1. Introduction
Since its invention, the optical microscope has become one of the most important observation devices in modern science and technology [
1,
2,
3,
4]. Optical microscopes, in general, enable observation of microscopic samples by focusing light using lenses and other optical devices based on the diffraction properties of light in the form of waves [
5,
6,
7]. The spatial resolution of such optical microscopes is limited to about half the wavelength resolution (200 nm) based on the diffraction theory formulated by Abbe [
3,
8]. Recently, the development of super-resolution fluorescence optical microscopy has made it possible to observe and analyse the location of fluorescently labelled proteins in cells with nano-level resolution [
3,
9,
10,
11,
12]. Thus, improvements in the resolution and functionality of optical microscopy have been directly related to advances in science and technology.
However, in addition to diffraction, light is known to exhibit scattering [
13]. For example, Rayleigh scattering occurs when light collides with and is absorbed and scattered by nitrogen and oxygen molecules in the atmosphere [
13,
14]. If Rayleigh scattering generated by such extremely small particles could be directly imaged, it should be possible to observe them with very high spatial resolution. To achieve this, a new optical system is needed that does not use lenses or other diffraction-based optical devices as used in conventional optical microscopes. Here, we report the development of a projection optical microscope (PROM) for direct observation of nanoparticles and nanostructures of 100 nm or less without the need for fluorescent labels and/or staining.
Light has particle properties in addition to wave properties. In 1905, Einstein’s light quantum hypothesis explained the photoelectric effect, in which electrons are emitted when light is shone on a metal surface, by assuming the particle nature of light as a photon [
15]. Recent theoretical research has suggested that the classical interference phenomenon, a characteristic of light waves, arises from a special phenomenon known as entanglement, which occurs between the quantum bright and dark states of photons [
16]. This theory suggests that the light interference phenomena can be interpreted as a quantum mechanism involving photons. Furthermore, recent theoretical research has also suggested that beam splitters may also be affected by quantum interference between the bright and dark states of photons [
17]. Therefore, in the PROM observation method described here, it is possible that the interference is reduced due to the breakdown of entanglement between the bright and dark states of photons diffused through nanoscale structures, enabling high-resolution observation.
2. Materials and Methods
2.1. Raman Microscopy and PROM System
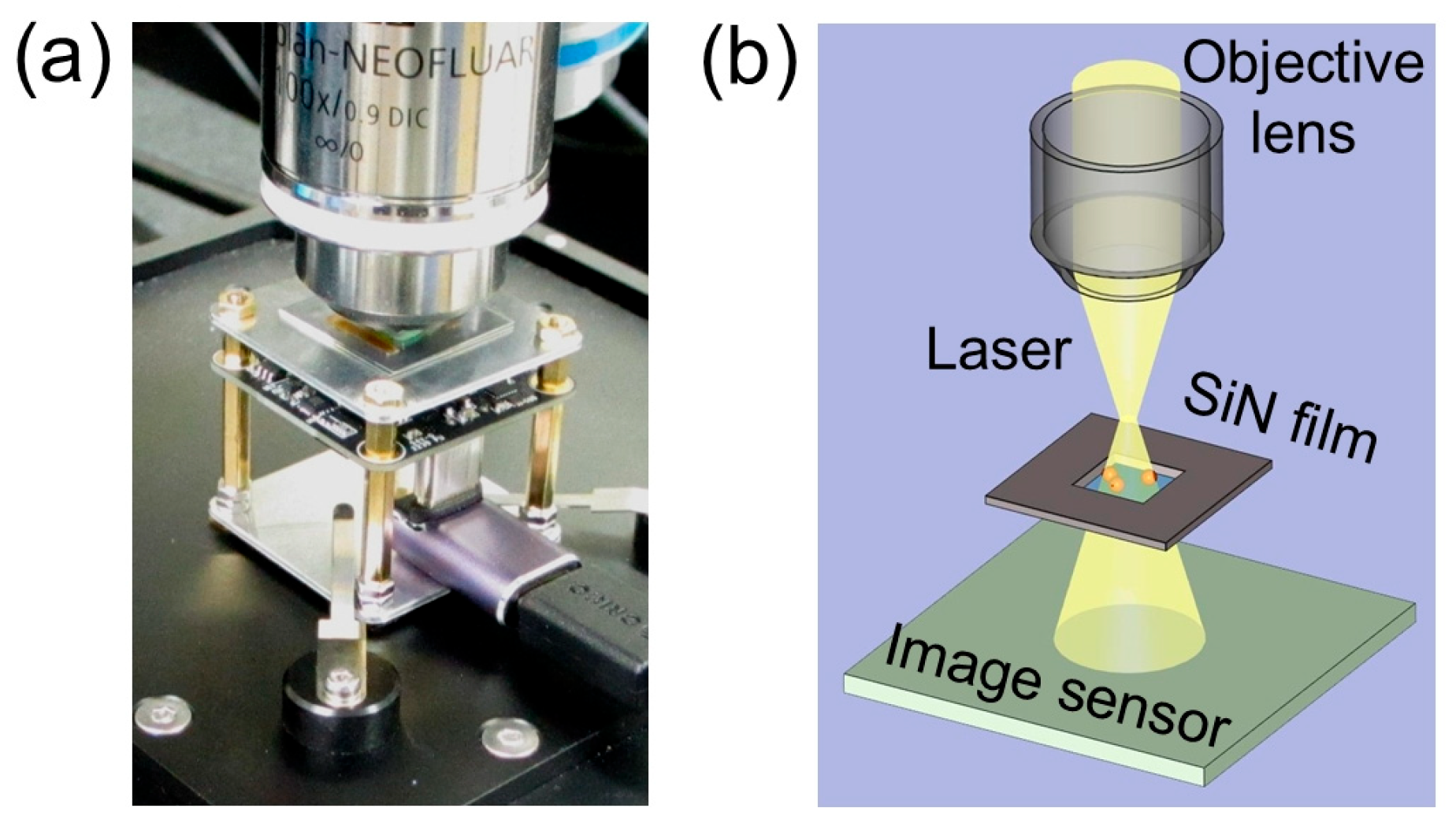
The samples on a silicon nitride (SiN) film (Silson Ltd., Southam, UK) were observed by a confocal laser Raman microscope (CLRM) (alpha300R, WITech, Ulm, Germany) using a 100× objective lens (Plan apo, Carl Zeiss, Oberkochen, Germany) and a 532 nm Nd-YAG laser. The CMOS image sensor (DFM37UX273-ML, The Imaging Source Co., Ltd., Bremen, Germany) was placed 5 mm below the focus position of the objective lens (
Figure 1a,b). The video output of the CMOS image sensor was recorded by a PC (Let’s Note CF-FV4SRCCP, Panasonic Co., Tokyo, Japan) using image capture software (ICCapture ver. 2.5.1571, The Imaging Source Co., Ltd., Bremen, Germany) via a USB 3.1 cable. This image sensor is capable of imaging colour at 1440 × 1080 pixels with a pixel size of 3.45 μm (CMOS IMX273, Sony Co., Tokyo, Japan). Videos of the samples were captured at 1440 × 1080 pixels at a colour image capture rate of 60–100 fps. Video files recorded in Mpeg4 format were transferred to a desktop PC (Endeavor Pro 9200, Epson direct Co., Nagano, Japan). Image analysis was performed using MATLAB R2023a (Math Works Inc., Natick, MA, USA) and plotted using Origin 2023J (Origin-Lab Co., Northampton, MA, USA).
To obtain PROM video data, the sample was first aligned with the focal position of the CLRM and the focus position was then moved by 3 to 10 μm above the sample for observation using PROM. For the PROM observation of PS spheres, ZnO, and sunscreen, a 10 × 10 μm area was raster scanned (50 × 50 vertical and horizontal lines) for 4 min using the 2D map imaging mode of CLRM, during which video images were continuously captured by the image sensor. For the observation of milk using PROM, the sample was fixed and the same position was captured on video for 1 min.
2.2. Preparation of PS Spheres
The PS spheres of 1 μm or 500 nm diameter suspended in aqueous buffer (Micromer, micromod Partikeltechnologie GmbH, Rostock, Germany) were diluted three times with ultrapure water, vortexed for 10 s and sonicated for 1 min. A sample of the sphere suspension (5 μL) was placed on a 50 nm thick SiN film (0.5 × 0.5 mm window) supported by a Si frame (5 × 5 mm, 0.2 mm thick, Silson Ltd., Southam, UK) after hydrophilization for 15 s (PIB-10, Vacuum Device Inc., Mito, Japan). The liquid of the suspension was absorbed by a piece of filter paper from the droplet. The sample was dried for 5 min at room temperature (23 °C). The SiN film with the PS spheres was attached to an aluminium sample stage with a 3 mm hole in the centre (
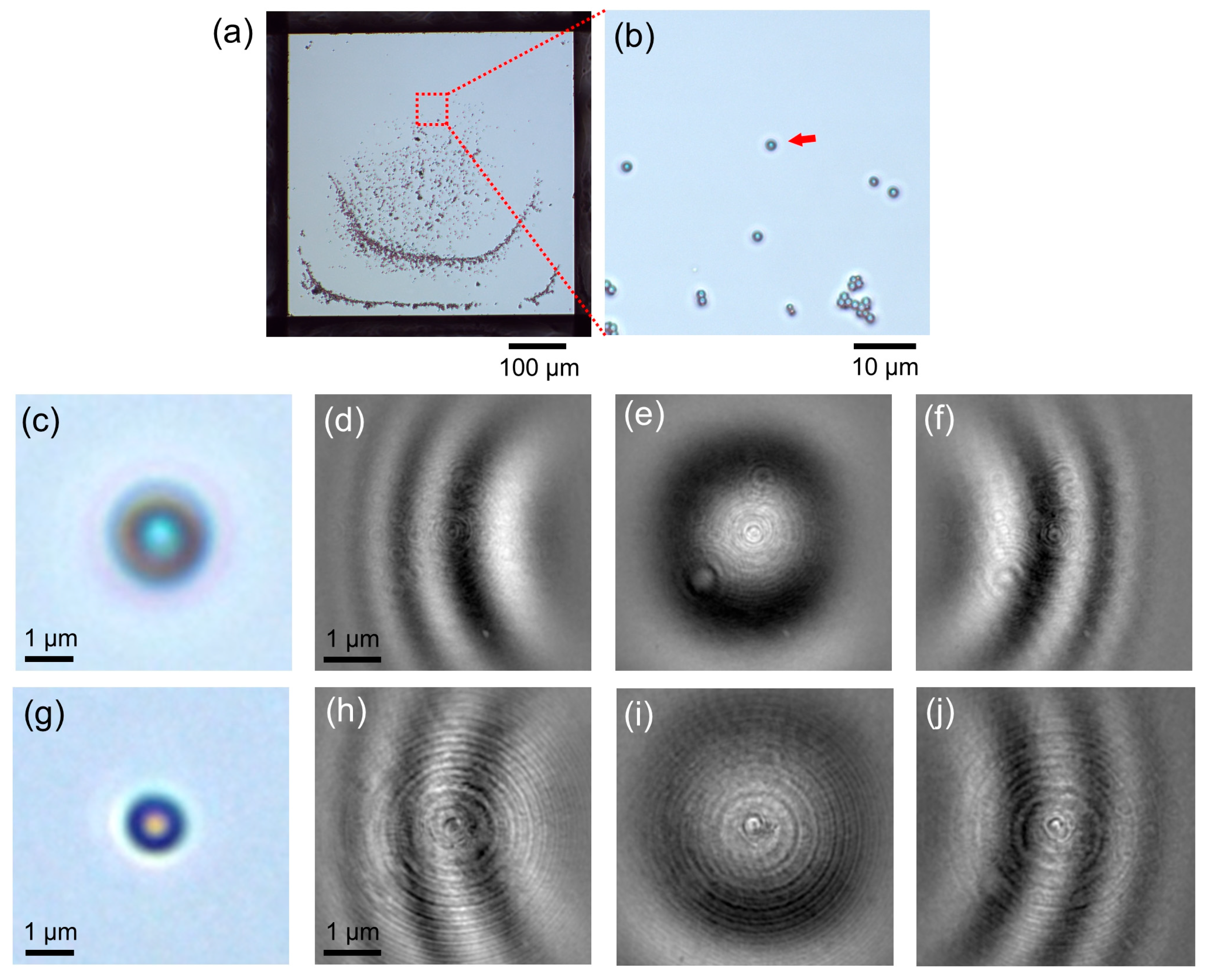
Figure 1a). For observation of the PS spheres by PROM, the focus position was set to 10 μm above the sphere sample, the laser light intensity was 0.1 mW, and video imaging was performed at 1440 × 1080 pixels at a speed of 60 frames per second (fps). When saving as an Mpeg4 file, the compression rate was set to 50MB/s.
2.3. ZnO Sample Preparation
ZnO particles of 20 nm diameter (260-01261, FUJIFILM Wako Pure Chemicals Co., Osaka, Japan) were suspended in pure water at a concentration of 10%/W and dispersed using an ultrasonic device for 2 min. 3 μL of this suspension was dropped onto the SiN film after hydrophilization. The excess liquid in the suspension was absorbed by filter paper and the sample was dried at room temperature (23 °C) for 5 min. The ZnO sample on the SiN film was attached to the centre of the cover glass with double-sided tape with the sample facing downwards and these were fixed to the sample stage.
For observation of the ZnO particles by PROM, the focus position was set to 3–10 μm above the sample, the laser light intensity was 0.1 mW, and the video imaging conditions were 1440 × 1080 pixels at a speed of 60 fps. When saving as an Mpeg4 file, the compression rate was set to 50 MB/s.
2.4. Sunscreen Sample Preparation
In the case of sunscreen containing TiO2 and ZnO nanoparticles (Sun play super cool, ROHTO Pharmaceutical Co., Ltd., Osaka, Japan), 2 µL of a sunscreen emulsion was dropped onto a tungsten-coated 50 nm thick SiN film (Silson Ltd., Southam, UK) that had been hydrophilized for 15 s. The SiN film with the sample was placed in a centrifuge holder and centrifuged at 2000 rpm for 1 min (MX300, Tommy Seiko Co., Tokyo, Japan) to thinly spread the sample on the SiN film.
For observation of the sunscreen sample by PROM, the focus position was set to 5 μm above the sample, the laser light intensity was 0.2 mW, and the video imaging conditions were 1440 × 1080 pixels at a speed of 60 fps. When saving as an Mpeg4 file, the compression rate was set to 50 MB/s.
2.5. Whole Milk Sample Preparation
The Si frame of a 50 nm thick SiN film was fixed to the cover glass with two strips of double-sided tape on both sides. Since the double-sided tape was 10 μm thick, there was a 10 μm gap between the SiN film and the cover glass. 2 μL of whole milk was dropped into the gap using an Eppendorf pipette (Eppendorf Co., Hamburg, Germany). The milk enters the gap between the cover glass and the SiN film due to surface tension. After that, the periphery of the SiN film chip was sealed with adhesive (RT30 ARALDITE, Huntsman Japan, Kobe, Japan) and kept at room temperature (23 °C) for 10 min. The milk suspension in its holder was fixed onto an aluminium sample stage with a 3 mm hole in the centre and was then observed using optical microscopy and PROM.
For observation of the milk sample by PROM, the focus position was set to 5 μm above the sample, the laser light intensity was 0.1 mW, and the video imaging conditions were 1440 × 1080 pixels at a speed of 100 fps. When saving as an Mpeg4 file, the compression rate was set to 50 MB/s.
2.6. Learning of Growing Neural Gas Network (GNG)
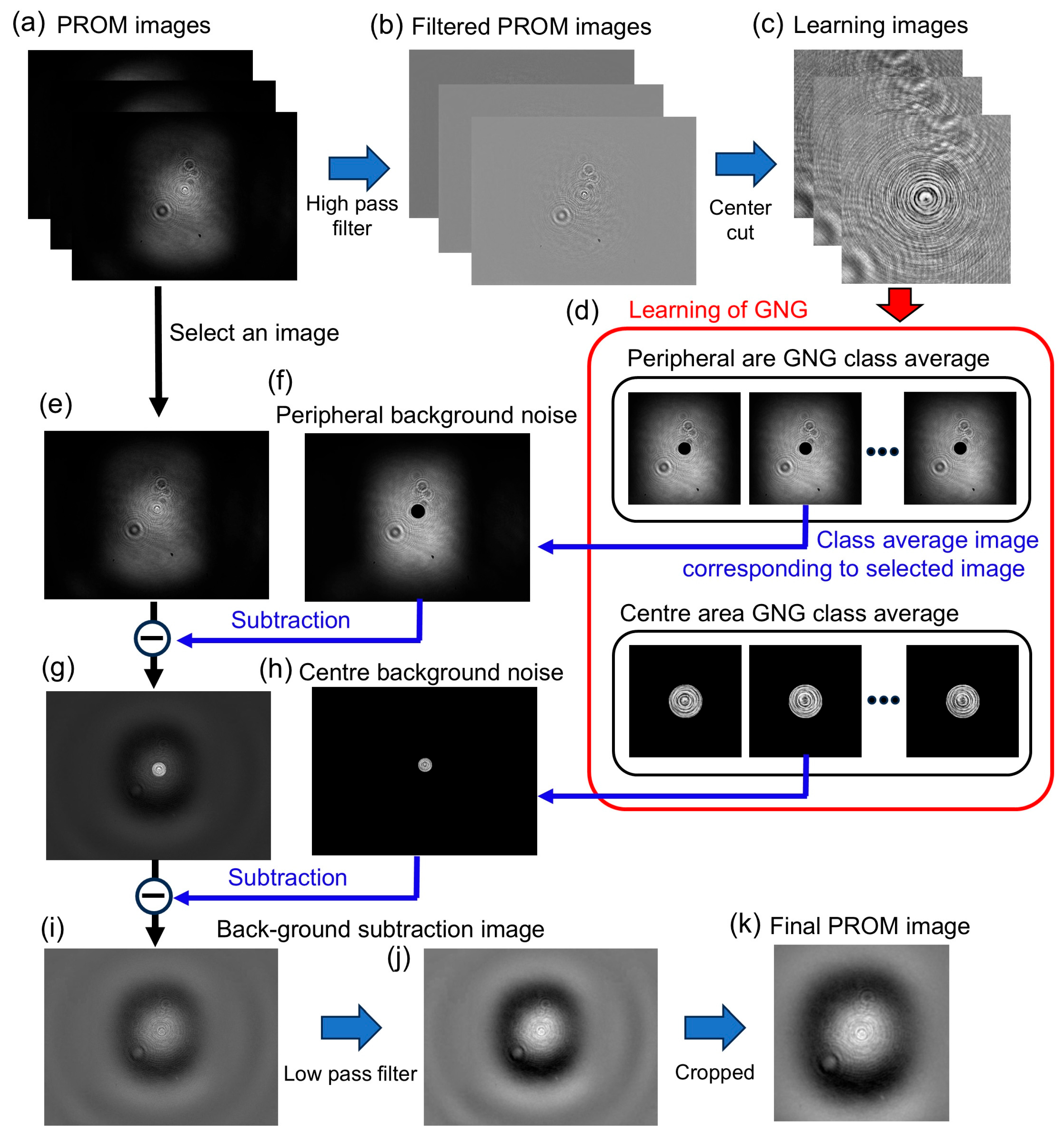
As the learning data for Growing Neural Gas Network (GNG) [
18,
19], we used an image obtained by extracting the high-frequency components of the PROM images and cutting out the central area of 400 × 400 pixels. The number of learning images for each sample was 14,350 for the PS spheres and the ZnO sample, 14,400 for the sunscreen sample, and 6000 for the milk sample. The number of GNG units was 100 units inside the PS sphere image and 50 units outside for the sphere samples, 20 units for the ZnO particles, 20 units for the sunscreen sample, and 10 units for the milk sample. The number of learning sessions was set to 200,000 iterations.
2.7. SEM and SE-ADM Observation of Samples
The ZnO sample on the SiN film with the Si frame was fixed on an aluminium stage with a conductive carbon tape and observed using a secondary electron image of the scanning electron microscope (SEM) (JSM7000F, JEOL, Tokyo, Japan). The ZnO sample was observed at an acceleration voltage of 4 kV and a current of 5–10 pA with a magnification of 20,000–40,000× and 1280 × 1024 pixel images were captured in 40 s.
For the scanning electron assisted dielectric microscopy (SE-ADM) observation [
20,
21] of the sunscreen sample, images of 1280 × 1020 pixels were captured over a period of 40 s at a magnification of 10,000–20,000× with an acceleration voltage of 10 kV and a current of 10 pA.
4. Discussion
In this study, we have demonstrated the feasibility of directly observing nano-scale particles and structures by irradiating samples with diffused light and directly capturing the images with an image sensor (
Figure 2 and
Figure 5). In conventional optical microscopes, images are magnified and observed by focusing diffracted light from submicron or larger structures using lenses [
5,
6,
7,
8]. In such conventional microscopes, the spatial resolution is limited by light diffraction [
3,
8]. However, nanoparticles can also scatter light at the molecular level [
13,
14]. In Rayleigh scattering, light is scattered even by very small molecules in the atmosphere [
13,
14]. If such Rayleigh scattering information could be directly obtained, it would be possible to achieve molecular-level spatial resolution. This method is independent of diffraction, so light diffraction limits do not come into play.
We have developed a PROM in which the sample is placed below the focus position of the objective lens of a CLRM, and an image sensor is installed further below (
Figure 1). With this method, interference fringes due to light diffraction and interference were indeed observed with particles larger than submicron (
Figure 2d–f,
Figure 8a). On the other hand, nanoparticles and nanostructures do not cause light diffraction but only Rayleigh scattering. Therefore, the incident light at the particle position is scattered, while that between the particles is transmitted, and the image sensor captures a projection image of the nanoparticle (
Figure 8b).
When laser light is irradiated onto a sample, random microstructures called speckle patterns can be seen in the transmitted and reflected light [
27,
28,
29]. The speckle pattern observation method is similar to the PROM method in the device configuration for projecting light onto the sample and detecting it. In speckle pattern detection, light with a small scattering angle, generally close to parallel light, is typically irradiated onto the sample, and this transmitted or reflected light is detected. On the other hand, with the PROM method, light focused by an objective lens near the sample is diffused over a wide angle and irradiated (
Figure 1a,b). Furthermore, the projected light is detected by a high-resolution CMOS image sensor placed directly below it. Therefore, it is considered that speckle patterns will hardly occur. These similarities and differences between speckle detection methods and the PROM method are thought to be key to elucidating the detection mechanism of the PROM method.
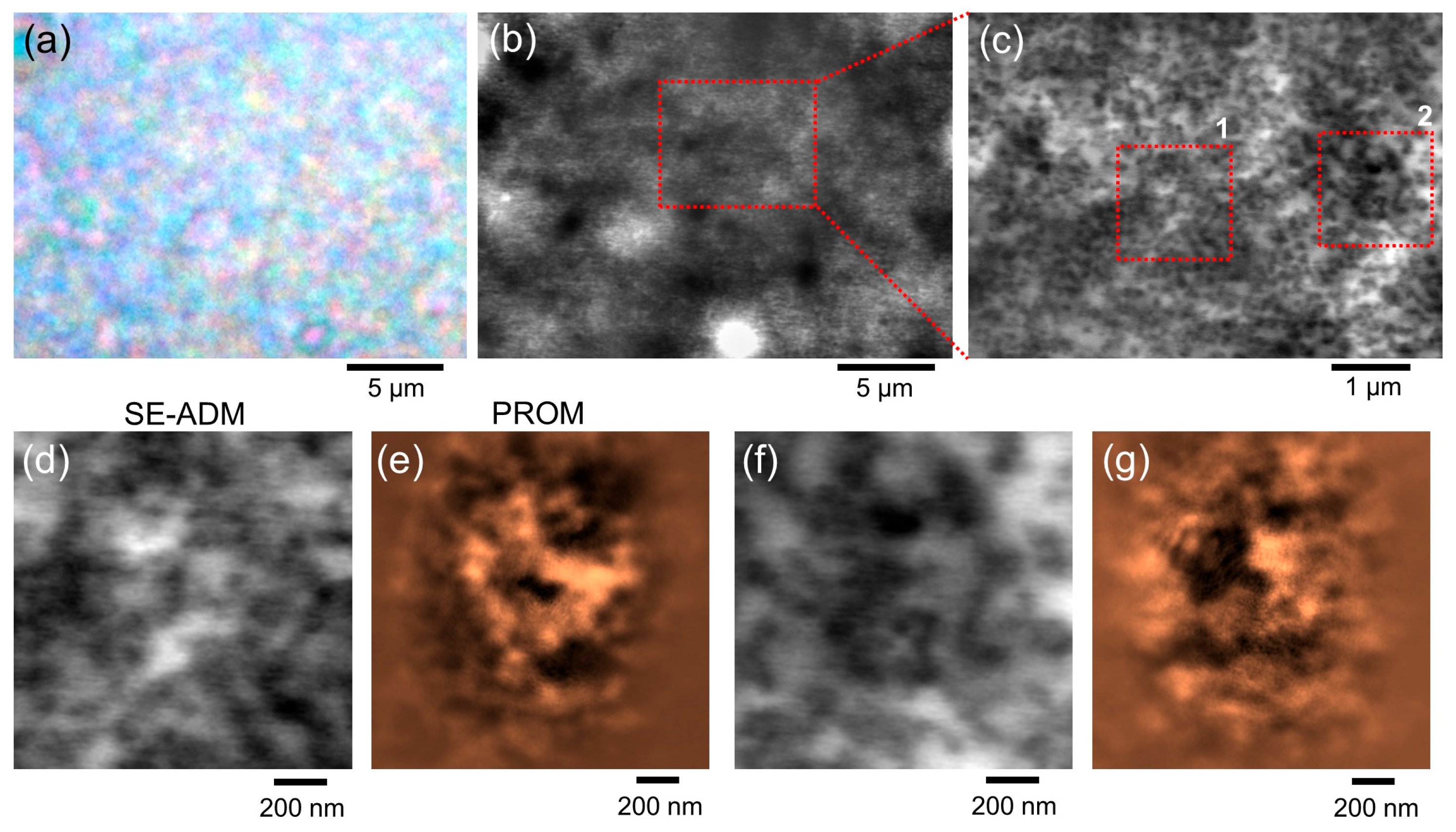
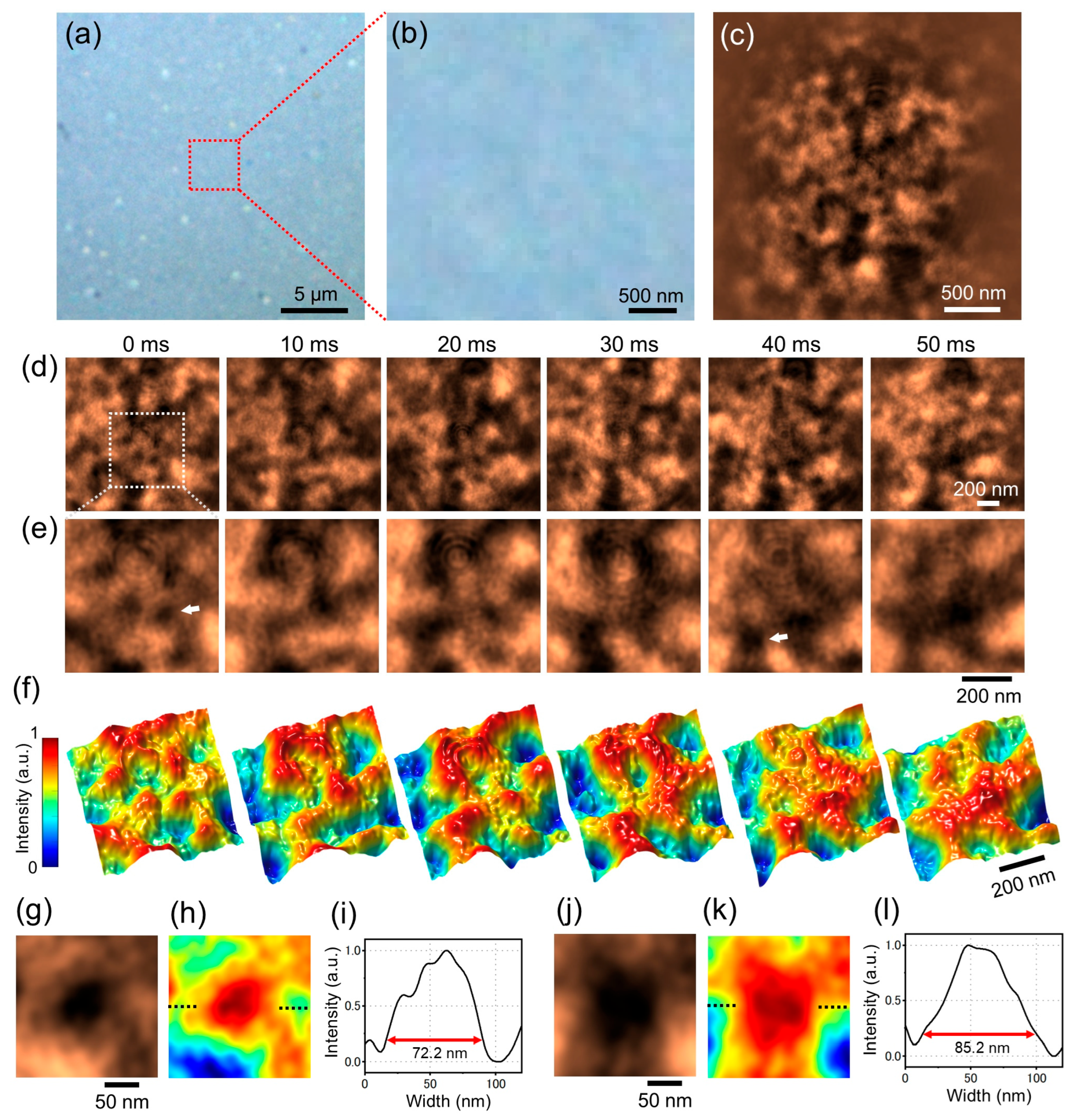
From the results using ZnO particles, the spatial resolution of the current PROM system was approximately 16.6 nm (
Figure 5o). The wavelength of the laser beam used was 532 nm and the spatial resolution due to Abbe diffraction using a 100× objective lens (NA 0.9) was approximately 300 nm. Therefore, PROM observation achieves a much higher spatial resolution than the diffraction limit. Furthermore, the spatial resolution and contrast can be improved by using shorter wavelengths. We predict that a resolution of less than 10 nm could be achieved by using a 400 nm laser beam.
The high spatial resolution achieved by PROMs may be related to the quantum mechanism of light. In particular, the reduction in the interference of light transmitted through nanoparticles and/or nanostructures, coupled with the manifestation of the particle property of light, may enable direct observation of nanoscale structures. Recent theoretical research on light interference has reported that it can be explained by entanglement between bright and dark states of photons [
16]. If the quantum interference of light can be reduced, it is thought that extremely high spatial resolution can be achieved in optical observation. One hypothesis for the high resolution of the PROM method is that when light passes through nano-level structures that cause Rayleigh scattering, the quantum entanglement between the bright and dark states of photons decreases, allowing the nano-level structure to be directly projected onto the image sensor. The PROM system in this paper uses a CMOS image sensor to detect the projected light, with each pixel consisting of a small photodiode [
30,
31]. Light detection in the photodiode is achieved through the quantum absorption of light and the excitation of electrons in the semiconductor [
31]. In other words, since the light detection process itself is performed based on the quantum mechanism of light rather than its wave property, the detection of projected images may depend significantly on the entanglement of the bright and dark states of photons. We plan to conduct more detailed research on these hypotheses in the future.
Several issues must be taken into account when using PROM. Since the sample is placed at a position where light is diffused and where the projected image is observed, there is a difference in the angle and distance of incident light between the centre and the periphery, causing distortion of the image. Thus, there is a slight structural difference between the images obtained with PROM compared to SEM or SE-ADM (
Figure 5 and
Figure 6). If the sample contains a micron-sized structure that causes diffraction (
Figure 2), diffracted and scattered light information is mixed and thus nanoscale structural information is mixed in the interference fringes. Furthermore, if the sample is thick, the projection magnification is higher at the top of the sample than at the bottom. This causes size variations in the observed image, even for spheres of the same diameter. Moreover, if the sample is thick and has a complex structure, the incident light is multiple-scattered and distortion may occur in the observed image. In addition, the effects of multiple scattering may increase background noise. Therefore, to suppress multiple scattering, the sample thickness must be relatively thin (less than a few tens of μm). To overcome these problems, a new image correction algorithm based on the observation position of the sample will have to be developed.
PROM detects the scattering information of the laser light transmitted through the sample, allowing direct observation with very high sensitivity. This leads to an improvement in time resolution. With the current system, it is possible to obtain up to 236 images per second at the full pixel size of 1440 × 1080. Nanoparticles in solution move very quickly, so a CMOS sensor capable of high-speed imaging is required. The CMOS sensor we currently use can capture images at a maximum of 236 frames per second, making it possible to directly observe nanoparticles of 100 nm or larger. However, particles smaller than this undergoes Brownian motion at a faster rate, requiring a camera element with an imaging speed of 1000 fps or higher. The use of an ultra-fast camera would enable the capture of more than 10,000 images per second. This would allow direct observation and analysis of nanoparticles moving at high speed in solution.