Remnant Cholesterol: From Pathophysiology to Clinical Implications in Type 1 Diabetes
Abstract
1. Introduction
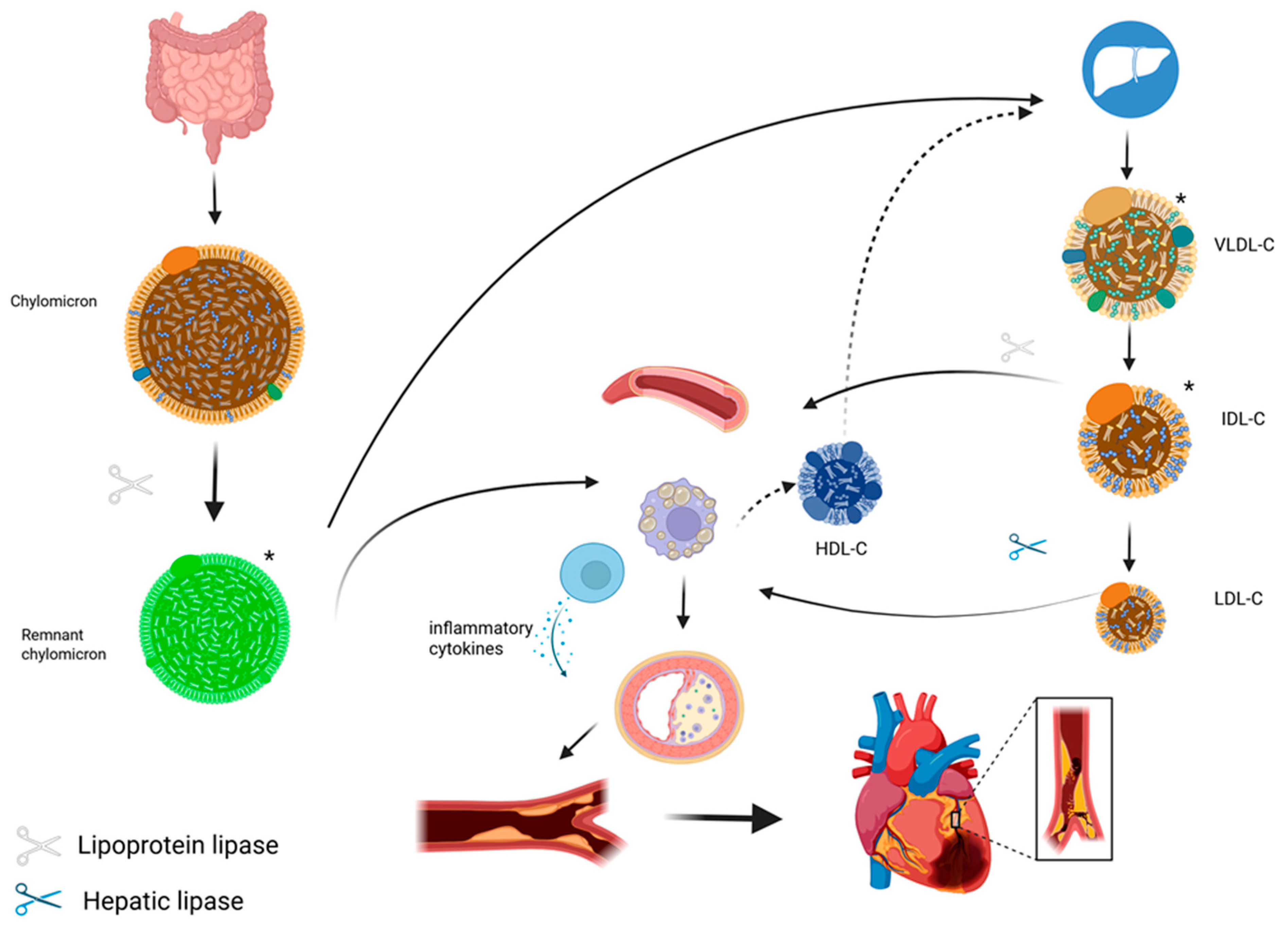
2. Pathophysiology of Remnant Cholesterol
2.1. Origin, Metabolism, and Quantification
2.2. Atherogenic and Inflammatory Mechanism
3. Epidemiological Evidence in the General Population and in Type 2 Diabetes
4. Physical Activity and Lifestyle Habits
5. Clinical Trials
6. Evidence in Type 1 Diabetes
7. Conclusions
Funding
Conflicts of Interest
References
- Castañer, O.; Pintó, X.; Subirana, I.; Amor, A.J.; Ros, E.; Hernáez, Á.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Estruch, R.; et al. Remnant Cholesterol, Not LDL Cholesterol, Is Associated with Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 76, 2712–2724. [Google Scholar] [CrossRef]
- Anette, V.; Marianne, B.; Anne, T.-H.; Anders, B.J.; Ruth, F.-S.; Børge, G.N. Remnant Cholesterol as a Causal Risk Factor for Ischemic Heart Disease. J. Am. Coll. Cardiol. 2013, 61, 427–436. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated Remnant Cholesterol Causes Both Low-Grade Inflammation and Ischemic Heart Disease, Whereas Elevated Low-Density Lipoprotein Cholesterol Causes Ischemic Heart Disease Without Inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.V.; Asselbergs, F.W.; Palmer, T.M.; Drenos, F.; Lanktree, M.B.; Nelson, C.P.; Dale, C.E.; Padmanabhan, S.; Finan, C.; Swerdlow, D.I.; et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J. 2015, 36, 539–550. [Google Scholar] [CrossRef]
- Do, R.; Willer, C.J.; Schmidt, E.M.; Sengupta, S.; Gao, C.; Peloso, G.M.; Gustafsson, S.; Kanoni, S.; Ganna, A.; Chen, J.; et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet. 2013, 45, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Pintó, X.; Fanlo, M.; Esteve, V.; Millán, J. Colesterol remanente, riesgo vascular y prevención de la arteriosclerosis. Clínica Investig. Arter. 2023, 35, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Langsted, A.; Nordestgaard, B.G. Remnant Cholesterol: Should it be a Target for Prevention of ASCVD? Curr. Atheroscler. Rep. 2025, 27, 44. [Google Scholar] [CrossRef]
- Varbo, A.; Nordestgaard, B.G. Directly measured vs. calculated remnant cholesterol identifies additional overlooked individuals in the general population at higher risk of myocardial infarction. Eur. Heart J. 2021, 42, 4833–4843. [Google Scholar] [CrossRef]
- Ridker, P.M.; Lüscher, T.F. Anti-inflammatory therapies for cardiovascular disease. Eur. Heart J. 2014, 35, 1782–1791. [Google Scholar] [CrossRef]
- Doi, T.; Langsted, A.; Nordestgaard, B.G. Dual elevated remnant cholesterol and C-reactive protein in myocardial infarction, atherosclerotic cardiovascular disease, and mortality. Atherosclerosis 2023, 379, 117141. [Google Scholar] [CrossRef]
- Amor, A.J.; Castelblanco, E.; Hernández, M.; Gimenez, M.; Granado-Casas, M.; Blanco, J.; Soldevila, B.; Esmatjes, E.; Conget, I.; Alonso, N.; et al. Advanced lipoprotein profile disturbances in type 1 diabetes mellitus: A focus on LDL particles. Cardiovasc. Diabetol. 2020, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Wadström, B.N.; Pedersen, K.M.; Wulff, A.B.; Nordestgaard, B.G. Elevated remnant cholesterol and atherosclerotic cardiovascular disease in diabetes: A population-based prospective cohort study. Diabetologia 2023, 66, 2238–2249. [Google Scholar] [CrossRef]
- Nordestgaard, B.G. A New Start for Triglycerides and Remnant Cholesterol—Nonfasting. Clin. Chem. 2017, 63, 1418–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, Y.; Wu, Y.; Qi, M.; Song, L.; Chen, B.; Wang, C.; Lu, J.; Yang, Y.; Zhang, X.; Cui, J.; et al. Associations of remnant cholesterol with cardiovascular and cancer mortality in a nationwide cohort. Sci. Bull. 2024, 69, 526–534. [Google Scholar] [CrossRef]
- Johansen, M.Ø.; Vedel-Krogh, S.; Nielsen, S.F.; Afzal, S.; Davey Smith, G.; Nordestgaard, B.G. Association of remnant cholesterol with unhealthy lifestyle and risk of coronary heart disease: A population-based cohort study. Lancet Reg. Health-Eur. 2025, 51, 101223. [Google Scholar] [CrossRef]
- Wadström, B.N.; Wulff, A.B.; Pedersen, K.M.; Jensen, G.B.; Nordestgaard, B.G. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: A cohort-based study. Eur. Heart J. 2022, 43, 3258–3269. [Google Scholar] [CrossRef] [PubMed]
- Wadström, B.N.; Pedersen, K.M.; Wulff, A.B.; Nordestgaard, B.G. Remnant Cholesterol, Not LDL Cholesterol, Explains Peripheral Artery Disease Risk Conferred by apoB: A Cohort Study. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1144–1155. [Google Scholar] [CrossRef]
- Grønholdt, M.-L.M.; Nordestgaard, B.G.; Nielsen, T.G.; Sillesen, H. Echolucent Carotid Artery Plaques Are Associated with Elevated Levels of Fasting and Postprandial Triglyceride-Rich Lipoproteins. Stroke 1996, 27, 2166–2172. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Zhang, H.; Chen, R.; Zhao, Y.; Zheng, Z. Impact of remnant cholesterol on short-term and long-term prognosis in patients with prediabetes or diabetes undergoing coronary artery bypass grafting: A large-scale cohort study. Cardiovasc. Diabetol. 2025, 24, 8. [Google Scholar] [CrossRef]
- Yu, D.; Wang, Z.; Zhang, X.; Qu, B.; Cai, Y.; Ma, S.; Zhao, Z.; Simmons, D. Remnant Cholesterol and Cardiovascular Mortality in Patients with Type 2 Diabetes and Incident Diabetic Nephropathy. J. Clin. Endocrinol. Metab. 2021, 106, 3546–3554. [Google Scholar] [CrossRef]
- Lin, A.; Nerlekar, N.; Rajagopalan, A.; Yuvaraj, J.; Modi, R.; Mirzaee, S.; Munnur, R.K.; Seckington, M.; Doery, J.C.G.; Seneviratne, S.; et al. Remnant cholesterol and coronary atherosclerotic plaque burden assessed by computed tomography coronary angiography. Atherosclerosis 2019, 284, 24–30. [Google Scholar] [CrossRef]
- Anagnostis, P.; Bitzer, J.; Cano, A.; Ceausu, I.; Chedraui, P.; Durmusoglu, F.; Erkkola, R.; Goulis, D.G.; Hirschberg, A.L.; Kiesel, L.; et al. Menopause symptom management in women with dyslipidemias: An EMAS clinical guide. Maturitas 2020, 135, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, K.; Bi, S.; Li, M.; Yao, Z. Higher remnant cholesterol increases the risk of coronary heart disease and diabetes in postmenopausal women. Front. Endocrinol. 2024, 15, 1475933. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, S.; Huang, Z.; Lu, X.; Li, J.; Wang, Y.; Wu, S.; Wu, Y.; Wu, Y.; Li, Y. Impact of remnant cholesterol to high-density lipoprotein cholesterol ratio on risk of incident ASCVD: The Kailuan prospective cohort study. Nutr. Metab. 2025, 22, 51. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Q.; Su, Y.; Wang, J.; Fang, Z.; Luo, F. Effects of physical activity on the levels of remnant cholesterol: A population-based study. J. Cell Mol. Med. 2024, 28, e18062. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Ye, J.; Guo, Q.; Wang, W.; Sun, Y.; Zeng, Q. Separate and combined associations of physical activity and obesity with lipid-related indices in non-diabetic and diabetic patients. Lipids Health Dis. 2019, 18, 49. [Google Scholar] [CrossRef]
- Igarashi, Y.; Akazawa, N.; Maeda, S. Effects of Aerobic Exercise Alone on Lipids in Healthy East Asians: A Systematic Review and Meta-Analysis. J. Atheroscler. Thromb. 2019, 26, 488–503. [Google Scholar] [CrossRef]
- Palazón-Bru, A.; Hernández-Lozano, D.; Gil-Guillén, V.F. Which Physical Exercise Interventions Increase HDL-Cholesterol Levels? A Systematic Review of Meta-analyses of Randomized Controlled Trials. Sports Med. 2021, 51, 243–253. [Google Scholar] [CrossRef]
- Kodama, S.; Tanaka, S.; Saito, K.; Shu, M.; Sone, Y.; Onitake, F.; Suzuki, E.; Shimano, H.; Yamamoto, S.; Kondo, K.; et al. Effect of Aerobic Exercise Training on Serum Levels of High-Density Lipoprotein Cholesterol: A Meta-analysis. Arch. Intern. Med. 2007, 167, 999–1008. [Google Scholar] [CrossRef]
- Sarzynski, M.A.; Burton, J.; Rankinen, T.; Blair, S.N.; Church, T.S.; Després, J.-P.; Hagberg, J.M.; Landers-Ramos, R.; Leon, A.S.; Mikus, C.R.; et al. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis 2015, 243, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramie, J.J.; Barber, J.L.; Sarzynski, M.A. Effects of exercise on HDL functionality. Curr. Opin. Lipidol. 2019, 30, 16–23. [Google Scholar] [CrossRef]
- Johnson, N.A.; Sachinwalla, T.; Walton, D.W.; Smith, K.; Armstrong, A.; Thompson, M.W.; George, J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009, 50, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Rector, R.S. Exercise Combats Hepatic Steatosis: Potential Mechanisms and Clinical Implications. Diabetes 2020, 69, 517–524. [Google Scholar] [CrossRef]
- The ACCORD Study Group. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef]
- Das Pradhan, A.; Glynn, R.J.; Fruchart, J.-C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride Lowering with Pemafibrate to Reduce Cardiovascular Risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. J. Am. Med. Assoc. 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.-M.; Eliasson, B.; Gudbjörnsdottir, S. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: A nationwide, register-based cohort study. Lancet 2018, 392, 477–486. [Google Scholar] [CrossRef]
- Pinés Corrales, P.J.; Sastre Marcos, J.; López Gallardo, G.; Fernández, J.M.; Antolín, S.H.; López, I.Q.; Del Val Zaballos, F.; López, J.G.; Alfaro Martínez, J.J. All-cause mortality and risk factors in patients with type 1 diabetes in Castilla-La Mancha, Spain. DIACAM1 2010–2020 study. Prim. Care Diabetes 2024, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Karmali, R.; Sipko, J.; Majid, M.; Bruemmer, D. Hyperlipidemia and Cardiovascular Disease in People with Type 1 Diabetes: Review of Current Guidelines and Evidence. Curr. Cardiol. Rep. 2023, 25, 435–442. [Google Scholar] [CrossRef] [PubMed]
- The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes: The DCCT/EDIC Study 30-Year Follow-up. Diabetes Care 2016, 39, 686–693. [Google Scholar] [CrossRef]
- Joshi, P.H.; Khokhar, A.A.; Massaro, J.M.; Lirette, S.T.; Griswold, M.E.; Martin, S.S.; Blaha, M.J.; Kulkarni, K.R.; Correa, A.; D’Agostino, R.B.; et al. Remnant Lipoprotein Cholesterol and Incident Coronary Heart Disease: The Jackson Heart and Framingham Offspring Cohort Studies. J. Am. Heart Assoc. 2016, 5, e002765. [Google Scholar] [CrossRef]
- Langsted, A.; Madsen, C.M.; Nordestgaard, B.G. Contribution of remnant cholesterol to cardiovascular risk. J. Intern. Med. 2020, 288, 116–127. [Google Scholar] [CrossRef]
- Capurso, C.; Capurso, A. From excess adiposity to insulin resistance: The role of free fatty acids. Vascul. Pharmacol. 2012, 57, 91–97. [Google Scholar] [CrossRef]
- Mangat, R.; Su, J.W.; Lambert, J.E.; Clandinin, M.T.; Wang, Y.; Uwiera, R.R.; Forbes, J.M.; Vine, D.F.; Cooper, M.E.; Mamo, J.C.; et al. Increased risk of cardiovascular disease in Type 1 diabetes: Arterial exposure to remnant lipoproteins leads to enhanced deposition of cholesterol and binding to glycated extracellular matrix proteoglycans. Diabet. Med. 2011, 28, 61–72. [Google Scholar] [CrossRef]
- Jansson Sigfrids, F.; Dahlström, E.H.; Forsblom, C.; Sandholm, N.; Harjutsalo, V.; Taskinen, M.-R.; Groop, P.-H. Remnant cholesterol predicts progression of diabetic nephropathy and retinopathy in type 1 diabetes. J. Intern Med. 2021, 290, 632–645. [Google Scholar] [CrossRef]
- Jansson Sigfrids, F.; Stechemesser, L.; Dahlström, E.H.; Forsblom, C.M.; Harjutsalo, V.; Weitgasser, R.; Taskinen, M.-R.; Groop, P.-H.; The FinnDiane Study Group. Apolipoprotein C-III predicts cardiovascular events and mortality in individuals with type 1 diabetes and albuminuria. J. Intern. Med. 2022, 291, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Jansson Sigfrids, F.; Lithovius, R.; Groop, P.-H.; Thorn, L.M. Lessons learned from the FinnDiane Study: Epidemiology and metabolic risk factors for diabetic kidney disease in type 1 diabetes. Diabet. Med. 2025, 42, e15431. [Google Scholar] [CrossRef]
- Raposo-López, J.J.; Tapia-Sanchiz, M.S.; Navas-Moreno, V.; Arranz Martín, J.A.; Marazuela, M.; Sebastián Valles, F. Association of remnant cholesterol with glycemic control and presence of microvascular complications in individuals with type 1 diabetes mellitus. Rev. Clínica Esp. Engl. Ed. 2023, 224, 43–47. [Google Scholar] [CrossRef]
- Sebastian-Valles, F.; Santiago-Redondo, A.; García-Artacho, E.; Sampedro-Nuñez, M.A.; Navas-Moreno, V.; Martín, J.A.A.; Marazuela, M. Impact of remnant cholesterol and triglycerides on diabetes foot and disease in type 1 diabetes: A propensity score-matched case-control study. J. Diabetes Complicat. 2025, 39, 109082. [Google Scholar] [CrossRef]
- Serés-Noriega, T.; Ortega, E.; Giménez, M.; Perea, V.; Boswell, L.; Mariaca, K.; Font, C.; Mesa, A.; Viñals, C.; Blanco, J. Advanced lipoprotein profile identifies atherosclerosis better than conventional lipids in type 1 diabetes at high cardiovascular risk. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1235–1244. [Google Scholar] [CrossRef]
- Puig-Jové, C.; Julve, J.; Castelblanco, E.; Julián, M.T.; Amigó, N.; Andersen, H.U.; Ahluwalia, T.S.; Rossing, P.; Mauricio, D.; Jensen, M.T.; et al. The novel inflammatory biomarker GlycA and triglyceride-rich lipoproteins are associated with the presence of subclinical myocardial dysfunction in subjects with type 1 diabetes mellitus. Cardiovasc. Diabetol. 2022, 21, 257. [Google Scholar] [CrossRef]
- Leroux, C.; Gingras, V.; Desjardins, K.; Brazeau, A.-S.; Strychar, I.; Rabasa-Lhoret, R. In adult patients with type 1 diabetes healthy lifestyle associates with a better cardiometabolic profile. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Jeon, J.; Baek, M.; Song, S.O.; Kim, J. Impact of statin treatment on cardiovascular risk in patients with type 1 diabetes: A population-based cohort study. J. Transl. Med. 2023, 21, 806. [Google Scholar] [CrossRef]
- Hero, C.; Rawshani, A.; Svensson, A.-M.; Franzén, S.; Eliasson, B.; Eeg-Olofsson, K.; Gudbjörnsdottir, S. Association Between Use of Lipid-Lowering Therapy and Cardiovascular Diseases and Death in Individuals with Type 1 Diabetes. Diabetes Care 2016, 39, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Ahmadizar, F.; Maitland-van der Zee, A.H. AdDIT Editorial comment—Challenges in medication treatment of renal and cardiovascular diseases and risk factors in adolescents with type 1 diabetes. Ann. Transl. Med. 2018, 6, 193. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes. Diabetes Care 2023, 47, S179–S218. [Google Scholar] [CrossRef]
| Authors | Population | Exposure | Outcome | Main Findings |
|---|---|---|---|---|
| Johansen et al., 2025 (Lancet Reg Health Eur) [15] | 104,867 adults from Copenhagen General Population Study, no CHD at baseline | Unhealthy lifestyle (smoking, inactivity, poor diet)→elevated remnant cholesterol | MI and CHD incidence | Remnant cholesterol explained 12–21% of excess CHD risk from unhealthy lifestyle, independent of LDL-C |
| Tian et al., 2024 (ChinaHEART) [14] | 3.4 million Chinese adults, prospective nationwide cohort | Baseline remnant cholesterol | CVD and cancer mortality | Higher remnant cholesterol increased CVD mortality (HR ~1.17) but inversely associated with some cancer mortality |
| Wadström et al., 2023 (Diabetologia) [12] | Contemporary Danish diabetes cohort (107,243 adults) | Remnant cholesterol vs. LDL-C | ASCVD (MI, stroke, PAD, CVD death) | Remnant cholesterol explained ~14–34% of excess ASCVD risk in diabetes, while LDL-C did not explain excess risk |
| Castañer et al., 2020 (PREDIMED trial) [1] | 6901 high-CVD-risk participants, 48% with diabetes | Baseline triglycerides and remnant cholesterol | Major adverse cardiovascular events (MACE) | Remnant cholesterol (HR 1.21 per 10 mg/dL) predicted MACE; LDL-C not predictive when adjusted |
| Li et al., 2025 (CABG cohort) [19] | 13,426 patients with diabetes or prediabetes undergoing CABG | Remnant cholesterol (continuous and categorical) | Perioperative AKI, MACCE, death, MI | Each 1-SD increase in remnant cholesterol raised MACCE risk (HR 1.07), MI (HR 1.11), death (HR 1.07) |
| Yu et al., 2021 [20] | 2282 T2D patients with CKD stage 3–5 | Baseline remnant cholesterol | CVD mortality (2 years) | Remnant cholesterol (OR 1.12 per 10 mg/dL) predicted CVD mortality, especially with LDL-C > 100 mg/dL |
| Lin et al., 2019 (Atherosclerosis) [21] | 587 patients undergoing CT coronary angiography | Remnant cholesterol (fasting lipid panel) | Coronary plaque burden (CT-LeSc > 5) | Remnant cholesterol independently predicted higher coronary plaque burden even with optimal LDL-C levels (OR ~3.9) |
| Wadström et al., 2022 (EHJ) [16] | 106,937 adults (Copenhagen General Population Study) and 13,974 (City Heart Study) | Calculated remnant cholesterol | Peripheral artery disease, MI, ischemic stroke | Elevated remnant cholesterol strongly associated with PAD (HR 4.9), MI (HR 4.2), stroke (HR 1.8); stronger effect for PAD |
| Wadström et al., 2024 (Arterioscler Thromb Vasc Biol) [17] | 93,461 adults with diabetes in Denmark (statin era) | Baseline remnant cholesterol apoB, and LDL-C | ASCVD (MI, stroke, PAD) | PAD risk was mainly driven by elevated remnants, whereas MI risk was explained by both remnants and LDL. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastian-Valles, F.; Montes Muñiz, Á.; Marazuela, M. Remnant Cholesterol: From Pathophysiology to Clinical Implications in Type 1 Diabetes. Endocrines 2025, 6, 46. https://doi.org/10.3390/endocrines6030046
Sebastian-Valles F, Montes Muñiz Á, Marazuela M. Remnant Cholesterol: From Pathophysiology to Clinical Implications in Type 1 Diabetes. Endocrines. 2025; 6(3):46. https://doi.org/10.3390/endocrines6030046
Chicago/Turabian StyleSebastian-Valles, Fernando, Álvaro Montes Muñiz, and Mónica Marazuela. 2025. "Remnant Cholesterol: From Pathophysiology to Clinical Implications in Type 1 Diabetes" Endocrines 6, no. 3: 46. https://doi.org/10.3390/endocrines6030046
APA StyleSebastian-Valles, F., Montes Muñiz, Á., & Marazuela, M. (2025). Remnant Cholesterol: From Pathophysiology to Clinical Implications in Type 1 Diabetes. Endocrines, 6(3), 46. https://doi.org/10.3390/endocrines6030046






