1. Introduction
Post-transplant lymphoproliferative disorder (PTLD) encompasses a wide spectrum of clinical conditions resulting from lymphoproliferation in patients receiving chronic immunosuppression for solid organ transplantation or allogeneic hematopoietic cell transplantation. These conditions can range from benign polyclonal lymphoproliferation, that can be managed with immunosuppression reduction alone, through to malignancies such as B cell lymphoma, T cell lymphoma, or Hodgkin’s lymphoma that require intensive chemotherapies [
1].
PTLD is commonly associated with the Epstein–Barr Virus (EBV). EBV is ubiquitous, with >90% of the adult population showing serological evidence of past infection (EBV IgG positive). Following acute infection, a small population of EBV-infected B cells downregulate viral antigen expression and largely manage to evade immune surveillance from cytotoxic T cells. These latently infected B cells persist throughout life but are of little consequence in a normal healthy population. However, in the setting of the post-transplant immunosuppression-related decrease in T cell immune surveillance, these cells can give rise to lymphoproliferative disorders such as PTLD [
2].
The risk of developing PTLD, particularly early in the post-transplant setting, is heavily influenced by EBV serostatus at the time of transplantation. EBV IgG-negative recipients (R−) who receive a mismatched graft from an EBV IgG-positive donor (D+), or who develop primary EBV infection post-transplantation, have no baseline EBV-specific T lymphocyte responses and are at risk of uncontrolled EBV infection, a high virion peak, and the subsequent amplification of B cell proliferation. It has been estimated that this leads to an increase in the risk of developing PTLD by a factor of 10–75 when compared with seropositive recipients [
1]. It is unsurprising, therefore, that the risk of PTLD is significantly higher in the pediatric population, who are more likely to be EBV naive (IgG negative) at the time of transplantation. Reflecting this clinical observation, the EBV genome is found in the majority (>90%) of B cell-PTLDs occurring within 12 months of transplantation, whereas those occurring later after transplant in an adult population are increasingly EBV negative [
2].
Another major determinant of risk is the type of organ transplanted. Intestinal transplant recipients have the highest risk (~20%), followed by lung (3–10%), heart (2–8%), liver (1–5.5%), pancreas (0.5–5%), and kidney transplants (0.8–2.5%) [
3,
4]. These graft-specific differences in disease incidence likely represent the overall magnitude of net immunosuppression, the different induction immunosuppressive regimes, and the amount of lymphoid tissue that accompanies the graft. With regard to isolated liver transplantation, studies that report specifically on adult recipients demonstrate an incidence of PTLD between 1.2% and 3.1% [
5].
The other significant risk factor, particularly with regard to late PTLD, is cumulative immunosuppression exposure. As most patients are prescribed a combination of medications, the contribution of each individual agent remains unclear. Induction immunosuppression has, however, been associated with a major role in the development of early PTLD, particularly OKT3 and alemtuzumab, whilst no increased risk is seen with interleukin 2 (IL2) receptor antagonists [
2].
Herein, we report the characteristics and outcomes of PTLD in a single adult liver transplant centre in Australia, with a case series of 1409 liver transplant recipients spanning close to 37 years.
2. Materials and Methods
A retrospective study was performed utilising data from a prospectively maintained database. Ethical approval for the study was granted by the Austin Health Research Ethics Committee (Approval ID: HREC/112740/Austin-2024), and the study was conducted in accordance with the Declaration of Helsinki.
In Australia, it is not uncommon for patients ≥16 years of age to be transplanted at the adult service, as is the practice at our centre. Therefore, all patients aged ≥16 years transplanted and managed at our liver transplant centre from the inception of the service (20 June 1988) were captured. Patients who received a liver transplant in combination with any other solid organ, be it simultaneous or sequential, were excluded from subsequent analysis.
The following data was recorded: patients diagnosed with PTLD (LT-PTLD), sex, age at transplantation, indication for liver transplantation, EBV serostatus at time of transplantation, time between transplantation and diagnosis of LT-PTLD (years), organs involved (visceral vs. nodal disease), EBV viral load at time of diagnosis (detectable vs. undetectable), histologic subtype of tumour (based upon the World Health Organization (WHO) classification system for tumours of hematopoietic and lymphoid tissues 2017) [
6], presence of EBV in tissue biopsy (defined as either positive EBV-encoded small RNAs in situ hybridisation (EBER-ISH) or immunohistochemistry targeting the latent membrane protein 1 (LMP1)), immunosuppression at the time of diagnosis, including any heightened immunosuppression in the three months prior to diagnosis, treatment received for LT-PTLD, and patient outcome, including time (years) from diagnosis to death, or censor date at time of data closure (31 December 2024) in surviving patients.
In our centre, over the entire study period, the standard immunosuppression protocol consisted of a calcineurin inhibitor (ciclosporin or tacrolimus) in combination with an immunomodulator (azathioprine or mycophenolate mofetil) and prednisolone. The prednisolone is discontinued at month three if possible. In recent years, basiliximab has been used for patients with impaired renal function at time of transplant, and everolimus for patients transplanted for hepatocellular carcinoma.
Continuous variables are presented as the median with interquartile range (IQR) and categorical data as the frequency with percentage. Between-group comparisons were investigated with the Mann–Whitney U test for continuous variables or the log-rank test for Kaplan–Meier probability of survival curves when investigating factors influencing mortality. A two-tailed p-value < 0.05 was considered statistically significant. Statistical analyses were conducted using Microsoft Excel version 16.96 (Microsoft, Redmond, WA, USA) and StataNow/SE 19.5 (StataCorp, College Station, TX, USA).
3. Results
3.1. Demographics
A total of 1465 patients transplanted ≥16 years of age were managed at our centre during the study period. Patients receiving a combined and/or sequential kidney transplant (n = 41), intestinal transplant (n = 8), pancreas transplant (n = 4), lung transplant (n = 2), and heart transplant (n = 1) were excluded from analysis, leaving a total of 1409 liver transplant recipients.
A total of 28 patients (n = 18, 64.3% male and n = 10, 35.7% female) were diagnosed with LT-PTLD during the study period, equating to an incidence of 2.0%. The median age at time of transplantation was 52.6 years (IQR 44–59), and the median age at time of diagnosis of LT-PTLD was 63.0 years (IQR 52–67.5). Only 2 (7.1%) patients were <18 years of age at the time of their transplant (16.3 years and 16.4 years). The median time between transplantation and diagnosis of PTLD was 11.4 years (IQR 3.4–6.2). There were only four (14.3%) episodes of early PTLD (diagnosis within 12 months of transplantation). The indication for liver transplantation and other patient demographics are presented in
Table 1. The case characteristics of each individual patient are presented in
Table 2.
3.2. Presentation of PTLD
The significant majority (n = 24, 85.7%) of patients presented with visceral disease, with the remaining four (14.3%) patients presenting with nodal-only disease. Of those with visceral disease, the most involved organ was the bowel (n = 9, 37.5%), followed by the central nervous system (CNS) (n = 6, 25%) and soft tissue (n = 4, 16.7%). There were single (4.2%) cases involving the bone, bone marrow, lung, and spleen. Only one case involved the liver allograft itself. The mode of presentation reflected the involved organs, such that patients with bowel disease presented with a range of symptoms including abdominal pain, gastrointestinal bleeding, bowel obstruction, and loss of weight. Those with primary CNS disease presented with focal neurological symptoms or seizures. A typical diagnostic computed tomography (CT) scan and positron emission tomography (PET) scan for a patient presenting with PTLD involving bowel are presented in
Figure 1, with the histology presented in
Figure 2.
3.3. Immunosuppression
No patients received induction immunosuppression with anti-thymocyte globulin, OKT3, or alemtuzumab, whilst just three (10.7%) received basiliximab. At the time of diagnosis of LT-PTLD 24 (85.7%), patients were prescribed a calcineurin inhibitor (tacrolimus n = 18, 75%, ciclosporin n = 6, 25%), whilst 18 (64.3%) were prescribed an antimetabolite (mycophenolate mofetil n = 14, 77.8%, azathioprine n = 4, 22.2%). Corticosteroids were used in only 10 (35.7%) patients, and a mammalian target of the rapamycin inhibitor in just 2 (7.1%) patients. Seven (25%) patients were maintained on triple immunosuppression at the time of LT-PTLD diagnosis.
Four (14.3%) of the patients had received heightened immunosuppression in the three months prior to LT-PTLD diagnosis. Three of these patients had liver allograft rejection requiring high-dose corticosteroids, whilst the remaining patient had a flare of ulcerative colitis, also requiring corticosteroids.
3.4. EBV Status
Consistent with the general population, most patients had evidence of previous EBV exposure (EBV IgG positive) at the time of transplantation (n = 24, 85.7%). All four of the patients who were EBV IgG negative (EBV naive) received a mismatched graft (D+/R−). Patients who were EBV naive at transplantation had a significantly shorter interval to diagnosis of LT-PTLD when compared to those with prior exposure (0.9 years, IQR 0.3–5.8, vs. 11.1 years, IQR 5.5–1.43,
p = 0.02). Aside from their earlier presentation of disease, the clinical features of these four patients were not dissimilar to the entire cohort, suggesting serostatus alone is most important. Two were on triple immunosuppression and only one was prescribed corticosteroids. Whilst two were under the age of 20 years at the time of transplantation, the remaining two were aged greater than 60 years. All four had diffuse large B cell lymphoma (DLBCL). One patient required surgical resection for an enterocolonic fistula. Three patients were treated with rituximab and chemotherapy, whilst the remaining patient had a resection followed by chemotherapy. The two older patients died from their disease (
Table 2).
Peripheral blood EBV viral load results were available at the time of LT-PTLD diagnosis in 26 (92.9%) patients. EBV was detectable in 16 (61.5%) of these patients and not detected in the remaining 10 (38.5%) patients. Of the four patients who were EBV naive at time of transplantation, three (75%) were found to have detectable EBV in peripheral blood at the time of their LT-PTLD diagnosis. There was no significant difference in time between transplantation to LT-PTLD diagnosis based on the detectable viral load (p = 0.17).
The results of EBV detection within the tissue biopsy were available for 25 (89.3%) patients. Of these, 13 (52%) had evidence of EBV detectable within the tumour (i.e., EBV-associated LT-PTLD), and the remaining 12 (48%) patients had no detectable EBV within the tumour. Importantly, whilst EBER-ISH was not available at our centre for the entire study period, all diagnoses of PTLD did occur during the period it was available; hence, all tissue biopsies were subjected to the most sensitive diagnostic technique. Latency between transplantation to LT-PTLD was no different based on detectable EBV within the tumour (p = 0.13). One patient had EBV detected in tissue but not in peripheral blood, and conversely one patient had EBV detected in peripheral blood but not in the tissue.
3.5. Histological Subtype of Tumour
The subtype was documented in 27 (96.4%) patients. The overwhelming majority of patients (n = 23, 85.2%) had DLBCL. There were single (3.7%) cases of florid follicular hyperplasia, plasmacytoma, Burkitt lymphoma, and Classic Hodgkin lymphoma LT-PTLD. There were no cases of T cell lymphoma. The morphological type did not influence time to diagnosis (p = 0.24).
3.6. Management
All patients had a reduction in maintenance immunosuppression following diagnosis. Our centre has no formal protocol for immunosuppression minimisation, which is primarily at the discretion of the treating physician. We do, however, endeavor to have at least a 50% reduction in net immunosuppression dosing at time of diagnosis of PTLD. Rituximab was prescribed in 18 (64.3%), with chemotherapy prescribed in 20 (71.4%) patients. Of the patients who received rituximab, 17 (94.4%) received this in combination with chemotherapy, whilst just a single (5.6%) patient received rituximab monotherapy. Aside from obtaining tissue for histological examination, only four (14.3%) patients required surgery, which was indicated in all cases for complications of bowel LT-PTLD (obstruction and perforation). One patient received palliative external beam radiotherapy for multiple cerebral tumours.
3.7. Outcome
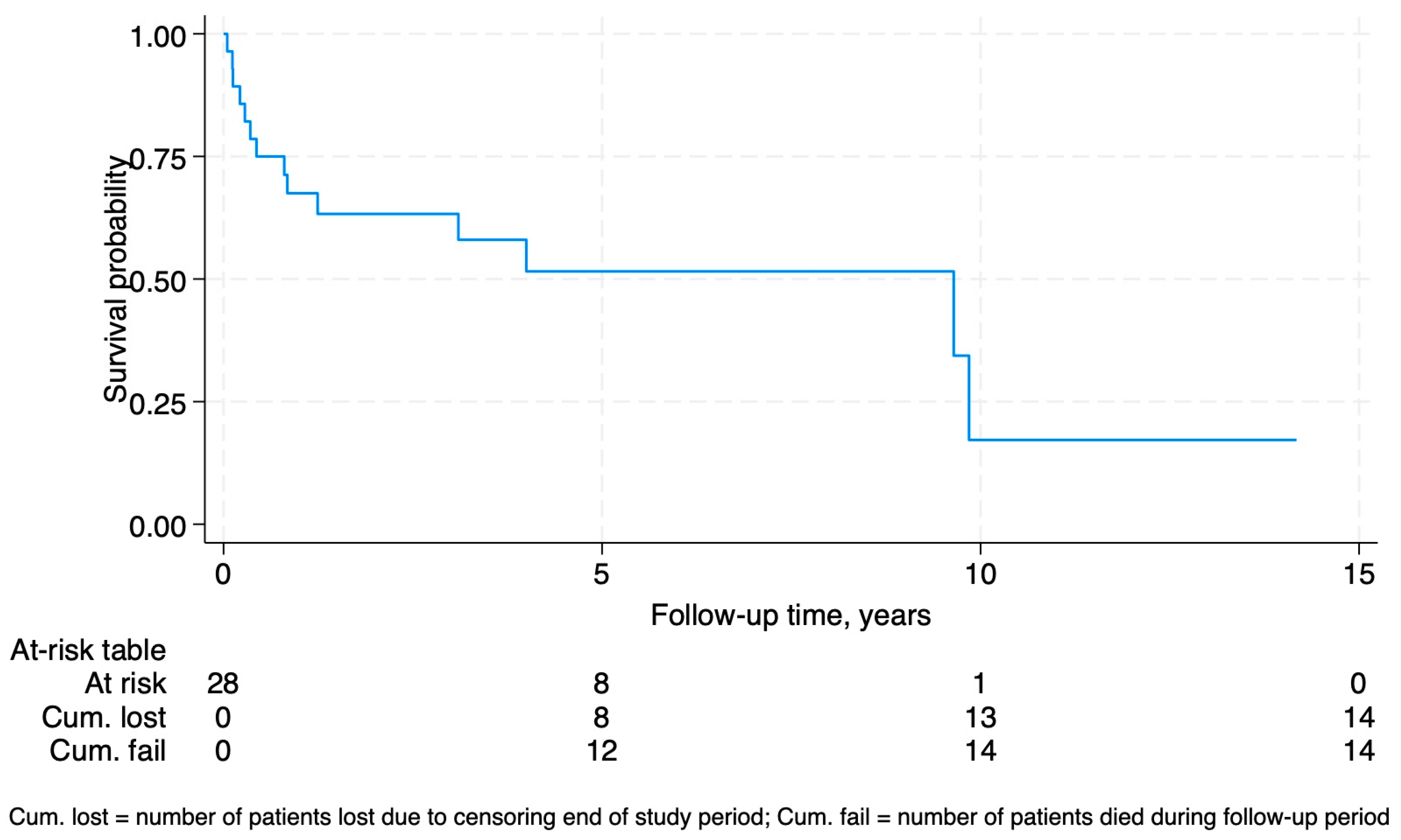
Fourteen (50%) of the patients with LT-PTLD died during the study period, with death directly attributed to LT-PTLD in 9 (64.3%). The median time from diagnosis of LT-PTLD to death was 0.6 years (IQR 0.2–2.6). Of the survivors, the median time from diagnosis to censor date was 3.6 years (IQR 1.8–6.6). Considering censoring for study completion, the survival probability following diagnosis was 67.5% at 12 months, 51.7% at 5 years, and 17.2% at 10 years (
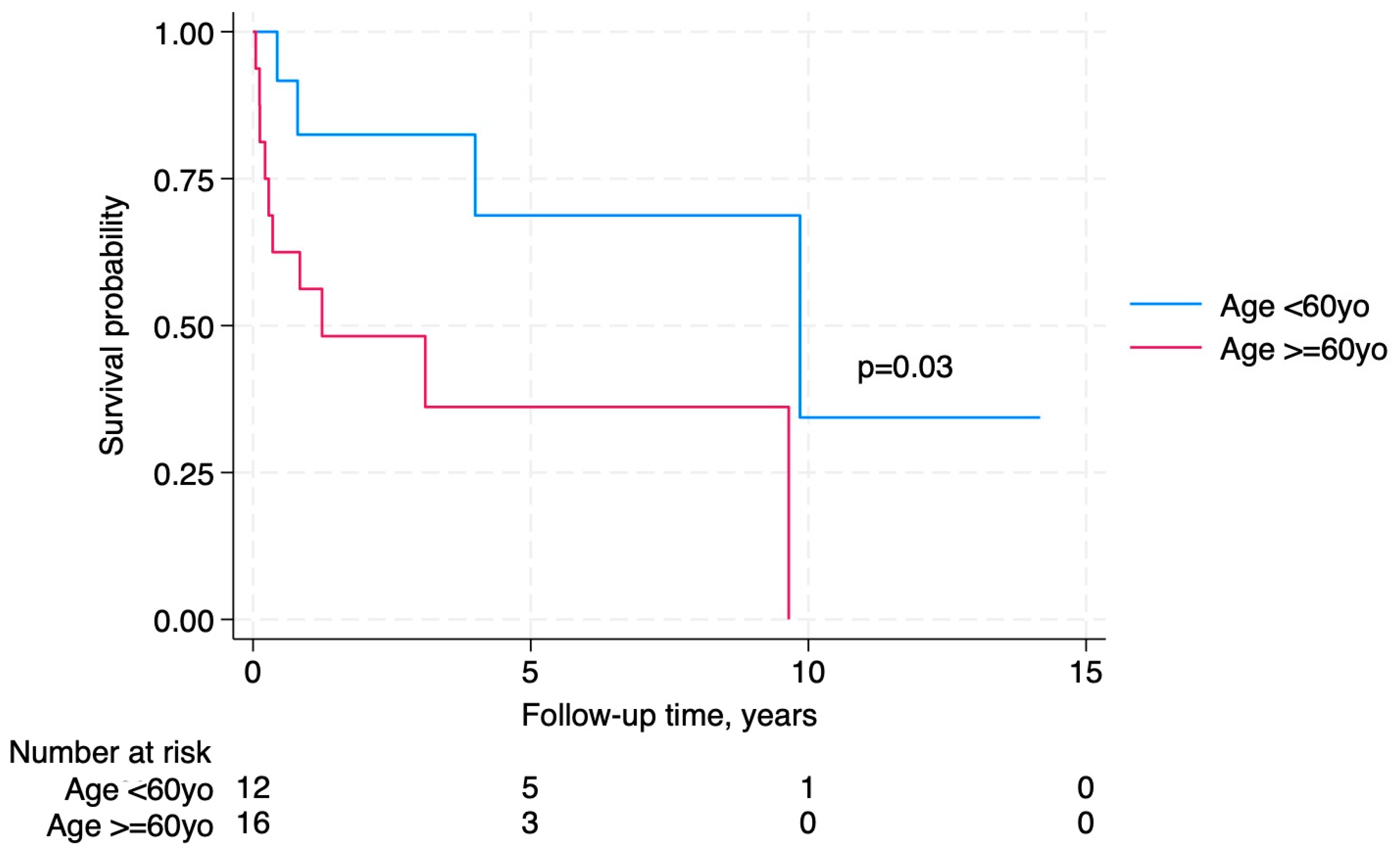
Figure 3). Older age at LT-PTLD diagnosis was significantly associated with worse survival (
Figure 4), whilst early vs. late LT-PTLD (
p = 0.60), calcineurin inhibitor type (
p = 0.30)
, triple immunosuppression at diagnosis (
p = 0.26), and EBV-associated LT-PTLD (
p = 0.33) or EBV viremia (
p = 0.39) were not predictive of survival.
4. Discussion
PTLD remains one of the most devastating complications of both solid organ and hematopoietic cell transplantation, with increasing incidence over the last two decades linked with the cumulative increase in organ transplants along with improved patient and graft survival.
The reported incidence of LT-PTLD is 1.0–5.5%, depending upon whether an adult or pediatric population is studied [
1,
5]. Tajima et al. reported in 2021 on their Japanese cohort of 1849 LT recipients, describing that the incidence of LT-PTLD in children (5.1%) was more than twice that seen in adults (2.3%) [
7]. Kremers et al. reported in 2006 their cohort that spanned an earlier era of (1985–2004). They described 37 cases of adult LT-PTLD occurring in 1206 LT recipients (incidence of 3.1%), with 59.5% of tumours EBV positive, with 85.3% monomorphic B cell lymphoma (77% of these DLBCL) [
8]. In this first reported study from Australia, we have demonstrated the incidence in adults to be 2.0%, with a vast majority late LT-PTLD, 52% of tumours EBV associated, and most of the monomorphic DLBCL subtype (85.2%).
Generally, EBV appears to be highly implicated in PTLD disease pathophysiology, particularly with regard to early PTLDs, where some analyses suggest >90% of cases are EBV associated [
2,
9,
10]. Further, reports primarily from large renal transplant registries indicate a biphasic pattern of disease incidence, with a second peak occurring 7–10 years post-transplant, where only ~50% of cases are EBV positive [
2,
10,
11].
Within the limitations of this single centre retrospective study, we have demonstrated a low incidence of LT-PTLD (2.0%) within an adult population. Consistent with other case series, we have again shown that adult disease appears to occur later in the post-transplant course (median 11.4 years) and is largely of the monomorphic DLBCL histologic subtype (85.2%) [
2,
7,
8]. It is unsurprising then that aside from immunosuppression dose reduction, rituximab and chemotherapy formed the backbone of patient management. Whilst rituximab was used in 64.3%, it is noteworthy that it was not available in Australia until 1998; hence, it was not a treatment option in the early era of this case series.
Even in this adult cohort, where 85.7% of LT-recipients were EBV IgG positive at time of diagnosis, EBV still appeared to have a significant association with LT-PTLD, with EBV found in peripheral blood and tumour tissue in 61.5% and 52%, respectively. Whilst the detection of EBV in blood or tissue did not influence the outcome, EBV serostatus at time of transplant appeared an important factor in diagnosis, as patients who were EBV naive at transplantation had a significantly shorter interval to the diagnosis of PTLD when compared to those with prior exposure (0.9 years vs. 11.1 years, p = 0.02).
Whilst many units advocate the use of routine preemptive monitoring of a peripheral blood EBV viral load to aid in the earlier diagnosis of PTLD, this practice remains controversial as the current level of evidence to support this is low [
2]. Based upon our case series, this practice is unlikely to be cost-effective in similar low-risk adult populations. Our low incidence of LT-PTLD (2.0%), along with a long period between transplantation and PTLD diagnosis (11.4 years), and the fact that only 61.5% of patients had EBV detectable in peripheral blood, suggests that routine surveillance would be of low diagnostic yield. Consistent with this observation, the guidelines published by the American Society of Transplantation Infectious Diseases Community of Practice do not recommend viral load surveillance in low-risk adult populations [
2]. Further, it currently remains unknown how to handle a positive EBV viral load in an otherwise asymptomatic recipient, and whether reflex reductions in immunosuppression will lead to lower PTLD incidence with acceptable rates of rejection. Within the liver transplant setting, routine viral load surveillance may have better utility when directed towards pediatric patients and those high-risk patients with EBV mismatch (D+/R−) at time of transplantation; however; this requires further investigation.
PTLD, unlike non-transplant related lymphomas, is often characterised by a high incidence of non-nodal disease. The gastrointestinal tract is involved in 20–30% of cases and the CNS in 5–20% [
1]. In our series, bowel (37.5%) and CNS (25%) were the two most involved organs, whilst only 14.3% presented with nodal disease.
Whilst post-transplant exogenous T cell-directed immunosuppression is undoubtedly implicated in the origin of PTLD, we found that there was no impact on survival based upon the type of calcineurin inhibitor used, nor the exposure to triple immunosuppression at time of diagnosis. This may in part be due to the universal reduction in immunosuppression in managing LT-PTLD in our centre.
Whilst 50% of the patients with LT-PTLD died during the study period, only 64.3% died as a direct result of the lymphoma. Older age was significantly associated with poorer survival, which likely represents the increase in both post-transplant survival and medical co-morbidity in transplant recipients in general, along with increased cumulative exposure to immunosuppression. One proposed explanation for the poor prognosis of PTLD in elderly patients is that, as the duration post-transplant increases, the required dosage of immunosuppressive agents may decrease, and in some cases, discontinuation might be feasible. This, in turn, raises the possibility that a subset of transplant recipients may be maintained on unnecessary or excessive immunosuppression. Consistent with this notion, a recent study looked at potential biomarkers to predict those patients who may be able to discontinue immunosuppression. Whilst the biomarker itself was not able to discriminate between patients who were able to wean immunosuppression and those that could not, the study did note that overall, 16.3% of the cohort managed to achieve operational tolerance at one year [
12].
In conclusion, LT-PTLD occurred in only 2.0% of this adult Australian cohort, at a median time of 11.4 years post-transplantation. Most cases were DLBCL and presented more commonly with visceral disease, with the bowel being the most involved organ. Despite EBV being found in over 50% of cases, its presence did not appear to impact survival. EBV serostatus at time of transplantation appeared to be of more relevance to timing of disease onset, as EBV-naive recipients developed PTLD at a much earlier post-transplant stage (0.9 years). Therefore, within an otherwise lower risk adult population, it may be those who are EBV naive that appeal as an appropriate target for routine pre-emptive EBV viral load surveillance in future studies. In contrast, routine EBV surveillance across the entire adult LT population would likely be of low yield and not cost-effective.