Real-World Utilization of Molnupiravir during the COVID-19 Omicron Surge in Israel
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Population
2.3. Variables
2.3.1. Outcomes following MOV Treatment
2.3.2. Characteristics of Patients in the Antiviral Treatment-Eligible Cohort and MOV-Treated Cohort
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Patient Characteristics
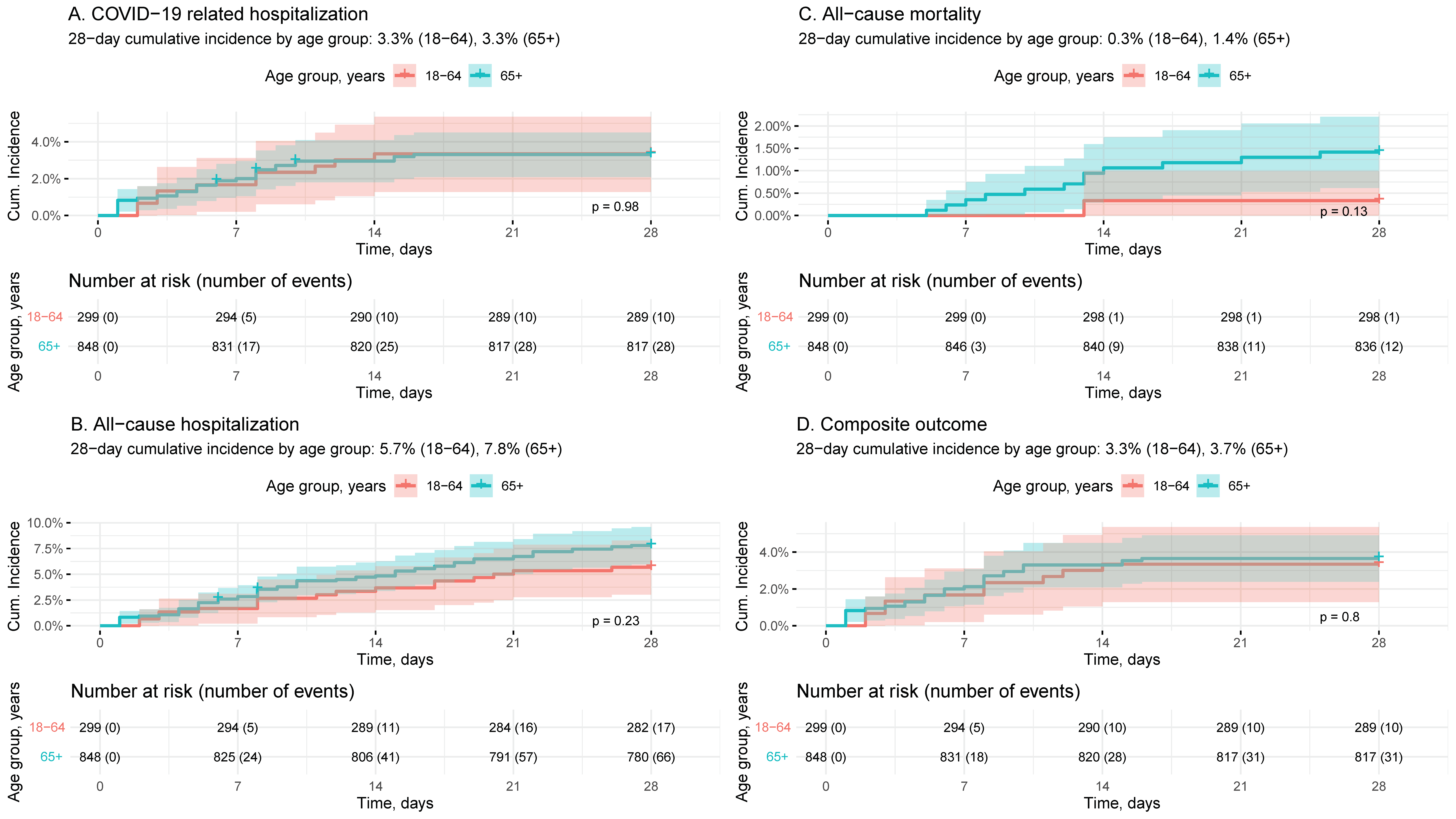
3.3. Outcomes following MOV Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, D.-Y.; Gu, Y.; Wheeler, B.; Young, H.; Holloway, S.; Sunny, S.-K.; Moore, Z.; Zeng, D. Effectiveness of COVID-19 vaccines over a 9-month period in North Carolina. N. Engl. J. Med. 2022, 386, 933–941. [Google Scholar] [CrossRef]
- Fang, X.; Li, S.; Yu, H.; Wang, P.; Zhang, Y.; Chen, Z.; Li, Y.; Cheng, L.; Li, W.; Jia, H.; et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: A systematic review and meta-analysis. Aging 2020, 12, 12493–12503. [Google Scholar] [CrossRef]
- Giannakoulis, V.G.; Papoutsi, E.; Siempos, I.I. Effect of Cancer on Clinical Outcomes of Patients With COVID-19: A Meta-Analysis of Patient Data. JCO Glob. Oncol. 2020, 6, 799–808. [Google Scholar] [CrossRef]
- Figliozzi, S.; Masci, P.G.; Ahmadi, N.; Tondi, L.; Koutli, E.; Aimo, A.; Stamatelopoulos, K.; Dimopoulos, M.A.; Caforio, A.L.P.; Georgiopoulos, G. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2020, 50, e13362. [Google Scholar] [CrossRef] [PubMed]
- Noor, F.M.; Islam, M.M. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J. Community Health 2020, 45, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Koirala, A.; Nicolopoulos, K.; Chiu, C.; Wood, N.; Britton, P.N. Vaccines for COVID-19: Where do we stand in 2021? Paediatr. Respir. Rev. 2021, 39, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Yek, C.; Warner, S.; Wiltz, J.L.; Sun, J.; Adjei, S.; Mancera, A.; Silk, B.J.; Gundlapalli, A.V.; Harris, A.M.; Boehmer, T.K.; et al. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged >/=18 Years Who Completed a Primary COVID-19 Vaccination Series-465 Health Care Facilities, United States, December 2020-October 2021. MMWR Morb Mortal Wkly Rep. 2022, 71, 19–25. [Google Scholar] [CrossRef]
- National Institutes of Health. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 1 May 2022).
- Ministry of Health. Medical Division. Drug Treatment in the Community for COVID-19 Patients, Whose Condition is Mild to Moderate—An Update. Available online: https://www.gov.il/he/Departments/policies/mr-106071122 (accessed on 1 May 2022).
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Bruno, G.; Giotta, M.; Perelli, S.; De Vita, G.; Bartolomeo, N.; Buccoliero, G.B. Early Access to Oral Antivirals in High-Risk Outpatients: Good Weapons to Fight COVID-19. Viruses 2022, 14, 2514. [Google Scholar] [CrossRef] [PubMed]
- Najjar-Debbiny, R.; Gronich, N.; Weber, G.; Khoury, J.; Amar, M.; Stein, N.; Goldstein, L.H.; Saliba, W. Effectiveness of Molnupiravir in High-Risk Patients: A Propensity Score Matched Analysis. Clin. Infect. Dis. 2023, 76, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Sagy, Y.W.; Battat, E.; Lavie, G.; Sergienko, R.; Friger, M.; Peretz, A.; Yaron, S.; Serby, D.; Hammerman, A. Molnupiravir use and Severe COVID-19 Outcomes during the Omicron Surge; Research Square: Durham, NC, USA, 2022. [Google Scholar] [CrossRef]
- De Vito, A.; Colpani, A.; Bitti, A.; Zauli, B.; Meloni, M.C.; Fois, M.; Denti, L.; Bacciu, S.; Marcia, C.; Maida, I. Safety and efficacy of molnupiravir in SARS-CoV-2-infected patients: A real-life experience. J. Med. Virol. 2022, 94, 5582–5588. [Google Scholar] [CrossRef]
- Kimata, M.; Watanabe, A.; Yanagida, Y.; Kinoshita, D.; Maekawa, S. Safety and Effectiveness of Molnupiravir (LAGEVRIO®) Capsules in Japanese Patients with COVID-19: Interim Report of Post-marketing Surveillance in Japan. Infect. Dis. Ther. 2023, 12, 1119–1136. [Google Scholar] [CrossRef] [PubMed]
- Streinu-Cercel, A.; Miron, V.D.; Oană, A.A.; Irimia, M.; Popescu, R.Ș.; Dărămuș, I.A.; Moțoi, M.M.; Ceapraga, G.J.; Săndulescu, O. Real-World Use of Molnupiravir in the Treatment of Outpatients with SARS-CoV-2 Infection—A Patient Profile Based on the Experience of a Tertiary Infectious Disease Center. Pharmaceuticals 2022, 15, 1065. [Google Scholar] [CrossRef] [PubMed]
- Israel Central Bureau of Statistics. Characterization and Classification of Geographic Units by the Socio-Economic Level of the Population 2015. Publication No. 1765. Available online: https://www.cbs.gov.il/he/publications/DocLib/2019/1765_socio_economic_2015/e_print.pdf (accessed on 1 May 2022).
- Rossman, H.; Shilo, S.; Meir, T.; Gorfine, M.; Shalit, U.; Segal, E. COVID-19 dynamics after a national immunization program in Israel. Nat. Med. 2021, 27, 1055–1061. [Google Scholar] [CrossRef]
- Chodick, G.; Heymann, A.D.; Shalev, V.; Kookia, E. The epidemiology of diabetes in a large Israeli HMO. Eur. J. Epidemiol. 2003, 18, 1143–1146. [Google Scholar] [CrossRef]
- Shalev, V.; Chodick, G.; Goren, I.; Silber, H.; Kokia, E.; Heymann, A.D. The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int. J. Cardiol. 2011, 152, 345–349. [Google Scholar] [CrossRef]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef]
- Weitzman, D.; Chodick, G.; Shalev, V.; Grossman, C.; Grossman, E. Prevalence and factors associated with resistant hypertension in a large health maintenance organization in Israel. Hypertension 2014, 64, 501–507. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 1 May 2022).
- FDA FactSheet. Available online: https://www.fda.gov/media/155050/download (accessed on 1 May 2022).
- Lexicomp. Available online: https://www.wolterskluwer.com/en/solutions/lexicomp (accessed on 1 May 2022).
- University of Liverpool. Available online: https://www.covid19-druginteractions.org/checker (accessed on 1 May 2022).
- Tene, L.; Chodick, G.; Fallach, N.; Ansari, W.; Distelman-Menachem, T.; Maor, Y. Describing COVID-19 Patients During The First Two Months of Paxlovid (Nirmatrelvir/Ritonavir) Initiation in a Large HMO in Israel. medRxiv 2022. [Google Scholar] [CrossRef]
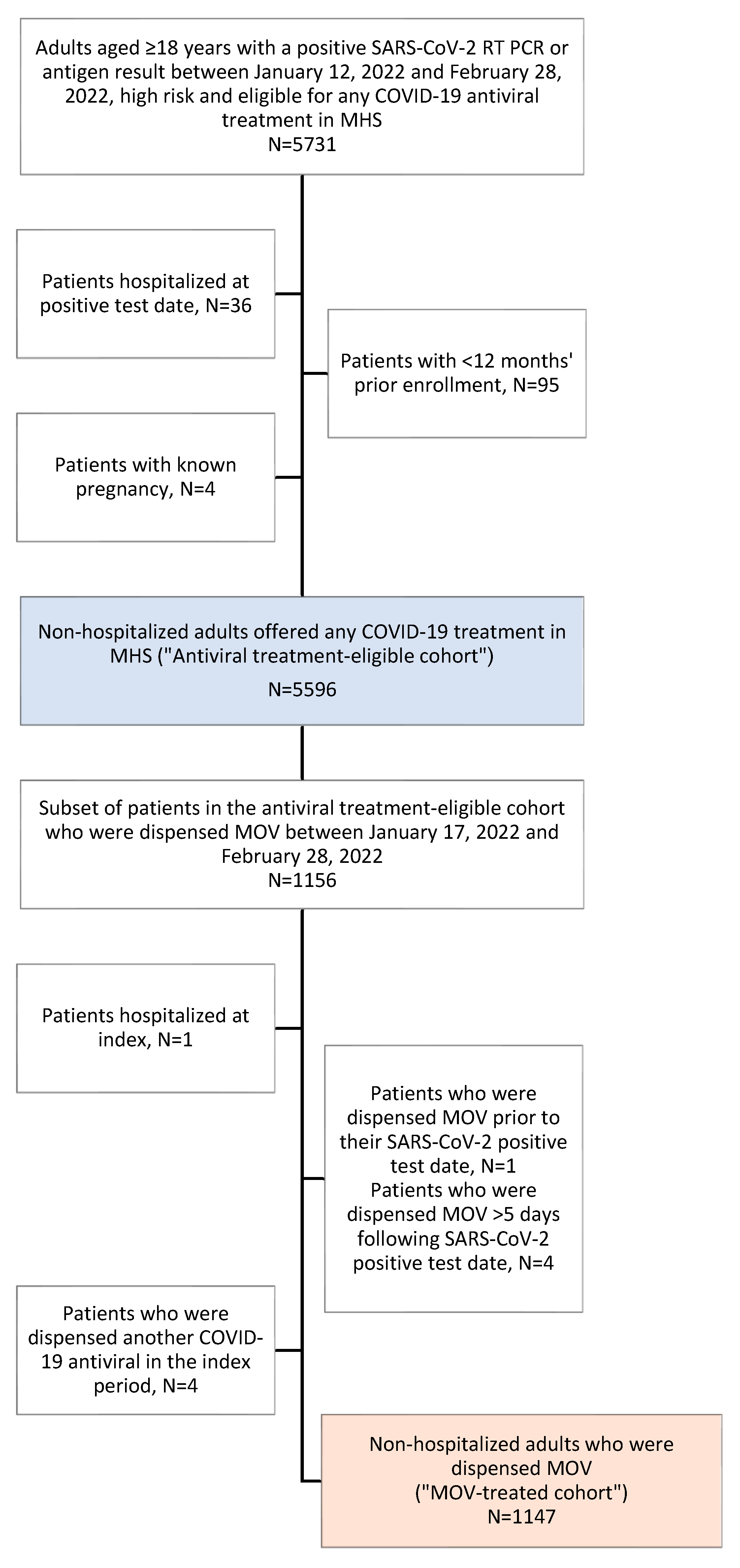
| Patient Characteristics | MOV-Treated Cohort, N = 1147 1 | Overall Antiviral Treatment-Eligible Cohort, N = 5596 2 |
|---|---|---|
| Age, years | ||
| Median (IQR) | 74.1 (64.3, 81.7) | 70.5 (61.1, 77.3) |
| Range | 21.0, 101.0 | 18.6, 103.2 |
| Age group, years | ||
| 18–64 | 299 (26.1%) | 1947 (34.8%) |
| 65+ | 848 (73.9%) | 3649 (65.2%) |
| Sex | ||
| Male | 610 (53.2%) | 2819 (50.4%) |
| Female | 537 (46.8%) | 2777 (49.6%) |
| Residential area | ||
| North | 219 (19.1%) | 1078 (19.3%) |
| Sharon | 231 (20.1%) | 1280 (22.9%) |
| South | 239 (20.8%) | 873 (15.6%) |
| Center | 192 (16.7%) | 1095 (19.6%) |
| Jerusalem and Shfela | 266 (23.2%) | 1270 (22.7%) |
| Socioeconomic status | ||
| Low | 227 (19.8%) | 900 (16.1%) |
| Med | 425 (37.1%) | 1910 (34.1%) |
| High | 494 (43.1%) | 2774 (49.6%) |
| Missing | 1 (0.1%) | 12 (0.2%) |
| COVID-19 vaccination, ever | 1048 (91.4%) | 4986 (89.1%) |
| Days since the last vaccine dose | ||
| N vaccinated (%) | 1048 (91.4%) | 4986 (89.1%) |
| Median (IQR) | 139.0 (33.0, 182.0) | 151.0 (32.0, 178.0) |
| Previous SARS-CoV-2 infection, ever | 39 (3.4%) | 217 (3.9%) |
| Previous SARS-CoV-2 infection, last 180 days | 7 (0.6%) | 29 (0.5%) |
| COVID-19 vaccination and/or infection | ||
| Unvaccinated (0 doses) without prior infection | 89 (7.8%) | 531 (9.5%) |
| Vaccinated (≥1 dose) and/or previously infected, with the most recent vaccine/infection >180 days prior | 280 (24.4%) | 1210 (21.6%) |
| Vaccinated (≥1 dose) and/or previously infected, with the most recent vaccine/infection ≤180 days prior | 778 (67.8%) | 3855 (68.9%) |
| BMI category (kg/m2) | ||
| Underweight (<18.5) | 10 (0.9%) | 40 (0.7%) |
| Normal (18.5–24.9) | 241 (21.0%) | 1154 (20.6%) |
| Overweight (25.0–29.9) | 418 (36.4%) | 2078 (37.1%) |
| Obese (30.0+) | 454 (39.6%) | 2170 (38.8%) |
| Missing | 24 (2.1%) | 154 (2.8%) |
| Comorbidities | ||
| Cancer | 375 (32.7%) | 1631 (29.1%) |
| Diabetes | 468 (40.8%) | 2130 (38.1%) |
| Cardiovascular disease | 654 (57.0%) | 2179 (38.9%) |
| Chronic kidney disease | 764 (66.6%) | 2658 (47.5%) |
| Hypertension | 928 (80.9%) | 3732 (66.7%) |
| Immunosuppression | 458 (39.9%) | 1730 (30.9%) |
| Liver disease | 62 (5.4%) | 306 (5.5%) |
| Neurological disorders | 465 (40.5%) | 2092 (37.4%) |
| Lung disease, any below | 184 (16.0%) | 739 (13.2%) |
| COPD | 137 (11.9%) | 530 (9.5%) |
| Asthma | 43 (3.7%) | 231 (4.1%) |
| Bronchiectasis | 16 (1.4%) | 60 (1.1%) |
| Cystic fibrosis | 0 (0.0%) | 1 (0.0%) |
| Interstitial lung disease | 0 (0.0%) | 2 (0.0%) |
| Pulmonary embolism | 19 (1.7%) | 32 (0.6%) |
| Pulmonary hypertension | 3 (0.3%) | 8 (0.1%) |
| Tuberculosis | 0 (0.0%) | 2 (0.0%) |
| Disaccharide/monosaccharide deficiencies and malabsorption | 6 (0.5%) | 21 (0.4%) |
| Sickle cell disease | 0 (0.0%) | 1 (0.0%) |
| Thalassemia | 0 (0.0%) | 5 (0.1%) |
| N comorbidities listed above 3 | ||
| N | 1147 | 5596 |
| Median (IQR) | 4.0 (3.0, 5.0) | 3.0 (2.0, 4.0) |
| Range | 0.0, 8.0 | 0.0, 9.0 |
| N comorbidities listed above ≥2 | 1076 (93.8%) | 4701 (84.0%) |
| Smoking, ever | 88 (7.7%) | 484 (8.6%) |
| eGFR 4, mL/min/1.73 m2 | ||
| 90+ | 175 (15.3%) | 1137 (20.3%) |
| 60–89 | 390 (34.0%) | 2023 (36.2%) |
| 30–59 | 275 (24.0%) | 780 (13.9%) |
| 15–29 | 43 (3.7%) | 62 (1.1%) |
| <15 | 13 (1.1%) | 17 (0.3%) |
| Patient Characteristics | Total N | Cumulative Incidence (95% CI), 28 Days Post-Index | |||
|---|---|---|---|---|---|
| A. COVID-19-Related Hospitalization | B. All-Cause Hospitalization | C. All-Cause Mortality | D. Composite Outcome 1 | ||
| Overall | 1147 | 3.3% (2.3%, 4.3%) | 7.2% (5.7%, 8.7%) | 1.1% (0.5%, 1.7%) | 3.6% (2.5%, 4.6%) |
| Age group, years | |||||
| 18–64 | 299 | 3.3% (1.3%, 5.4%) | 5.7% (3.0%, 8.3%) | 0.3% (0%, 1.0%) | 3.3% (1.3%, 5.4%) |
| 65+ | 848 | 3.3% (2.1%, 4.5%) | 7.8% (6.0%, 9.6%) | 1.4% (0.6%, 2.2%) | 3.7% (2.4%, 4.9%) |
| Sex | |||||
| Male | 610 | 3.0% (1.6%, 4.3%) | 7.1% (5.0%, 9.1%) | 1.6% (0.6%, 2.6%) | 3.4% (2.0%, 4.9%) |
| Female | 537 | 3.7% (2.1%, 5.3%) | 7.4% (5.2%, 9.6%) | 0.6% (0%, 1.2%) | 3.7% (2.1%, 5.3%) |
| COVID-19 vaccination, ≥1 dose | |||||
| No | 99 | 12% (5.5%, 18%) | 18% (10%, 25%) | 3.0% (0%, 6.3%) | 12% (5.5%, 18%) |
| Yes | 1048 | 2.5% (1.5%, 3.4%) | 6.2% (4.7%, 7.7%) | 1.0% (0.4%, 1.5%) | 2.8% (1.8%, 3.8%) |
| COVID-19 vaccination and/or previous SARS-CoV-2 infection | |||||
| Unvaccinated (0 doses) without prior infection | 89 | 12% (5.2%, 19%) | 19% (11%, 27%) | 3.4% (0%, 7.0%) | 12% (5.2%, 19%) |
| Vaccinated (≥1 dose) and/or previously infected, with the most recent vaccine/infection >180 days prior | 280 | 3.9% (1.6%, 6.2%) | 7.9% (4.7%, 11%) | 0.7% (0%, 1.7%) | 3.9% (1.6%, 6.2%) |
| Vaccinated (≥1 dose) and/or previously infected, with the most recent vaccine/infection ≤180 days prior | 778 | 2.1% (1.1%, 3.1%) | 5.7% (4.0%, 7.3%) | 1.0% (0.3%, 1.7%) | 2.4% (1.4%, 3.5%) |
| BMI category (kg/m2) | |||||
| Normal (18.5–24.9) | 241 | 5.4% (2.5%, 8.2%) | 11% (6.8%, 15%) | 1.2% (0%, 2.6%) | 5.8% (2.8%, 8.7%) |
| Underweight (<18.5) | 10 | 30% (0%, 53%) | 30% (0%, 53%) | 10.0% (0%, 27%) | 30% (0%, 53%) |
| Overweight (25.0–29.9) | 418 | 3.1% (1.4%, 4.8%) | 7.9% (5.3%, 10%) | 1.4% (0.3%, 2.6%) | 3.3% (1.6%, 5.1%) |
| Obese (30.0+) | 454 | 2.0% (0.7%, 3.3%) | 4.6% (2.7%, 6.5%) | 0.7% (0%, 1.4%) | 2.2% (0.8%, 3.5%) |
| Missing | 24 | 0% (0%, 0%) | 0% (0%, 0%) | 0% (0%, 0%) | 0% (0%, 0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weil, C.; Bergroth, T.; Eisenberg, A.; Whiteside, Y.O.; Caraco, Y.; Tene, L.; Chodick, G. Real-World Utilization of Molnupiravir during the COVID-19 Omicron Surge in Israel. Epidemiologia 2023, 4, 309-321. https://doi.org/10.3390/epidemiologia4030031
Weil C, Bergroth T, Eisenberg A, Whiteside YO, Caraco Y, Tene L, Chodick G. Real-World Utilization of Molnupiravir during the COVID-19 Omicron Surge in Israel. Epidemiologia. 2023; 4(3):309-321. https://doi.org/10.3390/epidemiologia4030031
Chicago/Turabian StyleWeil, Clara, Tobias Bergroth, Anna Eisenberg, Yohance Omar Whiteside, Yoseph Caraco, Lilac Tene, and Gabriel Chodick. 2023. "Real-World Utilization of Molnupiravir during the COVID-19 Omicron Surge in Israel" Epidemiologia 4, no. 3: 309-321. https://doi.org/10.3390/epidemiologia4030031
APA StyleWeil, C., Bergroth, T., Eisenberg, A., Whiteside, Y. O., Caraco, Y., Tene, L., & Chodick, G. (2023). Real-World Utilization of Molnupiravir during the COVID-19 Omicron Surge in Israel. Epidemiologia, 4(3), 309-321. https://doi.org/10.3390/epidemiologia4030031








