Seroprevalence and Risks Factors Associated with Coxiella burnetii Infection in Slaughterhouse Zebu Cattle (Bos indicus) from Northern Regions of Cameroon
Abstract
1. Introduction
2. Materials and Methods
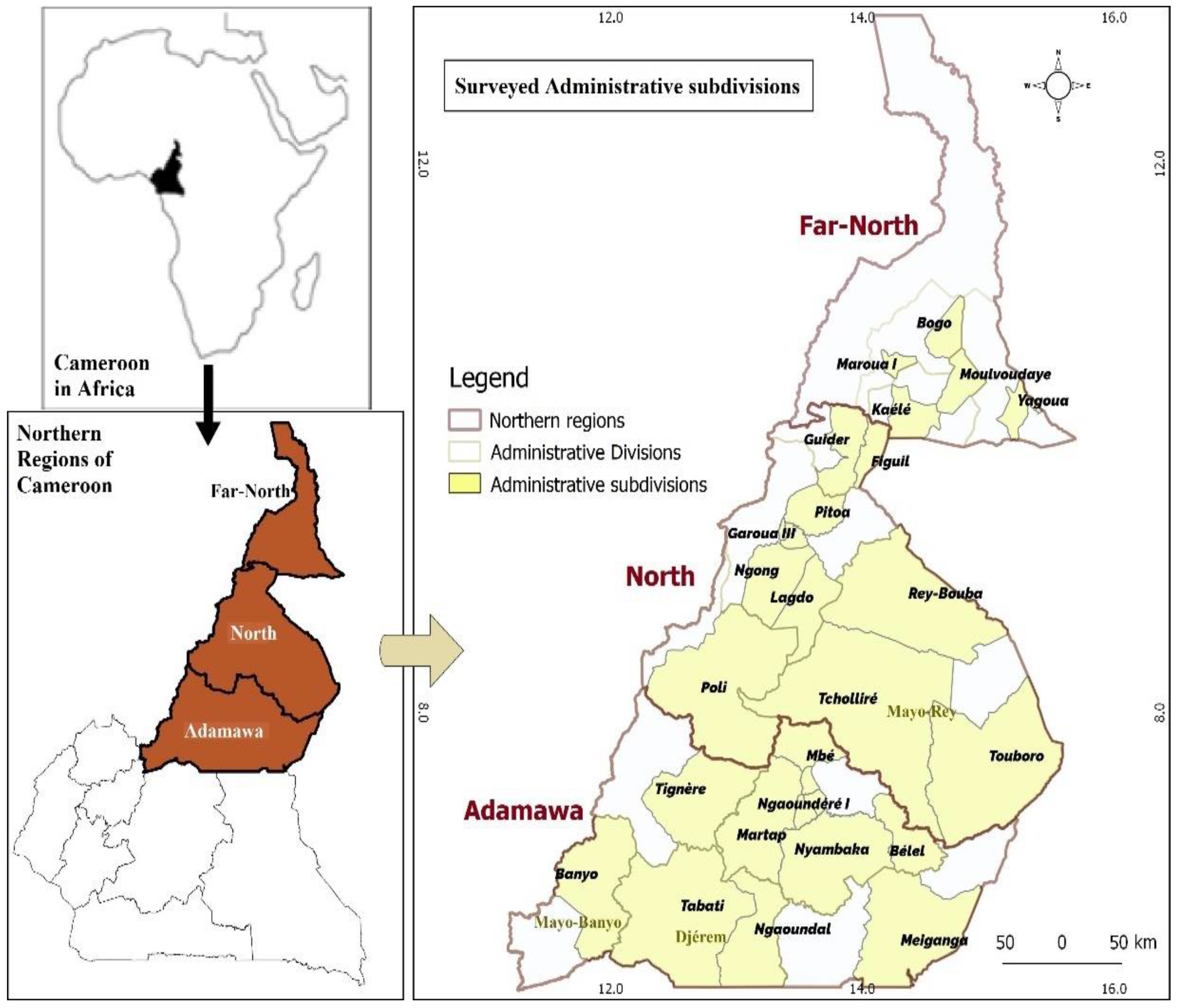
2.1. Study Area
2.2. Samples Size Determination
2.3. Specimen Collect Procedure
2.4. Laboratory Analysis and Interpretation
2.5. Statistical Analysis
3. Results
3.1. Seroprevalence of Coxiella burnetii in Northern Regions
3.2. Risks Factors of Coxiella burnetii in Northern Regions of Cameroon
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roest, H.I.J.; Tilburg, J.J.H.C.; van der Hoek, W.; Vellema, P.; Van Zijerveld, F.G.; Klaassen, C.H.W.; Raoult, D. The Q fever epidemic in The Netherlands: History, onset, response and reflection. Epidemiol. Infect. 2011, 139, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shome, R.; Deka, R.P.; Milesh, L.; Sahay, S.; Grace, D.; Lindahl, J.F. Coxiella seroprevalence and risk factors in large ruminants in Bihar and Assam. India. Acta Trop. 2019, 194, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tokue, Y.; Kikuchi, T.; Kobayashi, T.; Gomi, K.; Goto, I.; Shiraishi, H.; Fukushi, H.; Hirai, K.; Nukiwa, T.; et al. Prevalence of community-acquired respiratory tract infections associated with Q fever in Japan. Diagn. Microbiol. Infect. Dis. 2004, 48, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Van den Brom, R.; Van Engelen, E.; Luttikholt, S.; Moll, L.; Van Maanen, K.; Vellema, P. Coxiella burnetii in bulk tank milk samples from dairy goat and dairy sheep farms in The Netherlands in 2008. Vet. Rec. 2012, 170, 310. [Google Scholar] [CrossRef]
- Kersh, G.J.; Fitzpatrick, K.; Pletnikoff, K.; Brubaker, M.; Bruce, M.; Parkinson, A. Prevalence of serum antibodies to Coxiella burnetii in Alaska Native Persons from the Pribilof Islands. Zoonoses Public Health 2020, 67, 89–92. [Google Scholar] [CrossRef]
- Echeverría, G.; Reyna-Bello, A.; Minda-Aluisa, E.; Celi-Erazo, M.; Olmedo, L.; Garcia, H.A.; Garcia-Bereguiain, M.A.; De Waard, J.H. Serological evidence of Coxiella burnetii infection in cattle and farm workers: Is Q fever an underreported zoonotic disease in Ecuador? Infect. Drug Resist. 2019, 12, 701–706. [Google Scholar] [CrossRef]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef]
- Ghasemi, A.; Hajinezhad, M.R.; Esmaeili, S.; Mostafavi, E. Seroprevalence of Q Fever and Brucellosis in Domestic and Imported Cattle of Southeastern Iran. J. Med. Microbiol. Infect. Dis. 2018, 6, 48–52. [Google Scholar] [CrossRef]
- Min-Goo, S.; In-Ohk, O.; Young-Hoan, K.; Joong-Kew, K.; Oh-Deog, K.; Dongmi, K. Seroprevalence of Coxiella burnetii infection in cattle on Ulleung Island, Korea. Korean J. Vet. Res. 2018, 58, 147–151. [Google Scholar] [CrossRef]
- Adamu, S.G.; Kabi, J.; Umoh, R.J.U.; Raji, M.A. Seroprevalence of brucellosis and Q fever (Coxiellosis) in cattle herds in Maigana and Birnin Gwari agro-ecological zone of Kaduna State. Nigeria. Trop. Anim. Health Prod. 2018, 50, 1583–1589. [Google Scholar] [CrossRef]
- Djellata, N.; Yahimi, A.; Hanzen, C.; Saegerman, C.; Kaidi, R. Prevalence and factors associated with a higher or lower risk of exposure to Coxiella burnetii, Chlamydia abortus and Toxoplasma gondii in dairy cows that have aborted in Algeria. Rev. Sci. Tech. Off. Int. Epiz. 2019, 38, 775–786. [Google Scholar] [CrossRef]
- Cadmus, I.S.; Akporube, A.K.; Ola-Daniel, F.; Adelakun, D.O.; Akinseye, O.V. Seroprevalence and associated factors of brucellosis and Q-fever in cattle from Ibarapa area, Oyo state, South-western Nigeria. Pan Afr. Med. J. 2020, 36, 370. [Google Scholar] [CrossRef] [PubMed]
- Malal, E.M.; Karagül, K.S.; Akar, K.A.A. Investigation of Q fever seroprevalence in cattle in turkey. Kocaeli Üniversitesi Sağlık Bilimleri Derg. Özgün Araştırma 2021, 7, 98–102. [Google Scholar] [CrossRef]
- Mangena, M.; Gcebe, N.; Pierneef, R.; Thompson, N.P.; Adesiyun, A.A. Q Fever: Seroprevalence, Risk Factors in Slaughter Livestock and Genotypes of Coxiella burnetii in South Africa. Pathogens 2021, 10, 258. [Google Scholar] [CrossRef]
- Kelly, F.R.; Jennings, A.; Hunt, J.; Hamman, M.S.; Mazeri, S.; Nkongho, F.E.; Ngwa, N.V.; Tanya, V.; Sander, M.; Ndip, L.; et al. The epidemiology of bacterial zoonoses in pastoral and dairy cattle in Cameroon, Central Africa. Zoonoses Public Health 2021, 68, 781–793. [Google Scholar] [CrossRef]
- May, R.M.; Anderson, R.M. Population biology of infectious diseases: Part II. Nature 1979, 280, 455–461. [Google Scholar] [CrossRef]
- Cameroon Statistical Yearbook; Chapters 1 to 3; National Institute of Statistics: Cameroon, Yaounde, 2018; pp. 3–28.
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 2013; p. 1289. [Google Scholar]
- Scolamacchia, F.; Handel, I.G.; Fevre, E.M.; Morgan, K.L.; Tanya, V.N.; Bronsvoort, B.M.C. Serological patterns of brucellosis, leptospirosis and Q fever in Bos indicus cattle in Cameroon. PLoS ONE 2010, 5, e8623. [Google Scholar] [CrossRef]
- Mioni, R.S.M.; Costa, B.F.; Ribeiro, D.L.B.; Teixeira, R.S.W.; Pelicia, C.V.; Labruna, B.M.; Rousset, E.; Sidi-Boumedine, K.; Megid, J.T.R. Coxiella burnetii in slaughterhouses in Brazil: A public health concern. PLoS ONE 2020, 15, e241246. [Google Scholar] [CrossRef]
- Deressa, B.F.; Kal, O.D.; Gelalcha, D.B.; Magalhães, S.J.R. Seroprevalence of and risk factors for Q fever in dairy and slaughterhouse cattle of Jimma town, South Western Ethiopia. BMC Vet. Res. 2020, 16, 1–10. [Google Scholar] [CrossRef]
- Aljafar, A.; Salem, M.; Housawi, F.; Zaghawa, A.; Hegazy, Y. Seroprevalence and risk factors of Q-fever (C. burnetii infection) among ruminants reared in the eastern region of the Kingdom of Saudi Arabia. Trop. Anim. Health Prod. 2020, 52, 2631–2638. [Google Scholar] [CrossRef]
- Espí, A.; Cerro, D.A.; Oleaga, A.; Rodríguez-Pérez, M.; López, M.C.; Hurtado, A.; Rodríguez-Martínez, D.L.; Barandika, F.J.; García-Pérez, L.A. One Health Approach: An Overview of Q Fever in Livestock, Wildlife and Humans in Asturias (Northwestern Spain). Animals 2021, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Abbass, H.; Selim, K.A.S.; Sobhy, M.M.; El-Mokhtar, A.M.; Elhariri, M.; Abd-Elhafeez, H.H. High prevalence of Coxiella burnetii infection in humans and livestock in Assiut, Egypt: A serological and molecular survey. Vet. World 2020, 13, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Jarelnabi, A.A.; Alshaikh, M.A.; Bakhiet, A.O.; Omer, S.A.; Aljumaah, R.S.; Harkiss, G.D.; Mohammed, B.O.; Hussein, F.M. Seroprevalence of Q fever in farm animals in Saudi Arabia. Biomed. Res. 2018, 29, 1–5. [Google Scholar] [CrossRef]
- Megersa, B.; Biffa, D.; Niguse, F.; Rufael, T.; Asmare, K.; Skjerve, E. Cattle brucellosis in traditional livestock husbandry practice in southern and eastern Ethiopia, and its zoonotic implication. Acta Vet Scand. 2011, 53, 24. [Google Scholar] [CrossRef]
- Van den Brom, R.; Moll, L.; Van Schaik, G.; Vellema, P. Demography of Q fever seroprevalence in sheep and goats in The Netherlands in 2008. Prev. Vet. Med. 2013, 109, 76–82. [Google Scholar] [CrossRef]
- Sassa, M.A.; Wassa, D.R.; Awah, N.J. Prévalence et facteurs de risque des hémoparasites chez les petits ruminants abattus dans la ville de Ngaoundéré au Cameroun. Int. J. Biol. Chem. Sci. 2019, 13, 1157–1165. [Google Scholar] [CrossRef]
- Cetinkaya, B.; Kalender, H.; Ertas, H.B.; Muz, A.; Arslan, N.; Ongor, H.; Gurçay, M. Seroprevalence of coxiellosis in cattle, sheep and people in the East of Turkey. Vet. Rec. 2000, 146, 131–136. [Google Scholar] [CrossRef]
- Mc Caughey, C.; Murray, J.; Mckenna, J.; Menzies, F.; Mccullough, S.; O’neill, H.; Wyatt, D.; Cardwell, C.; Coyle, P. Coxiella burnetii (Q fever) seroprevalence in cattle. Epidemiol. Infect. 2010, 138, 21–27. [Google Scholar] [CrossRef]
- Ghanem-Zoubi, N.; Paul, M. Q fever during pregnancy: A narrative review. Clinic. Microbiol. Infect. 2019, 26, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Bauer, U.B.; Knittler, R.M.; Herms, L.T.; Frangoulidis, D.; Matthiesen, S.; Tappe, D.; Runge, M.; Ganter, M. Multispecies Q Fever Outbreak in a Mixed Dairy Goat and Cattle Farm Based on a New Bovine-Associated Genotype of Coxiella burnetii. Vet. Sci. 2021, 8, 252. [Google Scholar] [CrossRef]
| Characteristics | Regions | ||
|---|---|---|---|
| Adamawa | North | Far North | |
| Latitude | 5°–8° N | 8°–10° N | 10°–12° N |
| Longitude | 11°–14° E | 12°–14° E | 14°–15° E |
| Surface Area (km2) | 63,701 | 66,090 | 34,263 |
| Climate | Sudano Guinean | Soudanese | Sudano sahelian |
| Mean annual temperature (°C) | 22–25 | 21–34 | 25–35 |
| Mean annual precipitation (mm) | 900–1500 | 800–900 | 600–1000 |
| Number of slaughtered animals in 2018 (Head) | 110,495 | 76,677 | 62,598 |
| Northern Regions | Divisions | Slaughterhouse | Samples Analyzed | Positive | Prevalence (%) |
|---|---|---|---|---|---|
| Adamawa | Djerem | Tibati | 74 | 18 | 24.32 |
| Ngaoundal | 65 | 14 | 21.54 | ||
| Total | 139 | 32 | 23.02 | ||
| Faro et Deo | Tignere | 47 | 4 | 8.51 | |
| Mayo Banyo | Banyo | 111 | 28 | 25.23 | |
| Mbere | Meiganga | 98 | 44 | 44.90 | |
| Vina | Ngaoundere | 201 | 74 | 36.82 | |
| Belel | 51 | 16 | 31.37 | ||
| Martap | 44 | 6 | 13.64 | ||
| Nyambaka | 48 | 11 | 22.92 | ||
| Mbe | 62 | 18 | 29.03 | ||
| Total | 406 | 125 | 30.78 | ||
| Total | 801 | 233 | 29.09 | ||
| North | Benoue | Garoua | 144 | 29 | 20.14 |
| Ngong | 62 | 12 | 19.35 | ||
| Lagdo | 46 | 7 | 15.22 | ||
| Pitoa | 54 | 13 | 24.07 | ||
| Total | 306 | 61 | 19.93 | ||
| Faro | Poli | 51 | 3 | 5.88 | |
| Mayo louti | Figuil | 71 | 16 | 22.54 | |
| Guider | 68 | 17 | 25.00 | ||
| Total | 139 | 33 | 23.74 | ||
| Mayo Rey | Touboro | 92 | 21 | 22.83 | |
| Rey Bouba | 111 | 26 | 23.42 | ||
| Tchollire | 63 | 8 | 12.70 | ||
| Total | 266 | 55 | 20.68 | ||
| Total | 762 | 152 | 19.95 | ||
| Far North | Diamare | Maroua | 149 | 37 | 24.83 |
| Bogo | 69 | 10 | 14.49 | ||
| Total | 218 | 46 | 21.10 | ||
| Mayo Kani | Moulvoudaye | 66 | 19 | 28.79 | |
| Kaele | 82 | 12 | 14.63 | ||
| Total | 148 | 31 | 20.95 | ||
| Mayo Danay | Yagoua | 87 | 16 | 18.39 | |
| Total | 453 | 94 | 20.75 |
| Variable | Number Tested | Number Positive | Prevalence (%) | p Value | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| Regions | Far North | 453 | 94 | 20.75 | Referent | ||
| Adamawa | 801 | 233 | 29.09 | 0.02 * | 3.28 | 1.13–7.85 | |
| North | 762 | 152 | 19.95 | 0.49 | 3.02 | 1.06–6.90 | |
| Breed | Aku | 563 | 121 | 21.49 | Referent | ||
| Bokolo | 162 | 43 | 26.54 | 0.85 | 1.10 | 0.41–2.96 | |
| Djafun | 408 | 83 | 20.34 | 0.43 | 1.35 | 0.64–2.85 | |
| Gudali | 883 | 232 | 26.27 | 0.03 * | 2.52 | 1.06–5.99 | |
| Age (years) | [3,4,5] | 1074 | 175 | 14.62 | Referent | ||
| [6,7,8,9] | 672 | 235 | 34.97 | 0.03 * | 1.89 | 1.06–3.35 | |
| ≥10 | 270 | 69 | 25.56 | 0.30 | 1.47 | 0.71–3.05 | |
| BCS | [1,2] | 1481 | 178 | 12.02 | Referent | ||
| 3 | 402 | 232 | 57.71 | 0.64 | 0.91 | 0.36–2.33 | |
| [4,5] | 133 | 69 | 51.88 | 0.84 | 1.07 | 0.42–2.41 | |
| Physiological status (female) | Non pregnant | 1497 | 358 | 23.91 | Referent | ||
| Pregnant | 257 | 109 | 42.41 | 0.04 * | 1.71 | 1.01–2.90 | |
| Sex | Male | 262 | 12 | 4.58 | Referent | ||
| Female | 1754 | 467 | 26.62 | 0.002 * | 1.92 | 0.61–3.81 | |
| Season | Dry | 1293 | 258 | 19.95 | Referent | ||
| Rainy | 723 | 221 | 30.57 | 0.13 | 1.66 | 0.69–4.01 | |
| Factors | C. burnetii (95% CI) | p Value |
|---|---|---|
| Regions | 0.08 (0.01; 0.17) | 0.005 ** |
| Breed | 0.04 (−0.02; 0.11) | 0.09 |
| Age (years) | 0.03 (−0.03; 0.11) | 0.04 * |
| BCS | −0.01 (−0.06; 0.04) | 0.46 |
| Physiological status (female) | 0.11 (−0.05; 0.22) | 0.006 ** |
| Sex | 0.19 (0.08–0.32) | 0.001 ** |
| Season | −0.14 (−0.33–0.05) | 0.16 |
| Constant | 1.19 (0.73; 1.64) | 0.000 ** |
| N = 2016 | R2 = 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangue, C.T.; Kouamo, J.; Ngoula, F.; Tawali, L.P.M.; Talla, M.M.; Mbeba, L.Y.K.; Doumtsop, C.L.M.; Tangwa, B.V. Seroprevalence and Risks Factors Associated with Coxiella burnetii Infection in Slaughterhouse Zebu Cattle (Bos indicus) from Northern Regions of Cameroon. Epidemiologia 2022, 3, 434-442. https://doi.org/10.3390/epidemiologia3040033
Zangue CT, Kouamo J, Ngoula F, Tawali LPM, Talla MM, Mbeba LYK, Doumtsop CLM, Tangwa BV. Seroprevalence and Risks Factors Associated with Coxiella burnetii Infection in Slaughterhouse Zebu Cattle (Bos indicus) from Northern Regions of Cameroon. Epidemiologia. 2022; 3(4):434-442. https://doi.org/10.3390/epidemiologia3040033
Chicago/Turabian StyleZangue, Camille Teitsa, Justin Kouamo, Ferdinand Ngoula, Ludovic Pépin M’bapté Tawali, Mathias Mba Talla, Lionnel Yvan Kantchouet Mbeba, Claude Landry Makuetamang Doumtsop, and Bernard Viban Tangwa. 2022. "Seroprevalence and Risks Factors Associated with Coxiella burnetii Infection in Slaughterhouse Zebu Cattle (Bos indicus) from Northern Regions of Cameroon" Epidemiologia 3, no. 4: 434-442. https://doi.org/10.3390/epidemiologia3040033
APA StyleZangue, C. T., Kouamo, J., Ngoula, F., Tawali, L. P. M., Talla, M. M., Mbeba, L. Y. K., Doumtsop, C. L. M., & Tangwa, B. V. (2022). Seroprevalence and Risks Factors Associated with Coxiella burnetii Infection in Slaughterhouse Zebu Cattle (Bos indicus) from Northern Regions of Cameroon. Epidemiologia, 3(4), 434-442. https://doi.org/10.3390/epidemiologia3040033






