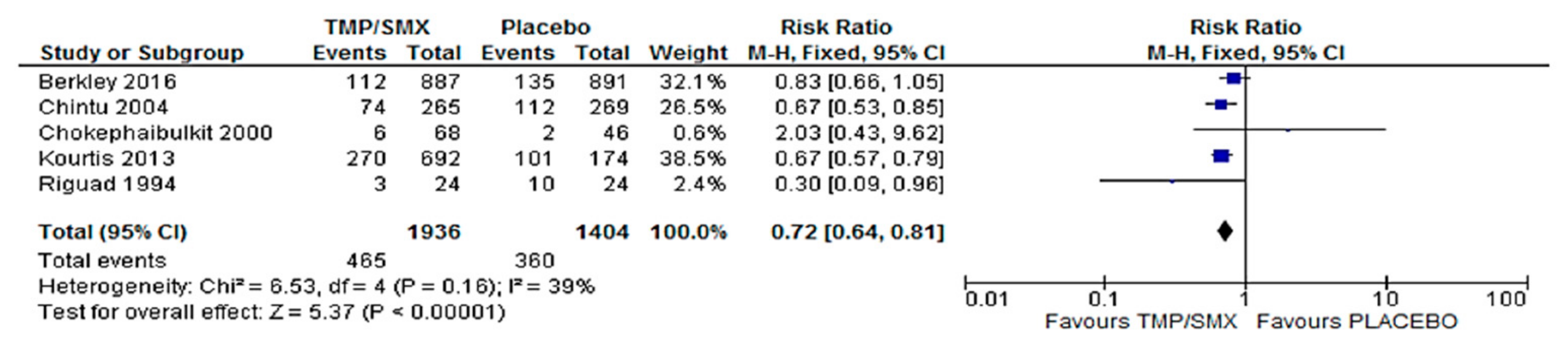
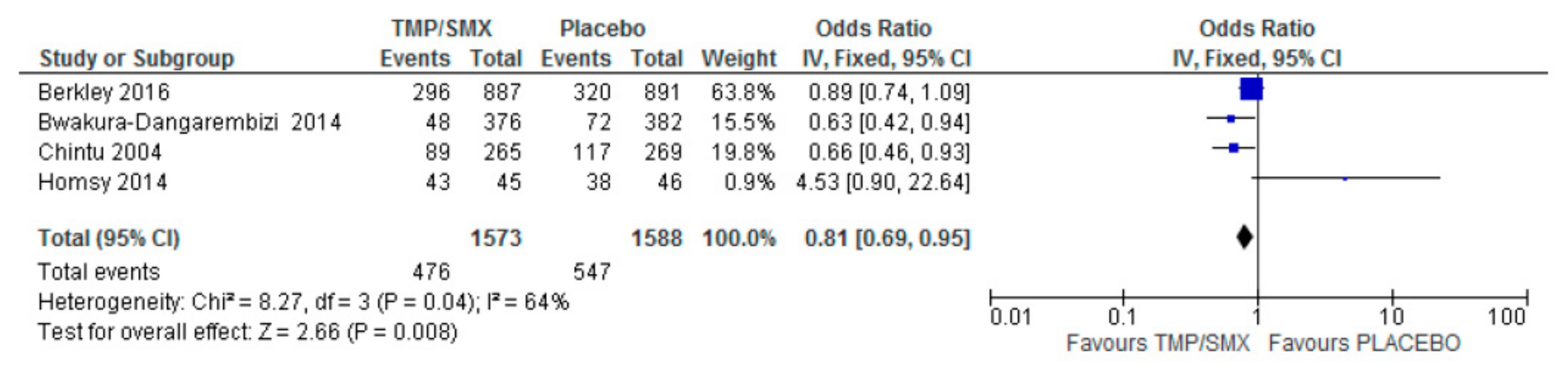
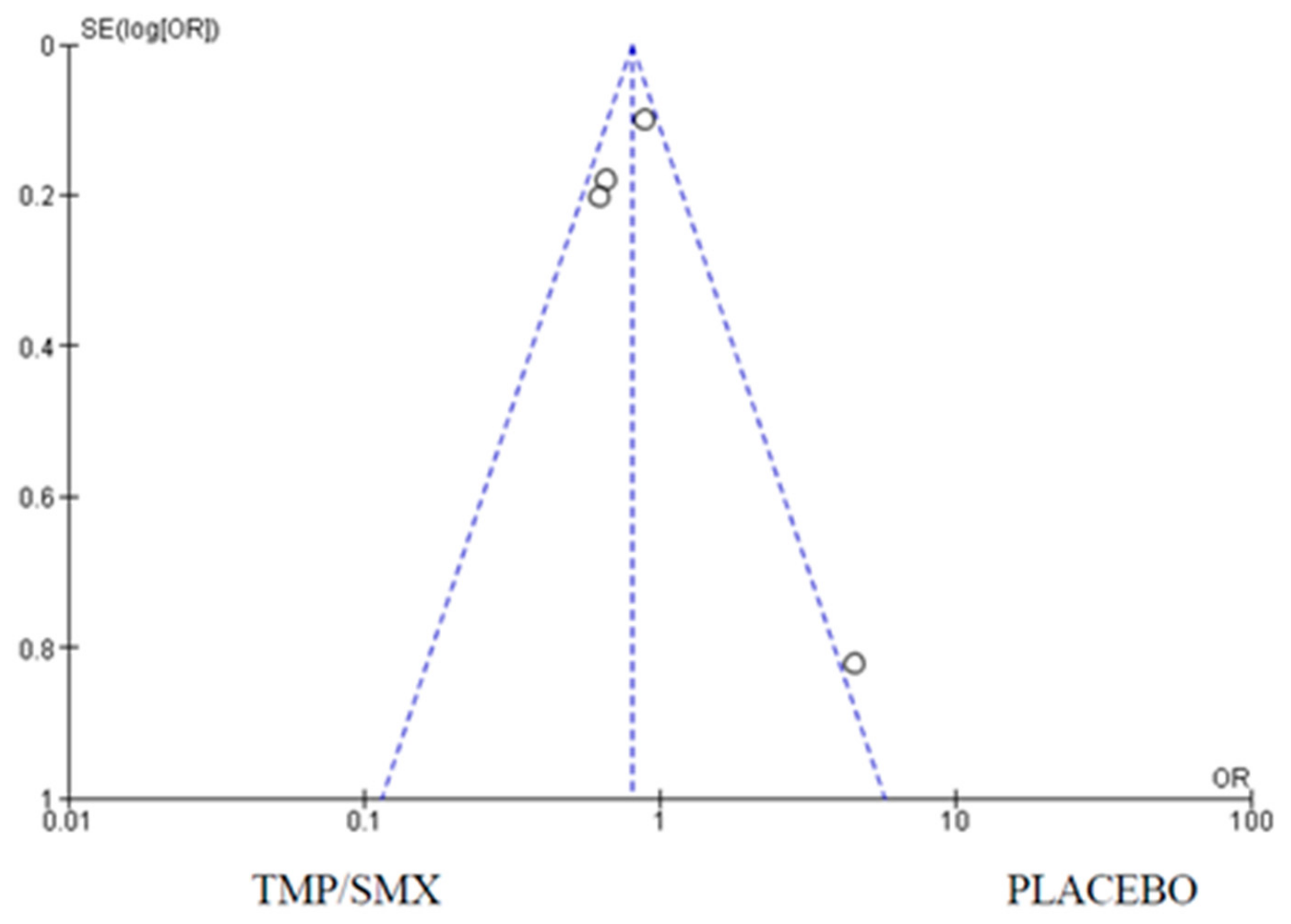
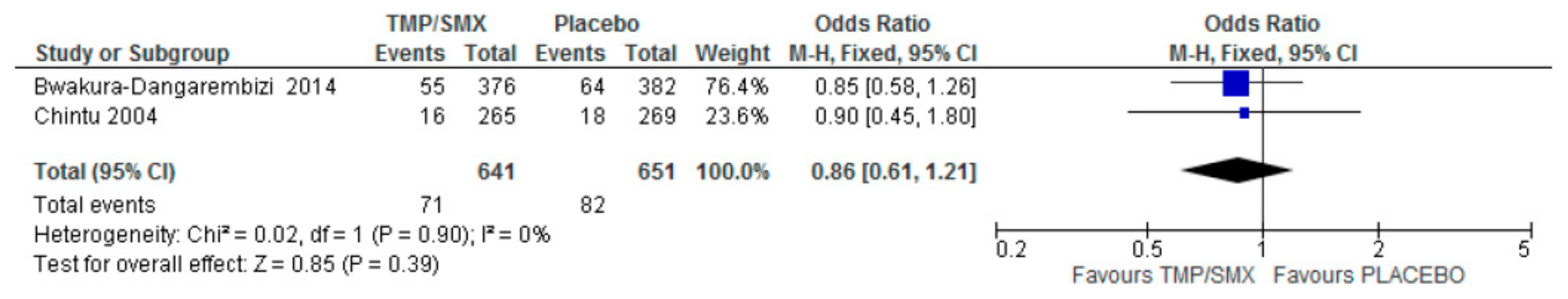
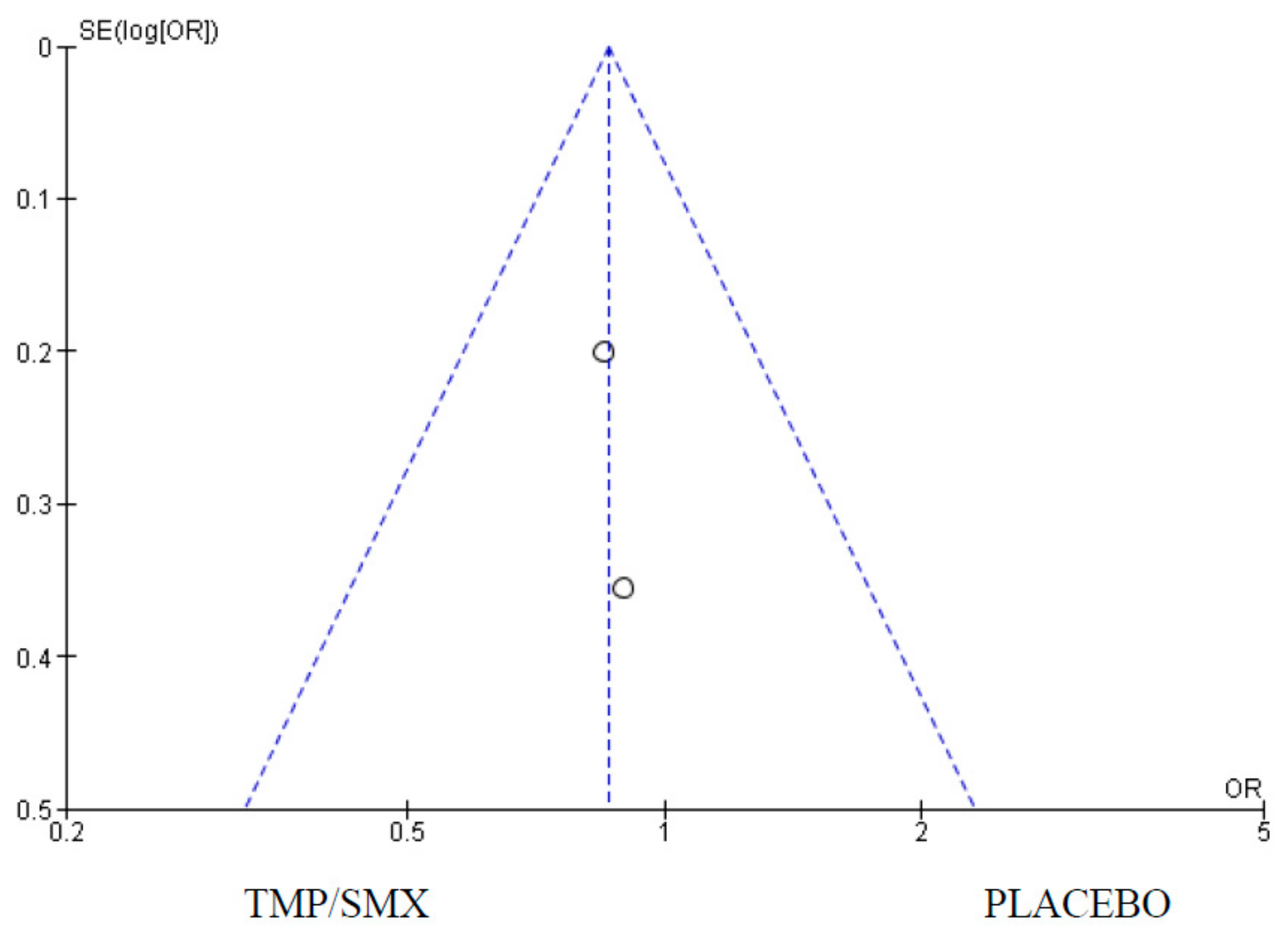
3.1. Mortality
3.2. Hospital Admission
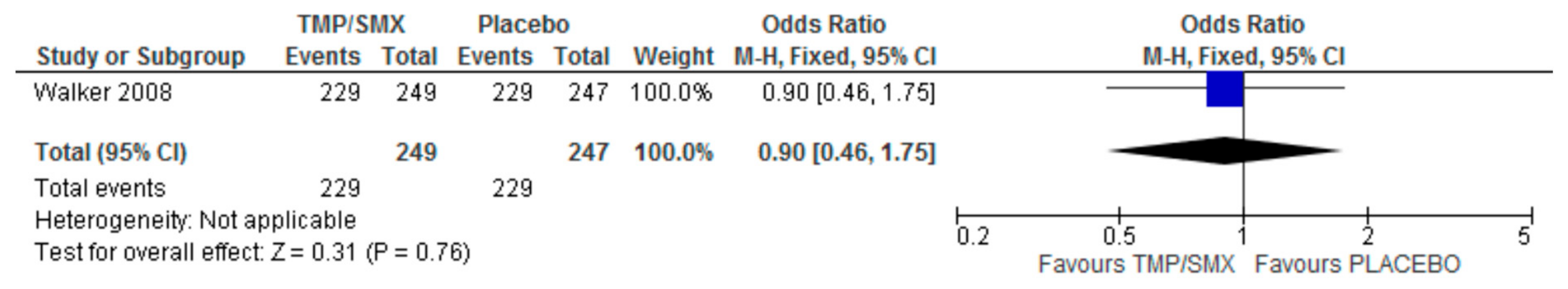
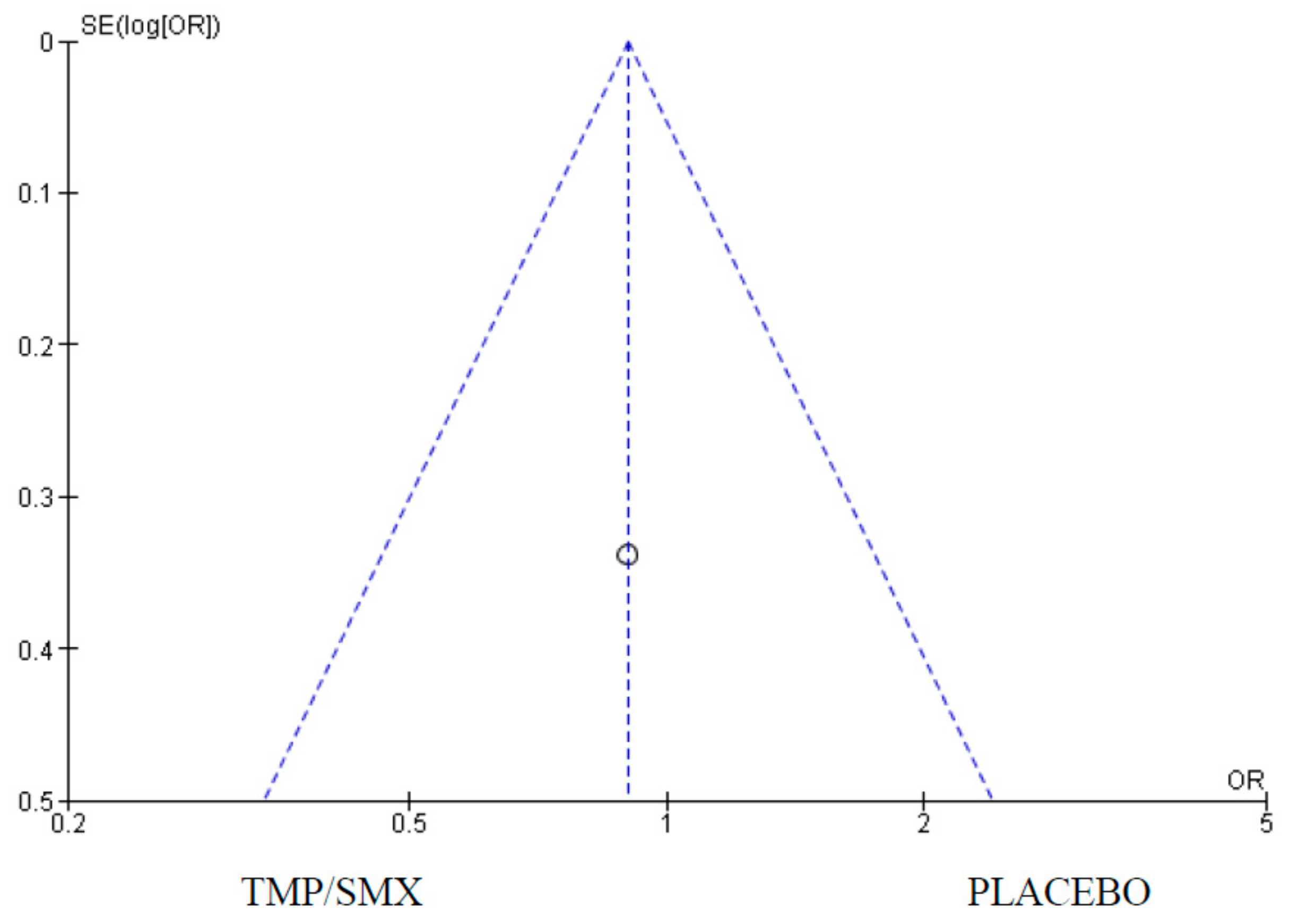
3.3. Adherence
3.4. Adverse Events
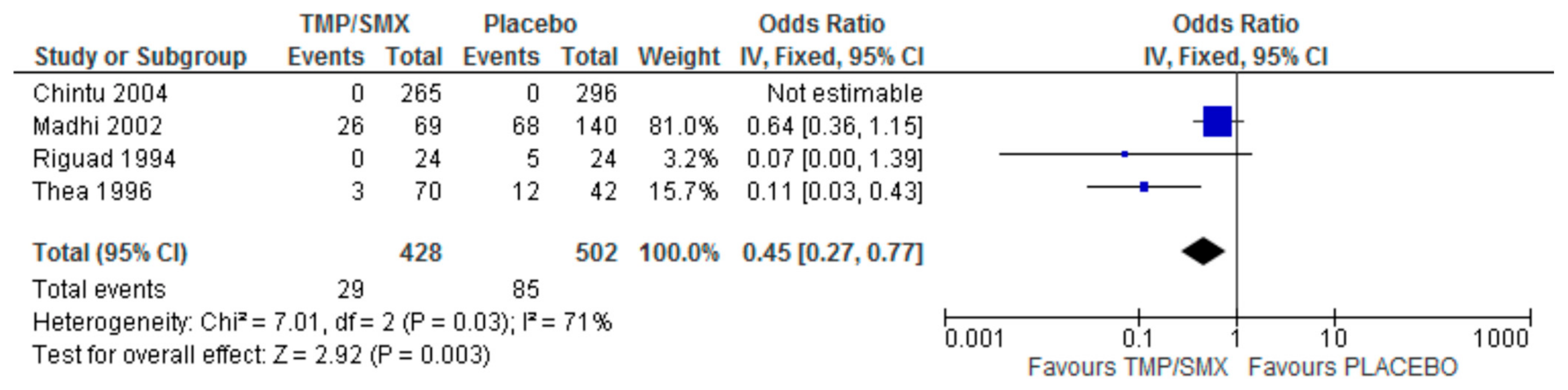
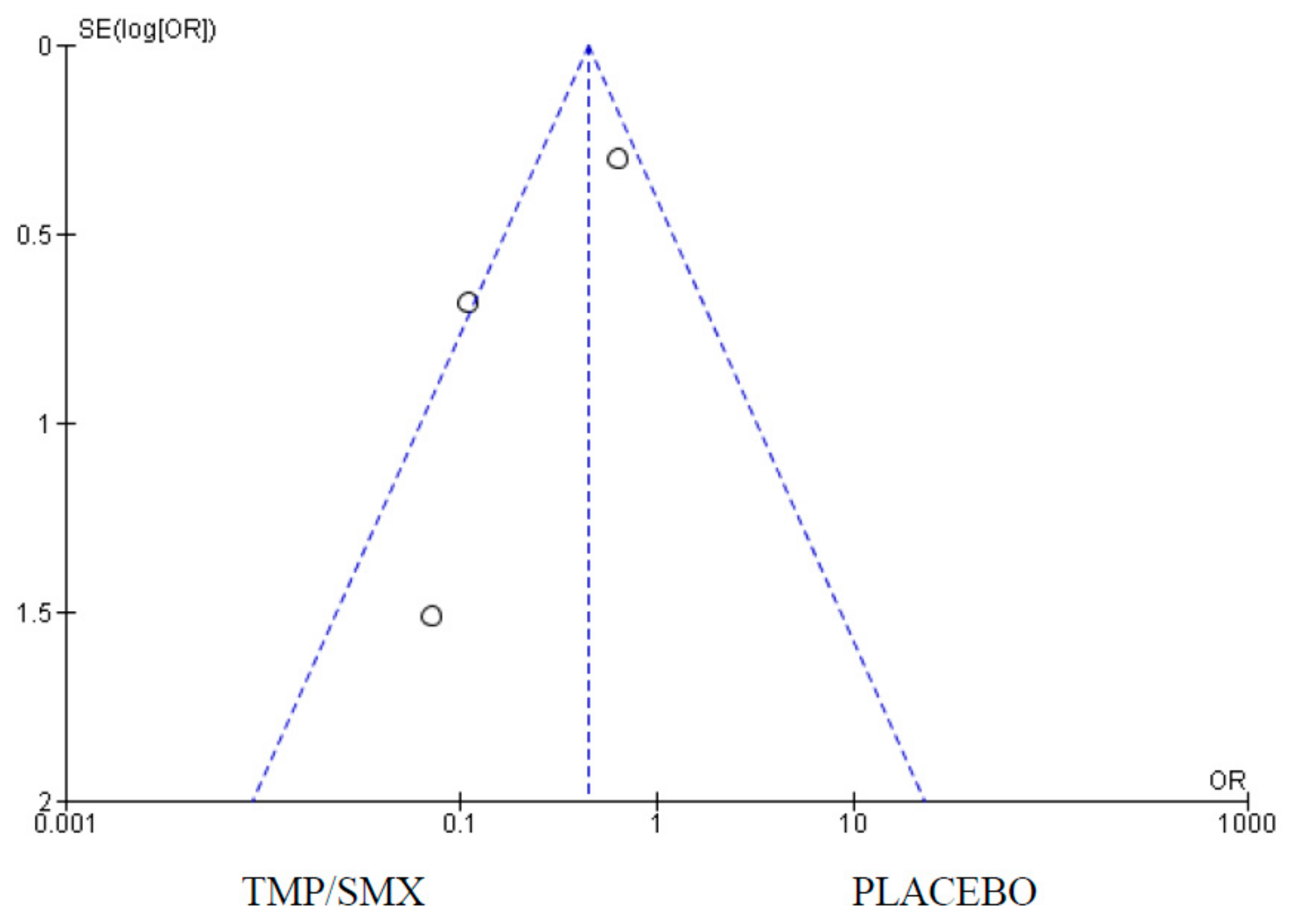
3.5. Pneumocystis jirovecii Isolation
4. Discussion
4.1. Mortality
4.2. Hospital Admission
4.3. Adherence
4.4. Adverse Events
4.5. Pneumocystis jirovecii Isolation
4.6. Limitations of the Systematic Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Why the HIV Epidemic Is Not Over. Available online: https://www.who.int/news-room/spotlight/why-the-hiv-epidemic-is-not-over (accessed on 2 October 2024).
- Vaillant, A.A.J.; Naik, R. HIV-1–Associated Opportunistic Infections; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lala, M.M. The ‘Pulmonary Diseases Spectrum’ in HIV Infected Children. Indian J. Tuberc. 2023, 70, S49–S58. [Google Scholar] [CrossRef] [PubMed]
- Zar, H.J. Prevention of HIV-Associated Respiratory Illness in Children in Developing Countries: Potential Benefits. Int. J. Tuberc. Lung Dis 2003, 7, 820–827. [Google Scholar] [PubMed]
- Huang, L.; Cattamanchi, A.; Davis, J.L.; Den Boon, S.; Kovacs, J.; Meshnick, S.; Miller, R.F.; Walzer, P.D.; Worodria, W.; Masur, H. HIV-Associated Pneumocystis Pneumonia. Proc. Am. Thorac. Soc. 2011, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Chokephaibulkit, K.; Chuachoowong, R.; Chotpitayasunondh, T.; Chearskul, S.; Vanprapar, N.; Waranawat, N.; Mock, P.; Shaffer, N.; Simonds, R.J. Evaluating a New Strategy for Prophylaxis to Prevent Pneumocystis Carinii Pneumonia in HIV-Exposed Infants in Thailand. Bangkok Collaborative Perinatal HIV Transmission Study Group. AIDS 2000, 14, 1563–1569. [Google Scholar] [CrossRef]
- Shetty, A.K. Perinatally Acquired HIV-1 Infection: Prevention and Evaluation of HIV-Exposed Infants. Semin. Pediatr. Infect. Dis. 2005, 16, 282–295. [Google Scholar] [CrossRef]
- Zar, H.J.; Madhi, S.A.; BCh, M. Childhood Pneumonia - Progress and Challenges. South African Med. J. 2006, 96, 890–899. [Google Scholar]
- Zachariah, R.; Harries, A.D.; Luo, C.; Bachman, G.; Graham, S.M. Scaling-up Co-Trimoxazole Prophylaxis in HIV-Exposed and HIV-Infected Children in High HIV-Prevalence Countries. Lancet. Infect. Dis. 2007, 7, 686–693. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Evans, C.; Slogrove, A.L.; Rehman, A.M.; Gibb, D.M.; Prendergast, A.J.; Penazzato, M. Co-trimoxazole Prophylaxis for Children Who Are HIV-exposed and Uninfected: A Systematic Review. J. Int. AIDS Soc. 2023, 26, e26079. [Google Scholar] [CrossRef]
- Church, J.A.; Fitzgerald, F.; Walker, A.S.; Gibb, D.M.; Prendergast, A.J. The Expanding Role of Co-Trimoxazole in Developing Countries. Lancet. Infect. Dis. 2015, 15, 327–339. [Google Scholar] [CrossRef]
- Chintu, C.; Bhat, G.J.; Walker, A.S.; Mulenga, V.; Sinyinza, F.; Lishimpi, K.; Farrelly, L.; Kaganson, N.; Zumla, A.; Gillespie, S.H.; et al. Co-Trimoxazole as Prophylaxis against Opportunistic Infections in HIV-Infected Zambian Children (CHAP): A Double-Blind Randomised Placebo-Controlled Trial. Lancet 2004, 364, 1865–1871. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook (accessed on 1 October 2024).
- Lepage, L.; Altman, D.G.; Schulz, K.F.; Moher, D.; Egger, M.; Davidoff, F.; Elbourne, D.; Gøtzsche, P.C.; Lang, T. The Revised CONSORT Statement for Reporting Randomized Trials: Explanation and Elaboration. Ann. Intern. Med. 2001, 134, 663–694. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Bwakura-Dangarembizi, M.; Kendall, L.; Bakeera-Kitaka, S.; Nahirya-Ntege, P.; Keishanyu, R.; Nathoo, K.; Spyer, M.J.; Kekitiinwa, A.; Lutaakome, J.; Mhute, T.; et al. A Randomized Trial of Prolonged Co-Trimoxazole in HIV-Infected Children in Africa. N. Engl. J. Med. 2014, 370, 41. [Google Scholar] [CrossRef]
- Rigaud, M.; Pollack, H.; Leibovitz, E.; Kim, K.; Persaud, D.; Kaul, A.; Lawrence, R.; Di John, D.; Borkowsky, W.; Krasinski, K. Efficacy of Primary Chemoprophylaxis against Pneumocystis Carinii Pneumonia during the First Year of Life in Infants Infected with Human Immunodeficiency Virus Type 1. J. Pediatr. 1994, 125, 476–480. [Google Scholar] [CrossRef]
- Homsy, J.; Dorsey, G.; Arinaitwe, E.; Wanzira, H.; Kakuru, A.; Bigira, V.; Muhindo, M.; Kamya, M.R.; Sandison, T.G.; Tappero, J.W. Protective Efficacy of Prolonged Co-Trimoxazole Prophylaxis in HIV-Exposed Children up to Age 4 Years for the Prevention of Malaria in Uganda: A Randomised Controlled Open-Label Trial. Lancet Glob. Heal. 2014, 2, e727–e736. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Wiener, J.; Kayira, D.; Chasela, C.; Ellington, S.R.; Hyde, L.; Hosseinipour, M.; Van Der Horst, C.; Jamieson, D.J. Health Outcomes of HIV-Exposed Uninfected African Infants. AIDS 2013, 27, 749. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.; Ismail, K.; O’Reilly, C.; Mancha, A.; Klugman, K.P. Ineffectiveness of Trimethoprim-Sulfamethoxazole Prophylaxis and the Importance of Bacterial and Viral Coinfections in African Children with Pneumocystis Carinii Pneumonia. Clin. Infect. Dis. 2002, 35, 1120–1126. [Google Scholar] [CrossRef]
- Thea, D.M.; Lambert, G.; Weedon, J.; Matheson, P.B.; Abrams, E.J.; Bamji, M.; Straus, W.L.; Thomas, P.A.; Krasinski, K.; Heagarty, M. Benefit of Primary Prophylaxis before 18 Months of Age in Reducing the Incidence of Pneumocystis Carinii Pneumonia and Early Death in a Cohort of 112 Human Immunodeficiency Virus-Infected Infants. New York City Perinatal HIV Transmission Collaborative Stu. Pediatrics 1996, 97, 59–64. [Google Scholar] [CrossRef]
- Walker, A.S.; Ford, D.; Mulenga, V.; Thomason, M.J.; Nunn, A.; Chintu, C.; Gibb, D.M.; Bangsberg, D.R. Adherence to Both Cotrimoxazole and Placebo Is Associated with Improved Survival among HIV-Infected Zambian Children. AIDS Behav. 2009, 13, 33–41. [Google Scholar] [CrossRef]
- Berkley, J.A.; Ngari, M.; Thitiri, J.; Mwalekwa, L.; Timbwa, M.; Hamid, F.; Ali, R.; Shangala, J.; Mturi, N.; Jones, K.D.J.; et al. Daily Co-Trimoxazole Prophylaxis to Prevent Mortality in Children with Complicated Severe Acute Malnutrition: A Multicentre, Double-Blind, Randomised Placebo-Controlled Trial. Lancet Glob. Heal. 2016, 4, e464–e473. [Google Scholar] [CrossRef]
- WHO. Guidelines on Co-Trimoxazole Prophylaxis for HIV-Related Infections among Children, Adolescents and Adults: Recommendations for a Public Health Approach; WHO Press: Geneva, Switzerland, 2006. [Google Scholar]
- Graham, S.M. Prophylaxis against Pneumocystis Carinii Pneumonia for HIV-Exposed Infants in Africa. Lancet 2002, 360, 1966–1968. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, K.; Swingler, G. Cotrimoxazole Prophylaxis for Opportunistic Infections in Adults with HIV. Cochrane Database Syst. Rev. 2003, 2003, CD003108. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.; Kuhn, L.; Spooner, E.; Mulol, H.; Goga, A.; Feucht, U.; Essack, S.Y.; Coutsoudis, A. Cotrimoxazole Guidelines for Infants Who Are HIV-Exposed but Uninfected: A Call for a Public Health and Ethics Approach to the Evidence. Lancet. Glob. Heal. 2022, 10, e1198–e1203. [Google Scholar] [CrossRef] [PubMed]
- Pressiat, C.; Mea-Assande, V.; Yonaba, C.; Treluyer, J.M.; Dahourou, D.L.; Amorissani-Folquet, M.; Blanche, S.; Eboua, F.; Ye, D.; Lui, G.; et al. Suboptimal Cotrimoxazole Prophylactic Concentrations in HIV-Infected Children According to the WHO Guidelines. Br. J. Clin. Pharmacol. 2017, 83, 2729–2740. [Google Scholar] [CrossRef]
- Ford, N.; Vitoria, M.; Penazzato, M.; Doherty, M.; Shubber, Z.; Meintjes, G.; Grinsztejn, B.; Eholie, S.; Mills, E.J.; Davies, M.A.; et al. Causes of Hospital Admission among People Living with HIV Worldwide: A Systematic Review and Meta-Analysis. Lancet HIV 2015, 2, e438–e444. [Google Scholar] [CrossRef]
| Study (First Author/Year) | Sample Size | Experiment/Control | Type of Study | Outcomes |
|---|---|---|---|---|
| Chintu 2004 [12] | 541 | Cotrimoxazole vs. placebo | Double-blinded RCT | Mortality; HR (95% CI) 0.57 (0.43–0.77) p = 0.0002 Hospital admission; HR (95% CI) 0.77 (0.62–0.95) p = 0.01 Pneumocystis Jarocki identification (molecular analysis); NIL from all 73 samples |
| Bwakura-Dangarembizi 2014 [16] | 758 | Continue cotrimoxazole (376) stop cotrimoxazole (382) | Open-label randomized parallel-gap trial | Hospitalization/deaths; HR (95% CI) 1.64 (1.14–2.37) p = 0.007 Hospitalization/deaths from pneumonias; HR (95% CI) 1.95 (1.15–3.32) p = 0.01 Grade 3 or 4 adverse effects; HR (95% CI) 1.20 (0.83–1.72) p = 0.33 Grade 4 adverse effects; HR (95% CI) 2.04 (0.99–4.22) p = 0.05 |
| Rigaud 1994 [17] | 48 | Cotrimoxazole vs. no cotrimoxazole (24/24) | Retrospective cohort | PCP occurrence cotrimoxazole; HR (95% CI) 0% (0–14.29%) No cotrimoxazole; HR (95% CI) 20.8% (7.1–42.2%) p = 0.049 Survival after 1 year; 92% of cotrimoxazole AND 74% of no cotrimoxazole Survival after 2 years; 87% of cotrimoxazole AND 59% of no cotrimoxazole. p = 0.035 |
| Homsy 2014 [18] | 203 | Continue cotrimoxazole vs. stop cotrimoxazole | Open- label RCT + 2 observational cohorts | Hospital admissions; 43 of 116 admissions in cotrimoxazole group AND 38 of 116 admissions in no cotrimoxazole group. Adverse effects All serious adverse events; IRR (95% CI) 0.91 (0.42–1.98). p = 0.81 |
| Kourtis 2013 [19] | 2250 | Cotrimoxazole vs. placebo | BAN RCT | Mortality; aHR 0.48. p = 0.03 |
| Madhi 2002 [20] | 216 | Cotrimoxazole vs. no cotrimoxazole | Prospective study | Mortality among children with PCP; HR (95 % CI) 98.6% (89.1–99.8%) Isolation of P. jiroveci; HR (95% CI) 36% (−15.4–64.5%) |
| Chokephaibulkit 2000 [6] | 395 | Continue cotrimoxazole vs. stop cotrimoxazole | Cohort | Mortality rate; RR 0.49 (0.10–2.34) p = 0.47 Incidence of pneumonia; RR 0.59 (0.25–1.41) p = 0.22 |
| Thea 1996 [21] | 112 | Cotrimoxazole vs. no cotrimoxazole | Retrospective Cohort | Incidence of PCP; un-adjustable risk of PCP in infants not receiving cotrimoxazole; 25% (12–39) Mortality; 28 deaths associated with PCP Likelihood of early death; RR (95% CI) 2.57 (1.1–6.1) |
| Walker 2008 [22] | 534 | Cotrimoxazole (249) vs. placebo (247) | CHAP randomized placebo-controlled trial | Adherence cotrimoxazole median (mean) = 92% (84%) Placebo median (mean) = 93% (82%) Rank sum Z = −0.17 p = 0.94 |
| Berkley 2016 [23] | 1778 | Cotrimoxazole vs. no cotrimoxazole | Multicenter double-blinded randomized placebo-controlled trial | Mortality; HR (95% CI) 0.90 (0.71–1.16) Hospital admissions; 57.1 per 100 CYO (95% CI 54.6–59.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agwu, A.O.; Egwu, C.O.; Okorocha, A.E.; Enyanwuma, I.; Amadi, C.C.; Okpokoro, E.; Akpabio, F.P.; Aguwa, C.O.; Onwu, D.; Nwokoro, O. The Efficacy of Cotrimoxazole for the Prevention of Pneumocystis jirovecii Pneumonia Among HIV-Exposed and Infected Children: A Systematic Review. Epidemiologia 2025, 6, 8. https://doi.org/10.3390/epidemiologia6010008
Agwu AO, Egwu CO, Okorocha AE, Enyanwuma I, Amadi CC, Okpokoro E, Akpabio FP, Aguwa CO, Onwu D, Nwokoro O. The Efficacy of Cotrimoxazole for the Prevention of Pneumocystis jirovecii Pneumonia Among HIV-Exposed and Infected Children: A Systematic Review. Epidemiologia. 2025; 6(1):8. https://doi.org/10.3390/epidemiologia6010008
Chicago/Turabian StyleAgwu, Anthony O., Chinedu Ogbonnia Egwu, Albert Egwu Okorocha, Ifeanyi Enyanwuma, Cyril C. Amadi, Evaezi Okpokoro, Francis Patrick Akpabio, Chukwuemeka Ogbonnaya Aguwa, Donatus Onwu, and Onyedikachi Nwokoro. 2025. "The Efficacy of Cotrimoxazole for the Prevention of Pneumocystis jirovecii Pneumonia Among HIV-Exposed and Infected Children: A Systematic Review" Epidemiologia 6, no. 1: 8. https://doi.org/10.3390/epidemiologia6010008
APA StyleAgwu, A. O., Egwu, C. O., Okorocha, A. E., Enyanwuma, I., Amadi, C. C., Okpokoro, E., Akpabio, F. P., Aguwa, C. O., Onwu, D., & Nwokoro, O. (2025). The Efficacy of Cotrimoxazole for the Prevention of Pneumocystis jirovecii Pneumonia Among HIV-Exposed and Infected Children: A Systematic Review. Epidemiologia, 6(1), 8. https://doi.org/10.3390/epidemiologia6010008







