Predictors of Vaccine Uptake among Migrants in the United States: A Rapid Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion, Exclusion, and Data Extraction
2.2. Search Strategy
2.3. Data Extraction
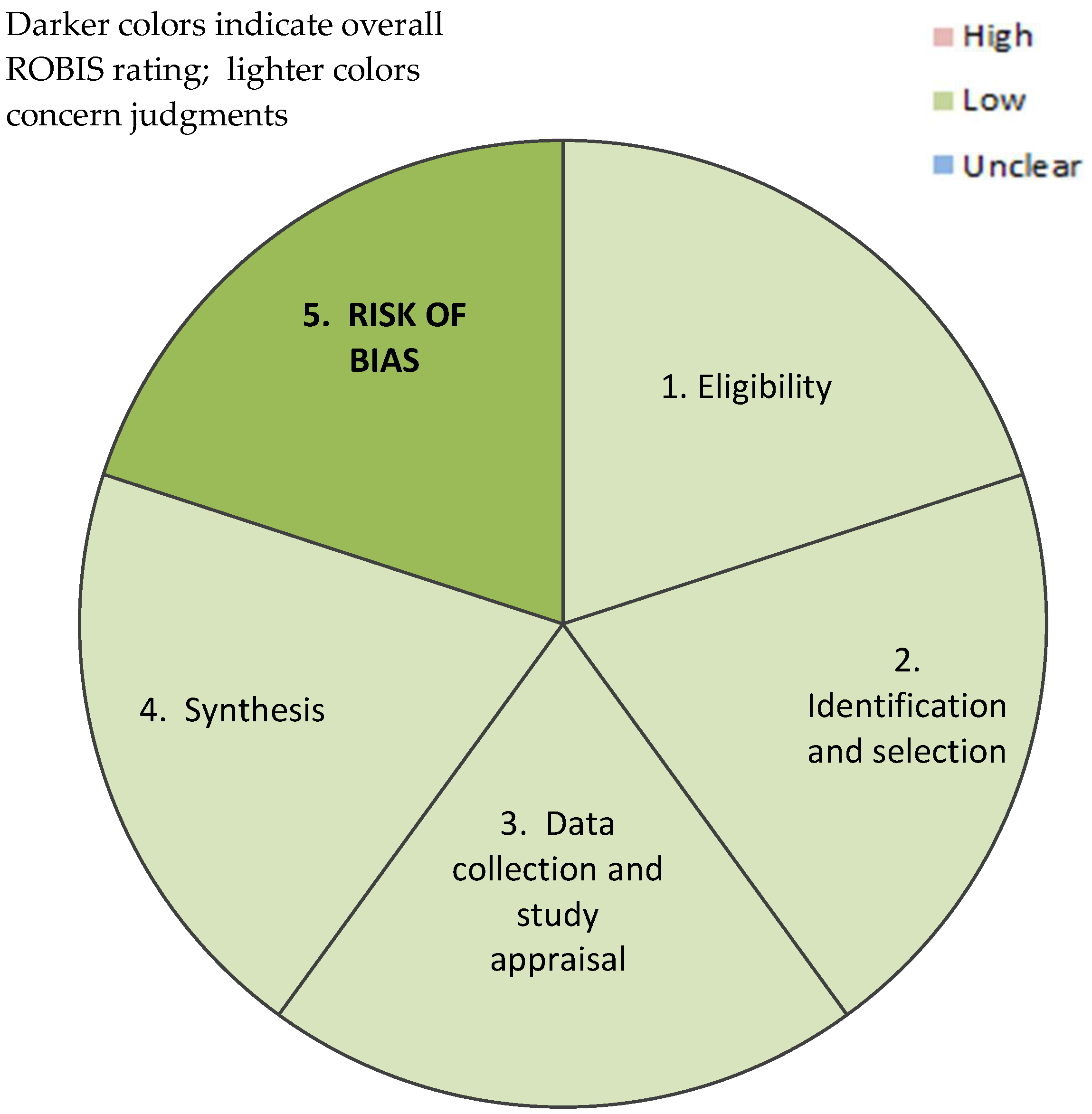
2.4. Risk Assessment
2.5. Data Synthesis and Analysis
3. Results
4. Discussion
4.1. Childhood Immunization
4.2. COVID-19 Vaccine
4.3. Seasonal Flu Vaccine
4.4. H1N1 Influenza Virus Vaccine
4.5. HPV Vaccine
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study Author(s) | Year(s) of Study | Study Design | Method | Vaccine | Study Population | Total Sample Size | Total Migrant Sample Size | Objective Addressed | Predictors Identified | |
|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (n) | (%) | ||||||||
| Sun et al. | 1996 | Cross-sectional | Survey | No particular vaccine noted | Children born in the US- vs. non-US-born | 270 | 211 | 78.1 | Vaccine uptake among US vs. non-US-born children in NYC | US residential duration (+) 1 Non-English primary language (−) Health insurance (+) Family Income (+) Immigration status (−) 2 |
| Page et al. | 2021 | Cross-sectional | Survey | COVID-19 | Undocumented migrants Age ≥ 16 years based in: US, Switzerland, Italy, and France | 812 | 142 from Baltimore 441 from Geneva 126 from Milano 103 from Paris | 17.5 54.3 15.5 12.7 | Perceived Accessibility Drivers and barriers for Demand | Gender (female) (+) Age (+) Comorbidity (+) Attitudes towards vaccination (+) |
| Victoria H. Buelow and Jennifer Van Hook | 2007 | Cross-sectional | Looking at childhood immunization from 2000–2003 (NHIS) | Combined 4:3:1:3:3 series 3 | Children 19 months to 5 years of the US-born mothers vs. non-US-born mothers who live with mothers and have exact dates of vaccination | 3947 | 1227 children of foreign-born mothers | 31.1 | The influence of parental immigration status, citizenship, and the residential duration on the completion of the childhood immunization series | Parental nativity (“composition”) (−) US citizenship (+) US residential duration (+) Health insurance (+) Socioeconomics (−) |
| Ejebe et al. | 2013 | Cross-sectional | Survey | Seasonal influenza | Adult Mexican migrants crossing at key transit points in Tijuana, Mexico | 2313 | 2313 | 100 | The uptake of the seasonal flu vaccine uptake | Gender (female) (+) comorbidity (+) Health insurance (+) |
| Zhang et al. | 2020–2021 | Cross-sectional | Survey | COVID-19 | Adult refugees | 435 | 166 from Bhutan 113 from Somalia 68 from Afghanistan 39 from South Sudan 34 from Burma/Myanmar | 38.2 26.0 15.6 9.0 7.8 | The acceptance of the COVID-19 vaccine and determinants of its uptake | Gender (male) (+) Employment as an essential worker (+) |
| Varan et al. | 2016 | Cross-sectional | Analysis of the National Immunization Survey 2010–2012 that assesses vaccination coverage among US children aged 19–35 months | Individual vaccines 4 and Combined 4:3:1:3*:3:1:4 series 6 | Children aged 19–35 months born in the US vs. non-US-born | 52,441 | 491 | 0.94 | Vaccination rate in the US children aged 19–35 months among different demographic characteristics | Nativity (−) Ethnicity/race (+) 5 Age of child (+) Study interview language (+) 7 Maternal education (+) Income: Poverty ratio (+) Midwest/ Southern region of residence (+) Health insurance (+) Birth order (−) Multiple health care providers (−) |
| Rodriguez-Lainz et al. | 2010 and 2012 | Cross-sectional | Survey | H1N1 pandemic influenza vaccine in 2010 Seasonal flu vaccine in 2012 | Adult migrant crossing the US–Mexico border in 2010 and 2012 in CA and TX | 559 participants in 2010 1423 participants in 2012 | 559 1423 | 100 100 | The uptake of the H1N1 pandemic flu vaccine in 2010 and seasonal flu vaccine in 2012 among the US–Mexico border crossers | Regarding H1N1 flu vaccine in 2010: Level of education (−) Residence location (Mexico vs. US border) Regarding seasonal flu in 2012: Comorbidity (+) Health insurance (+) Regarding both vaccines: Age (+) Frequency of border crossing (−) |
| Cofie et al. | 2019 | Cross-sectional | Analysis of pooled 2013–2017 National Health Interview Survey (NHIS) | HPV vaccine initiation | Black adults aged 18–37 years (the US-born vs. non-US-born; the study did not specify the country of birth) | 5246 | (n) was not specified Africa Mexico/ Caribbean Islands/South America | 54.5 33.1 | The HPV vaccine initiation among Black adults 18–37 years (the US-born vs. non-US-born) and its associated predictors | Gender (female) (+) Country of birth (+) 8 College degree (+) Age at HPV vaccine initiation (−) |
| Chuey et al. | 2021 | Longitudinal study | Analysis of pooled 2012–2013 to 2017–2018 National Health Interview Survey (NHIS) | Seasonal flu | Adults (the US-born vs. non-US-born) | 2012–2013 31,077 2013–2014 33,126 2014–2015 32,790 2015–2016 29,345 2016–2017 27,279 2017–2018 24,495 | 5989 6100 6067 4832 3936 3732 | 17.7 18.1 18.5 18.9 18.7 18.7 | The seasonal flu vaccine uptake among adults in the US using NHIS 2012–2018 | US citizenship (+) Nativity (−) 9 |
Appendix B
| Study | Random Sampling Method | Sample Size | Face/Construct Validity of the Survey | Appropriate Statistical Methods | Overall Rating | Comments |
|---|---|---|---|---|---|---|
| Sun et al. | Low concern | Low concern | Low concern | High concern | Low concern | The p-value was set to 0.10, and the tests were run multiple times, which might create type I error. |
| Page et al. | Medium concern | Medium concern | Low concern | Medium concern | Medium concern | The frequency of running the statistical tests might undermine the significance of some of the assessed variables. Additionally, the nonrandom sampling could lead to potential bias or issues with external validity. |
| Victoria H. Buelow and Jennifer Van Hook | Low concern | Low concern | Low concern | Low concern | Low concern | Not applicable. |
| Ejebe et al. | Low concern | Low concern | Low concern | Low concern | Low concern | Not applicable. |
| Zhang et al. | Medium concern | Low concern | High concern | Medium concern | Medium concern | The snowball sampling method with no response rate and no apparent check of interrater reliability (if there were even multiple raters) of participant responses for assignment to categories leads to concern about dependency of participants and potential bias in results. |
| Varan et al. | Low concern | Low concern | Low concern | Low concern | Low concern | Not applicable. |
| Rodriguez-Lainz et al. | Low concern | Low concern | Medium concern | High concern | Medium concern | Little verification of the survey itself along with collapsing data from multiple sites without weighing the data or correcting for multiple comparisons undercuts the significance of the results. |
| Cofie et al. | Low concern | Low concern | Low concern | Low concern | Low concern | Not applicable. |
| Chuey et al. | Low concern | Low concern | Low concern | High concern | Medium concern | Many types of tests run including linear regression and t-tests without any verification of the assumptions of those tests. Data was subdivided many times, which makes the few significant results questionable given the number of categories and comparisons. |
References
- Geoghegan, S.; O’Callaghan, K.P.; Offit, P.A. Vaccine Safety: Myths and Misinformation. Front. Microbiol. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Salmon, D.A.; Omer, S.B. Epidemiology of vaccine hesitancy in the United States. Hum. Vaccin. Immunother. 2013, 9, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Khubchandani, J.; Sharma, S.K.; Price, J.H.; Wiblishauser, M.; Sharma, M.K.; Webb, F.J. COVID-19 Vaccination Hesitancy in the United States: A Rapid National Assessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Bagasra, A.B.; Doan, S.; Allen, C.T. Racial differences in institutional trust and COVID-19 vaccine hesitancy and refusal. BMC Public Health 2021, 21, 2104. [Google Scholar] [CrossRef]
- Cofie, L.E.; Tailor, H.D.; Lee, M.H.; Xu, L. HPV vaccination uptake among foreign-born Blacks in the US: Insights from the National Health Interview Survey 2013–2017. Cancer Causes Control 2022, 33, 583–591. [Google Scholar] [CrossRef] [PubMed]
- CDC. Immigrant, Refugee, and Migrant Health. Available online: https://www.cdc.gov/immigrantrefugeehealth/about-irmh.html (accessed on 13 August 2022).
- CDC. Medical Examination: Frequently Asked Questions (FAQs). Available online: https://www.cdc.gov/immigrantrefugeehealth/about/medical-exam-faqs.html (accessed on 13 August 2022).
- CDC. Vaccines for Immigrants and Refugees. Available online: https://www.cdc.gov/vaccines/adults/rec-vac/immigrants-refugees.html#:~:text=immigrants%20will%20settleVaccination%20for%20Refugees,in%20the%20process%20of%20expansion (accessed on 13 August 2022).
- Kimmel, S.R.; Burns, I.T.; Wolfe, R.M.; Zimmerman, R.K. Addressing immunization barriers, benefits, and risks. J. Fam. Pract. 2007, 56, S61–S69. [Google Scholar] [PubMed]
- Anderson, E.L. Recommended solutions to the barriers to immunization in children and adults. Missouri Med. 2014, 111, 344–348. [Google Scholar]
- Pastula, D.M.; Copeland, M.J.; Hannan, M.C.; Rapaka, S.; Kitani, T.; Kleiner, E.; Showler, A.; Yuen, C.; Ferriman, E.M.; House, J.; et al. Two Cases of Monkeypox-Associated Encephalomyelitis—Colorado and the District of Columbia, July-August 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 71, 1212–1215. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Defining the Criteria for Including Studies and How They Will Be Grouped for the Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022. [Google Scholar]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.; Sangweni, B.; Butts, G.; Merlino, M. Comparisons of immunisation accessibility between non-US born and US-born children in New York City. Public Health 1998, 112, 405–408. [Google Scholar] [CrossRef]
- Varan, A.K.; Rodriguez-Lainz, A.; Hill, H.A.; Elam-Evans, L.D.; Yankey, D.; Li, Q. Vaccination Coverage Disparities Between Foreign-Born and U.S.-Born Children Aged 19–35 Months, United States, 2010–2012. J. Immigr. Minor. Health 2017, 19, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Buelow, V.H.; Van Hook, J. Timely immunization series completion among children of immigrants. J. Immigr. Minor. Health 2008, 10, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Page, K.R.; Genovese, E.; Franchi, M.; Cella, S.; Fiorini, G.; Tlili, R.; Salazar, S.; Duvoisin, A.; Cailhol, J.; Jackson, Y. COVID-19 vaccine hesitancy among undocumented migrants during the early phase of the vaccination campaign: A multicentric cross-sectional study. BMJ Open 2022, 12, e056591. [Google Scholar] [CrossRef]
- Rodriguez-Lainz, A.; DeSisto, C.; Waterman, S.; Wiedemann, M.S.; Moore, C.W.; Williams, W.W.; Moser, K. Influenza vaccination coverage among US-Mexico land border crossers: 2009 H1N1 pandemic and 2011–2012 influenza season. Travel Med. Infect. Dis. 2019, 27, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Chuey, M.R.; Hung, M.-C.; Srivastav, A.; Lu, P.-J.; Nguyen, K.H.; Williams, W.W.; Lainz, A.R. Influenza vaccination coverage among adults by nativity, race/ethnicity, citizenship, and language of the interview—United States, 2012–2013 through 2017–2018 influenza seasons. Am. J. Infect. Control 2022, 50, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Ejebe, I.H.; Zhang, X.; Rangel, M.G.; Martinez-Donate, A.P. Seasonal influenza vaccination among Mexican migrants traveling through the Mexico-US border region. Prev. Med. 2015, 71, 57–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chamnan, P.; Jaranit, K.; Weerawan, H.; Witaya, S.; Rakdaw, M.; Aree, M.; Saranath, L. Evaluation of Immunization Services for Children of Migrant Workers Along Thailand–Myanmar Border: Compliance with Global Vaccine Action Plan (2011–2020). Vaccines 2020, 8, 68. [Google Scholar] [CrossRef]
- Zhang, M.; Gurung, A.; Anglewicz, P.; Subedi, P.; Payton, C.; Ali, A.; Ibrahim, A.; Haider, M.; Hamidi, N.; Atem, J.; et al. Acceptance of COVID-19 Vaccine Among Refugees in the United States. Public Health Rep. 2021, 136, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Ravensbergen, S.J.; Nellums, L.B.; Hargreaves, S.; Stienstra, Y.; Friedland, J.S. National approaches to the vaccination of recently arrived migrants in Europe: A comparative policy analysis across 32 European countries. Travel Med. Infect. Dis. 2019, 27, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Abdi, I.; Gidding, H.; Leong, R.N.; Moore, H.C.; Seale, H.; Menzies, R. Vaccine coverage in children born to migrant mothers in Australia: A population-based cohort study. Vaccine 2021, 39, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, Q.; Luo, S.; Lou, L.; Qi, X.; Xie, S. Timeliness vaccination of measles containing vaccine and barriers to vaccination among migrant children in East China. PLoS ONE 2013, 8, e73264. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ma, R.; Zeng, Y.; Luo, F.; Zhang, J.; Hou, W. Immunization status and risk factors of migrant children in densely populated areas of Beijing, China. Vaccine 2010, 28, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Abela, M.; Melissa, K.; Lidija, S.; Mitchell, S.; Angela, D. Factors influencing refugees’ willingness to accept COVID-19 vaccines in Greater Sydney: A qualitative study. Aust. N. Z. J. Public Health 2022, 46, 502–510. [Google Scholar] [CrossRef]
- Belinda, J.L.; Stephanie, M.; Vicki, M.; Richard, B.; Meaghan, O.D.; Tadgh, M.; Angela, N. Factors associated with COVID-19 vaccine hesitancy amongst refugees in Australia. Eur. J. Psychotraumatology 2021, 12. [Google Scholar] [CrossRef]
- Salibi, N.; Abdulrahim, S.; El Haddad, M.; Bassil, S.; El Khoury, Z.; Ghattas, H.; McCall, S.J. COVID-19 vaccine acceptance in older Syrian refugees: Preliminary findings from an ongoing study. Prev. Med. Rep. 2021, 24, 101606. [Google Scholar] [CrossRef] [PubMed]
- May, S.; Ezinne, N.; Hye Young, C. Immigration enforcement exposures and COVID-19 vaccine intentions among undocumented immigrants in California. Prev. Med. Rep. 2022, 27. [Google Scholar] [CrossRef]
- Ogilvie, G.S.; Gordon, S.; Smith, L.W.; Albert, A.; Racey, C.S.; Booth, A.; Gottschlich, A.; Goldfarb, D.; Murray, M.C.M.; Galea, L.A.M.; et al. Intention to receive a COVID-19 vaccine: Results from a population-based survey in Canada. BMC Public Health 2021, 21, 1017. [Google Scholar] [CrossRef] [PubMed]
- Rezvi Rafiqul, A.H.M.; Hossen, M.S.; Huda, S.N. COVID-19 Vaccination and Undocumented Migrants. Asia Pac. J. Public Health 2021, 34, 298–299. [Google Scholar] [CrossRef]
- CDC. People at Higher Risk of Flu Complications. Available online: https://www.cdc.gov/flu/highrisk/index.htm (accessed on 9 August 2022).
- McFadden, S.M.; Demeke, J.; Dada, D.; Wilton, L.; Wang, M.; Vlahov, D.; Nelson, L.E. Confidence and Hesitancy During the Early Roll-out of COVID-19 Vaccines Among Black, Hispanic, and Undocumented Immigrant Communities: A Review. J. Urban Health 2022, 99, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Kang, J. Factors Associated with Influenza Vaccination Uptake among U.S. Adults: Focus on Nativity and Race/Ethnicity. Int. J. Environ. Res. Public Health 2021, 18, 5349. [Google Scholar] [CrossRef] [PubMed]
- Agénor, M.; Abboud, S.; Jazmine Garcia, D.; Pérez, A.E.; Peitzmeier, S.M.; Borrero, S. Intersectional nativity and racial/ethnic disparities in human papillomavirus vaccination initiation among U.S. women: A national population-based study. Cancer Causes Control 2018, 29, 927–936. [Google Scholar] [CrossRef]
- Pérez, A.E.; Agénor, M.; Gamarel, K.E.; Operario, D. Nativity Disparities in Human Papillomavirus Vaccination Among U.S. Adults. Am. J. Prev. Med. 2018, 54, 248–258. [Google Scholar] [CrossRef] [PubMed]
- WHO. Immunization Agenda 2030: A Global Strategy to Leave No One Behind. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030 (accessed on 8 August 2022).
- Shetty, A.K. Infectious Diseases among Refugee Children. Children 2019, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Gonzalez, I.M.; Perez, K.; Cheadle, A.D.; Jade, M.; Iverson, B.; Parchman, M.L. A Multicomponent Health Education Campaign Led by Community Health Workers to Increase Influenza Vaccination among Migrants and Refugees. J. Prim. Care Community Health 2021, 12, 21501327211055627. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Misra, S.M.; Zhou, F.; Sahni, L.C.; Boom, J.A.; Messonnier, M. Evaluating Partial Series Childhood Vaccination Services in a Mobile Clinic Setting. Clin. Pediatr. 2020, 59, 706–715. [Google Scholar] [CrossRef] [PubMed]
- USDA. The Special Supplemental Nutrition Program for Women, Infants and Children (WIC Program); United States Department of Agriculture: Washington, DC, USA, 2022.
- Thomas, T.N.; Kolasa, M.S.; Zhang, F.; Shefer, A.M. Assessing Immunization Interventions in the Women, Infants, and Children (WIC) Program. Am. J. Prev. Med. 2014, 47, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.M.; Lee, H.Y.; Pratt, R.; Vang, H.; Desai, J.R.; Dube, A.; Lightfoot, E. Facilitators and Barriers of Cervical Cancer Screening and Human Papilloma Virus Vaccination Among Somali Refugee Women in the United States: A Qualitative Analysis. J. Transcult. Nurs. 2019, 30, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Searle, K.; Galván, A.; Liebman, A.K.; Mann, E.M.; Kirsch, J.D.; Stauffer, W.M. Healthcare worker perspectives on COVID-19 vaccines: Implications for increasing vaccine acceptance among healthcare workers and patients. Vaccine 2022, 40, 2612–2618. [Google Scholar] [CrossRef] [PubMed]

| PICO | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Adult, adolescent, and child migrants (foreign-born) and children of migrants (under 18 years of age, with at least one migrant parent) residing in the US. | Migrant is not defined by country of origin or birth; data were not collected in the US; data were not collected within the specified timeframe; articles that are not primary research; articles not written in English. |
| Intervention | Predictors that significantly assess the COVID-19 vaccine and other vaccine uptake in migrant populations will be examined. Papers about variables that assess the likelihood of accepting COVID-19 vaccines and other vaccines among migrant populations will be considered (e.g., country of origin, age, income, integration into society, health belief, etc.). | This review will not consider interventions that influence vaccination. |
| Comparison | No control was selected for this review. | Not applicable. |
| Outcomes | Determinants of vaccine uptake in migrant populations. | Not applicable. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Janabi, T.; Petrillo, G.; Chung, S.; Pino, M. Predictors of Vaccine Uptake among Migrants in the United States: A Rapid Systematic Review. Epidemiologia 2022, 3, 465-481. https://doi.org/10.3390/epidemiologia3040035
Al Janabi T, Petrillo G, Chung S, Pino M. Predictors of Vaccine Uptake among Migrants in the United States: A Rapid Systematic Review. Epidemiologia. 2022; 3(4):465-481. https://doi.org/10.3390/epidemiologia3040035
Chicago/Turabian StyleAl Janabi, Taysir, Gianna Petrillo, Sunny Chung, and Maria Pino. 2022. "Predictors of Vaccine Uptake among Migrants in the United States: A Rapid Systematic Review" Epidemiologia 3, no. 4: 465-481. https://doi.org/10.3390/epidemiologia3040035
APA StyleAl Janabi, T., Petrillo, G., Chung, S., & Pino, M. (2022). Predictors of Vaccine Uptake among Migrants in the United States: A Rapid Systematic Review. Epidemiologia, 3(4), 465-481. https://doi.org/10.3390/epidemiologia3040035






