Unraveling the Genetic Link Between Endocrine Hormones and Psychiatric Disorders: An Atlas of Genetic Correlations
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Endocrine Hormones and Psychiatric Disorders for Analysis
2.2. Obtaining GWAS Summary Statistics for Endocrine Hormones and Psychiatric Disorders
2.3. Harmonizing GWAS Summary Statistics
2.4. Evaluating the Statistical Power for Calculating Genetic Correlations with Observed GWAS Sample Sizes
2.5. Computing Genetic Correlations Between Endocrine Hormone Metrics and Psychiatric Disorders
3. Results
3.1. Power Analysis for Detecting Genetic Correlations Using Observed GWAS Sample Sizes
3.2. The Shared Genetic Architecture of Endocrine Hormones
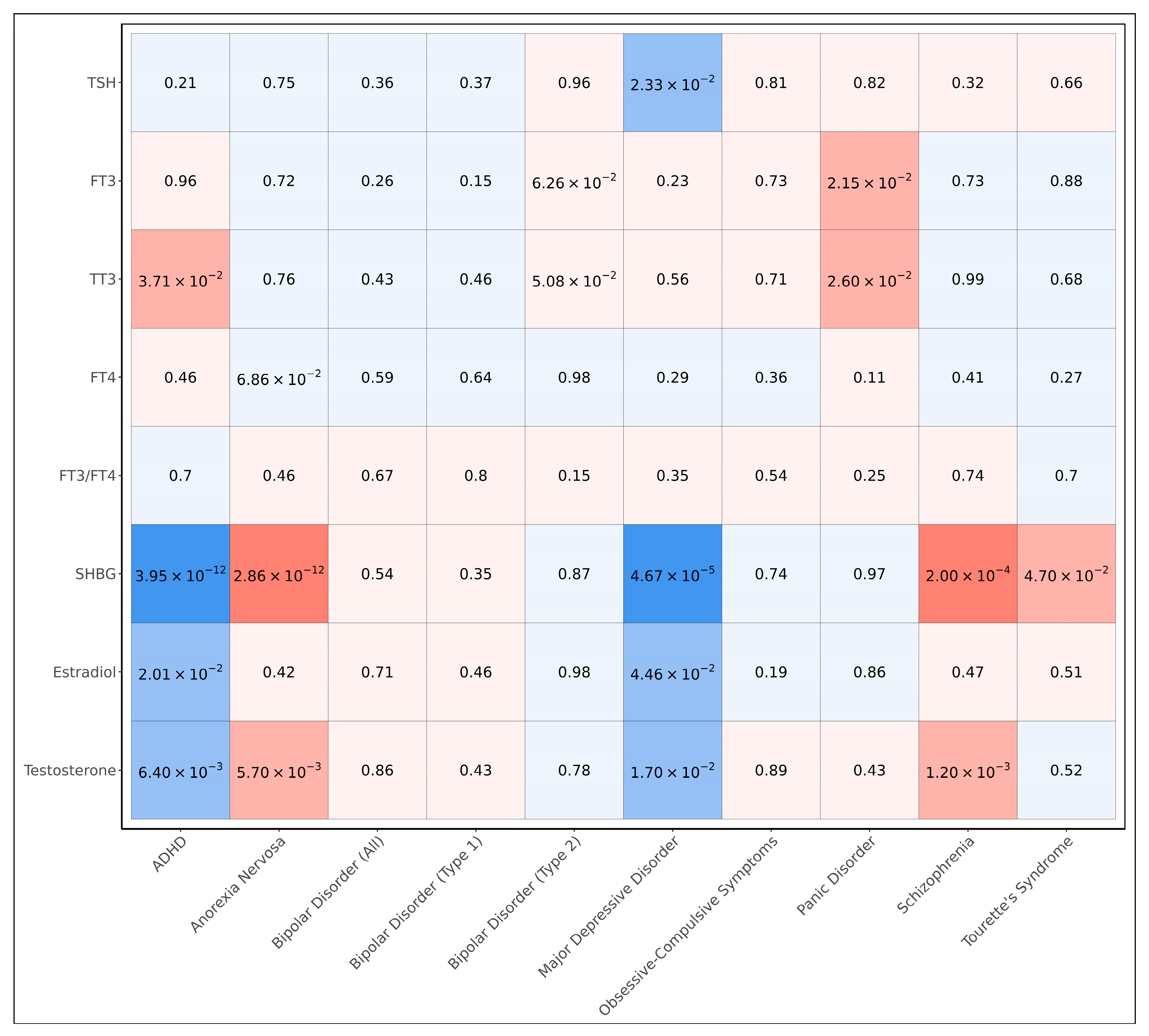
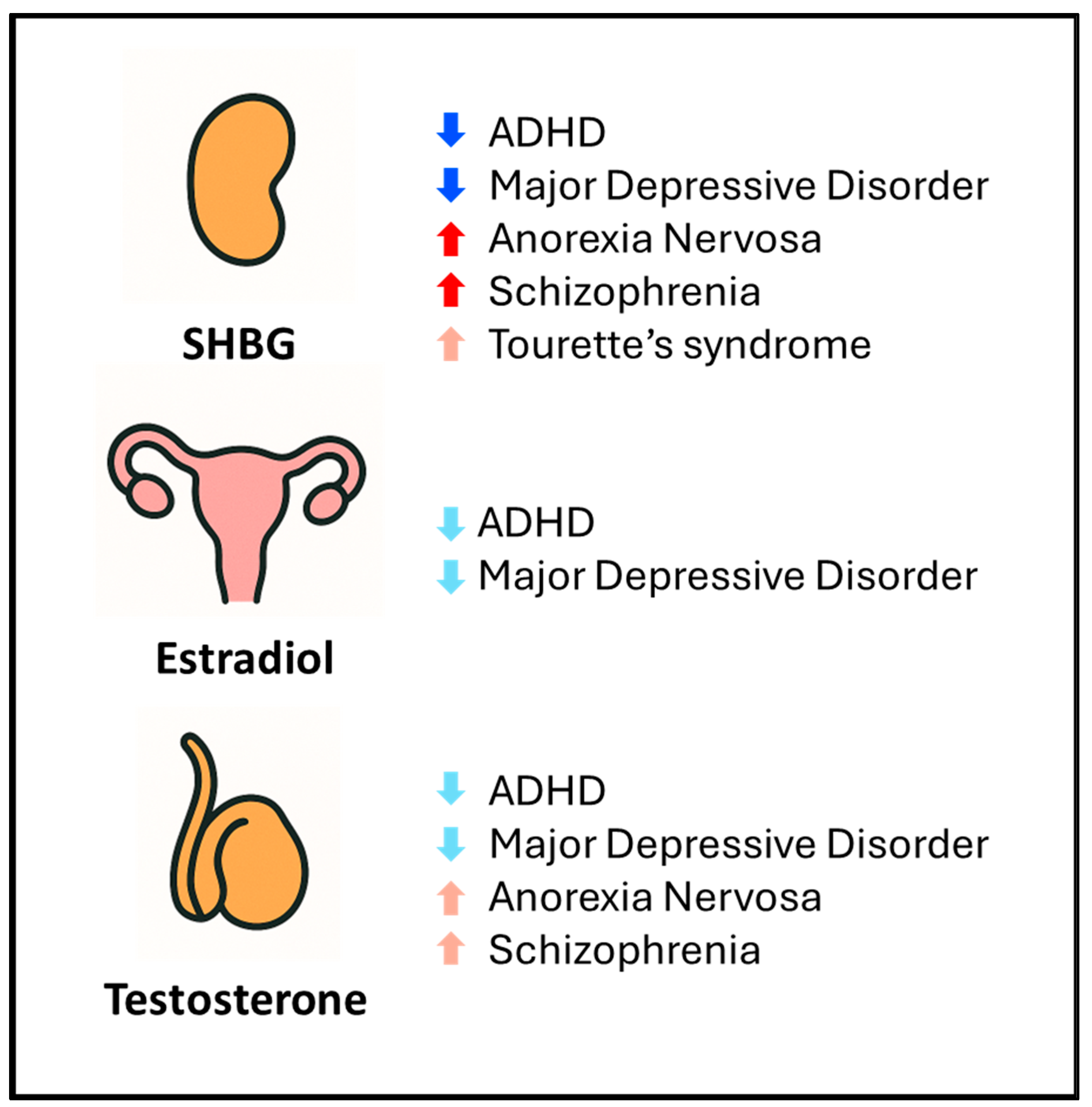
3.3. Genetic Correlations Between Endocrine Hormones and Psychiatric Disorders
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GWAS | Genome-wide association study |
| ADHD | Attention-deficit hyperactivity disorder |
| SHBG | Sex hormone-binding globulin |
| TSH | Thyroid-stimulating hormone |
| FT3 | Free triiodothyronine |
| TT3 | Total triiodothyronine |
| FT4 | Free thyroxine |
References
- Hiller-Sturmhöfel, S.; Bartke, A. The Endocrine System. Alcohol. Health Res. World 1998, 22, 153–164. [Google Scholar]
- Salvador, J.; Gutierrez, G.; Llavero, M.; Gargallo, J.; Escalada, J.; López, J. Endocrine Disorders and Psychiatric Manifestations. In Endocrinology and Systemic Diseases; Portincasa, P., Frühbeck, G., Nathoe, H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–35. [Google Scholar] [CrossRef]
- Feldman, A.Z.; Shrestha, R.T.; Hennessey, J.V. Neuropsychiatric Manifestations of Thyroid Disease. Endocrinol. Metab. Clin. N. Am. 2013, 42, 453–476. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M.; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef]
- Mauas, V.; Kopala-Sibley, D.C.; Zuroff, D.C. Depressive symptoms in the transition to menopause: The roles of irritability, personality vulnerability, and self-regulation. Arch. Womens Ment. Health 2014, 17, 279–289. [Google Scholar] [CrossRef]
- Studd, J. Hormone therapy for reproductive depression in women. Post. Reprod. Health 2014, 20, 132–137. [Google Scholar] [CrossRef]
- Barzilai, N.; Gabriely, I. Genetic Studies Reveal the Role of the Endocrine and Metabolic Systems in Aging. J. Clin. Endocrinol. Metab. 2010, 95, 4493–4500. [Google Scholar] [CrossRef]
- Tsuang, M.T.; Bar, J.L.; Stone, W.S.; Faraone, S.V. Gene-environment interactions in mental disorders. World Psychiatry 2004, 3, 73–83. [Google Scholar]
- Bulik-Sullivan, B.; Finucane, H.K.; Anttila, V.; Gusev, A.; Day, F.R.; Loh, P.-R.; Duncan, L.; Perry, J.R.B.; Patterson, N.; Robinson, E.B.; et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015, 47, 1236–1241. [Google Scholar] [CrossRef]
- van Rheenen, W.; Peyrot, W.J.; Schork, A.J.; Lee, S.H.; Wray, N.R. Genetic correlations of polygenic disease traits: From theory to practice. Nat. Rev. Genet. 2019, 20, 567–581. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The Hypothalamic-Pituitary-Adrenal Axis: Development, Programming Actions of Hormones, and Maternal-Fetal Interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Fekete, C.; Lechan, R.M. Central Regulation of Hypothalamic-Pituitary-Thyroid Axis Under Physiological and Pathophysiological Conditions. Endocr. Rev. 2014, 35, 159–194. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Ehlert, U. Hypothalamic-pituitary-thyroid (HPT) axis functioning in anxiety disorders. A systematic review. Depress. Anxiety 2018, 35, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sterenborg, R.B.T.M.; Steinbrenner, I.; Li, Y.; Bujnis, M.N.; Naito, T.; Marouli, E.; Galesloot, T.E.; Babajide, O.; Andreasen, L.; Astrup, A.; et al. Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications. Nat. Commun. 2024, 15, 888. [Google Scholar] [CrossRef]
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214. [Google Scholar] [CrossRef]
- Yu, D.; Sul, J.H.; Tsetsos, F.; Nawaz, M.S.; Huang, A.Y.; Zelaya, I.; Illmann, C.; Osiecki, L.; Darrow, S.M.; Hirschtritt, M.E.; et al. Interrogating the Genetic Determinants of Tourette’s Syndrome and Other Tic Disorders Through Genome-Wide Association Studies. Am. J. Psychiatry 2019, 176, 217–227. [Google Scholar] [CrossRef]
- Strom, N.I.; Burton, C.L.; Iyegbe, C.; Silzer, T.; Antonyan, L.; Pool, R.; Lemire, M.; Crowley, J.J.; Hottenga, J.-J.; Ivanov, V.Z.; et al. Genome-Wide Association Study of Obsessive-Compulsive Symptoms including 33,943 individuals from the general population. Mol. Psychiatry 2024, 29, 2714–2723. [Google Scholar] [CrossRef]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.-Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Demontis, D.; Walters, G.B.; Athanasiadis, G.; Walters, R.; Therrien, K.; Nielsen, T.T.; Farajzadeh, L.; Voloudakis, G.; Bendl, J.; Zeng, B.; et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 2023, 55, 198–208. [Google Scholar] [CrossRef]
- Mullins, N.; Forstner, A.J.; O’Connell, K.S.; Coombes, B.; Coleman, J.R.I.; Qiao, Z.; Als, T.D.; Bigdeli, T.B.; Børte, S.; Bryois, J.; et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 2021, 53, 817–829. [Google Scholar] [CrossRef]
- Howard, D.M.; Adams, M.J.; Clarke, T.-K.; Hafferty, J.D.; Gibson, J.; Shirali, M.; Coleman, J.R.I.; Hagenaars, S.P.; Ward, J.; Wigmore, E.M.; et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Forstner, A.J.; Awasthi, S.; Wolf, C.; Maron, E.; Erhardt, A.; Czamara, D.; Eriksson, E.; Lavebratt, C.; Allgulander, C.; Friedrich, N.; et al. Genome-wide association study of panic disorder reveals genetic overlap with neuroticism and depression. Mol. Psychiatry 2021, 26, 4179–4190. [Google Scholar] [CrossRef]
- Perez, G.; Barber, G.P.; Benet-Pages, A.; Casper, J.; Clawson, H.; Diekhans, M.; Fischer, C.; Gonzalez, J.N.; Hinrichs, A.S.; Lee, C.M.; et al. The UCSC Genome Browser database: 2025 update. Nucleic Acids Res. 2025, 53, D1243–D1249. [Google Scholar] [CrossRef]
- Auton, A.; Abecasis, G.R.; Altshuler, D.M.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Chakravarti, A.; Clark, A.G.; Donnelly, P.; Eichler, E.E.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef]
- Altshuler, D.M.; Gibbs, R.A.; Peltonen, L.; Altshuler, D.M.; Gibbs, R.A.; Peltonen, L.; Dermitzakis, E.; Schaffner, S.F.; Yu, F.; Peltonen, L.; et al. Integrating common and rare genetic variation in diverse human populations. Nature 2010, 467, 52–58. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.H.; Wray, N.R.; Goddard, M.E.; Visscher, P.M. GCTA-GREML accounts for linkage disequilibrium when estimating genetic variance from genome-wide SNPs. Proc. Natl. Acad. Sci. USA 2016, 113, E4579–E4580. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.K.; Loh, P.-R.; Finucane, H.K.; Ripke, S.; Yang, J.; Schizophrenia Working Group of the Psychiatric Genomics Consortium; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef]
- Ou, M.; Du, Z.; Jiang, Y.; Zhou, Q.; Yuan, J.; Tian, L.; Zhu, H. Causal relationship between schizophrenia and sex hormone binding globulin: A Mendelian randomization study. Schizophr. Res. 2024, 267, 528–533. [Google Scholar] [CrossRef]
- Petrikis, P.; Tigas, S.; Tzallas, A.T.; Karampas, A.; Papadopoulos, I.; Skapinakis, P. Sex hormone levels in drug-naïve, first-episode patients with psychosis. Int. J. Psychiatry Clin. Pract. 2020, 24, 20–24. [Google Scholar] [CrossRef]
- Wang, L.-J.; Lee, S.-Y.; Chou, M.-C.; Lee, M.-J.; Chou, W.-J. Dehydroepiandrosterone sulfate, free testosterone, and sex hormone-binding globulin on susceptibility to attention-deficit/hyperactivity disorder. Psychoneuroendocrinology 2019, 103, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Pascal, N.; Amouzou, E.K.S.; Sanni, A.; Namour, F.; Abdelmouttaleb, I.; Vidailhet, M.; Guéant, J.-L. Serum concentrations of sex hormone binding globulin are elevated in kwashiorkor and anorexia nervosa but not in marasmus1,2,3. Am. J. Clin. Nutr. 2002, 76, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Sun, Y.; Guo, S.; Zhou, Q.; Jiang, Y.; Shen, Y.; Zhou, Z.; Du, Z.; Zhou, H. Causal relationship between sex hormone-binding globulin and major depression: A Mendelian randomization study. Acta Psychiatr. Scand. 2023, 148, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; LeMahieu, A.M.; Stan, M.N.; Seshadri, A.; Ozerdem, A.; Pazdernik, V.; Haynes, T.L.; Daugherty, D.H.; Sundaresh, V.; Veldic, M.; et al. The Association Between Thyroid Stimulating Hormone And Depression: A Historical Cohort Study. Mayo Clin. Proc. 2023, 98, 1009–1020. [Google Scholar] [CrossRef]
- Kirkegaard, C.; Faber, J. The role of thyroid hormones in depression. Eur. J. Endocrinol. 1998, 138, 1–9. [Google Scholar] [CrossRef]
- Mason, G.A.; Walker, C.H.; Prange, A.J. L-Triiodothyronine: Is this Peripheral Hormone a Central Neurotransmitter? Neuropsychopharmacol. 1993, 8, 253–258. [Google Scholar] [CrossRef]
- Li, J.L. The Causal Role of Thyroid Hormones in Bipolar Disorders: A Two-Sample Mendelian Randomization Study. Endocrinol. Diabetes Metab. 2024, 7, e70009. [Google Scholar] [CrossRef]
- Chen, G.; Lv, H.; Zhang, X.; Gao, Y.; Liu, X.; Gu, C.; Xue, R.; Wang, Q.; Chen, M.; Zhai, J.; et al. Assessment of the relationships between genetic determinants of thyroid functions and bipolar disorder: A mendelian randomization study. J. Affect. Disord. 2022, 298 Pt A, 373–380. [Google Scholar] [CrossRef]
- Gan, Z.; Wu, X.; Chen, Z.; Liao, Y.; Wu, Y.; He, Z.; Yang, Z.; Zhang, Q. Rapid cycling bipolar disorder is associated with antithyroid antibodies, instead of thyroid dysfunction. BMC Psychiatry 2019, 19, 378. [Google Scholar] [CrossRef]
- Rowland, T.A.; Marwaha, S. Epidemiology and risk factors for bipolar disorder. Ther. Adv. Psychopharmacol. 2018, 8, 251–269. [Google Scholar] [CrossRef]
- Albiñana, C.; Zhu, Z.; Schork, A.J.; Ingason, A.; Aschard, H.; Brikell, I.; Bulik, C.M.; Petersen, L.V.; Agerbo, E.; Grove, J.; et al. Multi-PGS enhances polygenic prediction by combining 937 polygenic scores. Nat. Commun. 2023, 14, 4702. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.L. Unraveling the Genetic Link Between Endocrine Hormones and Psychiatric Disorders: An Atlas of Genetic Correlations. Endocrines 2025, 6, 32. https://doi.org/10.3390/endocrines6030032
Li JL. Unraveling the Genetic Link Between Endocrine Hormones and Psychiatric Disorders: An Atlas of Genetic Correlations. Endocrines. 2025; 6(3):32. https://doi.org/10.3390/endocrines6030032
Chicago/Turabian StyleLi, James L. 2025. "Unraveling the Genetic Link Between Endocrine Hormones and Psychiatric Disorders: An Atlas of Genetic Correlations" Endocrines 6, no. 3: 32. https://doi.org/10.3390/endocrines6030032
APA StyleLi, J. L. (2025). Unraveling the Genetic Link Between Endocrine Hormones and Psychiatric Disorders: An Atlas of Genetic Correlations. Endocrines, 6(3), 32. https://doi.org/10.3390/endocrines6030032





