Sex Differences in Renal Function: Participation of Gonadal Hormones and Prolactin
Abstract
1. Introduction
2. Morphological and Physiological Renal Differences between Genders
3. Role of Gender in Disease-Related Kidney Injury
3.1. Acute Kidney Injury
3.2. Chronic Kidney Disease
3.3. Renal Transplantation
3.4. Other Kidney-Related Diseases
4. Differences Associated with Factors Influencing Kidney Function
5. Influence of Sex Hormones in the Kidney
6. Influence of Prolactin in Kidney Function and Disease
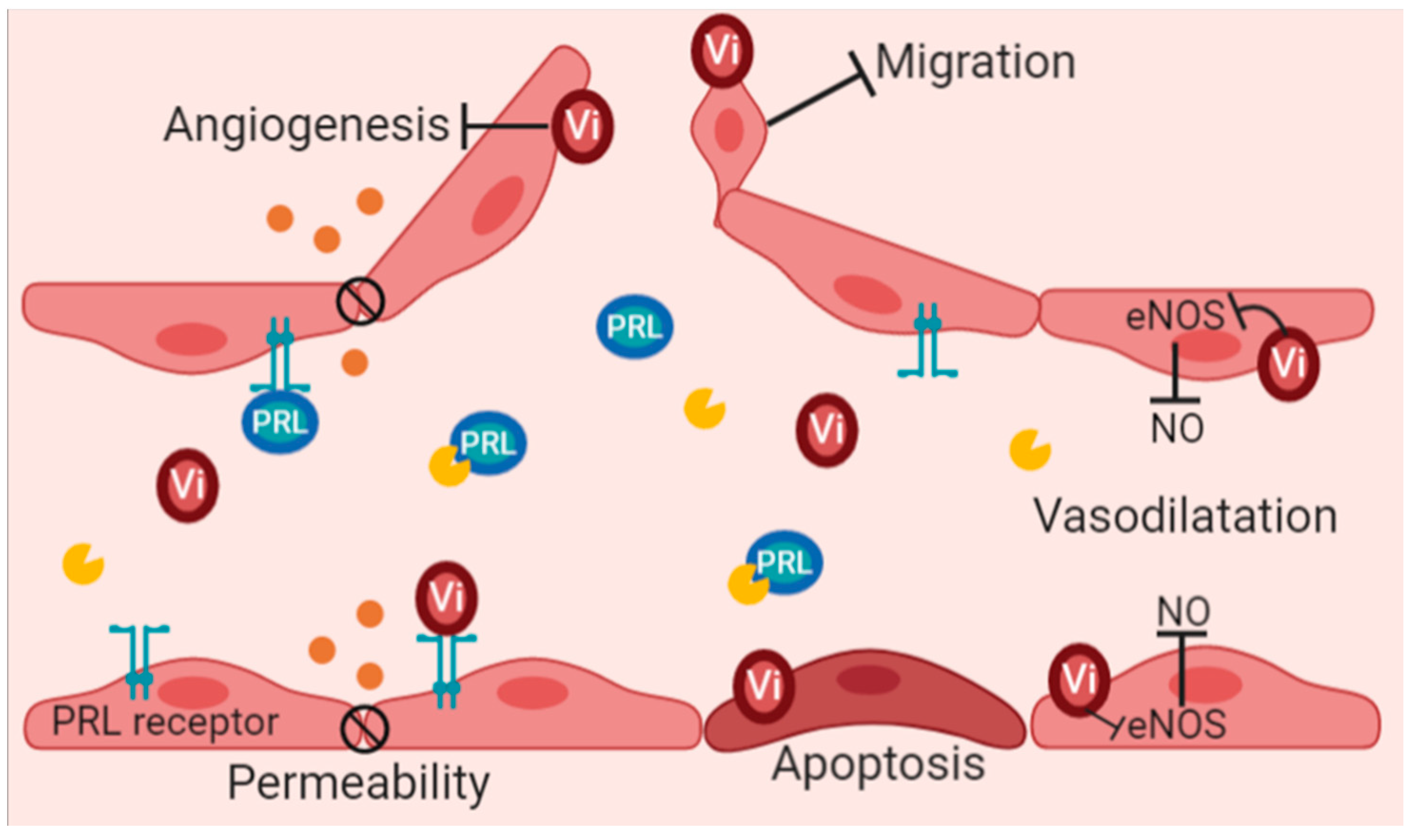
7. Vasoinhibins and Their Potential Role in Renal Function
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Bikbov, B.; Perico, N.; Remuzzi, G.; on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018, 139, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.D.S.; Roderick, P.J. Kidney disease in the Global Burden of Disease Study. Nat. Rev. Nephrol. 2019, 15, 193–194. [Google Scholar] [CrossRef]
- Palomino, J.; Echavarria, R.; Franco-Acevedo, A.; Moreno-Carranza, B.; Melo, Z. Opioids Preconditioning upon Renal Function and Ischemia-Reperfusion Injury: A Narrative Review. Medicina 2019, 55, 522. [Google Scholar] [CrossRef]
- Sabolić, I.; Asif, A.R.; Budach, W.E.; Wanke, C.; Bahn, A.; Burckhardt, G. Gender differences in kidney function. Pflügers Arch. Eur. J. Physiol. 2007, 455, 397–429. [Google Scholar] [CrossRef] [PubMed]
- Munger, K.; Baylis, C. Sex differences in renal hemodynamics in rats. Am. J. Physiol. Physiol. 1988, 254, F223–F231. [Google Scholar] [CrossRef] [PubMed]
- Miletić, D.; Željko, F.; Šustić, A.; Mozetič, V.; Stimac, D.; Žauhar, G. Sonographic measurement of absolute and relative renal length in adults. J. Clin. Ultrasound 1998, 26, 185–189. [Google Scholar] [CrossRef]
- Roseman, D.A.; Hwang, S.-J.; Oyama-Manabe, N.; Chuang, M.L.; O’Donnell, C.J.; Manning, W.J.; Fox, C.S. Clinical associations of total kidney volume: The Framingham Heart Study. Nephrol. Dial. Transplant. 2016, 32, 1344–1350. [Google Scholar] [CrossRef]
- Neugarten, J.; Kasiske, B.; Silbiger, S.R.; Nyengaard, J.R. Effects of sex on renal structure. Nephron 2002, 90, 139–144. [Google Scholar] [CrossRef]
- Koenig, H.; Goldstone, A.; Blume, G.; Lu, C.Y. Testosterone-mediated sexual dimorphism of mitochondria and lysosomes in mouse kidney proximal tubules. Science 1980, 209, 1023–1026. [Google Scholar] [CrossRef]
- Duann, P.; Lin, P.H. Mitochondria Damage and Kidney Disease. Adv. Exp. Med. Biol. 2017, 982, 529–551. [Google Scholar]
- Hommos, M.; Glassock, R.J.; Rule, A.D. Structural and Functional Changes in Human Kidneys with Healthy Aging. J. Am. Soc. Nephrol. 2017, 28, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A.; Montgomery, E.; Nightingale, P.; Peters, A.M.; Sheerin, N.; Wroe, A.C.; Lipkin, G.W. Glomerular filtration rate: New age- and gender- specific reference ranges and thresholds for living kidney donation. BMC Nephrol. 2018, 19, 336. [Google Scholar] [CrossRef]
- James, G.D.; Sealey, D.J.E.; Alderman, M.; Ljungman, S.; Mueller, F.B.; Pecker, M.S.; Laragh, J.H. A Longitudinal Study of Urinary Creatinine and Creatinine Clearance in Normal Subjects: Race, Sex, and Age Differences. Am. J. Hypertens. 1988, 1, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Perucca, J.; Bouby, N.; Valeix, P.; Bankir, L. Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R700–R705. [Google Scholar] [CrossRef]
- Godoy, D.A.; Alvarez, E.; Campi, V.; Soler, C.; Masotti, L.; Di Napoli, M. Diagnosis and therapy of polyuric states in patients with acute cerebral injury. Rev. Med. Chil. 2013, 141, 616–625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veiras, L.C.; Girardi, A.C.C.; Curry, J.; Pei, L.; Ralph, D.L.; Tran, A.; Castelo-Branco, R.C.; Pastor-Soler, N.; Arranz, C.T.; Yu, A.S.L. Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J. Am. Soc. Nephrol. JASN 2017, 28, 3504–3517. [Google Scholar] [CrossRef]
- Quigley, R. Androgens stimulate proximal tubule transport. Gend. Med. 2008, 5, S114–S120. [Google Scholar] [CrossRef] [PubMed]
- Quan, A.; Chakravarty, S.; Chen, J.-K.; Loleh, S.; Saini, N.; Harris, R.C.; Capdevila, J.; Quigley, R. Androgens augment proximal tubule transport. Am. J. Physiol. Physiol. 2004, 287, F452–F459. [Google Scholar] [CrossRef]
- Harris, A.N.; Lee, H.-W.; Osis, G.; Fang, L.; Webster, K.; Verlander, J.W.; Weiner, I.D. Differences in renal ammonia metabolism in male and female kidney. Am. J. Physiol. Physiol. 2018, 315, F211–F222. [Google Scholar] [CrossRef]
- Anton, F.M.; Garcia Puig, J.; Ramos, T.; Gonzalez, P.; Ordas, J. Sex differences in uric acid metabolism in adults: Evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism 1986, 35, 343–348. [Google Scholar] [CrossRef]
- Anzai, N.; Enomoto, A.; Endou, H. Renal urate handling: Clinical relevance of recent advances. Curr. Rheumatol. Rep. 2005, 7, 227–234. [Google Scholar] [CrossRef]
- Perry, G.M.L.; Scheinman, S.J.; Asplin, J.R. Effects of Sex on Intra-Individual Variance in Urinary Solutes in Stone-Formers Collected from a Single Clinical Laboratory. PLoS ONE 2013, 8, e53637. [Google Scholar] [CrossRef]
- Shoag, J.; Tasian, G.E.; Goldfarb, D.; Eisner, B.H. The New Epidemiology of Nephrolithiasis. Adv. Chronic Kidney Dis. 2015, 22, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Zhang, X.; Tang, Y.; Li, J. Upper urinary tract stone compositions: The role of age and gender. Int. Braz. J. Urol. 2020, 46, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Strope, S.A.; Wolf, J.S.; Hollenbeck, B.K. Changes in Gender Distribution of Urinary Stone Disease. Urology 2010, 75, 543–546.e1. [Google Scholar] [CrossRef] [PubMed]
- Worcester, E.M.; Bergsland, K.J.; Gillen, D.L.; Coe, F.L. Mechanism for higher urine pH in normal women compared with men. Am. J. Physiol. Physiol. 2018, 314, F623–F629. [Google Scholar] [CrossRef]
- Chen, H.-W.; Chen, Y.-C.; Yang, F.M.; Wu, W.-J.; Li, C.-C.; Chang, Y.-Y.; Chou, Y.-H. Mediators of the Effects of Gender on Uric Acid Nephrolithiasis: A Novel Application of Structural Equation Modeling. Sci. Rep. 2018, 8, 6077. [Google Scholar] [CrossRef]
- Palevsky, P.M.; Liu, K.D.; Brophy, P.D.; Chawla, L.; Parikh, C.R.; Thakar, C.V.; Tolwani, A.J.; Waikar, S.S.; Weisbord, S.D. KDOQI US Commentary on the 2012 KDIGO Clinical Practice Guideline for Acute Kidney Injury. Am. J. Kidney Dis. 2013, 61, 649–672. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Ballew, S.H.; Gansevoort, R.T.; Kimm, H.; Kovesdy, C.P.; Naimark, D.; Oien, C.; Smith, D.H.; Coresh, J.; et al. A Meta-analysis of the Association of Estimated GFR, Albuminuria, Age, Race, and Sex with Acute Kidney Injury. Am. J. Kidney Dis. 2015, 66, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Golestaneh, L. Female sex reduces the risk of hospital-associated acute kidney injury: A meta-analysis. BMC Nephrol. 2018, 19, 314. [Google Scholar] [CrossRef]
- Schulte-Steinberg, H.; Weninger, E.; Jokisch, D.; Hofstetter, B.; Misera, A.; Lange, V.; Stein, C. Intraperitoneal Versus Interpleural Morphine or Bupivacaine for Pain after Laparoscopic Cholecystectomy. Anesthesiology 1995, 82, 634–640. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, Z.; Cass, A.; Cole, L.; Finfer, S.; Gallagher, M.; McArthur, C.; McGuiness, S.; Myburgh, J.; Bellomo, R.; Mårtensson, J. Sex and mortality in septic severe acute kidney injury. J. Crit. Care 2019, 49, 70–76. [Google Scholar] [CrossRef]
- Neugarten, J.; Golestaneh, L.; Kolhe, N.V. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol. 2018, 19, 131. [Google Scholar] [CrossRef]
- Hodeify, R.; Megyesi, J.; Tarcsafalvi, A.; Mustafa, H.I.; Seng, N.S.H.L.; Price, P.M. Gender differences control the susceptibility to ER stress-induced acute kidney injury. Am. J. Physiol. Physiol. 2013, 304, F875–F882. [Google Scholar] [CrossRef]
- Boddu, R.; Fan, C.; Rangarajan, S.; Sunil, R.; Bolisetty, S.; Curtis, L.M. Unique sex- and age-dependent effects in protective pathways in acute kidney injury. Am. J. Physiol. Physiol. 2017, 313, F740–F755. [Google Scholar] [CrossRef]
- Kang, K.P.; Lee, J.E.; Lee, A.S.; Jung, Y.J.; Kim, D.; Lee, S.; Hwang, H.P.; Kim, W.; Park, S.K. Effect of gender differences on the regulation of renal ische-mia-reperfusion-induced inflammation in mice. Mol. Med. Rep. 2014, 9, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J. Gender Differences in Chronic Kidney Disease: Underpinnings and Therapeutic Implications. Kidney Blood Press. Res. 2010, 33, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-Y.; Chen, S.; Du, Y. Estrogen and estrogen receptors in kidney diseases. Ren. Fail. 2021, 43, 619–642. [Google Scholar] [CrossRef]
- Lima-Posada, I.; Bobadilla, N.A. Understanding the opposite effects of sex hormones in mediating renal injury. Nephrology 2021, 26, 217–226. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Wanner, C.; Metzger, T.; Heimbürger, O.; Mallamaci, F.; Tripepi, G.; Malatino, L.; Zoccali, C. Inflammation and outcome in end-stage renal failure: Does female gender constitute a survival advantage? Kidney Int. 2002, 62, 1791–1798. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef]
- Lima-Posada, I.; Portas-Cortés, C.; Pérez-Villalva, R.; Fontana, F.; Rodríguez-Romo, R.; Prieto, R.; Sánchez-Navarro, A.; Rodríguez-González, G.L.; Gamba, G.; Zambrano, E.; et al. Gender Differences in the Acute Kidney Injury to Chronic Kidney Disease Transition. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.; O’Callaghan, C.A.; Lasserson, D.; Hobbs, R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Markell, M. Impact of gender and gender disparities in patients with kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Jacobs-Cachá, C.; Soler, M.J. Sex hormones and their influence on chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. JASN 2000, 11, 319–329. [Google Scholar] [CrossRef]
- Pan, J.S.; Sheikh-Hamad, D. Mitochondrial dysfunction in acute kidney injury and sex-specific implications. Med. Res. Arch. 2019, 7. [Google Scholar] [CrossRef]
- Cobo, G.; Hecking, M.; Port, F.K.; Exner, I.; Lindholm, B.; Stenvinkel, P.; Carrero, J.J. Sex and gender differences in chronic kidney disease: Progression to end-stage renal disease and haemodialysis. Clin. Sci. 2016, 130, 1147–1163. [Google Scholar] [CrossRef]
- Zhang, G.; Kang, Y.; Zhou, C.; Cui, R.; Jia, M.; Hu, S.; Ji, X.; Yuan, J.; Cui, X.; Shi, G. Amelioratory Effects of Testosterone Propionate on Age-related Renal Fibrosis via Suppression of TGF-beta1/Smad Signaling and Activation of Nrf2-ARE Signaling. Sci Rep. 2018, 8, 10726. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, J.-M. Klotho and Postmenopausal Hormone Replacement Therapy in Women with Chronic Kidney Disease. J. Menopausal Med. 2018, 24, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Halbesma, N.; Brantsma, A.H.; Bakker, S.J.; Jansen, D.F.; Stolk, R.P.; De Zeeuw, D.; Jong, P.E.D.; Gansevoort, R.T.; PREVEND Study Group. Gender differences in predictors of the decline of renal function in the general population. Kidney Int. 2008, 74, 505–512. [Google Scholar] [CrossRef]
- Antlanger, M.; Noordzij, M.; Van De Luijtgaarden, M.; Carrero, J.J.; Palsson, R.; Finne, P.; Hemmelder, M.H.; Aresté-Fosalba, N.; Reisæter, A.V.; Cases, A.; et al. Sex Differences in Kidney Replacement Therapy Initiation and Maintenance. Clin. J. Am. Soc. Nephrol. 2019, 14, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Piras, D.; Masala, M.; Delitala, A.; Urru, S.A.M.; Curreli, N.; Balaci, L.; Ferreli, L.P.; Loi, F.; Atzeni, A.; Cabiddu, G.; et al. Kidney size in relation to ageing, gender, renal function, birthweight and chronic kidney disease risk factors in a general population. Nephrol. Dial. Transplant. 2020, 35, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Caplin, B.; Kumar, S.; Davenport, A. Patients’ perspective of haemodialysis-associated symptoms. Nephrol. Dial. Transplant. 2011, 26, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Kovesdy, C.P.; Nissenson, A.R.; Mehrotra, R.; Streja, E.; Van Wyck, D.; Greenland, S.; Kalantar-Zadeh, K. Association of Hemodialysis Treatment Time and Dose with Mortality and the Role of Race and Sex. Am. J. Kidney Dis. 2010, 55, 100–112. [Google Scholar] [CrossRef]
- Couchoud, C.; Kooman, J.; Finne, P.; Leivestad, T.; Stojceva-Taneva, O.; Ponikvar, J.B.; Collart, F.; Kramar, R.; de Fransicso, A.; Jager, K.J.; et al. From registry data collection to inter-national comparisons: Examples of haemodialysis duration and frequency. Nephrol. Dial. Transplant. 2009, 24, 217–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalantar-Zadeh, K.; Kovesdy, C.P.; Streja, E.; Rhee, C.M.; Soohoo, M.; Chen, J.L.; Molnar, M.Z.; Obi, Y.; Gillen, D.; Nguyen, D.V.; et al. Transition of care from pre-dialysis prelude to renal replacement therapy: The blueprints of emerging research in advanced chronic kidney disease. Nephrol. Dial. Transplant. 2017, 32, ii91–ii98. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kriesche, H.-U.; Ojo, A.O.; Leavey, S.F.; Hanson, J.A.; Leichtman, A.B.; Magee, J.C.; Cibrik, D.M.; Kaplan, B. Gender Differences in the Risk for Chronic Renal Allograft Failure. Transplantation 2001, 71, 429–432. [Google Scholar] [CrossRef]
- Baddiri, A.T.; Villanueva, R.T.; Cabanayan-Casasola, C.B. Impact of Age Difference, Sex Matching, and Body Mass Index Matching Between Donor and Recipient in Renal Transplant. Transplant. Proc. 2019, 51, 2568–2574. [Google Scholar] [CrossRef]
- Matter, Y.E.; Elhadedy, M.A.; Abbas, T.M.; Zahab, M.A.; Fouda, M.A.; Refaie, A.F.; Sheashaa, H.A.; Abbas, M.H.; Denewar, A.A.; Nagib, A.M. Impact of Sex Disparities on Outcomes of Liv-ing-Donor Kidney Transplant in Egypt: Data of 979 Patients. Exp. Clin. Transplant. 2018, 16, 133–137. [Google Scholar] [PubMed]
- Miller, A.J.; Kiberd, B.A.; Alwayn, I.P.; Odutayo, A.; Tennankore, K.K. Donor-Recipient Weight and Sex Mismatch and the Risk of Graft Loss in Renal Transplantation. Clin. J. Am. Soc. Nephrol. 2017, 12, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, T.; Iemura, Y.; Onishi, K.; Hori, S.; Nakai, Y.; Miyake, M.; Anai, S.; Torimoto, K.; Aoki, K.; Saka, T.; et al. Effect of Gender Differences on Transplant Kidney Function. Transplant. Proc. 2017, 49, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Santiago, E.V.; Silveira, M.R.; Araujo, V.E.; Farah Kde, P.; Acurcio Fde, A.; Ceccato, M. Gender in the allocation of organs in kidney transplants: Meta-analysis. Rev. Saude Publica 2015, 49, 68. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Cheng, J.; Huang, H.-F.; Shen, Y.; Jiang, Y.; Chen, J.-H. The effect of donor-recipient gender mismatch on short- and long-term graft survival in kidney transplantation: A systematic review and meta-analysis. Clin. Transplant. 2013, 27, 764–771. [Google Scholar] [CrossRef]
- Antus, B.; Yao, Y.; Song, E.; Liu, S.; Lutz, J.; Heemann, U. Opposite effects of testosterone and estrogens on chronic allograft nephropathy. Transpl. Int. 2002, 15, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A.; Döhler, B.; Stern, M.; Opelz, G. H-Y as a minor histocompatibility antigen in kidney transplantation: A retrospective cohort study. Lancet 2008, 372, 49–53. [Google Scholar] [CrossRef]
- Kim, S.J.; Gill, J.S. H-Y Incompatibility Predicts Short-Term Outcomes for Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2009, 20, 2025–2033. [Google Scholar] [CrossRef]
- Tan, J.C.; Kim, J.P.; Chertow, G.M.; Grumet, F.C.; Desai, M. Donor-recipient sex mismatch in kidney transplantation. Gend. Med. 2012, 9, 335–347.e2. [Google Scholar] [CrossRef]
- Shen, Y.; Cai, R.; Sun, J.; Dong, X.; Huang, R.; Tian, S.; Wang, S. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 2017, 55, 66–76. [Google Scholar] [CrossRef]
- Yu, M.K.; Lyles, C.R.; Bent-Shaw, L.A.; Young, B.A.; the Pathways Authors. Risk Factor, Age and Sex Differences in Chronic Kidney Disease Prevalence in a Diabetic Cohort: The Pathways Study. Am. J. Nephrol. 2012, 36, 245–251. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Theobald, H.; Journath, G.; Fritz, T. Gender differences in risk factor control and treatment profile in diabetes: A study in 229 swedish primary health care centres. Scand. J. Prim. Heal. Care 2004, 22, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gouni-Berthold, I.; Berthold, H.K.; Mantzoros, C.S.; Bohm, M.; Krone, W. Sex disparities in the treatment and control of cardio-vascular risk factors in type 2 diabetes. Diabetes Care 2008, 31, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.; Marphatia, A.A.; Cole, T.; McCoy, D. Associations of economic and gender inequality with global obesity prevalence: Understanding the female excess. Soc. Sci. Med. 2012, 75, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, I. Stronger associations of obesity with prehypertension and hypertension in young women than in young men. J. Hypertens. 2012, 30, 1423–1429. [Google Scholar] [CrossRef]
- Di Giosia, P.; Giorgini, P.; Stamerra, C.A.; Petrarca, M.; Ferri, C.; Sahebkar, A. Gender Differences in Epidemiology, Pathophysiology, and Treatment of Hypertension. Curr. Atheroscler. Rep. 2018, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in hypertension. Curr. Opin. Nephrol. Hypertens. 2018, 27, 176–181. [Google Scholar] [CrossRef]
- Tziomalos, K.; Giampatzis, V.; Baltatzi, M.; Efthymiou, E.; Psianou, K.; Papastergiou, N.; Magkou, D.; Bougatsa, V.; Savopoulos, C.; Hatzitolios, A.I. Sex-specific differences in cardio-vascular risk factors and blood pressure control in hypertensive patients. J. Clin. Hypertens 2014, 16, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xie, D.; Xu, X.; Qin, X.; Tang, G.; Wang, B.; Wang, Y.; Hou, F.; Xu, X.; Wang, X. Blood pressure and renal function decline: A 7-year prospective cohort study in middle-aged rural Chinese men and women. J. Hypertens. 2015, 33, 136–143. [Google Scholar] [CrossRef]
- Dubey, R.K.; Oparil, S.; Imthurn, B.; Jackson, E.K. Sex hormones and hypertension. Cardiovasc. Res. 2002, 53, 688–708. [Google Scholar] [CrossRef]
- Sullivan, J.C. Sex and the renin-angiotensin system: Inequality between the sexes in response to RAS stimulation and inhibition. Am. J. Physiol. Integr. Comp. Physiol. 2008, 294, R1220–R1226. [Google Scholar] [CrossRef]
- Hernandez Schulman, I.; Raij, L. Salt sensitivity and hypertension after menopause: Role of nitric oxide and angiotensin II. Am. J. Nephrol. 2006, 26, 170–180. [Google Scholar] [CrossRef]
- Seligman, V.A.; Lum, R.F.; Olson, J.L.; Li, H.; Criswell, L.A. Demographic differences in the development of lupus nephritis: A retrospective analysis. Am. J. Med. 2002, 112, 726–729. [Google Scholar] [CrossRef]
- O’Shaughnessy, M.; Hogan, S.L.; Thompson, B.D.; Coppo, R.; Fogo, A.B.; Jennette, J.C. Glomerular disease frequencies by race, sex and region: Results from the International Kidney Biopsy Survey. Nephrol. Dial. Transplant. 2018, 33, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Maroz, N.; Segal, M.S. Lupus nephritis and end-stage kidney disease. Am. J. Med. Sci. 2013, 346, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Chiu, W.-C.; Yang, T.-S.; Chen, C.-J.; Chen, Y.-C.; Lai, H.-M.; Yu, S.-F.; Su, Y.-J.; Cheng, T.-T. Age- and gender-related long-term renal outcome in patients with lupus nephritis. Lupus 2011, 20, 1135–1141. [Google Scholar] [CrossRef]
- Peng, W.; Tang, Y.; Tan, L.; Qin, W. Clinicopathological study of male and female patients with lupus nephritis: A retrospective study. Int. Urol. Nephrol. 2018, 50, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Cattran, D.C.; Reich, H.N.; Beanlands, H.J.; Miller, J.A.; Scholey, J.W.; Troyanov, S. The impact of sex in primary glomerulo-nephritis. Nephrol. Dial. Transplant. 2008, 23, 2247–2253. [Google Scholar] [CrossRef]
- Deng, W.; Tan, X.; Zhou, Q.; Ai, Z.; Liu, W.; Chen, W.; Yu, X.; Yang, Q. Gender-related differences in clinicopathological characteristics and renal outcomes of Chinese patients with IgA nephropathy. BMC Nephrol. 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Yang, H.-C.; Zuo, Y.; Fogo, A.B. Models of chronic kidney disease. Drug Discov. Today Dis. Model. 2010, 7, 13–19. [Google Scholar] [CrossRef]
- Gu, T.; Horová, E.; Möllsten, A.; Seman, N.A.; Falhammar, H.; Prázný, M.; Brismar, K.; Gu, H.F. IGF2BP2 and IGF2 genetic effects in diabetes and diabetic nephropathy. J. Diabetes Complicat. 2012, 26, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.-Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Hu, B.C.; Chu, S.L.; Wang, G.L.; Gao, P.J.; Zhu, D.L.; Wang, J.G. Association between genetic variation in transforming growth factors beta1 and beta3 and renal dysfunction in non-diabetic Chinese. Clin. Exp. Hypertens. 2008, 30, 121–131. [Google Scholar] [CrossRef]
- Nabrdalik, K.; Gumprecht, J.; Adamczyk, P.; Gorczynska-Kosiorz, S.; Zywiec, J.; Grzeszczak, W. Association of rs1800471 poly-morphism of TGFB1 gene with chronic kidney disease occurrence and progression and hypertension appearance. Arch. Med. Sci. 2013, 9, 230–237. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, X.; Long, J.; Chen, Y.; Xie, Y.; Liao, M.; Chen, J.; Tian, J.; Huang, S.; Tang, R.; et al. HIPK2 polymorphisms rs2058265, rs6464214, and rs7456421 were associated with kidney stone disease in Chinese males not females. Gene 2018, 653, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Banga, R.S.; Kapitsinou, P.; Ramaiah, M.; Lawrence, J.; Kambhampati, G.; Gruenwald, A.; Böttinger, E.; Glicklich, D.; Tellis, V.; et al. Human and Murine Kidneys Show Gender- and Species-Specific Gene Expression Differences in Response to Injury. PLoS ONE 2009, 4, e4802. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Rozowsky, J.S.; Laurenzi, I.J.; Petersen, P.H.; Zou, K.; Zhong, W.; Gerstein, M.; Snyder, M. Major Molecular Differences between Mammalian Sexes Are Involved in Drug Metabolism and Renal Function. Dev. Cell 2004, 6, 791–800. [Google Scholar] [CrossRef]
- Wang, L.; Song, J.; Wang, S.; Buggs, J.; Chen, R.; Zhang, J.; Wang, L.; Rong, S.; Li, W.; Wei, J.; et al. Cross-sex transplantation alters gene expression and enhances inflammatory response in the transplanted kidneys. Am. J. Physiol. Physiol. 2017, 313, F326–F338. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Knob, A.L.U.; et al. Association of Trypanolytic ApoL1 Variants with Kidney Disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef]
- Fedewa, S.A.; McClellan, W.M.; Judd, S.; Gutiérrez, O.M.; Crews, D.C. The association between race and income on risk of mortality in patients with moderate chronic kidney disease. BMC Nephrol. 2014, 15, 136. [Google Scholar] [CrossRef]
- Parsa, A.; Kao, W.L.; Xie, D.; Astor, B.C.; Li, M.; Hsu, C.-Y.; Feldman, H.I.; Parekh, R.S.; Kusek, J.W.; Greene, T.; et al. APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2183–2196. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.E.; Sawinski, D.; Reed, R.D.; Shelton, B.; MacLennan, P.A.; Kumar, V.; Mehta, S.; Mannon, R.B.; Gaston, R.; Julian, B.A.; et al. Apolipoprotein L1 and Chronic Kidney Disease Risk in Young Potential Living Kidney Donors. Ann. Surg. 2018, 267, 1161–1168. [Google Scholar] [CrossRef]
- Barbour, S.J.; Schachter, M.; Er, L.; Djurdjev, O.; Levin, A. A systematic review of ethnic differences in the rate of renal pro-gression in CKD patients. Nephrol. Dial. Transplant. 2010, 25, 2422–2430. [Google Scholar] [CrossRef]
- Laster, M.; Shen, J.I.; Norris, K.C. Kidney Disease Among African Americans: A Population Perspective. Am. J. Kidney Dis. 2018, 72, S3–S7. [Google Scholar] [CrossRef]
- Bryson, C.L.; Ross, H.J.; Boyko, E.J.; Young, B.A. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: Associations with diabetes and level of CKD. Am. J. Kidney Dis. 2006, 48, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Hughson, M.D.; Puelles, V.; Hoy, W.E.; Douglas-Denton, R.N.; Mott, S.A.; Bertram, J. Hypertension, glomerular hypertrophy and nephrosclerosis: The effect of race. Nephrol. Dial. Transplant. 2014, 29, 1399–1409. [Google Scholar] [CrossRef]
- Abdi, R.; Slakey, D.; Kittur, D.; Racusen, L.C. Heterogeneity of glomerular size in normal donor kidneys: Impact of race. Am. J. Kidney Dis. 1998, 32, 43–46. [Google Scholar] [CrossRef]
- Duru, O.K.; Li, S.; Jurkovitz, C.; Bakris, G.; Brown, W.; Chen, S.-C.; Collins, A.; Klag, M.; McCullough, P.A.; McGill, J.; et al. Race and Sex Differences in Hypertension Control in CKD: Results from the Kidney Early Evaluation Program (KEEP). Am. J. Kidney Dis. 2008, 51, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Pankewycz, O.; El-Ghoroury, M.; Shihab, F.; Wiland, A.; McCague, K.; Chan, L. Outcomes in African American Kidney Transplant Patients Receiving Tacrolimus and Mycophenolic Acid Immunosuppression. Transplantation 2013, 95, 566–572. [Google Scholar] [CrossRef]
- Eckhoff, D.E.; Young, C.J.; Gaston, R.S.; Fineman, S.W.; Deierhoi, M.H.; Foushee, M.T.; Brown, R.N.; Diethelm, A.G. Racial Disparities in Renal Allograft Survival: A Public Health Issue? J. Am. Coll. Surg. 2007, 204, 894–902. [Google Scholar] [CrossRef]
- Gibney, E.; Parikh, C.; Garg, A. Age, Gender, Race, and Associations With Kidney Failure Following Living Kidney Donation. Transplant. Proc. 2008, 40, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Schnitzler, M.A.; Garg, A.X.; Xiao, H.; Axelrod, D.; Tuttle-Newhall, J.E.; Brennan, D.C.; Segev, D.L. Race, Relationship and Renal Diagnoses After Living Kidney Donation. Transplantation 2015, 99, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Kucirka, L.M.; Grams, M.E.; Lessler, J.; Hall, E.C.; James, N.T.; Massie, A.B.; Montgomery, R.A.; Segev, D.L. Association of Race and Age with Survival Among Patients Undergoing Dialysis. JAMA 2011, 306, 620–626. [Google Scholar] [CrossRef]
- Crews, D.C.; Sozio, S.; Liu, Y.; Coresh, J.; Powe, N.R. Inflammation and the Paradox of Racial Differences in Dialysis Survival. J. Am. Soc. Nephrol. 2011, 22, 2279–2286. [Google Scholar] [CrossRef]
- Goyal, V. Changes with age in the human kidney. Exp. Gerontol. 1982, 17, 321–331. [Google Scholar] [CrossRef]
- Nyengaard, J.R.; Bendtsen, T.F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec. 1992, 232, 194–201. [Google Scholar] [CrossRef]
- Denic, A.; Lieske, J.C.; Chakkera, H.A.; Poggio, E.D.; Alexander, M.P.; Singh, P.; Kremers, W.K.; Lerman, L.O.; Rule, A.D. The Substantial Loss of Nephrons in Healthy Human Kidneys with Aging. J. Am. Soc. Nephrol. 2016, 28, 313–320. [Google Scholar] [CrossRef]
- Weinstein, J.R.; Anderson, S. The aging kidney: Physiological changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Pottel, H.; Hoste, L.; Yayo, E.; Delanaye, P. Glomerular Filtration Rate in Healthy Living Potential Kidney Donors: A Meta-Analysis Supporting the Construction of the Full Age Spectrum Equation. Nephron 2017, 135, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Boese, A.C.; Kim, S.C.; Yin, K.-J.; Lee, J.-P.; Hamblin, M.H. Sex differences in vascular physiology and pathophysiology: Estrogen and androgen signaling in health and disease. Am. J. Physiol. Circ. Physiol. 2017, 313, H524–H545. [Google Scholar] [CrossRef]
- Ngo, S.; Steyn, F.; McCombe, P. Gender differences in autoimmune disease. Front. Neuroendocr. 2014, 35, 347–369. [Google Scholar] [CrossRef] [PubMed]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; da Silva, F.B.; Ribeiro, R.F., Jr.; Stefanon, I. Sex hormones in the cardiovascular system. Horm. Mol. Biol. Clin. Investig. 2014, 18, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Sathish, V.; Martin, Y.N.; Prakash, Y. Sex steroid signaling: Implications for lung diseases. Pharmacol. Ther. 2015, 150, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Hoermann, R.; Fui, M.N.T.; Zajac, J.D.; Ierino, F.L.; Roberts, M.A. Sex steroids levels in chronic kidney disease and kidney transplant recipients: Associations with disease severity and prediction of mortality. Clin. Endocrinol. 2014, 82, 767–775. [Google Scholar] [CrossRef]
- Harris, A.N.; Lee, H.-W.; Verlander, J.W.; Weiner, I.D. Testosterone modulates renal ammonia metabolism. Am. J. Physiol. Physiol. 2020, 318, F922–F935. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.Y.; Giribabu, N.; Salleh, N. Effects of gonadectomy and testosterone treatment on aquaporin expression in the kidney of normotensive and hypertensive rats. Exp. Biol. Med. 2017, 242, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-J.; Dimke, H.; Schoeber, J.P.; Hsu, S.-C.; Lin, S.-H.; Chu, P.; Hoenderop, J.G.; Bindels, R.J. Testosterone increases urinary calcium excretion and inhibits expression of renal calcium transport proteins. Kidney Int. 2010, 77, 601–608. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Nakashima, A.; Arver, S.; Parini, P.; Lindholm, B.; Bárány, P.; Heimbürger, O.; Stenvinkel, P. Prevalence and clinical implications of tes-tosterone deficiency in men with end-stage renal disease. Nephrol. Dial. Transplant. 2011, 26, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Tehrani, F.R.; Rahmati, M.; Soudmand, S.A.; Behboudi-Gandevani, S.; Sabet, Z.; Azizi, F. Low serum testosterone levels and the incidence of chronic kidney disease among male adults: A prospective population-based study. Andrology 2019, 8, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Qureshi, A.R.; Parini, P.; Arver, S.; Lindholm, B.; Bárány, P.; Heimbürger, O.; Stenvinkel, P. Low Serum Testosterone Increases Mortality Risk among Male Dialysis Patients. J. Am. Soc. Nephrol. 2009, 20, 613–620. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Kerr, H.; Askar, M.; Goldfarb, D.A.; Schold, J. Low testosterone at time of transplantation is independently as-sociated with poor patient and graft survival in male renal transplant recipients. J. Urol. 2014, 192, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Soljancic, A.; Ruiz, A.L.; Chandrashekar, K.; Maranon, R.; Liu, R.; Reckelhoff, J.F.; Juncos, L.A. Protective role of testosterone in ischemia-reperfusion-induced acute kidney injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R951–R958. [Google Scholar] [CrossRef] [PubMed]
- Patil, C.N.; Wallace, K.; Lamarca, B.D.; Moulana, M.; Lopez-Ruiz, A.; Soljancic, A.; Juncos, L.A.; Grande, J.P.; Reckelhoff, J.F. Low-dose testosterone protects against renal ischemia-reperfusion injury by increasing renal IL-10-to-TNF-α ratio and attenuating T-cell infiltration. Am. J. Physiol. Physiol. 2016, 311, F395–F403. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kil, I.S.; Seok, Y.M.; Yang, E.S.; Kim, D.K.; Lim, D.G.; Park, J.-W.; Bonventre, J.V.; Park, K.M. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J. Biol. Chem. 2006, 281, 20349–20356. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, J.I.; Ahn, Y.; Bonventre, A.J.; Bonventre, J.V. Testosterone Is Responsible for Enhanced Susceptibility of Males to Ischemic Renal Injury. J. Biol. Chem. 2004, 279, 52282–52292. [Google Scholar] [CrossRef]
- Metcalfe, P.; Leslie, J.A.; Campbell, M.T.; Meldrum, D.R.; Hile, K.L.; Meldrum, K.K. Testosterone exacerbates obstructive renal injury by stimulating TNF-α production and increasing proapoptotic and profibrotic signaling. Am. J. Physiol. Metab. 2008, 294, E435–E443. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Gandolfo, M.T.; Salvatore, F.; Villaggio, B.; Gianiorio, F.; Traverso, P.; Deferrari, G.; Garibotto, G. Testosterone promotes apoptotic damage in human renal tubular cells. Kidney Int. 2004, 65, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Lateef, S.M.; El-Sayed, E.M.; Mansour, A.M.; Salama, S.A. The protective role of estrogen and its receptors in gentami-cin-induced acute kidney injury in rats. Life Sci. 2019, 239, 117082. [Google Scholar] [CrossRef]
- Ikeda, M.; Swide, T.; Vayl, A.; Lahm, T.; Anderson, S.; Hutchens, M.P. Estrogen administered after cardiac arrest and cardio-pulmonary resuscitation ameliorates acute kidney injury in a sex- and age-specific manner. Crit. Care 2015, 19, 332. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chang, C.Y.; Chang, S.T.; Chen, S.H. 17beta-Estradiol Accelerated Renal Tubule Regeneration in Male Rats After Is-chemia/Reperfusion-Induced Acute Kidney Injury. Shock 2016, 46, 158–163. [Google Scholar] [CrossRef]
- El-Gendy, A.A.; Elsaed, W.M.; Abdallah, H.I. Potential role of estradiol in ovariectomy-induced derangement of renal endo-crine functions. Ren. Fail. 2019, 41, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.; Berho, M.; Korach, K.; Doublier, S.; Lupia, E.; Striker, G.; Karl, M. Gender-specific effects of endogenous testosterone: Female α-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007, 72, 464–472. [Google Scholar] [CrossRef]
- Doublier, S.; Lupia, E.; Catanuto, P.; Periera-Simon, S.; Xia, X.; Korach, K.; Berho, M.; Elliot, S.J.; Karl, M. Testosterone and 17beta-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011, 79, 404–413. [Google Scholar] [CrossRef]
- Dixon, A.; Maric, C. 17beta-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-beta protein expression and signaling. Am. J. Physiol. Ren. Physiol. 2007, 293, F1678–F1690. [Google Scholar] [CrossRef]
- Negulescu, O.; Bognar, I.; Lei, J.; Devarajan, P.; Silbiger, S.; Neugarten, J. Estradiol reverses TGF-beta1-induced mesangial cell apoptosis by a casein kinase 2-dependent mechanism. Kidney Int. 2002, 62, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Mankhey, R.W.; Wells, C.C.; Bhatti, F.; Maric, C. 17beta-Estradiol supplementation reduces tubulointerstitial fibrosis by in-creasing MMP activity in the diabetic kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R769–R777. [Google Scholar] [CrossRef]
- Kummer, S.; Jeruschke, S.; Wegerich, L.V.; Peters, A.; Lehmann, P.; Seibt, A.; Mueller, F.; Koleganova, N.; Halbenz, E.; Schmitt, C.P.; et al. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in vitro and in vivo. PLoS ONE 2011, 6, e27457. [Google Scholar] [CrossRef]
- Hutchens, M.P.; Fujiyoshi, T.; Komers, R.; Herson, P.S.; Anderson, S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am. J. Physiol. Physiol. 2012, 303, F377–F385. [Google Scholar] [CrossRef]
- Shibata, Y.; Takaoka, M.; Maekawa, D.; Kuwahara, C.; Matsumura, Y. Involvement of nitric oxide in the suppressive effect of 17beta-estradiol on endothelin-1 overproduction in ischemic acute renal failure. J. Cardiovasc. Pharmacol. 2004, 44, S459–S461. [Google Scholar] [CrossRef] [PubMed]
- Satake, A.; Takaoka, M.; Nishikawa, M.; Yuba, M.; Shibata, Y.; Okumura, K.; Kitano, K.; Tsutsui, H.; Fujii, K.; Kobuchi, S.; et al. Protective effect of 17beta-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008, 73, 308–317. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Yin, Y.; Fang, Y.; Zhao, J.; Chen, J. Estrogen induces endothelial progenitor cells proliferation and mi-gration by estrogen receptors and PI3K-dependent pathways. Microvasc. Res. 2008, 75, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, N.; Pathak, D.; Bedi, P.M.S. Estradiol attenuates ischemia reperfusion-induced acute kidney injury through PPAR-gamma stimulated eNOS activation in rats. Mol. Cell. Biochem. 2019, 453, 1–9. [Google Scholar] [CrossRef]
- Lakzaei, H.; Safari, T.; Komeili, G.R. Interaction of Sex Hormones and the Renin-Angiotensin System in Ovariectomized Rats Subjected to Ischemia-Reperfusion Induction. Adv. Biomed. Res. 2019, 8, 64. [Google Scholar] [CrossRef]
- Nematbakhsh, M.; Nasri, H.; Talebi, A.; Pilehvarian, A.-A.; Safari, T.; Eshraghi-Jazi, F.; Haghighi, M.; Ashrafi, F.; Pezeshki, Z. Evidence against protective role of sex hormone estrogen in cisplatin-induced nephrotoxicity in ovarectomized rat model. Toxicol. Int. Former. Indian J. Toxicol. 2013, 20, 43–47. [Google Scholar] [CrossRef]
- Müller, V.; Szabó, A.; Viklicky, O.; Gaul, I.; Pörtl, S.; Philipp, T.; Heemann, U.W. Sex hormones and gender-related differences: Their influence on chronic renal allograft rejection. Kidney Int. 1999, 55, 2011–2020. [Google Scholar] [CrossRef]
- Antus, B.; Liu, S.; Yao, Y.; Zou, H.; Song, E.; Lutz, J.; Heemann, U. Effects of progesterone and selective oestrogen receptor modulators on chronic allograft nephropathy in rats. Nephrol. Dial. Transplant. 2004, 20, 329–335. [Google Scholar] [CrossRef][Green Version]
- Hughes, G.C.; Martin, D.; Zhang, K.; Hudkins, K.L.; Alpers, C.E.; Clark, E.A.; Elkon, K.B. Decrease in glomerulonephritis and Th1-associated autoantibody production after progesterone treatment in NZB/NZW mice. Arthritis Rheum. 2009, 60, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Sandhi, J.; Singh, J.P.; Kaur, T.; Ghuman, S.S.; Singh, A.P. Involvement of progesterone receptors in ascorbic acid–mediated protection against ischemia-reperfusion–induced acute kidney injury. J. Surg. Res. 2014, 187, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.E.; Kanyicska, B.; Lerant, A.; Nagy, G. Prolactin: Structure, Function, and Regulation of Secretion. Physiol. Rev. 2000, 80, 1523–1631. [Google Scholar] [CrossRef] [PubMed]
- Pickford, G.E.; Phillips, J.G. Prolactin, a Factor in Promoting Survival of Hypophysectomized Killifish in Fresh Water. Science 1959, 130, 454–455. [Google Scholar] [CrossRef]
- Lam, T. Prolactin and hydromineral regulation in fishes. Gen. Comp. Endocrinol. 1972, 3, 328–338. [Google Scholar] [CrossRef]
- Loretz, C.A.; Bern, H.A. Prolactin and Osmoregulation in Vertebrates. Neuroendocrinology 1982, 35, 292–304. [Google Scholar] [CrossRef]
- Sakai, Y.; Hiraoka, Y.; Ogawa, M.; Takeuchi, Y.; Aiso, S. The prolactin gene is expressed in the mouse kidney. Kidney Int. 1999, 55, 833–840. [Google Scholar] [CrossRef]
- Mountjoy, K.; Cowden, E.A.; Dobbie, J.W.; Ratcliffe, J.G. Prolactin Receptors in the Rat Kidney. J. Endocrinol. 1980, 87, 47–54. [Google Scholar] [CrossRef]
- Evan, A.P.; Palmer, G.C.; Lucci, M.S.; Solomon, S. Prolactin-induced stimulation of rat renal adenylate cyclase and autoradiographic localization to the distal nephron. Nephron 1977, 18, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Emmanouel, D.S.; Fang, V.S.; Katz, A.I. Prolactin metabolism in the rat: Role of the kidney in degradation of the hormone. Am. J. Physiol. Physiol. 1981, 240, F437–F445. [Google Scholar] [CrossRef] [PubMed]
- Stier, C.T.; Cowden, E.A.; Friesen, H.G.; Allison, M.E.M. Prolactin and the Rat Kidney: A Clearance and Micropuncture Study. Endocrinology 1984, 115, 362–367. [Google Scholar] [CrossRef]
- Horrobin, D.; Lloyd, I.; Lipton, A.; Burstyn, P.; Durkin, N.; Muiruri, K. Actions of Prolactin on Human Renal Function. Lancet 1971, 298, 352–354. [Google Scholar] [CrossRef]
- Lucci, M.S.; Bengele, H.H.; Solomon, S. Suppressive action of prolactin on renal response to volume expansion. Am. J. Physiol. Content 1975, 229, 81–85. [Google Scholar] [CrossRef]
- Marshall, S.; Gelato, M.; Meites, J. Serum Prolactin Levels and Prolactin Binding Activity in Adrenals and Kidneys of Male Rats After Dehydration, Salt Loading, and Unilateral Nephrectomy. Exp. Biol. Med. 1975, 149, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, F.; Crambert, S.; Eklöf, A.-C.; Lundquist, A.; Hansell, P.; Holtbäck, U. Prolactin, a natriuretic hormone, interacting with the renal dopamine system. Kidney Int. 2005, 68, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Crambert, S.; Sjöberg, A.; Eklöf, A.-C.; Ibarra, F.; Holtbäck, U. Prolactin and dopamine 1-like receptor interaction in renal proximal tubular cells. Am. J. Physiol. Physiol. 2010, 299, F49–F54. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Kledzik, G.; Gelato, M.; Campbell, G.; Meites, J. Effects of estrogen and testosterone on specific prolactin binding in the kidneys and adrenals of rats. Steroids 1976, 27, 187–195. [Google Scholar] [CrossRef]
- Morrissey, S.E.; Newth, T.; Rees, R.; Barr, A.; Shora, F.; Laycock, J.F. Renal effects of recombinant prolactin in anaesthetized rats. Eur. J. Endocrinol. 2001, 145, 65–71. [Google Scholar] [CrossRef][Green Version]
- Bussieres, L.; Laborde, K.; Dechaux, M.; Sachs, C. Effects of prolactin on Na−K-ATPase activity along the rat nephron. Pflügers Arch. Eur. J. Physiol. 1987, 409, 182–187. [Google Scholar] [CrossRef]
- Rojas, L.; Reyes-Castro, L.A.; Ramírez, V.; Bautista-Pérez, R.; Rafael, C.; Castañeda-Bueno, M.; Meade, P.; Heros, P.D.L.; Arroyo-Garza, I.; Bernard, V.; et al. Ovarian hormones and prolactin increase renal NaCl cotransporter phosphorylation. Am. J. Physiol. Physiol. 2015, 308, F799–F808. [Google Scholar] [CrossRef]
- Handelsman, D.J. Hypothalamic-Pituitary Gonadal Dysfunction in Renal Failure, Dialysis and Renal Transplantation. Endocr. Rev. 1985, 6, 151–182. [Google Scholar] [CrossRef]
- Cowden, E.A.; Ratcliffe, W.A.; Ratcliffe, J.G.; Dobbie, J.W.; Kennedy, A.C. Hyperprolactinaemia in Renal Disease. Clin. Endocrinol. 1978, 9, 241–248. [Google Scholar] [CrossRef]
- Lo, J.C.; Beck, G.J.; Kaysen, G.A.; Chan, C.T.; Kliger, A.S.; Rocco, M.V.; Chertow, G.M.; for the FHN Study. Hyperprolactinemia in end-stage renal disease and effects of frequent hemodialysis. Hemodial. Int. 2017, 21, 190–196. [Google Scholar] [CrossRef]
- Dobbie, J.; Mountjoy, K.; Cowden, E.; Allison, M.; Ratcliffe, J. Prolactin Status in Experimentally Induced Acute Renal Failure in the Rat. Nephron 1981, 27, 316–319. [Google Scholar] [CrossRef]
- Falconer, I.R.; Vacek, A.T. Degradation of 125I-labelled prolactin in the rabbit: Effect of nephrectomy and prolactin infusion. J. Endocrinol. 1983, 99, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Sievertsen, G.D.; Lim, V.S.; Nakawatase, C.; Frohman, L.A. Metabolic Clearance and Secretion Rates of Human Prolactin in Normal Subjects and in Patients with Chronic Renal Failure. J. Clin. Endocrinol. Metab. 1980, 50, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, D.G.; Topcu, G.; Ozener, C.; Akalin, S.; Sirikci, O. Macroprolactin does not contribute to elevated levels of prolactin in patients on renal replacement therapy. Clin. Endocrinol. 2005, 63, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Peces, R.; Horcajada, C.; López-Novoa, J.; Frutos, M.; Casado, S.; Hernando, L. Hyperprolactinemia in Chronic Renal Failure: Impaired Responsiveness to Stimulation and Suppression. Nephron 1981, 28, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Grzeszczak, W.; Kokot, F.; Wiecek, A.; Zukowska-Szczechowska, E. Prolactin secretion in kidney transplant patients. Int. Urol. Nephrol. 1990, 22, 567–571. [Google Scholar] [CrossRef]
- Hou, S.H.; Grossman, S.; Molitch, M.E. Hyperprolactinemia in Patients with Renal Insufficiency and Chronic Renal Failure Requiring Hemodialysis or Chronic Ambulatory Peritoneal Dialysis. Am. J. Kidney Dis. 1985, 6, 245–249. [Google Scholar] [CrossRef]
- Marshall, S.; Huang, H.H.; Kledzik, G.S.; Campbell, G.A.; Meites, J. Glucocorticoid Regulation of Prolactin Receptors in Kidneys and Adrenals of Male Rats. Endocrinology 1978, 102, 869–875. [Google Scholar] [CrossRef]
- Carrero, J.J.; Kyriazis, J.; Sonmez, A.; Tzanakis, I.; Qureshi, A.R.; Stenvinkel, P.; Saglam, M.; Stylianou, K.; Yaman, H.; Taslipinar, A.; et al. Prolactin Levels, Endothelial Dysfunction, and the Risk of Cardiovascular Events and Mortality in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2011, 7, 207–215. [Google Scholar] [CrossRef]
- Reuwer, A.Q.; Nowak-Sliwinska, P.; Mans, L.A.; van der Loos, C.M.; von der Thusen, J.H.; Twickler, M.T.; Spek, C.A.; Goffin, V.; Griffioen, A.W.; Borensztajn, K.S. Functional consequences of prolactin signalling in endothelial cells: A potential link with angiogenesis in pathophysiology? J. Cell Mol. Med. 2012, 16, 2035–2048. [Google Scholar] [CrossRef]
- Yang, X.; Meyer, K.; Friedl, A. STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis. J. Biol. Chem. 2013, 288, 21184–21196. [Google Scholar] [CrossRef]
- Triebel, J.; Bertsch, T.; Bollheimer, C.; Rios-Barrera, D.; Pearce, C.F.; Hüfner, M.; De La Escalera, G.M.; Clapp, C. Principles of the prolactin/vasoinhibin axis. Am. J. Physiol. Integr. Comp. Physiol. 2015, 309, R1193–R1203. [Google Scholar] [CrossRef]
- González, C.; Rosas-Hernández, H.; Jurado-Manzano, B.; Ramirez-Lee, M.A.; Salazar-García, S.; Martinez-Cuevas, P.P.; Velarde-Salcedo, A.J.; Morales-Loredo, H.; Espinosa-Tanguma, R.; Ali, S.F.; et al. The prolactin family hormones regulate vascular tone through NO and prostacyclin production in isolated rat aortic rings. Acta Pharmacol. Sin. 2015, 36, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Goldhar, A.S.; Vonderhaar, B.K.; Trott, J.F.; Hovey, R.C. Prolactin-induced expression of vascular endothelial growth factor via Egr-1. Mol. Cell. Endocrinol. 2005, 232, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Clapp, C.; Aranda, J.; Gonzalez, C.; Jeziorski, M.C.; Martinez de la Escalera, G. Vasoinhibins: Endogenous regulators of angiogenesis and vascular function. Trends Endocrinol. Metab. 2006, 17, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Piwnica, D.; Touraine, P.; Struman, I.; Tabruyn, S.; Bolbach, G.; Clapp, C.; Martial, J.A.; Kelly, P.A.; Goffin, V. Cathepsin D Processes Human Prolactin into Multiple 16K-Like N-Terminal Fragments: Study of Their Antiangiogenic Properties and Physiological Relevance. Mol. Endocrinol. 2004, 18, 2522–2542. [Google Scholar] [CrossRef]
- Clapp, C.; Martial, J.A.; Guzman, R.C.; Rentier-Delure, F.; Weiner, R.I. The 16-kilodalton N-terminal fragment of human prolactin is a potent inhibitor of angiogenesis. Endocrinology 1993, 133, 1292–1299. [Google Scholar] [CrossRef]
- D’Angelo, G.; Struman, I.; Martial, J.; Weiner, R.I. Activation of mitogen-activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kDa N-terminal fragment of prolactin. Proc. Natl. Acad. Sci. USA 1995, 92, 6374–6378. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kunz, J.; Lin, S.-H.; Yu-Lee, L.-Y. 16-kDa Prolactin Inhibits Endothelial Cell Migration by Down-Regulating the Ras-Tiam1-Rac1-Pak1 Signaling Pathway. Cancer Res. 2007, 67, 11045–11053. [Google Scholar] [CrossRef]
- Martini, J.F.; Piot, C.; Humeau, L.M.; Struman, I.; Martial, J.A.; Weiner, R.I. The antiangiogenic factor 16K PRL induces programmed cell death in endothelial cells by caspase activation. Mol. Endocrinol. 2000, 14, 1536–1549. [Google Scholar] [CrossRef]
- Gonzalez, C.; Corbacho, A.M.; Eiserich, J.P.; Garcia, C.; Lopez-Barrera, F.; Morales-Tlalpan, V.; Barajas-Espinosa, A.; Diaz-Muñoz, M.; Rubio, R.; Lin, S.H.; et al. 16K-prolactin inhibits activation of endothelial nitric oxide synthase, intracellular calcium mobilization, and endothelium-dependent vasorelaxation. Endocrinology 2004, 145, 5714–5722. [Google Scholar] [CrossRef]
- Garcia, C.; Nunez-Anita, R.E.; Thebault, S.; Arredondo Zamarripa, D.; Jeziorsky, M.C.; Martinez de la Escalera, G.; Clapp, C. Requirement of phosphorylatable endothelial nitric oxide synthase at Ser-1177 for vasoinhibin-mediated inhibition of endothelial cell migration and proliferation in vitro. Endocrine 2014, 45, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Baldocchi, R.A.; Tan, L.; Nicoll, C.S. Processing of rat prolactin by rat tissue explants and serum in vitro. Endocrinology 1992, 130, 1653–1659. [Google Scholar] [CrossRef]
- Clapp, C.; Sears, P.S.; Nicoll, C.S. Binding Studies with Intact Rat Prolactin and a 16K Fragment of the Hormone. Endocrinology 1989, 125, 1054–1059. [Google Scholar] [CrossRef]
- Leaños-Miranda, A.; Márquez-Acosta, J.; Cárdenas-Mondragón, G.M.; Chinolla-Arellano, Z.L.; Rivera-Leaños, R.; Bermejo-Huerta, S.; Romero-Arauz, J.F.; Alvarez-Jiménez, G.; Ramos-León, J.C.; Ulloa-Aguirre, A. Urinary Prolactin as a Reliable Marker for Preeclampsia, Its Severity, and the Occurrence of Adverse Pregnancy Outcomes. J. Clin. Endocrinol. Metab. 2008, 93, 2492–2499. [Google Scholar] [CrossRef]
- Jourde-Chiche, N.; Fakhouri, F.; Dou, L.; Bellien, J.; Burtey, S.; Frimat, M.; Jarrot, P.-A.; Kaplanski, G.; Le Quintrec, M.; Pernin, V.; et al. Endothelium structure and function in kidney health and disease. Nat. Rev. Nephrol. 2019, 15, 87–108. [Google Scholar] [CrossRef]
- González, C.; Parra, A.; Ramírez-Peredo, J.; Garcia, C.; Rivera, J.C.; Macotela, Y.; Aranda, J.; Lemini, M.; Arias, J.; Ibargüengoitia, F.; et al. Elevated vasoinhibins may contribute to endothelial cell dysfunction and low birth weight in preeclampsia. Lab. Investig. 2007, 87, 1009–1017. [Google Scholar] [CrossRef]
- Cruz, M.; Cosío, G.; Jeziorski, M.C.; Vargas-Barroso, V.; Aguilar, M.B.; Cárabez, A.; Berger, P.; Saftig, P.; Arnold, E.; Thebault, S.; et al. Cathepsin D Is the Primary Protease for the Generation of Adenohypophyseal Vasoinhibins: Cleavage Occurs within the Prolactin Secretory Granules. Endocrinology 2009, 150, 5446–5454. [Google Scholar] [CrossRef]
- Lkhider, M.; Castino, R.; Bouguyon, E.; Isidoro, C.; Ollivier-Bousquet, M. Cathepsin D released by lactating rat mammary epithelial cells is involved in prolactin cleavage under physiological conditions. J. Cell Sci. 2004, 117, 5155–5164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozkayar, N.; Piskinpasa, S.; Akyel, F.; Turgut, D.; Bulut, M.; Turhan, T.; Dede, F. Relation between serum cathepsin D levels and endothelial dysfunction in patients with chronic kidney disease. Nefrologia 2015, 35, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.; Cocchiaro, P.; Oakley, F.; Howarth, R.; Callaghan, K.; Leslie, J.; Luli, S.; Wood, K.M.; Genovese, F.; Sheerin, N.S.; et al. Inhibition of lysosomal protease cathepsin D reduces renal fibrosis in murine chronic kidney disease. Sci. Rep. 2016, 6, 20101. [Google Scholar] [CrossRef]
- Cocchiaro, P.; Fox, C.; Tregidgo, N.W.; Howarth, R.; Wood, K.M.; Situmorang, G.R.; Pavone, L.M.; Sheerin, N.S.; Moles, A. Lysosomal protease cathepsin D; a new driver of apoptosis during acute kidney injury. Sci. Rep. 2016, 6, 27112. [Google Scholar] [CrossRef]
- Suzuki, C.; Tanida, I.; Ohmuraya, M.; Trejo, J.A.O.; Kakuta, S.; Sunabori, T.; Uchiyama, Y. Lack of Cathepsin D in the Renal Proximal Tubular Cells Resulted in Increased Sensitivity against Renal Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2019, 20, 1711. [Google Scholar] [CrossRef]
- Yamamoto-Nonaka, K.; Koike, M.; Asanuma, K.; Takagi, M.; Trejo, J.A.O.; Seki, T.; Hidaka, T.; Ichimura, K.; Sakai, T.; Tada, N.; et al. Cathepsin D in Podocytes Is Important in the Pathogenesis of Proteinuria and CKD. J. Am. Soc. Nephrol. 2016, 27, 2685–2700. [Google Scholar] [CrossRef] [PubMed]
- Moulton, B.C.; Khan, S. Progestin and Estrogen Control of Cathepsin D Expression and Processing in Rat Uterine Luminal Epithelium and Stroma-Myometrium. Exp. Biol. Med. 1992, 201, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Moulton, B.C. Progesterone and estrogen control of rates of synthesis of uterine cathepsin D. J. Biol. Chem. 1980, 255, 7474–7479. [Google Scholar] [CrossRef]
- Li, S.A.; Liao, D.Z.; Yazlovitskaya, E.M.; Pantazis, C.G.; Li, J.J. Induction of cathepsin D protein during estrogen carcinogenesis: Possible role in estrogen-mediated kidney tubular cell damage. Carcinog 1997, 18, 1375–1380. [Google Scholar] [CrossRef]
- Augereau, P.; Miralles, F.; Cavailles, V.; Gaudelet, C.; Parker, M.; Rochefort, H. Characterization of the proximal estro-gen-responsive element of human cathepsin D gene. Mol. Endocrinol. 1994, 8, 693–703. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Acevedo, A.; Echavarria, R.; Melo, Z. Sex Differences in Renal Function: Participation of Gonadal Hormones and Prolactin. Endocrines 2021, 2, 185-202. https://doi.org/10.3390/endocrines2030019
Franco-Acevedo A, Echavarria R, Melo Z. Sex Differences in Renal Function: Participation of Gonadal Hormones and Prolactin. Endocrines. 2021; 2(3):185-202. https://doi.org/10.3390/endocrines2030019
Chicago/Turabian StyleFranco-Acevedo, Adriana, Raquel Echavarria, and Zesergio Melo. 2021. "Sex Differences in Renal Function: Participation of Gonadal Hormones and Prolactin" Endocrines 2, no. 3: 185-202. https://doi.org/10.3390/endocrines2030019
APA StyleFranco-Acevedo, A., Echavarria, R., & Melo, Z. (2021). Sex Differences in Renal Function: Participation of Gonadal Hormones and Prolactin. Endocrines, 2(3), 185-202. https://doi.org/10.3390/endocrines2030019






