Retrospective Analysis of Vitamin D Levels in Girls with Idiopathic Central Precocious Puberty: A Potential Role in Pubertal Activation?
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference Values for Anthropometric and Biochemical Parameters
2.2. Ethical Issues
2.3. Statistical Analysis
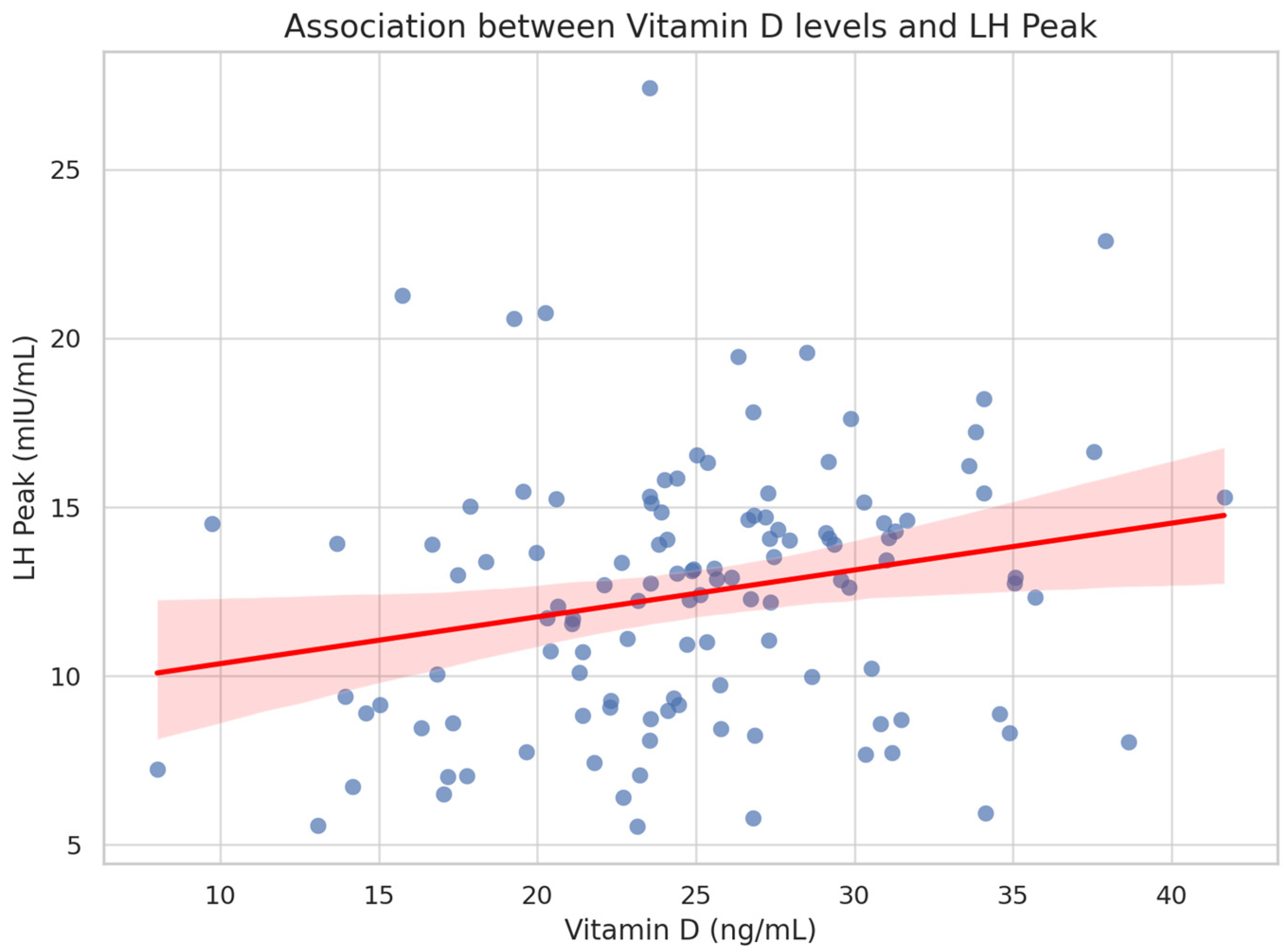
3. Results
3.1. Clinical and Auxological Characteristics by Vitamin D Status
3.2. Correlation Analysis
3.3. Multiple Linear Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cantas-Orsdemir, S.; Eugster, E.A. Update on central precocious puberty: From etiologies to outcomes. Expert Rev. Endocrinol. Metab. 2019, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; So, C.H.; Lee, H.S.; Lim, J.S.; Hwang, J.S. Prevalence of pathological brain lesions in girls with central precocious puberty: Possible overestimation? J. Korean Med. Sci. 2018, 33, e329. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.H. Precocious puberty: Types, pathogenesis and updated management. Cureus 2023, 15, e47485. [Google Scholar] [CrossRef] [PubMed]
- Cassio, A.; Cacciari, Ε.; Zucchini, S.; Balsamo, A.; Diegoli, M.; Orsini, F. Central precocious puberty: Clinical and imaging aspects. J. Pediatr. Endocrinol. Metab. 2000, 13, 703–708. [Google Scholar] [CrossRef]
- Pellegrin, M.C.; Marzin, C.; Monasta, L.; Tamaro, G.; Vidonis, V.; Vittori, G.; Faleschini, E.; Barbi, E.; Tornese, G. A short-duration gonadotropin-releasing hormone stimulation test for the diagnosis of central precocious puberty. Medicina 2023, 60, 24. [Google Scholar] [CrossRef]
- Sodero, G.; Cipolla, C.; Camporesi, A.; Martino, L.; Costa, S.; Cannioto, Z.; Frassanito, P.; Tamburrini, G.; Veredice, C.; Maggio, L.; et al. Endocrinologic dysfunctions and neuropsychiatric sequelae in pediatric patients with a history of central nervous system infection (Endless): A prospective monocentric study. Pediatr. Infect. Dis. J. 2025, 44, 310–317. [Google Scholar] [CrossRef]
- Moise-Silverman, J.; Silverman, L.A. A review of the genetics and epigenetics of central precocious puberty. Front. Endocrinol. 2022, 13, 1029137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tajima, T. Genetic causes of central precocious puberty. Clin. Pediatr. Endocrinol. 2022, 31, 101–109. [Google Scholar] [CrossRef]
- Shim, Y.S.; Lee, H.S.; Hwang, J.S. Genetic factors in precocious puberty. Clin. Exp. Pediatr. 2022, 65, 172–181. [Google Scholar] [CrossRef]
- Savastio, S.; Cinquatti, R.; Tagliaferri, F.; Rabbone, I.; Bona, G. Vitamin D effects and endocrine diseases. Minerva Pediatr. 2020, 72, 326–339. [Google Scholar] [CrossRef]
- Kılınç, S.; Atay, E.; Ceran, Ö.; Atay, Z. Evaluation of vitamin D status and its correlation with gonadal function in children at mini-puberty. Clin. Endocrinol. 2019, 90, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.N. Vitamin D in toddlers, preschool children, and adolescents. Ann. Nutr. Metab. 2020, 76 (Suppl. S2), 30–41. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080S–1086S. [Google Scholar] [CrossRef]
- Ganmaa, D.; Bromage, S.; Khudyakov, P.; Erdenenbaatar, S.; Delgererekh, B.; Martineau, A.R. Influence of vitamin D supplementation on growth, body composition, and pubertal development among school-aged children in an area with a high prevalence of Vitamin D deficiency. JAMA Pediatr. 2023, 177, 32–41. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.J.; Shim, Y.S.; Jeong, H.R.; Kwon, E.; Hwang, J.S. Associations between serum vitamin D levels and precocious puberty in girls. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 91–95. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, H.; Guo, J. Risk factors for precocious puberty: A systematic review and meta-analysis. Psychoneuroendocrinology 2025, 176, 107427. [Google Scholar] [CrossRef]
- Sessa, L.; Rotunno, G.; Sodero, G.; Pane, L.C.; Rendeli, C.; Maresca, G.; Rigante, D.; Cipolla, C. Predictive value of transabdominal pelvic ultrasonography for the diagnosis of central precocious puberty: A single-center observational retrospective study. Clin. Pediatr. Endocrinol. 2024, 33, 199–206. [Google Scholar] [CrossRef]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- Guo, D.; Ning, X.; Bai, T.; Tan, L.; Zhou, Y.; Guo, Z.; Li, X. Interaction between Vitamin D homeostasis, gut microbiota, and central precocious puberty. Front. Endocrinol. 2024, 15, 1449033. [Google Scholar] [CrossRef]
- Calcaterra, V.; Magenes, V.C.; Tagi, V.M.; Grazi, R.; Bianchi, A.; Cena, H.; Zuccotti, G.; Fabiano, V. Association between vitamin D levels, puberty timing, and age at menarche. Children 2023, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Crafa, A.; Cannarella, R.; Cannarella, V.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Retrospective real world study on vitamin D supplementation: Looking for the most effective molecule and its frequency of use. Clin. Nutr. 2025, 47, 265–274. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Xiao, Y.; Cao, N.-N.; Wang, Y.-F.; Zhang, H.-R.; Jiang, S.-Q. Correlation between serum vitamin D level and uterine volume in girls with idiopathic central precocious puberty. J. Pediatr. Endocrinol. Metab. 2024, 37, 144–149. [Google Scholar] [CrossRef]
- Gan, D.-M.; Fang, J.; Zhang, P.-P.; Zhao, Y.-D.; Xu, Y.-N. Serum 25-hydroxyvitamin D levels and the risk of idiopathic central precocious puberty in girls. Clinics 2023, 78, 100244. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Vitamin D status and parathyroid hormone assessment in girls with central precocious puberty. J. Endocrinol. Investig. 2022, 45, 2069–2075. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, R.; Jiang, Y.; Zhang, Y.; Liu, C.; Yu, F.; Zhou, W. Novel serological biomarker models composed of bone turnover markers, vitamin D, and estradiol and their auxiliary diagnostic value in girls with idiopathic central precocious puberty. Bone 2022, 154, 116221. [Google Scholar] [CrossRef]
- Villa, P.; Cipolla, C.; Amar, I.; Sodero, G.; Pane, L.C.; Ingravalle, F.; Pontecorvi, A.; Scambia, G. Bone mineral density and body mass composition measurements in premenopausal anorexic patients: The impact of lean body mass. J. Bone Miner. Metab. 2024, 42, 134–141. [Google Scholar] [CrossRef]
- Chen, M.; Eugster, E.A. Central precocious puberty: Update on diagnosis and treatment. Pediatr. Drugs 2015, 17, 273–281. [Google Scholar] [CrossRef]
- Cipolla, C.; Sodero, G.; Cammisa, I.; Colonna, A.T.; Giuliano, S.; Amar, I.D.; Biton, R.R.; Scambia, G.; Villa, P. The impact of glucocorticoids on bone health and growth: Endocrine and non-endocrine effects in children and young patients. Minerva Pediatr. 2023, 75, 896–904. [Google Scholar] [CrossRef]
- Sodero, G.; Cipolla, C.; Rigante, D.; Arzilli, F.; Mercuri, E.M. Pubertal induction therapy in pediatric patients with Duchenne muscular dystrophy. J. Pediatr. Endocrinol. Metab. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Kaya, A. An examination of the effects of leuprolide acetate used in the treatment of central precocious puberty on bone mineral density and 25-hydroxy vitamin D. West Indian Med. J. 2015, 64, 104–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Zhu, X.; Wang, Y.; Yan, S.; Li, D.; Cui, W. The association between vitamin D levels and precocious puberty: A meta-analysis. J. Pediatr. Endocrinol. Metab. 2020, 33, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, X.; Yan, F.; Cui, Y.; Song, Y.; Yan, S.; Cui, W. Does vitamin D have a potential role in precocious puberty? A meta-analysis. Food Funct. 2023, 14, 5301–5310. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, D.; Gao, H. An updated meta-analysis of the relationship between vitamin D levels and precocious puberty. Front. Endocrinol. 2023, 14, 1298374. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.; Harris, S.S. Does vitamin D affect timing of menarche? Nutr. Rev. 2013, 71, 189–193. [Google Scholar] [CrossRef]
- De Bénazé, G.D.; Brauner, R.; Souberbielle, J.-C. There is no association between vitamin D status and characteristics of central precocious puberty in girls. Eur. J. Pediatr. 2017, 176, 1677–1680. [Google Scholar] [CrossRef]
- Harmon, Q.E.; Kissell, K.; Jukic, A.M.Z.; Kim, K.; Sjaarda, L.; Perkins, N.J.; Umbach, D.M.; Schisterman, E.F.; Baird, D.D.; Mumford, S.L. Vitamin D and Reproductive Hormones Across the Menstrual Cycle. Hum. Reprod. 2020, 35, 413–423. [Google Scholar] [CrossRef]
- Sodero, G.; Agresti, P.; Triarico, S.; Romano, A.; Mastrangelo, S.; Attinà, G.; Maurizi, P.; Cipolla, C.; Ruggiero, A. Growth hormone replacement therapy in pediatric brain tumor survivors. Minerva Pediatr. 2022, 74, 340–348. [Google Scholar] [CrossRef]
- Calcaterra, V.; Klersy, C.; Vinci, F.; Regalbuto, C.; Dobbiani, G.; Montalbano, C.; Pelizzo, G.; Albertini, R.; Larizza, D. Rapid progressive central precocious puberty: Diagnostic and predictive value of basal sex hormone levels and pelvic ultrasound. J. Pediatr. Endocrinol. Metab. 2020, 33, 785–791. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Wang, Y.; Wu, Y.; Li, X.; Li, R.; Fang, Y.; Bai, H.; Luo, P.; Yuan, Y. The value of luteinizing hormone basal values and sex hormone-binding globulin for early diagnosis of rapidly progressive central precocious puberty. Front. Endocrinol. 2024, 14, 1273170. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.M.; Del Giudice, M.M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
| Parameter | <20 ng/mL (n = 47) | 20–30 ng/mL (n = 50) | >30 ng/mL (n = 25) | p-Value |
|---|---|---|---|---|
| Age at diagnosis (years) | 6.95 ± 0.63 | 6.92 ± 0.63 | 6.99 ± 0.73 | 0.86 |
| Height (cm) | 122.1 ± 5.8 | 121.3 ± 6.4 | 122.6 ± 5.9 | 0.74 |
| Height SDS | 1.18 ± 0.78 | 1.05 ± 0.81 | 1.12 ± 0.84 | 0.63 |
| Weight (kg) | 25.6 ± 4.9 | 25.0 ± 5.2 | 24.5 ± 4.6 | 0.48 |
| Weight SDS | 0.71 ± 0.89 | 0.64 ± 1.11 | 0.59 ± 1.06 | 0.62 |
| BMI | 17.1 ± 2.0 | 16.9 ± 2.1 | 16.6 ± 1.9 | 0.40 |
| BMI SDS | 0.81 ± 0.86 | 0.75 ± 1.17 | 0.60 ± 0.92 | 0.39 |
| Growth velocity SDS | 1.60 ± 0.65 | 1.44 ± 0.63 | 1.53 ± 0.68 | 0.27 |
| Basal LH (mIU/mL) | 0.42 ± 0.27 | 0.46 ± 0.30 | 0.50 ± 0.28 | 0.19 |
| Basal FSH (mIU/mL) | 2.62 ± 1.15 | 2.76 ± 1.08 | 2.88 ± 1.14 | 0.37 |
| LH Peak (mIU/mL) | 11.18 ± 4.40 | 12.61 ± 3.76 | 12.48 ± 4.34 | 0.16 |
| LH/FSH ratio | 1.46 ± 0.59 | 1.64 ± 1.45 | 1.86 ± 1.13 | 0.06 |
| Variable | Spearman’s Rho | p-Value |
|---|---|---|
| LH Peak (mIU/mL) | 0.23 | 0.037 |
| LH/FSH ratio | 0.11 | 0.31 |
| Basal LH (mIU/mL) | 0.15 | 0.10 |
| BMI SDS | −0.08 | 0.45 |
| Growth velocity SDS | 0.07 | 0.56 |
| Height SDS | 0.05 | 0.64 |
| Age at diagnosis | −0.02 | 0.80 |
| Predictor | β Coefficient | Standard Error | t-Value | p-Value |
|---|---|---|---|---|
| Intercept | 10.42 | 4.31 | 2.42 | 0.017 |
| Vitamin D (ng/mL) | 0.125 | 0.059 | 2.12 | 0.036 |
| BMI SDS | −0.002 | 0.345 | −0.01 | 0.995 |
| Growth velocity SDS | −0.068 | 0.566 | −0.12 | 0.905 |
| Age at diagnosis | −0.163 | 0.569 | −0.29 | 0.775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodero, G.; Moscogiuri, L.A.; Camporeale, A.; Meoli, A.; Comes, F.; Passoforte, P.; Perrone, G.; Villirillo, A.; Lezzi, M. Retrospective Analysis of Vitamin D Levels in Girls with Idiopathic Central Precocious Puberty: A Potential Role in Pubertal Activation? Endocrines 2025, 6, 33. https://doi.org/10.3390/endocrines6030033
Sodero G, Moscogiuri LA, Camporeale A, Meoli A, Comes F, Passoforte P, Perrone G, Villirillo A, Lezzi M. Retrospective Analysis of Vitamin D Levels in Girls with Idiopathic Central Precocious Puberty: A Potential Role in Pubertal Activation? Endocrines. 2025; 6(3):33. https://doi.org/10.3390/endocrines6030033
Chicago/Turabian StyleSodero, Giorgio, Luigi Antonio Moscogiuri, Anna Camporeale, Aniello Meoli, Fabio Comes, Paola Passoforte, Giacomo Perrone, Antonietta Villirillo, and Marilea Lezzi. 2025. "Retrospective Analysis of Vitamin D Levels in Girls with Idiopathic Central Precocious Puberty: A Potential Role in Pubertal Activation?" Endocrines 6, no. 3: 33. https://doi.org/10.3390/endocrines6030033
APA StyleSodero, G., Moscogiuri, L. A., Camporeale, A., Meoli, A., Comes, F., Passoforte, P., Perrone, G., Villirillo, A., & Lezzi, M. (2025). Retrospective Analysis of Vitamin D Levels in Girls with Idiopathic Central Precocious Puberty: A Potential Role in Pubertal Activation? Endocrines, 6(3), 33. https://doi.org/10.3390/endocrines6030033







