SARS-CoV-2 in Kidney Transplant Recipients: A Systematic Review
Abstract
:1. Introduction
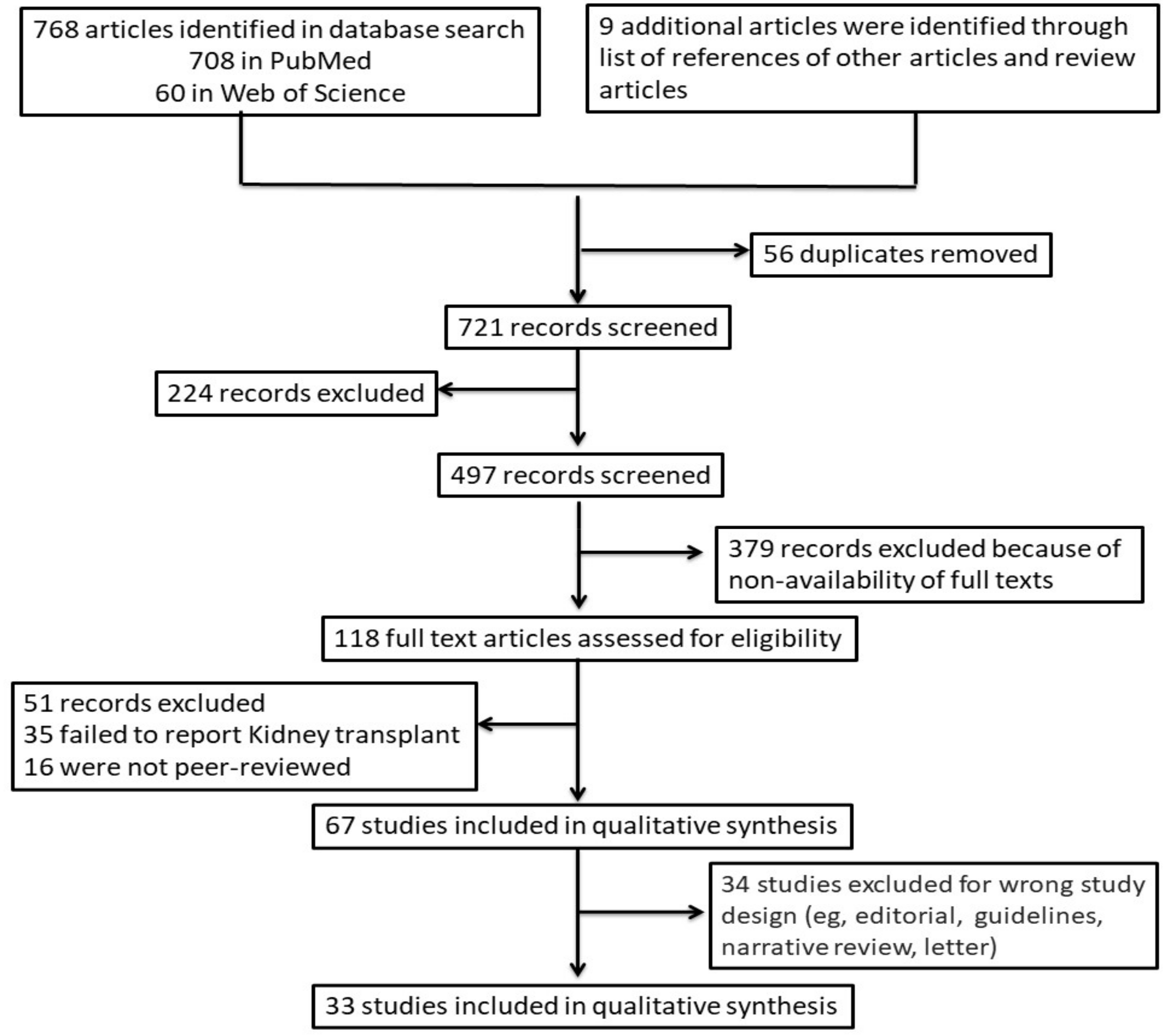
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
3. Results
3.1. Clinical Presentation
3.2. Treatment and Immunosuppressant Adjustments
3.3. Mortalities in Kidney Transplant Recipients Due to COVID-19 Infection
3.4. Vaccinating Kidney Transplant Recipients
3.5. Comparing the Impact of COVID-19 among CKD, Kidney Transplant and General Population Patients
3.6. Transplantation during COVID-19 Pandemic
3.7. Occurrence of COVID-19 among Vaccinated Kidney Transplant Patients
4. Implications for the Future
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: What next? Lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Toapanta, N.; Torres, I.B.; Sellarés, J.; Chamoun, B.; Serón, D.; Moreso, F. Kidney transplantation and COVID-19 renal and patient prognosis. Clin. Kidney J. 2021, 14, i21–i29. [Google Scholar] [CrossRef] [PubMed]
- John Hopkins University & Medicine. Coronavirus Resource Center. Available online: https://origin-coronavirus.jhu.edu/ (accessed on 8 June 2021).
- Benjamin, O.; Lappin, S.L. End-stage renal disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499861/ (accessed on 27 November 2021).
- Pereira, M.R.; Mohan, S.; Cohen, D.J.; Husain, S.A.; Dube, G.K.; Ratner, L.E.; Arcasoy, S.; Aversa, M.M.; Benvenuto, L.J.; Dadhania, D.M.; et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am. J. Transplant. 2020, 20, 1800–1808. [Google Scholar] [CrossRef] [PubMed]
- Global Observatory on Donation and Transplantation. Available online: http://www.transplant-observatory.org/data-charts-and-tables/ (accessed on 27 February 2021).
- Lentine, K.L.; Mannon, R.B.; Josephson, M.A. Practicing with Uncertainty: Kidney Transplantation During the COVID-19 Pandemic. Am. J. Kidney Dis. 2021, 77, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Amer, H.; Anglicheau, D.; Ascher, N.; Baan, C.; Battsetset, G.; Bat-Ireedui, B.; Berney, T.; Betjes, M.; Bichu, S.; et al. Global Transplantation COVID Report March. Transplantation 2020, 104, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Salvalaggio, P.R.; Ferreira, G.F.; Caliskan, Y.; Vest, L.S.; Schnitzler, M.A.; de Sandes-Freitas, T.V.; Moura, L.R.; Lam, N.N.; Maldonado, R.A.; Loupy, A.; et al. An International survey on living kidney donation and transplant practices during the COVID-19 pandemic. Transpl. Infect. Dis. 2021, 23, e13526. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. People with Certain Medical Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (accessed on 24 May 2021).
- Raja, M.A.; Mendoza, M.A.; Villavicencio, A.; Anjan, S.; Reynolds, J.M.; Kittipibul, V.; Fernandez, A.; Guerra, G.; Camargo, J.F.; Simkins, J.; et al. COVID-19 in solid organ transplant recipients: A systematic review and meta-analysis of current literature. Transplant. Rev. 2021, 35, 100588. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Bhalla, A.; Azhar, A.; Tsujita, M.; Talwar, M.; Balaraman, V.; Sodhi, A.; Kadaria, D.; Eason, J.D.; Hayek, S.S.; et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am. J. Transplant. 2020, 20, 3061–3071. [Google Scholar] [CrossRef]
- Elias, M.; Pievani, D.; Randoux, C.; Louis, K.; Denis, B.; DeLion, A.; Le Goff, O.; Antoine, C.; Greze, C.; Pillebout, E.; et al. COVID-19 Infection in Kidney Transplant Recipients: Disease Incidence and Clinical Outcomes. J. Am. Soc. Nephrol. 2020, 31, 2413–2423. [Google Scholar] [CrossRef]
- Webster, A.C.; Woodroffe, R.C.; Taylor, R.S.; Chapman, J.R.; Craig, J. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: Meta-analysis and meta-regression of randomised trial data. BMJ 2005, 331, 810. [Google Scholar] [CrossRef] [Green Version]
- Woodle, E.S.; First, M.R.; Pirsch, J.; Shihab, F.; Gaber, A.O.; Van Veldhuisen, P.; Corticosteroid Withdrawal Study Group. A Prospective, Randomized, Double-Blind, Placebo-Controlled Multicenter Trial Comparing Early (7 Day) Corticosteroid Cessation Versus Long-Term, Low-Dose Corticosteroid Therapy. Ann. Surg. 2008, 248, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Brennan, D.C.; Daller, J.A.; Lake, K.D.; Cibrik, D.; Del Castillo, D. Rabbit Antithymocyte Globulin versus Basiliximab in Renal Transplantation. N. Engl. J. Med. 2006, 355, 1967–1977. [Google Scholar] [CrossRef] [Green Version]
- Matas, A.J.; Kandaswamy, R.; Humar, A.; Payne, W.D.; Dunn, D.L.; Najarian, J.S.; Gruessner, R.W.G.; Gillingham, K.J.; McHugh, L.E.; Sutherland, D.E.R. Long-term Immunosuppression, Without Maintenance Prednisone, After Kidney Transplantation. Ann. Surg. 2004, 240, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Jandovitz, N.; Hirsch, J.S.; Nair, G.; Abate, M.; Bhaskaran, M.; Grodstein, E.; Berlinrut, I.; Hirschwerk, D.; Cohen, S.L.; et al. COVID-19 in kidney transplant recipients. Am. J. Transplant. 2020, 20, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Jit, M.; Warren-Gash, C.; Guthrie, B.; Wang, H.H.X.; Mercer, S.W.; Sanderson, C.; McKee, M.; Troeger, C.; Ong, K.L.; et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob. Health 2020, 8, e1003–e1017. [Google Scholar] [CrossRef]
- Barutcu Atas, D.; Aydin Sunbul, E.; Velioglu, A.; Tuglular, S. The association between perceived stress with sleep quality, insomnia, anxiety and depression in kidney transplant recipients during COVID-19 pandemic. PLoS ONE 2021, 16, e0248117. [Google Scholar] [CrossRef]
- Oto, O.A.; Ozturk, S.; Turgutalp, K.; Arici, M.; Alpay, N.; Merhametsiz, O.; Sipahi, S.; Ogutmen, M.B.; Yelken, B.; Altiparmak, M.R.; et al. Predicting the outcome of COVID-19 infection in kidney transplant recipients. BMC Nephrol. 2021, 22, 1–16. [Google Scholar] [CrossRef]
- Gandolfini, I.; Delsante, M.; Fiaccadori, E.; Zaza, G.; Manenti, L.; Degli Antoni, A.; Peruzzi, L.; Riella, L.V.; Cravedi, P.; Maggiore, U. COVID-19 in kidney transplant recipients. Am. J. Transplant. 2020, 20, 1941–1943. [Google Scholar] [CrossRef]
- Mamode, N.; Ahmed, Z.; Jones, G.; Banga, N.; Motallebzadeh, R.; Tolley, H.; Marks, S.; Stojanovic, J.; Khurram, M.A.; Thuraisingham, R.; et al. Mortality Rates in Transplant Recipients and Transplantation Candidates in a High-prevalence COVID-19 Environment. Transplantation 2021, 105, 212–215. [Google Scholar] [CrossRef]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. Covid-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef]
- Rinaldi, M.; Bartoletti, M.; Bussini, L.; Pancaldi, L.; Pascale, R.; Comai, G.; Morelli, M.; Ravaioli, M.; Cescon, M.; Cristini, F.; et al. COVID-19 in solid organ transplant recipients: No difference in survival compared to general population. Transpl. Infect. Dis. 2021, 23, e13421. [Google Scholar] [CrossRef]
- Abrishami, A.; Samavat, S.; Behnam, B.; Arab-Ahmadi, M.; Nafar, M.; Taheri, M.S. Clinical Course, Imaging Features, and Outcomes of COVID-19 in Kidney Transplant Recipients. Eur. Urol. 2020, 78, 281–286. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Yuan, Q.; Xia, Q.-X.; Zeng, X.-P.; Peng, J.-T.; Liu, J.; Xiao, X.-Y.; Jiang, G.-S.; Xiao, H.-Y.; et al. Identification of Kidney Transplant Recipients with Coronavirus Disease. Eur. Urol. 2020, 77, 742–747. [Google Scholar] [CrossRef]
- Chavarot, N.; Gueguen, J.; Bonnet, G.; Jdidou, M.; Trimaille, A.; Burger, C.; Amrouche, L.; Weizman, O.; Pommier, T.; Aubert, O.; et al. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am. J. Transplant. 2021, 21, 1285–1294. [Google Scholar] [CrossRef]
- Caillard, S.; Chavarot, N.; Francois, H.; Matignon, M.; Greze, C.; Kamar, N.; Gatault, P.; Thaunat, O.; Legris, T.; Frimat, L.; et al. Is COVID-19 infection more severe in kidney transplant recipients? Arab. Archaeol. Epigr. 2021, 21, 1295–1303. [Google Scholar] [CrossRef]
- Kute, V.B.; Bhalla, A.K.; Guleria, S.; Ray, D.S.; Bahadur, M.M.; Shingare, A.; Hegde, U.; Gang, S.; Raju, S.; Patel, H.V.; et al. Clinical Profile and Outcome of COVID-19 in 250 Kidney Transplant Recipients: A Multicenter Cohort Study from India. Transplantation 2021, 105, 851–860. [Google Scholar] [CrossRef]
- Cucchiari, D.; Guillen, E.; Cofan, F.; Torregrosa, J.; Esforzado, N.; Revuelta, I.; Ventura-Aguiar, P.; Oppenheimer, F.; Bayés, B.; Marcos, M. Ángeles; et al. Taking care of kidney transplant recipients during the COVID-19 pandemic: Experience from a medicalized hotel. Clin. Transplant. 2021, 35, e14132. [Google Scholar] [CrossRef]
- Shrivastava, P.; Prashar, R.; Khoury, N.; Patel, A.; Yeddula, S.; Kitajima, T.; Nagai, S.; Samaniego, M. Acute Kidney Injury in a Predominantly African American Cohort of Kidney Transplant Recipients With COVID-19 Infection. Transplantation 2021, 105, 201–205. [Google Scholar] [CrossRef]
- Elhadedy, M.A.; Marie, Y.; Halawa, A. COVID-19 in Renal Transplant Recipients: Case Series and a Brief Review of Current Evidence. Nephron 2020, 145, 192–198. [Google Scholar] [CrossRef]
- Cravedi, P.; Mothi, S.S.; Azzi, Y.; Haverly, M.; Farouk, S.S.; Pérez-Sáez, M.J.; Redondo-Pachón, M.D.; Murphy, B.; Florman, S.; Cyrino, L.G.; et al. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am. J. Transplant. 2020, 20, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Giorgakis, E.; Zehtaban, S.P.; Stevens, A.E.; Bhusal, S.; Burdine, L. COVID-19 in solid organ transplant recipients. Transpl. Infect. Dis. 2021, 23, e13419. [Google Scholar] [CrossRef]
- Banerjee, D.; Popoola, J.; Shah, S.; Ster, I.C.; Quan, V.; Phanish, M. COVID-19 infection in kidney transplant recipients. Kidney Int. 2020, 97, 1076–1082. [Google Scholar] [CrossRef]
- Dheir, H.; Sipah, S.; Yaylaci, S.; Cetin, E.S.; Genc, A.B.; First, N.; Koroglu, M.; Muratdagi, G.; Tomak, Y.; Suner, K.O.; et al. Clinical course of COVID-19 disease in immunosuppressed renal transplant patients. Turk. J. Med. Sci. 2021, 51, 428–434. [Google Scholar] [CrossRef]
- Craig-Schapiro, R.; Salinas, T.; Lubetzky, M.; Abel, B.T.; Sultan, S.; Lee, J.R.; Kapur, S.; Aull, M.J.; Dadhania, D.M. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Arab. Archaeol. Epigr. 2021, 21, 1576–1585. [Google Scholar] [CrossRef]
- Azzi, Y.; Parides, M.; Alani, O.; Loarte-Campos, P.; Bartash, R.; Forest, S.; Colovai, A.; Ajaimy, M.; Liriano-Ward, L.; Pynadath, C.; et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020, 98, 1559–1567. [Google Scholar] [CrossRef]
- Coll, E.; Fernández-Ruiz, M.; Sánchez-Álvarez, J.E.; Martínez-Fernández, J.R.; Crespo, M.; Gayoso, J.; Bada-Bosch, T.; Oppenheimer, F.; Moreso, F.; López-Oliva, M.O.; et al. COVID-19 in transplant recipients: The Spanish experience. Am. J. Transplant. 2021, 21, 1825–1837. [Google Scholar] [CrossRef]
- Fung, M.; Nambiar, A.; Pandey, S.; Aldrich, J.M.; Teraoka, J.; Freise, C.; Roberts, J.; Chandran, S.; Hays, S.R.; Bainbridge, E.; et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transpl. Infect. Dis. 2021, 23. [Google Scholar] [CrossRef]
- Naeem, S.; Gohh, R.; Bayliss, G.; Cosgrove, C.; Farmakiotis, D.; Merhi, B.; Morrissey, P.; Osband, A.; Bailey, J.A.; Sweeney, J.; et al. Successful recovery from COVID-19 in three kidney transplant recipients who received convalescent plasma therapy. Transpl. Infect. Dis. 2021, 23, e13451. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R.; Joshi, S.R.; Misra, A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102146. [Google Scholar] [CrossRef]
- Raut, A.; Huy, N.T. Rising incidence of mucormycosis in patients with COVID-19: Another challenge for India amidst the second wave? Lancet Respir. Med. 2021, 9, e77. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Fungal Disease. Available online: https://www.cdc.gov/fungal/diseases/mucormycosis/index.html (accessed on 24 May 2021).
- Honavar, S.G.; Sen, M.; Lahane, S.; Lahane, T.P.; Parekh, R. Mucor in a Viral Land: A Tale of Two Pathogens. Indian J. Ophthalmol. 2021, 69, 244–252. [Google Scholar] [CrossRef]
- Deb, A.K.; Sarkar, S.; Gokhale, T.; Choudhury, S.S. COVID-19 and orbital mucormycosis. Indian J. Ophthalmol. 2021, 69, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Mutya, V.S.S.; Thomas, A.; Rai, G.; Reddy, B.; Anithakumari, A.M.; Ray, S.; Anand, V.T.; Vishwanth, S.; Hegde, R. A case series of invasive mucormycosis in patients with COVID-19 infection. Int. J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 867–870. [Google Scholar] [CrossRef]
- Satish, D.; Joy, D.; Ross, A. Mucormycosis coinfection associated with global COVID-19: A case series from India. Int. J. Otorhinolaryngol. Head Neck Surg. 2021, 7, 815–820. [Google Scholar] [CrossRef]
- Moorthy, A.; Gaikwad, R.; Krishna, S.; Hegde, R.; Tripathi, K.K.; Kale, P.G.; Rao, P.S.; Haldipur, D.; Bonanthaya, K. SARS-CoV-2, Uncontrolled Diabetes and Corticosteroids—An Unholy Trinity in Invasive Fungal Infections of the Maxillofacial Region? A Retrospective, Multi-centric Analysis. J. Maxillofac. Oral Surg. 2021, 20, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Grover, M.; Bhargava, S.; Samdani, S.; Kataria, T. Post coronavirus disease mucormycosis: A deadly addition to the pandemic spectrum. J. Laryngol. Otol. 2021, 135, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.K.; Ghazarian, Z.; Cendrowski, K.D.; Persichino, J.G. Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Med. Mycol. Case Rep. 2021, 32, 64–67. [Google Scholar] [CrossRef]
- Hanley, B.; Naresh, K.; Roufosse, C.; Nicholson, A.G.; Weir, J.; Cooke, G.S.; Thursz, M.; Manousou, P.; Corbett, R.; Goldin, R.; et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 2020, 1, e245–e253. [Google Scholar] [CrossRef]
- Kanwar, A.; Jordan, A.; Olewiler, S.; Wehberg, K.; Cortes, M.; Jackson, B. A Fatal Case of Rhizopus azygosporus Pneumonia Following COVID-19. J. Fungi 2021, 7, 174. [Google Scholar] [CrossRef]
- Karimi-Galougahi, M.; Arastou, S.; Haseli, S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID-19). Int. Forum Allergy Rhinol. 2021, 11, 1029–1030. [Google Scholar] [CrossRef]
- Bajpai, D.; Deb, S.; Bose, S.; Gandhi, C.; Modi, T.; Katyal, A.; Saxena, N.; Patil, A.; Thakare, S.; Pajai, A.E.; et al. Recovery of kidney function after AKI because of COVID-19 in kidney transplant recipients. Transpl. Int. 2021, 34, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Mahalingasivam, V.; Craik, A.; Tomlinson, L.A.; Ge, L.; Hou, L.; Wang, Q.; Yang, K.; Fogarty, D.G.; Keenan, C. A Systematic Review of COVID-19 and Kidney Transplantation. Kidney Int. Rep. 2021, 6, 24–45. [Google Scholar] [CrossRef] [PubMed]
- Keating, B.J.; Mukhtar, E.H.; Elftmann, E.D.; Eweje, F.R.; Gao, H.; Ibrahim, L.I.; Kathawate, R.G.; Lee, A.C.; Li, E.H.; Moore, K.A.; et al. Early detection of SARS-CoV-2 and other infections in solid organ transplant recipients and household members using wearable devices. Transpl. Int. 2021, 34, 1019–1031. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Convalescent Plasma vs Human Immunoglobulin to Treat COVID-19 Pneumonia. Available online: https://clinicaltrials.gov/ct2/show/study/NCT04381858 (accessed on 3 June 2021).
- Jager, K.J.; Kramer, A.; Chesnaye, N.C.; Couchoud, C.; Sánchez-Álvarez, J.E.; Garneata, L.; Collart, F.; Hemmelder, M.H.; Ambühl, P.; Kerschbaum, J.; et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020, 98, 1540–1548. [Google Scholar] [CrossRef]
- Torreggiani, M.; Blanchi, S.; Fois, A.; Fessi, H.; Piccoli, G.B. Neutralizing SARS-CoV-2 antibody response in dialysis patients after the first dose of the BNT162b2 mRNA COVID-19 vaccine: The war is far from being won. Kidney Int. 2021, 99, 1494–1496. [Google Scholar] [CrossRef]
- US Government. Centers for Disease Control and Prevention. Influenza. Vaccine Effectiveness—How Well Does the Flu Vaccine Work? Available online: Https://www.Cdc.Gov/flu/vaccines-work/vaccineeffect.Html (accessed on 24 May 2021).
- European Centre for Disease Control and Prevention. COVID-19 Vaccination and Prioritisation Strategies in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-vaccination-and-prioritisation-strategies-eueea. (accessed on 24 May 2021).
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Weak anti–SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021, 99, 1487–1489. [Google Scholar] [CrossRef]
- Heldman, M.R.; Limaye, A.P. SARS-CoV-2 Vaccines in Kidney Transplant Recipients: Will They Be Safe and Effective and How Will We Know? J. Am. Soc. Nephrol. 2021, 32, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Stucchi, R.S.; Lopes, M.H.; Kumar, D.; Manuel, O. Vaccine Recommendations for Solid-Organ Transplant Recipients and Donors. Transplantation 2018, 102, S72–S80. [Google Scholar] [CrossRef]
- Ramesh, V.; Kute, V.B.; Agarwal, S.K.; Prakash, J.; Guleria, S.; Shroff, S.; Sharma, A.; Varma, P.; Prasad, N.; Sahay, M.; et al. NOTTO COVID-19 vaccine guidelines for transplant recipients. Indian J. Nephrol. 2021, 31, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, A.; Anders, H.-J.; Fernandez-Juárez, G.M.; Floege, J.; Goumenos, D.; Segelmark, M.; Tesar, V.; Turkmen, K.; van Kooten, C.; Bruchfeld, A.; et al. Recommendations for the use of COVID-19 vaccines in patients with immune-mediated kidney diseases. Nephrol. Dial. Transplant. 2021, 36, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- USA Centers for Disease Control and Prevention. Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html#SARS-CoV-2-infection (accessed on 21 May 2021).
- Aslam, S.; Goldstein, D.R.; Vos, R.; Gelman, A.E.; Kittleson, M.M.; Wolfe, C.; Danziger-Isakov, L. COVID-19 vaccination in our transplant recipients: The time is now. J. Hear. Lung Transplant. 2021, 40, 169–171. [Google Scholar] [CrossRef]
- Billany, R.E.; Selvaskandan, H.; Adenwalla, S.F.; Hull, K.L.; March, D.S.; Burton, J.O.; Bishop, N.C.; Carr, E.J.; Beale, R.; Tang, J.W.; et al. Seroprevalence of antibody to S1 spike protein following vaccination against COVID-19 in patients receiving hemodialysis: A call to arms. Kidney Int. 2021, 99, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Attias, P.; Sakhi, H.; Rieu, P.; Soorkia, A.; Assayag, D.; Bouhroum, S.; Nizard, P.; El Karoui, K. Antibody response to the BNT162b2 vaccine in maintenance hemodialysis patients. Kidney Int. 2021, 99, 1490–1492. [Google Scholar] [CrossRef] [PubMed]
- VCU Health. COVID-19 Vaccines and Transplant Patients: Is Vaccine Safe? Available online: https://www.vcuhealth.org/news/covid-19/covid-19-vaccines-and-transplant-patients-is-it-safe (accessed on 23 May 2021).
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 99, 1498–1500. [Google Scholar] [CrossRef] [PubMed]
- De Meester, J.; De Bacquer, D.; Naesens, M.; Meijers, B.; Couttenye, M.M.; De Vriese, A.S.; For the NBVN Kidney Registry Group. Incidence, Characteristics, and Outcome of COVID-19 in Adults on Kidney Replacement Therapy: A Regionwide Registry Study. J. Am. Soc. Nephrol. 2021, 32, 385–396. [Google Scholar] [CrossRef]
- Negahdaripour, M.; Shafiekhani, M.; Moezzi, S.M.I.; Amiri, S.; Rasekh, S.; Bagheri, A.; Mosaddeghi, P.; Vazin, A. Administration of COVID-19 Vaccines in ImmunocompromisedPatients. Int. Immunopharmacol. 2021, 99, 108021. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Nasr, S.H.; Larsen, C.P.; Kemper, A.; Ormsby, A.H.; Williamson, S.R. COVID-19–Associated Collapsing Focal Segmental Glomerulosclerosis: A Report of 2 Cases. Kidney Med. 2020, 2, 493–497. [Google Scholar] [CrossRef]
- Lei, S.; Jiang, F.; Su, W.; Chen, C.; Chen, J.; Mei, W.; Zhan, L.-Y.; Jia, Y.; Zhang, L.; Liu, D.; et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EclinicalMedicine 2020, 21, 100331. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Anglicheau, D.; Matignon, M.; Durrbach, A.; Greze, C.; Frimat, L.; Thaunat, O.; Legris, T.; Moal, V.; Westeel, P.F.; et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020, 98, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Chiang, P.-Y.; Werbel, W.A.; Durand, C.M.; Avery, R.K.; Getsin, S.; Jackson, K.R.; Kernodle, A.B.; Rasmussen, S.E.V.P.; Massie, A.B.; et al. Early impact of COVID-19 on transplant center practices and policies in the United States. Arab. Archaeol. Epigr. 2020, 20, 1809–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravanan, R.; Callaghan, C.J.; Mumford, L.; Ushiro-Lumb, I.; Thorburn, D.; Casey, J.; Friend, P.; Parameshwar, J.; Currie, I.; Burnapp, L.; et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Arab. Archaeol. Epigr. 2020, 20, 3008–3018. [Google Scholar] [CrossRef] [PubMed]
- Am. Society of Transplantation Surgeon (ASTS). Re-Engaging Organ Transplantation in the COVID-19 Era. Available online: https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/re-engaging-organ-transplantation-in-the-covid-19-era#.YK4KN6gzbIV (accessed on 21 May 2021).
- Am. Society of Transplantation (AST). COVID-19 Information. Available online: https://www.myast.org/covid-19-information# (accessed on 21 May 2021).
- The Transplantation Society. Transplant Infectious Disease. Available online: https://tts.org/tid-about/tid-presidents-message/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians (accessed on 21 May 2021).
- Kanchi, P.; Sambandam, S.; Siddhan, R.; Soundappan, S.; Vaseekaran, V.P.; Gupta, A. Successful kidney transplantation after COVID-19 infection in two cases. Nefrología 2021, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Aster CMI Hospital. Can I Undergo Kidney Transplant during COVID-19? Available online: https://www.asterbangalore.com/blog/kidney-transplant-during-covid-kidney-transplant-hospital-in-bangalore-india-aster-cmi-53 (accessed on 21 May 2021).
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Immunogenicity of a Single Dose of SARS-CoV-2 Messenger RNA Vaccine in Solid Organ Transplant Recipients. J. Am. Med. Assoc. 2021, 325, 1784. [Google Scholar] [CrossRef]
- Haskin, O.; Ashkenazi-Hoffnung, L.; Ziv, N.; Borovitz, Y.; Dagan, A.; Levi, S.; Koren, G.; Hamdani, G.; Levi-Erez, D.; Landau, D.; et al. Serological Response to the BNT162b2 COVID-19 mRNA Vaccine in Adolescent and Young Adult Kidney Transplant Recipients. Transplantation 2021, 105, e226–e233. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. J. Am. Med. Assoc. 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Marinaki, S.; Adamopoulos, S.; Degiannis, D.; Roussos, S.; Pavlopoulou, I.D.; Hatzakis, A.; Boletis, I.N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021. ahead of print. [Google Scholar] [CrossRef]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody response to SARS-CoV-2 mRNA vaccine among kidney transplant recipients: A prospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1173.e1–1173.e4. [Google Scholar] [CrossRef]
- Caillard, S.; Chavarot, N.; Bertrand, D.; Kamar, N.; Thaunat, O.; Moal, V.; Masset, C.; Hazzan, M.; Gatault, P.; Sicard, A.; et al. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021, 100, 477–479. [Google Scholar] [CrossRef]
- Meshram, H.S.; Kute, V.B.; Shah, N.; Chauhan, S.; Navadiya, V.V.; Patel, A.H.; Patel, H.V.; Engineer, D.; Banerjee, S.; Rizvi, J.; et al. COVID-19 in Kidney Transplant Recipients Vaccinated With Oxford–AstraZeneca COVID-19 Vaccine (Covishield): A Single-center Experience From India. Transplantation 2021, 105, e100–e103. [Google Scholar] [CrossRef]
- Aslam, S.; Adler, E.; Mekeel, K.; Little, S.J. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl. Infect. Dis. 2021, 23, e13705. [Google Scholar] [CrossRef]
- Ali, N.M.; Alnazari, N.; Mehta, S.A.; Boyarsky, B.; Avery, R.K.; Segev, D.L.; Montgomery, R.A.; Stewart, Z.A. Development of COVID-19 Infection in Transplant Recipients After SARS-CoV-2 Vaccination. Transplantation 2021, 105, e104–e106. [Google Scholar] [CrossRef] [PubMed]
- Tau, N.; Yahav, D.; Schneider, S.; Rozen-Zvi, B.; Abu Sneineh, M.; Rahamimov, R. Severe consequences of COVID-19 infection among vaccinated kidney transplant recipients. Am. J. Transplant. 2021, 21, 2910–2912. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Chiang, T.P.-Y.; Ou, M.T.; Werbel, W.A.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to the Janssen COVID-19 Vaccine in Solid Organ Transplant Recipients. Transplantation 2021, 105, e82–e83. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; -Vargas, G.G.; Gallais, F.; Gantner, P.; Cognard, N.; Olagne, J.; Velay, A.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; et al. Strong antibody response after a first dose of a SARS-CoV-2 mRNA-based vaccine in kidney transplant recipients with a previous history of COVID-19. Arab. Archaeol. Epigr. 2021, 21, 3808–3810. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Clarke, C.; Brown, J.; Cox, A.; Gleeson, S.; Guckian, M.; Randell, P.; Pria, A.D.; Lightstone, L.; Xu, X.-N.; et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet 2021, 397, 1178–1181. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals after mRNA vaccination. Sci. Immunol. 2021, 6, 6950. [Google Scholar] [CrossRef]
| Study | Place | Sample Size (N) * | Clinical Presentation # | Treatments # | Immunosuppression Adjustment # | Mortality |
|---|---|---|---|---|---|---|
| Abreshami et al., 2020 [28] | Iran | 12 | fever, cough, myalgia, headache, shortness of breath, gastrointestinal symptoms | HCQ, LR, AB, Ig | Decrease in MMF/AZT, MMF and CNI | 8 |
| Akalin et al., 2020 [26] | USA | 36 | fever, cough, myalgia, diarrhea, shortness of breath | HCQ, AZ, TL, LL | Withdrawal of F and AMB | 10 |
| Azzi et al., 2020 [41] | USA | 229 | - | HCQ, AB, RD, TL, CP, AK, Ig, LL, SL, AC | Withdrawal of AMB, CNI | 47 |
| Banerjee et al., 2020 [38] | UK | 7 | fever, cough, diarrhea, emesis, shortness of breath | - | Withdrawal of MMF and FK | 1 |
| Caillard et al., 2021 [31] | France | 273 | fever, cough, diarrhea, headache, shortness of breath, loss of smell/taste | HCQ, AZ, LR, OR, TL, RD, AB, AF | Withdrawal of CNI, mTOR, AMB and BC | - |
| Chavarot et al., 2021 [30] | France | 100 | fever, cough, myalgia, diarrhea, shortness of breath, loss of smell/taste | HCQ, AZ, TL | Withdrawal of CNI, AMB and BC | 26 |
| Coll et al., 2021 [42] | Spain | 375 | HCQ, AZ, AK, AV | CNI, AMB and mTOR adjustments | 103 | |
| Cravedi et al., 2020 [36] | USA | 144 | fever, myalgia, diarrhea, shortness of breath, | HCQ, AB, TL, RD, LR, DC, DR | Withdrawal of FK, MMF | 46 |
| Cucchiari et al., 2020 [33] | Spain | 28 | fever, cough, shortness of breath, gastrointestinal symptoms, loss of smell/taste | HCQ, AZ, LR, TL, Steroids | Withdrawal of MPA/mTOR and CNI | 5 |
| Dheir et al., 2021 [39] | Turkey | 20 | fever, cough, shortness of breath, myalgia, diarrhea | HCQ, FR, DX, ORCP, AB | Withdrawal of AMB, CNI, mTOR | 2 |
| Elhadedy et al., 2020 [35] | UK | 8 | fever, cough, shortness of breath | - | Discontinued MMF, increase/decrease in FK | No death |
| Elias et al., 2020 [14] | France | 66 | fever, cough, diarrhea, shortness of breath, loss of smell/taste | HCQ, TL, EL | Withdrawal of MMF/MPA/AZ, CNI | 16 |
| Fung et al., 2021 [43] | USA | 4 | fever, cough, diarrhea, fatigue, shortness of breath | HCQ, LR, TL, AB, RD, CP, Steroids | Withdrawal of MPA, FK, MPA | No death |
| Gandolfini et al., 2020 [24] | Italy | 2 | fever, myalgia, diarrhea, shortness of breath | HCQ, AB, LR, DC, RD | Withdrawal of Tac and MMF | 1 |
| Giorgakis et al., 2020 [37] | USA | 4 | fever, cough, loss of smell/taste, emesis, throat pain, fatigue, headache, loss of appetite, rhinorrhea | HCQ, AZ, TL | Decrease in FK, MMF, MPA, CNI | 1 |
| Kute et al., 2021 [32] | India | 250 | fever, cough, myalgia, fatigue, headache, emesis, diarrhea, shortness of breath, gastrointestinal symptoms, loss of smell/taste, throat pain, Z, rhinorrhea, loss of appetite, altered mental state | HCQ, AZ, FR, RD, CP, Ig | Withdrawal of and decrease in AMB, decrease in CNI and increase in PS | 29 |
| Mamode et al., 2021 [25] | UK | KTx = 121, W/L = 52 | fever, cough, myalgia, fatigue, headache, emesis, diarrhea, shortness of breath, | - | - | KTx-36 W/L-12 |
| Naeem et al., 2020 [44] | USA | 3 | fever, chills, fatigue, diarrhea, shortness of breath, emesis, gastrointestinal symptoms | CP, CFT, AZ, VM, PT, RD | Withdrawal of MMF, AZT | No deaths |
| Nair et al., 2020 [19] | USA | 10 | fever, cough, chills, myalgia, nasal congestion, fatigue, headache, emesis, diarrhea, shortness of breath | HCQ, AZ | Decrease in MPA, MMF and FK | 3 |
| Oto et al., 2021 [23] | Turkey | 109 | fever, cough, myalgia, fatigue, headache, diarrhea, shortness of breath, throat pain | HCQ, OR, LR, FR, GC, TL, AK, AP | - | 14 |
| Rinaldi et al., 2020 [27] | Italy | 24 (22 KTx) | fever, cough, diarrhea, shortness of breath | HCQ, AZ, DC, TL, Steroids | 4 (30 days) | |
| Schapiro et al., 2021 [40] | USA | KTx = 80 W/L = 56 | fever, cough, diarrhea, emesis, headache, fatigue, myalgia, shortness of breath, | HCQ, AZ, RD, TL, SX, CP, DY | Withdrawal of, increase or decrease in MMF | KTx = 13 W/L = 19 |
| Shrivastava et al., 2021 [34] | USA | 39 | fever, cough, diarrhea, headache, altered mental state, hypoxia | HCQ, TL | Withdrawal of or decrease in AMB and CNI | 9 |
| Zhang et al., 2020 [29] | China | 5 | fever, cough, myalgia, fatigue | OR, AB, Ig | Decrease in GC, MMF and CNI | No deaths |
| Patient | Age (Years) | Time from Tx | Vaccine | No. of Doses | Time from Vaccine (Days) | Clinical Presentation | Severity of COVID-19 | Treatments | Outcomes | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| I. | 71 | 192 | Oxford-AstraZeneca | 2 | 20 | Fever, cough, shortness of breath | - | TF, DX, RD, MV | Died | [95] |
| II. | 51 | 18 | Oxford-AstraZeneca | 2 | 13 | Fever, cough, diarrhea | - | AZ, DX, CP, MV | Ventilator | [95] |
| III. | 46 | 108 | Oxford-AstraZeneca | 2 | 23 | Fever, cough, shortness of breath, weakness | - | AZ, DX, RD | Ventilator, dialysis dependent | [95] |
| IV. | 67 | 72 | Oxford-AstraZeneca | 2 | 8 | cough | - | AZ | Recovered | [95] |
| V. | 67 | 72 | BNT162b2 | 2 | 72 | Diarrhea | Moderate | RD | Recovered | [96] |
| VI. | 44 | 16 | BNT162b2 | 2 | 11 | Fever, cough, shortness of breath | Severe | RD, DX | Recovered | [97] |
| VII. | 68 | 16 | mRNA-1273 | 2 | 4 | Cough, weakness | Mild | None | Recovered | [97] |
| VIII. | 58 | 19 | Ad26.COV2.S | 2 | 19 | Diarrhea | Mild | MAB, RD | Inpatient | [97] |
| IX. | 72 | 2.5 | BNT162b2 | 2 | 20 | Fever, cough, diarrhea | Mild–moderate | MAB | Recovered | [97] |
| X. | 27 | 11 | BNT162b2 | 2 | 43 | Cough | Mild | MAB | Recovered | [97] |
| XI. | 69 | 18 | BNT162b2 | 2 | 25 | Fever, shortness of breath | Severe | RD, DX | Inpatient | [97] |
| XII. | 71 | 24 | mRNA-1273 | 2 | 18 | Fever, diarrhea, vomiting | Severe | RD, DX, MAB, CP | Recovered | [97] |
| XIII. | 59 | 47 | mRNA-1273 | 2 | 36 | Headache, body ache, weakness | Mild | None | Recovered | [97] |
| XIV. | 54 | 48 | BNT162b2 | 2 | 45 | Weakness | Mild | None | Recovered | [97] |
| XV. | 52 | 53 | BNT162b2 | 2 | 40 | Cough, body aches | Mild–moderate | MAB | Recovered | [97] |
| XVI. | 55 | 83 | BNT162b2 | 1 | 7 | - | - | None | Recovered | [98] |
| XVII. | 58 | 38 | BNT162b2 | 1 | 14 | - | - | None | Recovered | [98] |
| XVIII. | 55 | 58 | BNT162b2 | 1 | 14 | - | Critical | RD, DX | Died | [98] |
| XIX. | 60 | 48 | BNT162b2 | 1 | 18 | - | - | None | Recovered | [98] |
| XX. | 51 | 5 | BNT162b2 | 1 | 21 | - | Critical | RD, DX, CP | Died | [98] |
| XXI. | 57 | 49 | BNT162b2 | 1 | 22 | - | - | None | Recovered | [98] |
| XXII. | 42 | 64 | BNT162b2 | 1 | 24 | - | Critical | DX, CP | Died | [98] |
| XXIII. | 51 | 23 | BNT162b2 | 2 | 4 | - | Mild | BAM | Recovered | [98] |
| XXIV. | 35 | 27 | BNT162b2 | 2 | 9 | - | Severe | None | Recovered | [98] |
| XXV. | 34 | 13 | BNT162b2 | 2 | 12 | - | Mild | None | Recovered | [98] |
| XXVI. | 34 | 16 | BNT162b2 | 2 | 12 | - | Mild | None | Recovered with elevated creatinine | [98] |
| XXVII. | 62 | 246 | BNT162b2 | 2 | 25 | - | Critical | RD, DX, CP | Died | [98] |
| XXVIII. | 64 | 25 | BNT162b2 | 2 | 33 | - | Severe | RD, DX, CP | Recovered | [98] |
| XXIX. | 49 | 7 | BNT162b2 | 2 | 35 | - | Severe | DX, CP | Recovered | [98] |
| XXX. | 65 | 41 | BNT162b2 | 2 | 36 | - | Critical | RD, DX | Died | [98] |
| XXXI. | 26 | 154 | BNT162b2 | 2 | 38 | - | - | None | Recovered | [98] |
| XXXII. | 40 | 237 | BNT162b2 | 2 | 43 | - | - | None | Recovered | [98] |
| XXXIII. | 77 | 9 | BNT162b2 | 2 | 46 | - | Severe | DX | Recovered | [98] |
| XXXIV. | 78 | 59 | BNT162b2 | 2 | 52 | - | Mild | DX | Recovered | [98] |
| XXXV. | 72 | 94 | BNT162b2 | 2 | 53 | - | Critical | RD, DX, CP | Died | [98] |
| XXXVI. | 68 | 23 | BNT162b2 | 2 | 53 | - | - | None | Recovered | [98] |
| XXXVII. | 57 | 121 | BNT162b2 | 2 | 54 | - | - | None | Recovered | [98] |
| XXXVIII. | 63 | 69 | BNT162b2 | 2 | 73 | - | Severe | - | In hospital | [98] |
| XXXIX. | 26 | 250 | BNT162b2 | 2 | 85 | - | - | None | Recovered | [98] |
| XL. | 70 | 45 | BNT162b2 | 2 | - | - | Critical | RD, CP | Died | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Rana, R.; Rana, D.S.; Gupta, A.; Sachdeva, M.P. SARS-CoV-2 in Kidney Transplant Recipients: A Systematic Review. Transplantology 2022, 3, 33-48. https://doi.org/10.3390/transplantology3010004
Kumar N, Rana R, Rana DS, Gupta A, Sachdeva MP. SARS-CoV-2 in Kidney Transplant Recipients: A Systematic Review. Transplantology. 2022; 3(1):33-48. https://doi.org/10.3390/transplantology3010004
Chicago/Turabian StyleKumar, Naveen, Rashmi Rana, Devinder Singh Rana, Anurag Gupta, and Mohinder Pal Sachdeva. 2022. "SARS-CoV-2 in Kidney Transplant Recipients: A Systematic Review" Transplantology 3, no. 1: 33-48. https://doi.org/10.3390/transplantology3010004
APA StyleKumar, N., Rana, R., Rana, D. S., Gupta, A., & Sachdeva, M. P. (2022). SARS-CoV-2 in Kidney Transplant Recipients: A Systematic Review. Transplantology, 3(1), 33-48. https://doi.org/10.3390/transplantology3010004






