Abstract
The growing disparity between the demand for donor hearts and their availability has reignited interest in donation after circulatory death (DCD) heart transplantation. Historically, DCD heart transplantation has been overshadowed by donation after brain death (DBD) due to ethical and preservation challenges. However, recent advancements in procurement techniques allow for evaluation of the donor heart and enable the broader utilization of DCD donors. While challenges remain, early outcomes suggest comparable survival rates between DCD and DBD heart transplantation. This review provides a comprehensive overview of the historical evolution, current practices, and future directions of DCD heart transplantation. Here, we emphasize its potential to expand the heart donor pool and alleviate the organ shortage crisis.
1. Introduction
Organ transplantation offers a lifesaving solution to patients with end-stage organ failure [1]. However, the demand for donor organs continues to exceed the supply. This has led to a global crisis, with thousands of patients on organ waitlists currently awaiting organ donation [2].
Initially, organ donation was performed after the irreversible cessation of circulatory and respiratory functions [3]. This is now termed donation after circulatory death (DCD) [4]. However, with the establishment of brain death criteria in 1968, a new method of organ procurement was introduced: donation after brain death (DBD) [5,6]. DBD is the process of organ donation from individuals who have been declared brain dead. This diagnosis is made based on strict neurological criteria which include the absence of brainstem reflexes and the inability to breath independently [7]. Since its conception, DBD has been the cornerstone of organ transplantation as it allows for the procurement of organs while maintaining artificial circulation [8]. This increases organ viability and function, reducing complications in the organ recipient. Thus, regardless of its historic precedence compared to DBD, DCD has been seen as less favorable.
However, the shortage of donor organs has led to an increased interest in DCD [9,10]. In 1995, the First International Workshop on DCD categorized DCD into controlled (CDCD) and uncontrolled (UDCD) donation. CDCD involves the planned withdrawal of life-sustaining treatment, while UDCD occurs after unexpected cardiac arrest [11]. Despite initial challenges in this form of procurement, advancements in protocols and surgical techniques have significantly improved outcomes. Now, the graft survival rates for DCD organs approach those of DBD organs [12,13].
This review aims to provide a comprehensive overview of DCD heart transplantation, exploring its historical evolution, current practices, preservation methods, outcomes, and future directions. By addressing these key areas, we aim to highlight the critical role of DCD donors in modern heart transplantation and its potential to bridge the gap between organ supply and demand.
2. Historical Context of DCD Donation
DCD predates brain death criteria and was the original form of organ donation. In the 1930s, Yuri Vooronoy performed the first documented human-to-human transplant using a kidney from a recently deceased donor. His work served to lay the foundation for modern transplant practices [14].
After World War II, researchers like René Küss advanced transplantation techniques using DCD donors. Despite the ethical concerns with using organs from executed prisoners, the experiments provided insights into the management of a disease with no other viable options [15]. Around the same time, David Hume in Boston achieved six-month graft survival without immunosuppression using DCD donors [16]. These efforts culminated in Joseph Murray’s breakthrough kidney transplant between identical twins in 1954, demonstrating the potential for long-term graft survival [17].
The rise of DBD, enabled by the introduction of brain death criteria, better organ viability, and reduced ischemic injury, led it to overshadow DCD. However, organ shortages have led to an increased interest in DCD. The Maastricht classification, introduced in 1995, categorized DCD into four types: dead on arrival (category 1), unsuccessful resuscitation (category 2), awaiting cardiac death (category 3), and cardiac arrest in brain dead donors (category 4). Controlled DCD (category 3) has become the most commonly used type [6] The controlled environment and careful planning allow for better organ retrieval and reduced ischemic injury. Advances in technique have further improved outcomes and narrowed the gap between DCD and DBD graft survival rates. This has helped solidify the role of DCD donation in modern transplantation.
3. Overview of DCD Heart Donation
More than 5000 heart transplants are performed annually worldwide. However, there are an estimated 50,000 candidates on waiting lists for a donor heart. Simply put, the number of available donor organs continues to limit the number of transplants that can be performed. The total number of heart transplants increased by 53% between 2011 and 2022, and this growth was supported in part by the adoption of DCD hearts [18]. The first successful DCD heart transplant in the United States was performed at Duke University in 2019 [19]. Heart transplantation was the last solid organ transplantation to begin utilizing DCD donors, a transition made possible through technological advancements and the successes observed in DCD kidney, liver, and lung transplantations [20].
Unlike DBD, DCD organs are not able to be fully assessed prior to the irreversible cessation of circulatory and respiratory functions [21]. This has been reported to result in lower-quality organs and fewer organs being obtained per donor compared to DBD [22]. However, advancements in organ preservation, protocols, and surgical techniques have significantly improved the viability and outcomes of DCD organ transplantation [23]. As a result, DCD is being utilized more and is contributing to the effort to meet the demand for organ donation.
4. Procurement Techniques for DCD Heart Transplantation
Organ procurement and preservation is critical to the success of donation. Whereas the process is streamlined in DBD donors due to controlled organ evaluation and procurement procedures, this is not the case in DCD donors. Pre-procurement organ evaluation is limited in DCD donation as it is not always possible to obtain pre-procurement testing (e.g., cardiac catheterization) to fully evaluate a patient’s heart. Even when procuring the heart, its function cannot be assessed until the initiation of Normothermic Regional Perfusion (NRP) or placement on the Organ Care System (OCS) (Transmedics, Andover, MA, USA). Moreover, the process of DCD donation involves a period of functional warm ischemic time, which subjects the heart to potential ischemic damage [21]. However, several innovative techniques have been developed to minimize ischemic injury, assess heart function, and maintain organ viability in DCD donors.
4.1. Withdrawal Process and Functional Warm Ischemic Time
Currently, only controlled DCD donation is utilized for heart transplantation. The process of donation begins with the withdrawal of life support. The patient’s circulatory and respiratory functions are then closely monitored until circulatory arrest is confirmed [24]. At this point, a predetermined standoff period begins. This period ranges from two to five minutes and is institution-dependent. It ensures the irreversible cessation of cardiac and respiratory activity before heart procurement can begin [25]. Certification of death is institution-dependent and typically performed by an anesthesiologist or critical care physician. While longer standoff times lead to increased ischemic duration, these small variations generally do not significantly impact outcomes unless the ischemic time approaches the program’s established cutoff threshold for acceptance.
A critical factor in DCD donation that does not exist in DBD donors is functional warm ischemia time (fWIT) [26]. This is the period during which organs remain with low oxygenated blood supply or without oxygenated blood supply after withdrawal of life support. Peripheral oxygen saturation < 70% or systolic arterial blood pressure < 50 mmHg marks the onset of this time. This period ends at the time of reperfusion or cold flush, depending on the organ procurement technique. This conventional definition of fWIT has been adapted from DCD liver transplantation. Emerging clinical data suggest this definition is overly restrictive for hearts. Therefore, many institutions now prioritize hemodynamic criteria rather than peripheral oxygen saturation to define fWIT onset in heart transplantation. A fWIT < 30 min is currently widely utilized as the accepted threshold to proceed with heart procurement, as a prolonged fWIT can significantly impact organ viability and post-transplant outcomes [21,27].
Unlike controlled DCD, uncontrolled DCD (uDCD) is not currently utilized for heart transplantation. Normally, uDCD is performed following witnessed cardiac arrest, where CPR is initiated to allow transport to the hospital. Once in the hospital, if no further resuscitative measures are deemed viable, CPR is stopped, and the patient is declared dead. Due to the challenges of preserving organs before irreversible ischemic injury occurs, this method has not been adopted for heart transplantation.
4.2. DCD Procurement Techniques: NRP vs. DPP
The two primary techniques utilized for DCD heart procurement are Normothermic Regional Perfusion (NRP) and Direct Procurement and Perfusion (DPP) [28]. A comparison of the NRP and DPP procurement timelines is depicted in Figure 1.
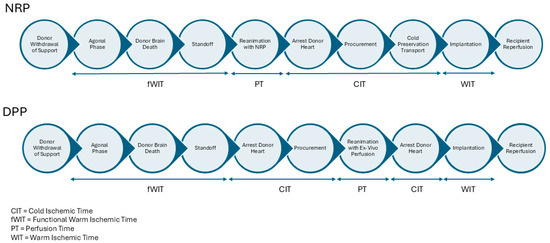
Figure 1.
Timeline of NRP and DPP procurement techniques in DCD heart transplantation.
4.3. Normothermic Regional Perfusion (NRP)
In the NRP technique, the chest is rapidly opened, the right atrium (venous) and aorta (arterial) are cannulated, and a modified extracorporeal membrane oxygenation (ECMO) circuit with a reservoir or cardiopulmonary bypass circuit is utilized to restore perfusion to the donor’s visceral organs [29,30]. The cerebral vessels are clamped or ligated to ensure no cerebral blood flow is reestablished [31]. The restoration of flow allows for the heart to recover in a near-physiological state, enabling a more accurate assessment of its function before transplantation [32]. With the use of inotropes, vasopressors, or dilators, a mean perfusion pressure between 60 and 80 mmhg is maintained. Cardiac contractility, electrolytes, and serum lactate are all measured to assess the function of the heart. If contractility is adequate, NRP is weaned and the heart is procured in a manner similar to DBD donation [33,34].
4.4. Direct Procurement and Perfusion (DPP)
The DPP method involves rapidly opening the chest, cannulating the aorta, applying a distal cross clamp, delivering cardioplegia, and removing the heart for immediate placement on the OCS device [35]. This device provides oxygenated blood and nutrients, allowing the heart to recover and be evaluated in a controlled environment [36]. Unlike NRP, DPP avoids reinstituting circulation within the donor’s body, circumventing the potential reperfusion of the brain and other organs [35]. The OCS device is based on retrograde coronary perfusion with normothermic oxygenated blood. The heart is maintained in a beating but unloaded state. While the OCS device allows for recovery teams to evaluate contractility in an unloaded state and monitor lactate consumption, this technique does not allow for full functional evaluation, as true cardiac output and contractility cannot be directly assessed.
5. Ethical Considerations in NRP/DCD
The use of NRP in DCD heart transplantation has sparked ethical debate, particularly regarding the reinstitution of circulation in the donor’s thoracic cavity [37]. According to the dead donor rule (DDR), which is not a legal doctrine but an ethical principle, organs cannot be removed until the patient is considered dead [38]. In the case of DCD, the donor is declared dead when circulation ceases following the withdrawal of life-sustaining treatment. Critics argue that restoring circulation in NRP, even regionally, may blur the lines between circulatory death and brain death, challenging the declaration of death in DCD [39]. To mitigate this concern, cerebral perfusion is explicitly avoided through the surgical clamping or ligation of head vessels during NRP [31]. However, ongoing discussions continue regarding the public’s perception of this technique and the ethical implications of altering the definition of death.
In contrast, the DPP technique avoids these concerns due to the lack of circulation in the donor [35]. Regardless, DPP presents logistical challenges, such as the need for rapid procurement and immediate device availability to minimize ischemic injury. It also lacks the potential for in vivo heart assessment [36].
6. Preservation Methods in DCD
Organ preservation is critical to the success of heart donation [40]. In DCD, several innovative techniques have been developed to minimize ischemic injury and maintain organ viability. Following procurement with NRP, static cold storage is most often utilized. Traditionally, a cooler with ice was used, but new technologies such as the Sherpapak (Paragonix Technologies, Cambridge, MA, USA) allow for temperature-controlled preservation. On the other hand, organs procured using DPP are currently preserved using the OCS in the United States. There are reports of NRP organs being preserved using OCS, but this is less common.
6.1. NRP Preservation with Static Storage
During NRP, after the donor heart has been inspected, it typically cooled and flushed with a preservation solution similar to that used in DBD procurement [41]. Once explanted, the heart is transported in static storage, either on ice or on the SherpaPak. These passive preservation methods rely on hypothermia to reduce metabolic activity and ischemic damage [42]. While hypothermia does not halt cellular metabolism, it does slow the rate of this [43]. Storing organs in an ice-filled cooler was the traditional preservation strategy due to its simplicity and cost-effectiveness. However, there are unpredictable temperature fluctuations which are not ideal for storage. It has been found that storing hearts at temperatures below the optimal range results in potential tissue damage from freezing myocardium [44].
On the other hand, the SherpaPak system allows for the regulated storage of hearts between 4 °C and 8 °C and can maintain organ temperatures within this range for 30 h [44,45]. After harvesting, the donor heart is placed into a sterile inner canister filled with cold cardioplegia. The inner canister is then placed into a larger canister that is surrounded with cooling packs in the rapid outer transport shell, which maintains the ideal temperature [46]. This method not only mitigates damage due to fluctuating temperatures but also allows for longer-distance transportation.
6.2. DPP Preservation with the Organ Care System (OCS)
Unlike NRP, DPP hearts cannot be assessed in the donor following arrest. Therefore, the use of cold, static storage is avoided in DPP procurement. Instead, to perfuse, assess and preserve the heart, the OCS device is used. This method allows for normothermic reperfusion on a beating heart in a near-physiological, unloaded state, which enables the continuous monitoring of metabolic and functional parameters [47]. However, the left ventricle is vented so the true ejection fracture cannot be accurately evaluated, although an assessment of the heart’s gross functionality is possible.
A key advantage of OCS is its ability to assess lactate consumption. Similarly to its use in evaluating splanchnic organ function, its application in cardiac transplantation provides insight into myocardial metabolic recovery, helping guide decisions on organ acceptance [40].
Compared to static cold storage, patients who underwent DBD heart transplantation after heart preservation with OCS had similar 30-day, 1-year, and 2-year survival [48]. Similarly, the PROCEED II trial found no difference between OCS and cold-storage preservation in 30-day mortality, ICU length of stay, severe rejection, or serious cardiac events in DBD heart transplantation. However, they did find that OCS perfused organs were associated with longer preservation times [49]. No study directly comparing ischemic and non-ischemic preservation in DCD hearts has been performed.
7. Cost Considerations
There is a significant difference in the cost of organs retrieved by the NRP and DPP techniques. The prior literature has suggested that DCD NRP procurement costs USD 186,418 while a DPP procurement costs USD 280,051. The USD 93,000 savings in favor of NRP can be partially attributed to the cost of the OCS device (~USD 65,000) which is included in the DPP cost [50].
8. Outcomes of DCD Heart Transplantation
Organ-specific and general complications underscore the critical importance of optimizing preservation techniques to improve outcomes in DCD organ transplantation. The outcomes and challenges of organ transplantation differ significantly between donation after brain death (DBD) and donation after circulatory death (DCD). These differences are particularly evident in primary graft dysfunction (PGD).
DCD heart transplants show a higher incidence of severe biventricular PGD when compared to DBD recipients. Reported rates of severe PGD are as high as 19% for DCD recipients versus 7.4% for DBD recipients [51]. Another study found that severe PGD at 24 h post transplant was higher in DCD at 9.5% than DBD (5.1%) recipients [52]. Despite this, DCD recipients with severe PGD often experience shorter durations of mechanical circulatory support and shorter hospital stays compared to similar DBD recipients [51]. Despite an increased incidence of PGD, a landmark clinical trial found comparable six-month survival between DCD and DBD heart transplants [53].
Considering the recent adoption of DCD heart transplantation, long-term outcomes are not well known. A recent meta-analysis examining over 900 DCD and 7200 DBD heart transplants reported that 6- and 12-month survival rates were similar between both groups, at 93% and 91%, respectively. Importantly, the five-year survival rate for DCD recipients was reported at 89.5%, which was higher than the 80.6% observed in DBD recipients [54]. Another meta-analysis, which included a pooled cohort analysis of 1219 donor/recipient pairs in DCD heart transplantation, estimated survival rates at 1, 3, and 5 years to be 92.4%, 85.3%, and 85.3%, respectively [55].
Regardless of these results, early reports suggest a learning curve with DCD transplantation. In the United States, 1-year survival was worse in low-volume centers (85.7%) when compared to medium- and high-volume centers (93%) [56]. Other studies show that promising results could be achieved through expanding the donor pool in DCD heart donation by pushing the boundaries of fWIT and preservation time [57,58].
9. Global Perspective on DCD Heart Transplantation
DCD heart transplantation has been adopted at varying levels across the globe, with some countries integrating it into their routine transplant programs and others yet to implement it. In nations like the United Kingdom, the Netherlands, and Australia, DCD makes a significant contribution to overall deceased organ donation, while other regions, including parts of Latin America and Russia, face legal or logistical restrictions that limit its use. The approach to DCD procurement also varies, with some countries primarily utilizing controlled DCD (cDCD), such as the UK and Australia, while others, like France and Spain, focus on uncontrolled DCD (uDCD). Some, such as the Netherlands, incorporate both forms [59].
Latin America has recorded 12,374 heart transplants across 16 countries from 1968 to 2022, with Brazil as the leading contributor. Although Brazil’s population is 65% that of the United States, it performs only 10% of the heart transplants completed in the U.S. The number of heart transplants in Brazil has remained stable over the past decade, averaging 350 transplants per year. However, DCD heart transplantation has not been implemented in Brazil due to legal and regulatory restrictions [60].
In the United States, 11,625 heart transplants were performed between December 2019 and September 2023, with 792 (7%) utilizing DCD allografts. Among these, 249 (31%) were procured using DCD-NRP, while 543 (69%) were obtained via DCD-DPP. The use of DCD hearts has grown significantly, increasing from 2% of all transplants in December 2019 to 11% in 2023. Notably, DCD-NRP usage has surged, making up over one-third of all DCD heart procurements by September 2023 [61].
In the United Kingdom, the Joint Innovation Fund (JIF) pilot program, supported by NHS Blood and Transplant (NHSBT) and NHS England (NHSE), was launched to expand access to DCD hearts across all transplant centers. Between September 2020 and February 2022, 215 DCD hearts were offered, with 98 (46%) accepted, leading to 50 successful DCD heart transplants. During this period, 179 DBD heart transplants were also performed. The integration of DCD donors increased total heart transplants in the UK by 28%, with early post-transplant survival rates comparable to those of DBD recipients [62,63].
In Russian, DCD heart transplantation is legally permitted, with national legislation and guidelines in place. However, the mandatory no-touch period is 30 min. In other countries this time varies significantly, ranging from 5 min in 13 countries to 20 min in Italy [64].
Lastly, in Australia, 74 DCD heart transplants have been performed since 2014, using direct procurement followed by normothermic machine perfusion. Over the past two years, DCD transplants have accounted for nearly 30% of all heart transplants in Australia, with outcomes comparable to those obtained using DBD transplants. The successful integration of DCD into Australia’s transplant program highlights its potential as a major contributor to heart transplantation worldwide [65].
DCD heart transplantation varies significantly across the world, with differences in adoption rates, legal frameworks, procurement techniques, and outcome measures. This reflects the diverse challenges and approaches among countries. Global collaboration, standardization of best practices, and a deeper understanding of DCD transplantation are essential to expanding its adoption in heart transplantation and maximizing its potential to address the growing need for donor hearts.
Registry Data
Three major national registries provide key insights into the outcomes and expansion of DCD heart transplantation: the United Network for Organ Sharing (UNOS) in the United States, NHS Blood and Transplant (NHSBT) in the United Kingdom, and the Australia and New Zealand Cardiothoracic Organ Transplant Registry (ANZCOTR) [66].
The United States has seen a rapid increase in DCD heart transplants, as did the UK and Australia. While survival outcomes remain strong across all three registries, DCD recipients experience higher rates of PGD. Registry data confirm that DCD heart transplantation is a viable and expanding strategy, with ongoing refinements in donor selection and post-transplant management expected to further improve outcomes [67]. In addition to tracking survival, registries play a critical role in identifying risk factors, standardizing best practices, and guiding research to optimize long-term graft function and patient selection.
10. Future Directions in DCD
Given the recent adoption of DCD heart transplantation, efforts are still underway to optimize procurement techniques and preservation devices. Currently, factors like standoff time, the allowance of NRP and preservation strategies differ not only by country but by institution. Some, like New York University, are pioneering their own protocols in which NRP is used on an in-house donor [68]. Others, like those in Stanford University, are assessing whether the beating heart implant technique is a safe and effective way to avoid additional ischemic time. In this method, the donor heart receives uninterrupted coronary perfusion during implant. This reduces ischemic time and, in hearts preserved with the OCS device, it also prevents a second arrest in an effort to minimize potential additional ischemic injury [69]. Lastly, ongoing clinical trials are assessing heart-preserving technology, including a non-ischemic heart preservation (NIHP) method which uses a continuous cold preservation solution to preserve hearts at 8 °C [70].
Hypothermic Oxygenated Perfusion (HOPE) is a promising preservation method that offers theoretical advantages over normothermic perfusion. Unlike OCS, HOPE preserves the heart in a non-beating state. Early clinical experience from Australia and Europe has shown promising results [71,72]. In the U.S., the XVIVO PRESERVE Heart Study has completed enrollment, and the results will be published soon. Future advancements may involve DPP followed by HOPE, allowing for extended-criteria DCD hearts to be considered for transplantation.
Expanding the Donor Pool
In order to expand DCD heart transplantation, the donor pool must be increased. To achieve this, the current boundaries of donor selection criteria must be pushed while maintaining safety and optimizing outcomes. Some key factors under investigation include donor age, warm ischemic time (WIT), and the use of donors with left ventricular hypertrophy (LVH).
Studies are needed to determine the optimal upper limits for these and other criteria. Research should focus on identifying the maximum safe donor age, the longest tolerable WIT before irreversible myocardial injury occurs, and the degree of LVH that can be accepted without compromising post-transplant function. Additionally, other donor characteristics, such as pre-existing comorbidities, hemodynamic stability before circulatory arrest, and metabolic markers of organ viability, may further refine the selection criteria. By systematically evaluating these factors, transplant programs can safely expand the donor pool while ensuring favorable long-term outcomes for recipients.
11. Conclusions
The adoption of DCD heart transplantation has recently expanded. Successful procurement techniques such as DPP and NRP, as well as novel preservation devices, have resulted in an exponential growth in DCD heart transplantation. Early results are promising and suggest DCD to be non-inferior to DBD heart transplantation, though more investigation is needed to explore the higher incidence of PGD in DCD recipients. With the increasing need for donor hearts, DCD transplantation offers a novel and safe approach that will increase the donor pool and decrease the waitlist time for patients with end-stage heart disease.
Author Contributions
Conceptualization, B.E.F. and T.S.; writing—original draft preparation, A.D.; writing—review and editing, A.D., K.S.H., M.U., T.S. and B.E.F.; supervision, T.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Grinyó, J.M. Why is organ transplantation clinically important? Cold Spring Harb. Perspect. Med. 2013, 3, a014985. [Google Scholar] [CrossRef] [PubMed]
- Abouna, G.M. Organ shortage crisis: Problems and possible solutions. Transplant. Proc. 2008, 40, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Nordham, K.D.; Ninokawa, S. The history of organ transplantation. Bayl. Univ. Med. Cent. Proc. 2021, 35, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Manara, A.R.; Murphy, P.G.; O’Callaghan, G. Donation after circulatory death. Br. J. Anaesth. 2012, 108 (Suppl. S1), i108–i121. [Google Scholar] [CrossRef]
- Machado, C. The first organ transplant from a brain-dead donor. Neurology 2005, 64, 1938–1942. [Google Scholar] [CrossRef]
- A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA 1968, 205, 337–340.
- Walter, K. Brain Death. JAMA 2020, 324, 1116. [Google Scholar] [CrossRef]
- Mascia, L.; Mastromauro, I.; Viberti, S.; Vincenzi, M.; Zanello, M. Management to optimize organ procurement in brain dead donors. Minerva Anestesiol. 2009, 75, 125–133. [Google Scholar]
- Rajab, T.K.; Singh, S.K. Donation After Cardiac Death Heart Transplantation in America Is Clinically Necessary and Ethically Justified. Circ. Heart Fail. 2018, 11, e004884. [Google Scholar] [CrossRef]
- McKellar, S.H.; Durham, L.A., 3rd; Scott, J.P.; Cassivi, S.D. Successful lung transplant from donor after cardiac death: A potential solution to shortage of thoracic organs. Mayo Clin. Proc. 2010, 85, 150–152. [Google Scholar] [CrossRef]
- Kootstra, G.; Daemen, J.H.; Oomen, A.P. Categories of non-heart-beating donors. Transplant. Proc. 1995, 27, 2893–2894. [Google Scholar] [PubMed]
- Kizilbash, S.J.; Evans, M.D.; Chavers, B.M. Survival Benefit of Donation After Circulatory Death Kidney Transplantation in Children Compared With Remaining on the Waiting List for a Kidney Donated After Brain Death. Transplantation 2022, 106, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Dubbeld, J.; Hoekstra, H.; Farid, W.; Ringers, J.; Porte, R.J.; Metselaar, H.J.; Baranski, A.G.; Kazemier, G.; Berg, A.P.v.D.; van Hoek, B. Similar liver transplantation survival with selected cardiac death donors and brain death donors. Br. J. Surg. 2010, 97, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.C.; Nair, R.; Behera, V. Kidney transplantation: The journey across a century. Med. J. Armed Forces India 2023, 79, 631–637. [Google Scholar] [CrossRef]
- Schultheiss, D.; Jardin, A. René Küss (1913–2006)—A transplant pioneer in Paris. Transplant. Proc. 2013, 45, 1220–1223. [Google Scholar] [CrossRef]
- Hume, D.M.; Merrill, J.P.; Miller, B.F.; Thorn, G.W. Experiences with renal homotransplantation in the human: Report of nine cases. J. Clin. Investig. 1955, 34, 327–382. [Google Scholar] [CrossRef]
- Merrill, J.P.; Murray, J.E.; Harrison, J.H.; Guild, W.R. Successful homotransplantation of the human kidney between identical twins. JAMA 1956, 160, 277–282. [Google Scholar] [CrossRef]
- Ishlt Fast Facts. Default. Available online: https://www.ishlt.org/education-and-publications/resource/ishlt-fast-facts (accessed on 25 December 2024).
- Walker, B. Giving Life: Duke Surgeons Perform First Donation After Circulatory Death Heart Transplant in the United States; Duke Department of Surgery: Durham, NC, USA, 2020; Available online: https://surgery.duke.edu/news/giving-life-duke-surgeons-perform-first-donation-after-circulatory-death-heart-transplant (accessed on 25 December 2024).
- Gardiner, D.; Charlesworth, M.; Rubino, A.; Madden, S. The rise of organ donation after circulatory death: A narrative review. Anaesthesia 2020, 75, 1215–1222. [Google Scholar] [CrossRef]
- Sánchez-Cámara, S.; Asensio-López, M.C.; Royo-Villanova, M.; Soler, F.; Jara-Rubio, R.; Garrido-Peñalver, J.F.; Pinar, E.; Hernández-Vicente, Á.; Hurtado, J.A.; Lax, A.; et al. Critical warm ischemia time point for cardiac donation after circulatory death. Am. J. Transplant. 2022, 22, 1321–1328. [Google Scholar] [CrossRef]
- Tuttle-Newhall, J.E.; Krishnan, S.M.; Levy, M.F.; McBride, V.; Orlowski, J.P.; Sung, R.S. Organ donation and utilization in the United States: 1998–2007. Am. J. Transplant. 2009, 9 Pt 2, 879–893. [Google Scholar] [CrossRef]
- Condello, I. Advancements in Donation after Circulatory Death Heart Procurement and Preservation: A Comprehensive Review of Recent Innovations. Surg. Technol. Int. 2024, 44, 230–234. [Google Scholar] [CrossRef] [PubMed]
- White, C.W.; Messer, S.J.; Large, S.R.; Conway, J.; Kim, D.H.; Kutsogiannis, D.J.; Nagendran, J.; Freed, D.H. Transplantation of Hearts Donated after Circulatory Death. Front. Cardiovasc. Med. 2018, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Shemie, S.D.; Baker, A.J.; Knoll, G.; Wall, W.; Rocker, G.; Howes, D.; Davidson, J.; Pagliarello, J.; Chambers-Evans, J.; Cockfield, S.; et al. National recommendations for donation after cardiocirculatory death in Canada: Donation after cardiocirculatory death in Canada. CMAJ 2006, 175, S1. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Simpkins, C.E.; Montgomery, R.A.; Locke, J.E.; Segev, D.L.; Maley, W.R. Factors Affecting Graft Survival After Liver Transplantation from Donation After Cardiac Death Donors. Transplantation 2006, 82, 1683–1688. [Google Scholar] [CrossRef]
- Brann, A.; Jackson, B.; White, R.; Sharaf, K.; Cookish, D.; Gernhofer, Y.; Adler, E.; Urey, M.; Pretorius, V.; Kearns, M. Impact of functional warm ischemic time on short-term outcomes in donation after circulatory death heart transplantation. J. Heart Lung Transplant. 2023, 42, S94. [Google Scholar] [CrossRef]
- Ran, G.; Wall, A.E.; Narang, N.; Khush, K.K.; Hoffman, J.R.; Zhang, K.C.; Parker, W.F. Post-transplant survival after normothermic regional perfusion versus direct procurement and perfusion in donation after circulatory determination of death in heart transplantation. J. Heart Lung Transplant. 2024, 43, 954–962. [Google Scholar] [CrossRef]
- Messer, S.; Page, A.; Colah, S.; Axell, R.; Parizkova, B.; Tsui, S.; Large, S. Human heart transplantation from donation after circulatory-determined death donors using normothermic regional perfusion and cold storage. J. Heart Lung Transplant. 2018, 37, 865–869. [Google Scholar] [CrossRef]
- Ali, A.; White, P.; Dhital, K.; Ryan, M.; Tsui, S.; Large, S. Cardiac recovery in a human non-heart-beating donor after extracorporeal perfusion: Source for human heart donation? J. Heart Lung Transplant. 2009, 28, 290–293. [Google Scholar] [CrossRef]
- Dalsgaard, F.F.; Moeslund, N.; Zhang, Z.L.; Pedersen, M.; Qerama, E.; Beniczky, S.; Ryhammer, P.; Ilkjær, L.B.; Erasmus, M.; Eiskjær, H.M. Clamping of the Aortic Arch Vessels During Normothermic Regional Perfusion After Circulatory Death Prevents the Return of Brain Activity in a Porcine Model. Transplantation 2022, 106, 1763–1769. [Google Scholar] [CrossRef]
- Iyer, A.; Gao, L.; Doyle, A.; Rao, P.; Cropper, J.R.; Soto, C.; Dinale, A.; Kumarasinghe, G.; Jabbour, A.; Hicks, M.; et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. Am. J. Transplant. 2015, 15, 371–380. [Google Scholar] [CrossRef]
- Macdonald, P.; Dhital, K. Heart transplantation from donation-after-circulatory-death (DCD) donors: Back to the future-Evolving trends in heart transplantation from DCD donors. J. Heart Lung Transplant. 2019, 38, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Alamouti-Fard, E.; Garg, P.; Wadiwala, I.J.; Yazji, J.H.; Alomari, M.; Hussain, W.A.; Elawady, M.S.; Jacob, S. Normothermic Regional Perfusion is an Emerging Cost-Effective Alternative in Donation After Circulatory Death (DCD) in Heart Transplantation. Cureus 2022, 14, e26437. [Google Scholar] [CrossRef] [PubMed]
- Smail, H.; Garcia-Saez, D.; Stock, U.; Ahmed-Hassan, H.; Bowles, C.; Zych, B.; Mohite, P.N.; Maunz, O.; Simon, A.R. Direct Heart Procurement After Donation After Circulatory Death with Ex Situ Reperfusion. Ann. Thorac. Surg. 2018, 106, e211–e214. [Google Scholar] [CrossRef]
- Wang, L.; MacGowan, G.A.; Ali, S.; Dark, J.H. Ex situ heart perfusion: The past, the present, and the future. J. Heart Lung Transplant. 2021, 40, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Shemie, S.D.; Torrance, S.; Wilson, L.; Hornby, L.; MacLean, J.; Mohr, J.; Gillrie, C.; Badiwala, M.V.; Baker, A.; Freed, D.H.; et al. Heart donation and transplantation after circulatory determination of death: Expert guidance from a Canadian consensus building process. Don et transplantation cardiaques après un décès circulatoire: Évaluation d’experts issus d’un processus canadien d’établissement de consensus. Can. J. Anaesth. 2021, 68, 661–671. [Google Scholar] [CrossRef]
- Truog, R.D.; Miller, F.G. The dead donor rule and organ transplantation. N. Engl. J. Med. 2008, 359, 674–675. [Google Scholar] [CrossRef]
- Entwistle, J.W.; Drake, D.H.; Fenton, K.N.; Smith, M.A.; Sade, R.M.; Cardiothoracic Ethics Forum. Normothermic regional perfusion: Ethical issues in thoracic organ donation. J. Thorac. Cardiovasc. Surg. 2022, 164, 147–154. [Google Scholar] [CrossRef]
- Hess, N.R.; Ziegler, L.A.; Kaczorowski, D.J. Heart Donation and Preservation: Historical Perspectives, Current Technologies, and Future Directions. J. Clin. Med. 2022, 11, 5762. [Google Scholar] [CrossRef]
- Camp, P.C. Heart transplantation: Donor operation for heart and lung transplantation. Oper. Tech. Thorac. Cardiovasc. Surg. 2010, 15, 125–137. [Google Scholar] [CrossRef]
- Fuller, B.; Guibert, E.; Rodriguez, J. Lessons from natural cold-induced dormancy to organ preservation in medicine and biotechnology: From the ‘backwoods to the bedside’. In Dormancy and Resistance to Harsh Environments, Topics in Current Genetics; Lubens, E., Cerda, J., Clark, M., Eds.; Springer: Berlin, Germany, 2010; pp. 253–278. [Google Scholar]
- Buckberg, G.D. Myocardial temperature management during aortic clamping for cardiac surgery: Protection, preoccupation, and perspective. J. Thorac. Cardiovasc. Surg. 1991, 102, 895–903. [Google Scholar] [CrossRef]
- Horch, D.F.; Mehlitz, T.; Laurich, O.; Abel, A.; Reuter, S.; Pratschke, H.; Neuhaus, P.; Wesslau, C. Organ transport temperature box: Multicenter study on transport temperature of organs. Transplant. Proc. 2002, 34, 2320. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Cartoux, C.; Caus, T.; Sciaky, M.; Cozzone, P.J. The influence of temperature on metabolic and cellular protection of the heart during long-term ischemia: A study using P-31 magnetic resonance spectroscopy and biochemical analyses. Cryobiology 1998, 37, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Michel, S.G.; LaMuraglia Ii, G.M.; Madariaga, M.L.; Anderson, L.M. Innovative cold storage of donor organs using the Paragonix Sherpa Pak ™ devices. Heart Lung Vessel. 2015, 7, 246–255. [Google Scholar] [PubMed]
- Center for Devices and Radiological Health. Organ Care System (OCS) Heart System—P180051/S001; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/medical-devices/recently-approved-devices/organ-care-system-ocs-heart-system-p180051s001 (accessed on 26 December 2024).
- Koerner, M.M.; Ghodsizad, A.; Schulz, U.; El Banayosy, A.; Koerfer, R.; Tenderich, G. Normothermic ex vivo allograft blood perfusion in clinical heart transplantation. Heart Surg. Forum 2014, 17, E141–E145. [Google Scholar] [CrossRef]
- Ardehali, A.; Esmailian, F.; Deng, M.; Soltesz, E.; Hsich, E.; Naka, Y.; Mancini, D.; Camacho, M.; Zucker, M.; Leprince, P.; et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015, 385, 2577–2584. [Google Scholar] [CrossRef]
- Urban, M.; Ryan, T.R.; Um, J.Y.; Siddique, A.; Castleberry, A.W.; Lowes, B.D. Financial impact of donation after circulatory death heart transplantation: A single-center analysis. Clin. Transplant. 2024, 38, e15296. [Google Scholar] [CrossRef]
- Ayer, A.; Truby, L.K.; Schroder, J.N.; Casalinova, S.; Green, C.L.; Bishawi, M.A.; Bryner, B.S.; Milano, C.A.; Patel, C.B.; Devore, A.D. Improved Outcomes in Severe Primary Graft Dysfunction After Heart Transplantation Following Donation After Circulatory Death Compared With Donation After Brain Death. J. Card. Fail. 2023, 29, 67–75. [Google Scholar] [CrossRef]
- Cho, P.D.; Kim, S.T.; Zappacosta, H.; White, J.P.; McKay, S.; Biniwale, R.; Ardehali, A. Severe primary graft dysfunction in heart transplant recipients using donor hearts after circulatory death: United States experience. J. Heart Lung Transplant. 2025, 44, 760–769. [Google Scholar] [CrossRef]
- Schroder, J.N.; Patel, C.B.; DeVore, A.D.; Bryner, B.S.; Casalinova, S.; Shah, A.; Smith, J.W.; Fiedler, A.G.; Daneshmand, M.; Silvestry, S.; et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N. Engl. J. Med. 2023, 388, 2121–2131. [Google Scholar] [CrossRef]
- Jolliffe, J.; Brookes, J.; Williams, M.; Walker, E.; Jansz, P.; Watson, A.; MacDonald, P.; Smith, J.; Bennetts, J.; Boffini, M.; et al. Donation after circulatory death transplantation: A systematic review and meta-analysis of outcomes and methods of donation. Ann. Cardiothorac. Surg. 2025, 14, 11–27. [Google Scholar] [CrossRef]
- Muston, B.T.; Lo, W.; Eranki, A.; Boffini, M.; Loforte, A. Cardiac transplantation in controlled donation after circulatory death: A meta-analysis of long-term survival using reconstructed time-to-event data. Ann. Cardiothorac. Surg. 2025, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Potel, K.N.; Tam, D.Y.; Chen, Q.; Emerson, D.; Bowdish, M.; Chikwe, J.; Megna, D.; Catarino, P. Impact of institutional volume on outcomes following DCD heart transplantation in the United States. J. Heart Lung Transplant. 2024, 43, S13. [Google Scholar] [CrossRef]
- Hong, Y.; Hess, N.R.; Ziegler, L.A.; Chu, D.; Yoon, P.D.; Bonatti, J.O.; Serna-Gallegos, D.R.; Sultan, I.; Kaczorowski, D.J. Can we safely expand the donation after circulatory death donor heart pool by extending the donor age limit? J. Thorac. Cardiovasc. Surg 2025, 169, 658–666.e3. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hanna, Y.; Fajardo, R.; Tessmann, P.; Hermsen, J.; Xia, Y. The impact of functional warm ischemia time in DCD heart transplantation. J. Heart Lung Transplant. 2024, 43, S259–S260. [Google Scholar] [CrossRef]
- Ortega-Deballon, I.; Hornby, L.; Shemie, S.D. Protocols for uncontrolled donation after circulatory death: A systematic review of international guidelines, practices and transplant outcomes. Crit. Care 2015, 19, 268. [Google Scholar] [CrossRef]
- Loyaga-Rendon, R.Y.; Acharya, D. Heart transplantation in south america: Risk factors, challenges, and opportunities. JHLT Open 2025, 7, 100186. [Google Scholar] [CrossRef]
- Bakhtiyar, S.S.; Sakowitz, S.; Mallick, S.; Curry, J.; Benharash, P. Heart Transplantation After Donation After Circulatory Death: Early United States Experience. Ann. Thorac. Surg. 2024, 118, 484–493. [Google Scholar] [CrossRef]
- Messer, S.; Rushton, S.; Simmonds, L.; Macklam, D.; Husain, M.; Jothidasan, A.; Large, S.; Tsui, S.; Kaul, P.; Baxter, J.; et al. A national pilot of donation after circulatory death (DCD) heart transplantation within the United Kingdom. J. Heart Lung Transplant. 2023, 42, 1120–1130. [Google Scholar] [CrossRef]
- NHS Choices. Available online: https://www.odt.nhs.uk/deceased-donation/best-practice-guidance/donation-after-circulatory-death/ (accessed on 20 March 2025).
- Lomero, M.; Gardiner, D.; Coll, E.; Haase-Kromwijk, B.; Procaccio, F.; Immer, F.; Gabbasova, L.; Antoine, C.; Jushinskis, J.; Lynch, N.; et al. Donation after circulatory death today: An updated overview of the European landscape. Transpl. Int. 2020, 33, 76–88. [Google Scholar] [CrossRef]
- Joshi, Y.; Scheuer, S.; Chew, H.; Qiu, M.R.; Soto, C.M.; Villanueva, J.; Gao, L.; Doyle, A.M.; Takahara, S.; Jenkinson, C.M.; et al. Heart Transplantation From DCD Donors in Australia: Lessons Learned From the First 74 Cases. Transplantation 2023, 107, 361–371. [Google Scholar] [CrossRef]
- Tarzia, V.; Ponzoni, M.; Azzolina, D.; Vedovelli, L.; Pradegan, N.; Gregori, D.; Gerosa, G. Heart transplantation from donation after circulatory death: A meta-analysis of national registries. Ann. Cardiothorac. Surg. 2024, 13, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.; Wang, K.; MacLean, C.; Villanueva, J.; Gao, L.; Watson, A.; Iyer, A.; Connellan, M.; Granger, E.; Jansz, P.; et al. The Rapidly Evolving Landscape of DCD Heart Transplantation. Curr. Cardiol. Rep. 2024, 26, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- James, L.; LaSala, V.R.; Hill, F.; Ngai, J.Y.; Reyentovich, A.; Hussain, S.T.; Gidea, C.; Piper, G.L.; Galloway, A.C.; Smith, D.E.; et al. Donation after circulatory death heart transplantation using normothermic regional perfusion:The NYU Protocol. JTCVS Tech. 2022, 17, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Ruaengsri, C.; Guenthart, B.A.; Shudo, Y.; Wang, H.; Ma, M.R.; MacArthur, J.W.; Hiesinger, W.; Woo, Y.J. Beating Heart Transplant Procedures Using Organs From Donors With Circulatory Death. JAMA Netw. Open 2024, 7, e241828. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT04066127 (accessed on 26 December 2024).
- Rega, F.; Lebreton, G.; Para, M.; Michel, S.; Schramm, R.; Begot, E.; Vandendriessche, K.; Kamla, C.; Gerosa, G.; Berman, M.; et al. Hypothermic oxygenated perfusion of the donor heart in heart transplantation: The short-term outcome from a randomised, controlled, open-label, multicentre clinical trial. Lancet 2024, 404, 670–682, Erratum in Lancet 2024, 404, 1644. https://doi.org/10.1016/S0140-6736(24)02313-4. [Google Scholar] [CrossRef]
- McGiffin, D.C.; Kure, C.E.; Macdonald, P.S.; Jansz, P.C.; Emmanuel, S.; Marasco, S.F.; Doi, A.; Merry, C.; Larbalestier, R.; Shah, A.; et al. Hypothermic oxygennated perfusion (HOPE) safely and effectively extends acceptable donor heart preservation times: Results of the Australian and New Zealand trial. J. Heart Lung Transplant. 2024, 43, 485–495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).