Artificial Intelligence-Based Epileptic Seizure Prediction Strategies: A Review
Abstract
1. Introduction
- Safety. Accurate seizure prediction can help individuals take appropriate safety measures, such as avoiding potentially hazardous activities during high-risk periods [16].
- Economic impact. Forecasting epileptic seizures can help reduce healthcare costs associated with emergency room visits and hospitalizations, as people with epilepsy have a 9% probability of requiring hospitalization due to seizure-related injuries [16].
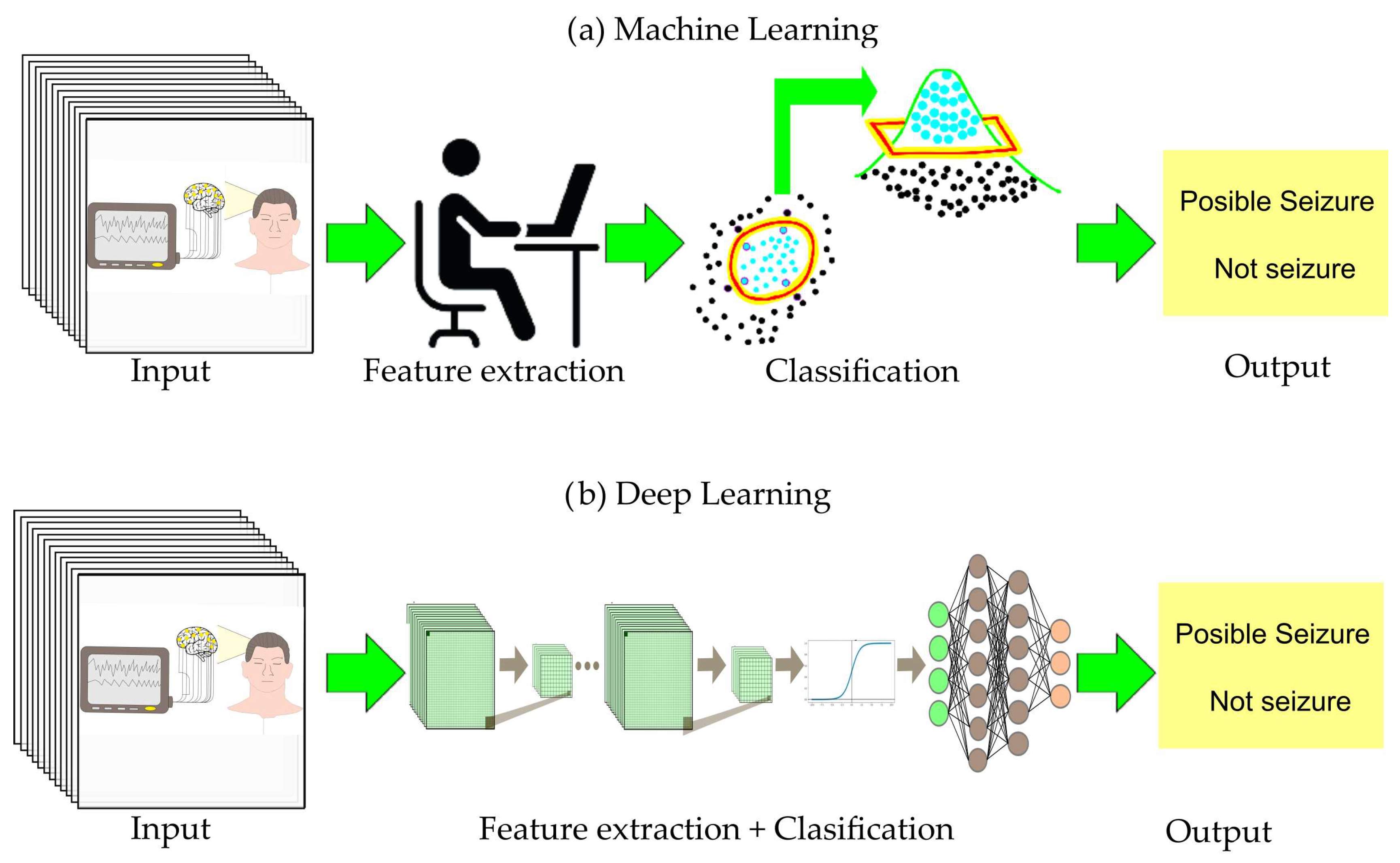
2. Background General
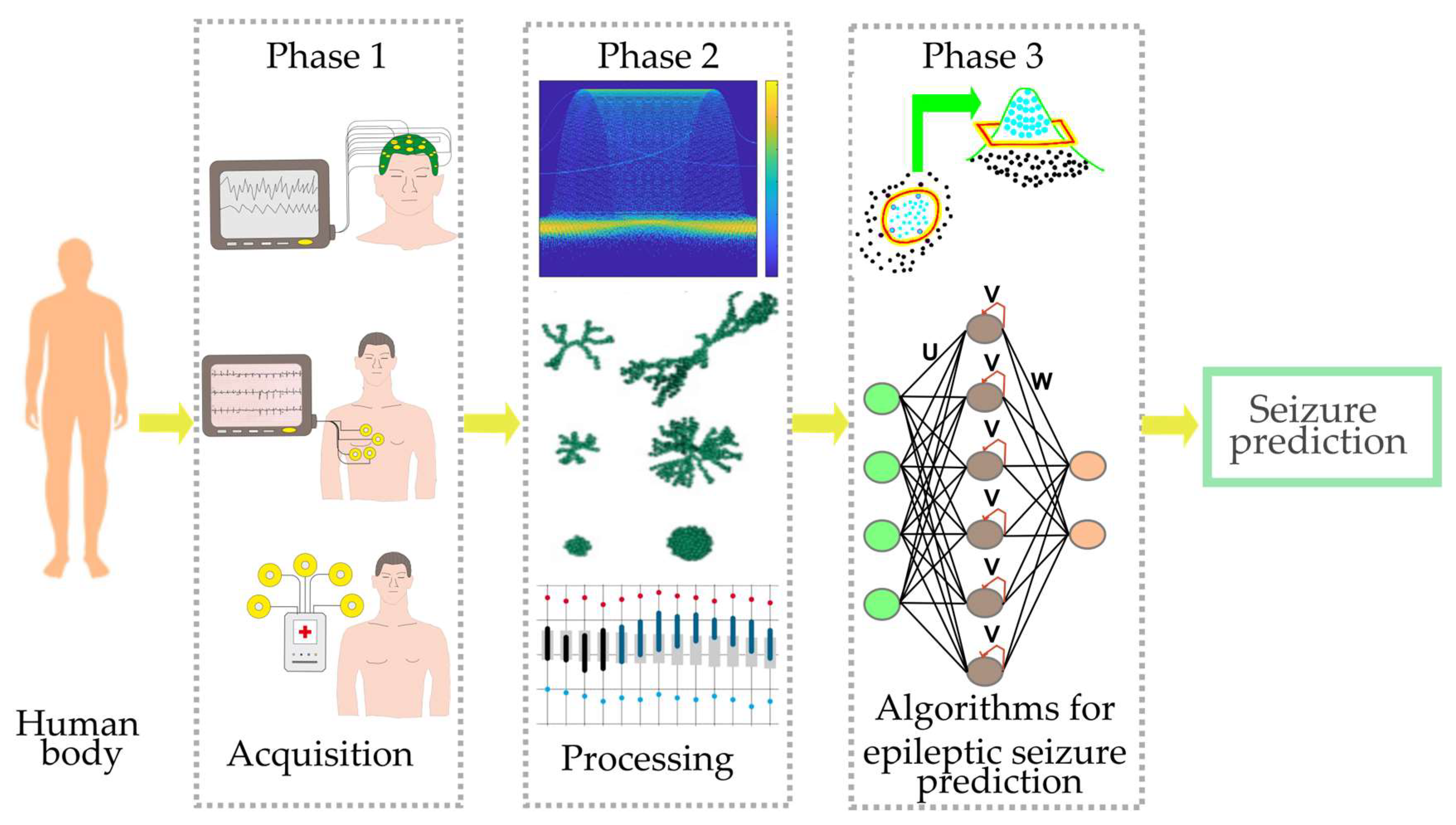
- Phase 1, signal acquisition. This initial phase is detailed by first outlining the bioelectrical signals commonly used for seizure prediction. This is followed by a discussion of some available datasets. Finally, a brief discussion of devices developed for seizure prediction is presented.
- Phase 2, signal processing. This phase covers the core components of processing acquired signals, specifically feature extraction techniques and feature selection methods, which are critical for optimizing classifier performance.
- Phase 3, classification. This final phase presents a concise discussion of ML and DL algorithms applied to seizure prediction, including a comparative analysis of their respective advantages and disadvantages.
2.1. Phase 1, Signal Acquisition
2.1.1. Bioelectrical Signals Commonly Used for Seizure Prediction
2.1.2. Available Datasets
2.1.3. Devices for Epileptic Seizure Prediction
2.2. Phase 2, Signal Processing
2.2.1. Features Extraction Techniques
- (a)
- TD features are known for having the lowest computational burden since they do not require any transformation domain signal transformation [49]. TD techniques can capture temporal patterns and signal variations by analyzing waveforms over specific time intervals. The most employed techniques include (1) statistical methods (e.g., variance, mean, standard deviation, among others), (2) energy-related features, (3) the number of zero-crossings, (4) histogram analysis, (5) the number of slope sign changes, and (6) waveform patterns [49,50]. It should be noted that these techniques reveal the internal dynamics of the signal over time [50]. TD features help distinguish between preictal and interictal states to identify seizure-related features [51,52].
- (b)
- FD features are obtained from the signal in the frequency domain, which is generated by applying the Fourier Transform (FT), or one of its variants, to the signal in the time domain [53]. Once the transformation is carried out, spectral power analysis is performed to extract features, providing information about the signal’s energy distribution and power characteristics. FD techniques are known for enhancing predictive capabilities by assessing a broader range of signal properties, consequently raising the reliability and accuracy rates of epilepsy prediction in bioelectrical signals [52,54].
- (c)
- TFD algorithms allow for the analysis of physiological signals that exhibit both time-variant and transient characteristics [52], as their mathematical framework is well-suited for such applications [55]. In general, TFD algorithms enable the following: (1) a comprehensive analysis of dynamic behavior over time and across different frequency bands, (2) improved time resolution for detecting suspicious activities, and (3) effective analysis of transient events or changes in signal dynamics occurring over short time intervals; features are typically obtained using techniques that represent time and frequency simultaneously, such as the Wavelet Transform and its variants, the Short-Time Fourier Transform (STFT), and empirical mode decomposition along with its different versions. These algorithms have been used to develop methods capable of predicting epileptic events based on bioelectrical signals with reasonable accuracy [55,56,57,58].
- (d)
- NF were applied to signals that exhibit a nonlinear and chaotic nature, characterized by patterns that repeat across different scales [59,60]. To capture these complex dynamics and patterns, it is necessary to employ algorithms specifically designed for this purpose [60]. The most commonly used NF techniques include Lyapunov exponents and fractal estimation algorithms, such as Higuchi’s method, Box dimension, and detrended fluctuation analysis [60]. These techniques provide a more comprehensive and detailed analysis of physiological signals compared to linear methods, enabling the development of more effective classification schemes [61].
- (e)
- HOSF analysis is a nonlinear method that can handle higher-order data and provide comprehensive signal characterization, as it preserves both the phase and magnitude of the frequency components [62]. Moreover, this technique generates smoother spectral lines, allowing it to be effectively applied to weak and noisy signals [5]. These techniques have been employed in sEEG signals to predict an epileptic seizure [5,63]. In particular, HOSFs offer a robust framework for analyzing complex and nonlinear bio signals, allowing meaningful characteristics from signals such as sEEG, iEEG, ECG, EMG, and general bio signals to be extracted. These characteristics provide deeper insights into physiological processes and the high-order spectral features in extracting valuable information from bio signals that traditional methods (e.g., FT, statistical features, cross-correlation, among others) may not capture [64].
2.2.2. Feature Selection
2.3. Phase 3, Classification
- Supervised algorithms: These require labeled data, which are employed during the training and validation stages to develop the classification strategy.
- Unsupervised algorithms: These do not require labeled data; instead, the algorithm clusters data with similar features during its training stage.
- Data required for the training stage: DL techniques usually require more data for training than ML methods due to the supervised training algorithms typically employed.
- Computational load: ML techniques generally require fewer computational resources compared to DL techniques. Therefore, when the computational load becomes a critical factor in algorithm selection, a good balance between computational demand and resulting accuracy should be achieved.
- Training time: DL algorithms usually require more training time since they process a large amount of data to achieve optimal results. Conversely, ML algorithms can be trained in less time. However, the selection criteria often depend on the presence of noise in the data.
2.3.1. ML-Based Algorithms for Epileptic Seizure Prediction
- (a)
- The Support Vector Machine (SVM) algorithm is a well-known classification strategy that aims to separate two different classes using hyperplanes. During the training stage, the algorithm determines the parameters of two hyperplanes that maximize the separation between the classes, typically represented as a linear boundary [72,73]. However, when the data cannot be linearly separated, the algorithm applies a kernel function to map the data into a higher-dimensional space where linear separation becomes feasible. For this purpose, radial basis function, polynomial, and linear kernels are commonly employed [74].
- (b)
- The K-nearest Neighbors (KNN) algorithm has been extensively utilized in numerous studies by researchers around the world to predict epileptic seizures [75,76,77]. This algorithm is a simple yet effective ML classifier that relies on recent training examples. KNN assigns multiclass labels based on two factors: the number of nearest neighbors (K) to the data point being classified, and the selection of K [78,79]. By calculating the distance between the new data vector and all existing vectors, the model is approximated [80].
- (c)
- Decision Tree (DT) is an effective classifier that provides a straightforward and adaptable implementation, as it can be programmed using a series of if-else rules [81]. Reasonable accuracy can be achieved if the feature sets do not exhibit a significant degree of overlap [82,83]. These tests are repeated until a terminal node (leaf node) is reached [82]. Once a leaf node is reached, the tree predicts the associated outcome, completing the classification. In other words, the classifier operates by taking an object described by a set of properties as input, which are used to build a classification tree model, where decisions at each stage are determined by previous branching operations.
Advantages and Disadvantages of the ML Classifiers
2.3.2. DL-Based Algorithms for Epileptic Seizure Prediction
- (a)
- Convolutional Neural Networks (CNNs) are bioinspired algorithms capable of extracting relevant features without requiring human assistance [86,88]. A CNN classifier can be developed as follows: (1) the selection and number of convolutional layers must be determined, as they set the dimensionality of the input layers; next, (2) the selection of kernel size, number of filters, stride, padding, and the number of pooling layers must be made to reduce dimensionality and computational complexity while retaining essential features; after that, (3) it is necessary to define the activation functions, and (4) the fully connected layers that define the classifier output [70,86,88]. It should be pointed out that the selection of the filters used in the convolutional layers determines the classifier’s accuracy [86]; hence, they must be carefully determined.
- (b)
- Recurrent Neural Networks (RNNs) are characterized by being a type of neural network that is well-suited for analyzing data with temporal patterns [89]. The stages of this classifier are (1) the input layer, whose size is determined by the time-series sequences, (2) the number and size of the recurrent layers, which define the classifier’s ability to capture the temporal dependencies of the training data, (3) the use of dropout layers between recurrent layers to prevent overfitting, and (4) a fully connected layer added to obtain the classification result. An important aspect to highlight is that the selection of the number of hidden layers, neurons, and activation functions in these layers plays a crucial role in determining classification accuracy [90,91].
- (c)
- Transformer-based methods (TBMs) models combine the strengths of recurrent architecture with attention mechanisms to enhance the specificity and sensitivity of the resulting models. One key advantage of TBMs is their capability to perform parallelization during training, which makes the process faster and more efficient. Additionally, TBMs mitigate the vanishing gradient problem, resulting in easier training and the development of classifiers with a higher resistance to uncertainty [92]. The process begins by passing the inputs through a positional encoding layer, which includes a multi-head self-attention mechanism. Then, in step two, a dropout and normalization layer are applied to enhance generalization capabilities. Step three involves a fully connected feed-forward network, followed by step four, where a decoder layer, similar in structure to the encoder, incorporates a multi-head attention mechanism that attends to the encoder output, ensuring that the result depends only on the known outputs [93,94]. Various TBM variants have emerged, including encoder-only transformers, bidirectional encoder representations from transformers, decoder-only transformers, star-transformers, BigBird, and generative pre-trained transformers, among others.
Advantages and Disadvantages of the DL Algorithms
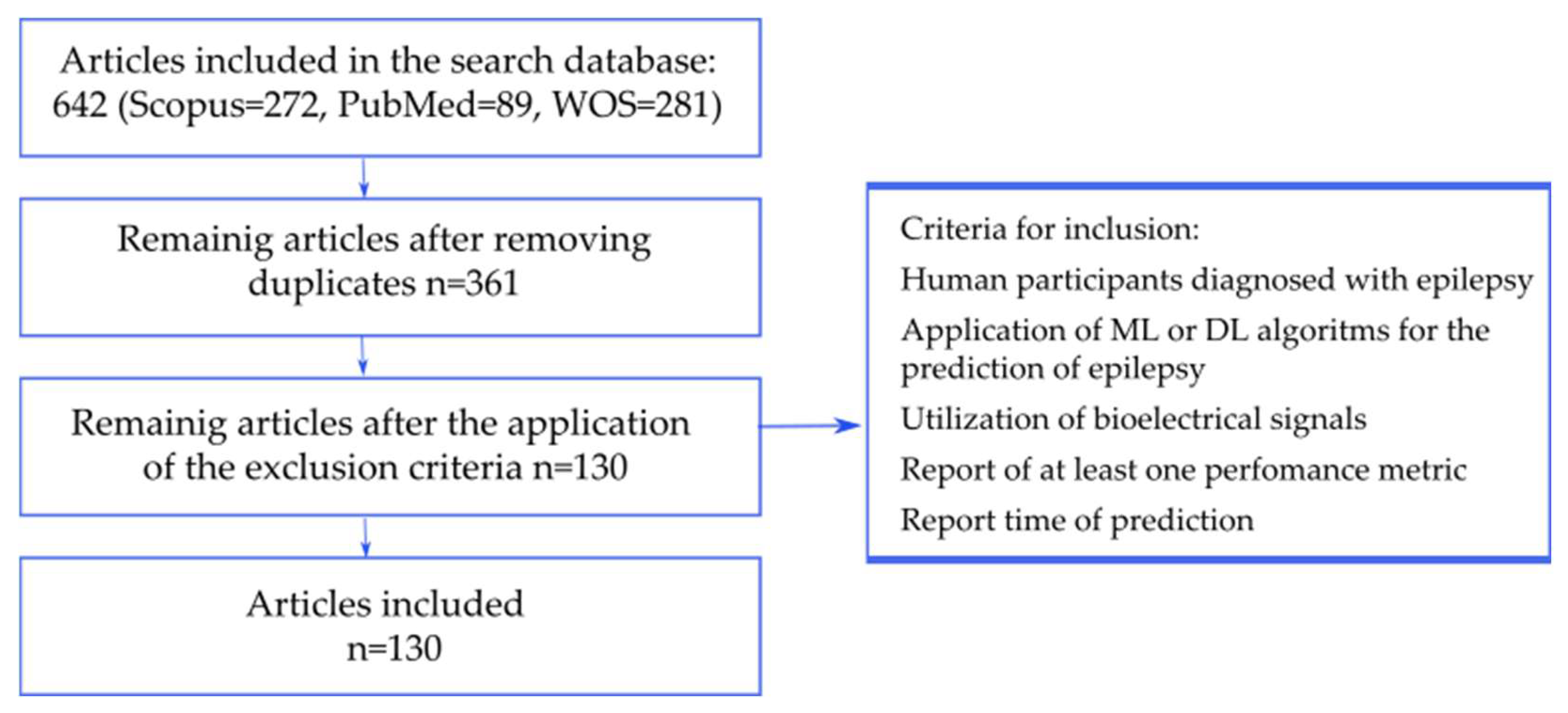
3. Review Methodology
- Population: Human participants diagnosed with epilepsy.
- Objective: Application of ML or DL algorithms for the prediction of epileptic seizures (not limited to ictal detection/classification).
- Data: Utilization of bioelectrical signals (e.g., iEEG, sEEG, ECG, PPG, or multimodal approaches).
- Outcomes: Reporting of at least one performance metric (e.g., accuracy, sensitivity, specificity, AUC) and the time of prediction (seizure prediction horizon (SPH)).
4. ML and DL in Epilepsy Seizure Prediction
4.1. ML in Epilepsy Seizure Prediction
4.1.1. SVM
4.1.2. K-Nearest Neighbors (KNN)
4.1.3. Decision Tree (DT)
4.2. Epileptic Seizure Prediction Algorithms Using DL Methods
4.2.1. CNNs
4.2.2. Recurrent Neural Networks (RNNs)
4.2.3. Transformer-Based Methods (TBMs)
5. Future Perspectives
6. Conclusions
6.1. Advantages
- The observed trend is for models to learn nonlinear and multiscale structures directly from signals, which reduces the reliance on manually created features and improves discriminative performance.
- The architectures discussed in this review jointly capture spatial/spectral structure and temporal dependencies, improving the detection of subtle preictal patterns.
- It is observed that integrating sEEG/iEEG with other physiological signals (e.g., ECG, PPG) improves the robustness and validity of the application in real-world conditions.
6.2. Opportunity Areas
- Future studies could more systematically characterize out-of-distribution performance by prioritizing patient-independent, multi-site evaluations.
- The seizure prediction studies would benefit from a core, consistently reported metric set so that results can be compared fairly across studies and settings.
- To strengthen the methodological rigor of the studies centered in seizure epilepsy prediction, authors that reported works in this area might consider subject-disjoint splits, nested model selection, and clear documentation of preprocessing and hyperparameter search, together with an explicit description of the techniques used to mitigate overfitting.
- Providing learning curves, variance across repeated-run studies would help disentangle architectural contributions from data-centric effects and clarify how models perform in the area.
- Routine disclosure of computing budgets, memory, and training/inference time would enable more transparent and equitable comparisons among methods and facilitate deployment planning.
- Due to clinical utility hinges on timely warnings, it would be helpful for all studies with “seizure prediction” in their title to consistently report the SPH alongside performance metrics to avoid mixing with works that realized classification or detection of epileptic seizure models, increasingly learn nonlinear, multiscale representations directly from bio signals, and jointly model spatial/spectral and temporal structure, yielding stronger discrimination and better detection of subtle preictal patterns.
- Multimodal integration combining sEEG/iEEG with physiological streams (e.g., ECG, PPG) enhances robustness and ecological validity, supporting performance in real-world conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Accelerometry |
| AI | Artificial Intelligence |
| asEMG | Arm Surface Electromyography |
| AUC | Area Under the Curve |
| beEEG | Behind-the-Ear EEG |
| BiLSTM | Bidirectional Long Short-Term Memory |
| CHB-MIT | Children’s Hospital Boston–Massachusetts Institute of Technology |
| CNN | Convolutional Neural Network |
| DL | Deep Learning |
| DFN | Deep Feedforward Network |
| DT | Decision Tree |
| DWT | Discrete Wavelet Transform |
| EDA | Electrodermal Activity |
| ECG | Electrocardiogram |
| EEG | Electroencephalography |
| FD | Frequency domain |
| FPR | False-Positive Rate |
| FT | Fourier Transform |
| GDP | Gross Domestic Product |
| HOSF | High-Order Spectral Features |
| HRV | Heart Rate Variability |
| iEEG | Intracranial Electroencephalogram |
| KAES | Kaggle American Epilepsy Society |
| KNN | K-Nearest Neighbors |
| LSTM | Long Short-Term Memory |
| ML | Machine Learning |
| NB | Naïve Bayes |
| NF | Nonlinear features |
| SPH | Seizure Prediction Horizon |
| PPG | Photoplethysmography |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RF | Random Forest |
| RNN | Recurrent Neural Network |
| sEEG | Scalp Electroencephalogram |
| SpO2 | Oxygen Saturation |
| STFT | Short-Time Fourier Transform |
| SNUH | Seoul National University Hospital |
| SVM | Support Vector Machine |
| TBM | Transformer-Based Methods |
| TD | Time Domain |
| TFD | Time-Frequency Domain |
| TUH | Temple University Hospital |
References
- Shoeb, A. Application of Machine Learning to Epileptic Seizure Onset Detection and Treatment. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2009. [Google Scholar]
- Siuly, S.; Li, Y. Discriminating the Brain Activities for Brain-Computer Interface Applications Through the Optimal Allocation-Based Approach. Neural Comput. Appl. 2015, 26, 799–811. [Google Scholar] [CrossRef]
- Truccolo, W.; Donoghue, J.A.; Hochberg, L.R.; Eskandar, E.N.; Madsen, J.R.; Anderson, W.S.; Brown, E.N.; Halgren, E.; Cash, S.S. Single-Neuron Dynamics in Human Focal Epilepsy. Nat. Neurosci. 2011, 14, 635–643. [Google Scholar] [CrossRef]
- Trinka, E.; Rainer, L.J.; Granbichler, C.A.; Zimmermann, G.; Leitinger, M. Mortality, and Life Expectancy in Epilepsy and Status Epilepticus—Current Trends and Future Aspects. Front. Epidemiol. 2023, 3, 1081757. [Google Scholar] [CrossRef]
- Mahmoodian, N.; Haddadnia, J.; Illanes, A.; Boese, A.; Friebe, M. Seizure Prediction with Cross-Higher-Order Spectral Analysis of EEG Signals. Signal Image Video Process 2020, 14, 821–828. [Google Scholar] [CrossRef]
- Riccio, C.; Martone, A.; Zazzaro, G.; Pavone, L. Training Datasets for Epilepsy Analysis: Preprocessing and Feature Extraction from Electroencephalography Time Series. Data 2024, 9, 61. [Google Scholar] [CrossRef]
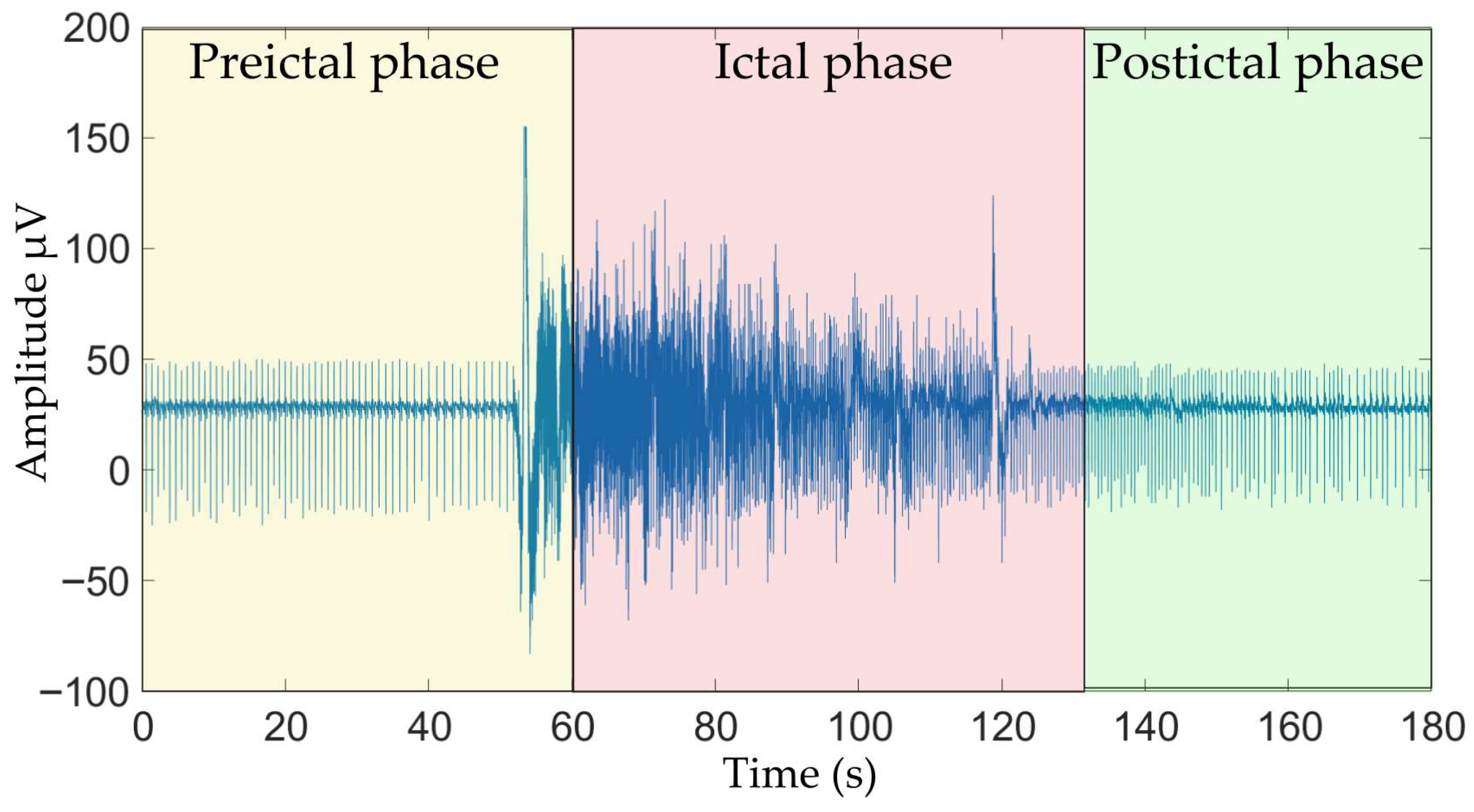
- Fisher, R.S.; Scharfman, H.E.; de Curtis, M. How Can We Identify Ictal and Interictal Abnormal Activity? In Issues in Clinical Epileptology: A View from the Bench; Springer: Dordrecht, The Netherlands, 2014; pp. 3–23. [Google Scholar]
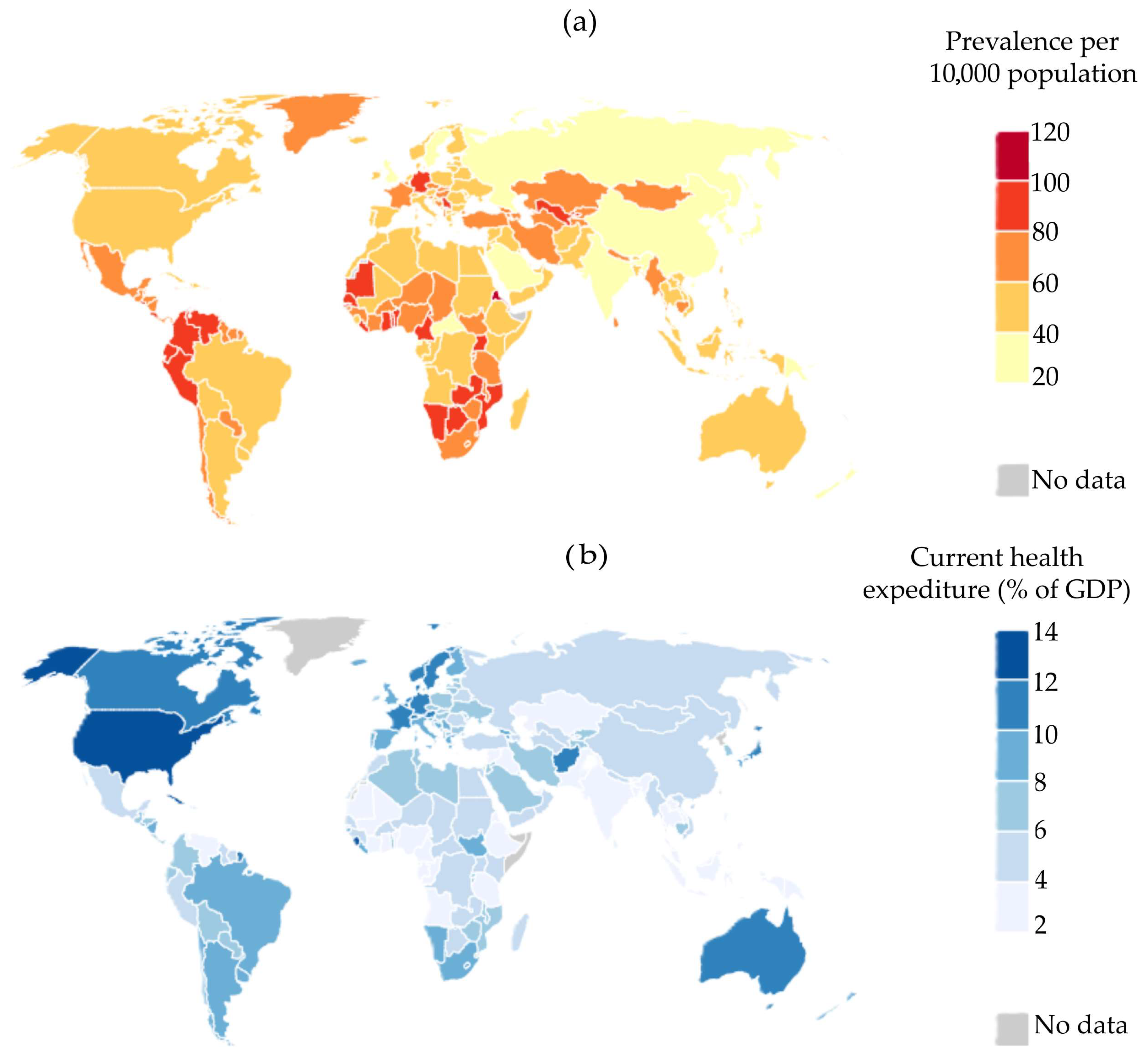
- Espinosa-Jovel, C.; Toledano, R.; Aledo-Serrano, Á.; García-Morales, I.; Gil-Nagel, A. Epidemiological Profile of Epilepsy in Low Income Populations. Seizure 2018, 56, 67–72. [Google Scholar] [CrossRef]
- Muhigwa, A.; Preux, P.M.; Gérard, D.; Marin, B.; Boumediène, F.; Ntamwira, C.; Tsai, C.H. Comorbidities of Epilepsy in Low and Middle-Income Countries: Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 9015. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Giussani, G.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Adib, M.G.; Agrawal, S.; Alahdab, F.; Awasthi, A.; et al. Global, Regional, and National Burden of Epilepsy, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 357–375. [Google Scholar] [CrossRef] [PubMed]
- World Bank. Population. Available online: https://databank.worldbank.org/reports.aspx?source=2&series=SP.POP.TOTL&country= (accessed on 8 June 2024).
- World Bank Current Health Expenditure (% of GDP). Available online: https://databank.worldbank.org/reports.aspx?source=2&series=SH.XPD.CHEX.GD.ZS&country= (accessed on 8 June 2024).
- Purnima, P.S.; Suresh, M. Machine Learning Models for Epileptic Seizure Prediction. In Proceedings of the 2023 International Conference on Inventive Computation Technologies (ICICT), Lalitpur, Nepal, 26–28 April 2023; pp. 135–141. [Google Scholar]
- Charsouei, S. Investigating Non-Pharmacological Treatments for Psychological Problems Associated with Epilepsy: A Narrative Review. Health Technol. Assess. Action 2021, 5, 1–12. [Google Scholar] [CrossRef]
- Jane, M.; Catharyn, T.L.; Andrea, M.S.; Larisa, M.S. Quality of Life and Community Resources. In Epilepsy Across the Spectrum: Promoting Health and Understanding; National Academies Press (US): Washington, DC, USA, 2012; p. 568. ISBN 978-0-309-25507-3. [Google Scholar]
- Ripatti, L.; Puustinen, L.; Rautava, P.; Koivisto, M.; Haataja, L. Impact of Epilepsy on the Risk of Hospital-Treated Injuries in Finnish Children. Epilepsy Behav. Rep. 2023, 21, 100587. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Lehnertz, K.; Richardson, M.P.; Schelter, B.; Zaveri, H.P. Seizure Prediction—Ready for a New Era. Nat. Rev. Neurol. 2018, 14, 618–630. [Google Scholar] [CrossRef]
- Martinek, R.; Ladrova, M.; Sidikova, M.; Jaros, R.; Behbehani, K.; Kahankova, R.; Kawala-Sterniuk, A. Advanced Bioelectrical Signal Processing Methods: Past, Present and Future Approach—Part I: Cardiac Signals. Sensors 2021, 21, 5186. [Google Scholar] [CrossRef]
- Blinowska, K.; Durka, P. Electroencephalography (EEG). In Wiley Encyclopedia of Biomedical Engineering; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Parvizi, J.; Kastner, S. Promises and Limitations of Human Intracranial Electroencephalography. Nat. Neurosci. 2018, 21, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Shah, S.U.; Villa-Lopez, E.; Murillo, M.; Arenas, N.; Oshima, K.; Chang, R.-K.; Lauzon, M.; Guo, X.; Pillutla, P. Comparison of Electrocardiogram Quality and Clinical Interpretations Using Prepositioned ECG Electrodes and Conventional Individual Electrodes. J. Electrocardiol. 2020, 59, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Leutmezer, F.; Schernthaner, C.; Lurger, S.; Pötzelberger, K.; Baumgartner, C. Electrocardiographic Changes at the Onset of Epileptic Seizures. Epilepsia 2003, 44, 348–354. [Google Scholar] [CrossRef]
- Bhagubai, M.; Vandecasteele, K.; Swinnen, L.; Macea, J.; Chatzichristos, C.; De Vos, M.; Van Paesschen, W. The Power of ECG in Semi-Automated Seizure Detection in Addition to Two-Channel behind-the-Ear EEG. Bioengineering 2023, 10, 491. [Google Scholar] [CrossRef]
- Zambrana-Vinaroz, D.; Vicente-Samper, J.M.; Manrique-Cordoba, J.; Sabater-Navarro, J.M. Wearable Epileptic Seizure Prediction System Based on Machine Learning Techniques Using ECG, PPG and EEG Signals. Sensors 2022, 22, 9372. [Google Scholar] [CrossRef]
- Amengual-Gual, M.; Ulate-Campos, A.; Loddenkemper, T. Status Epilepticus Prevention, Ambulatory Monitoring, Early Seizure Detection and Prediction in at-Risk Patients. Seizure 2019, 68, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mason, F.; Scarabello, A.; Taruffi, L.; Pasini, E.; Calandra-Buonaura, G.; Vignatelli, L.; Bisulli, F. Heart Rate Variability as a Tool for Seizure Prediction: A Scoping Review. J. Clin. Med. 2024, 13, 747. [Google Scholar] [CrossRef]
- Djemal, A.; Bouchaala, D.; Fakhfakh, A.; Kanoun, O. Epileptic Seizure Motion Classification Based on SEMG and Artificial Neural Network. In Proceedings of the 2021 International Workshop on Impedance Spectroscopy (IWIS), Online, 29 September–1 October 2021; pp. 141–145. [Google Scholar]
- Eltrass, A.S.; Ghanem, N.H. A New Automated Multi-Stage System of Non-Local Means and Multi-Kernel Adaptive Filtering Techniques for EEG Noise and Artifacts Suppression. J. Neural Eng. 2021, 18, 36023. [Google Scholar] [CrossRef]
- Karoly, P.; Cook, M.; Kuhlmann, L.; Freestone, D.; Grayden, D.; Nurse, E.; Lai, A.; Payne, D.; D’souza, W.; Seneviratne, U.; et al. Melbourne NeuroVista Seizure Prediction Trial. Univ. Melb. Dataset 2018. [Google Scholar] [CrossRef]
- Kuhlmann, L.; Karoly, P.; Freestone, D.R.; Brinkmann, B.H.; Temko, A.; Barachant, A.; Li, F.; Titericz, G.J.; Lang, B.W.; Lavery, D.; et al. Epilepsyecosystem.Org: Crowd-Sourcing Reproducible Seizure Prediction with Long-Term Human Intracranial EEG. Brain 2018, 141, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Ihle, M.; Feldwisch-Drentrup, H.; Teixeira, C.A.; Witon, A.; Schelter, B.; Timmer, J.; Schulze-Bonhage, A. EPILEPSIAE—A European Epilepsy Database. Comput. Methods Programs Biomed. 2012, 106, 127–138. [Google Scholar] [CrossRef]
- Andrzejak, R.G.; Schindler, K.; Rummel, C. Nonrandomness, Nonlinear Dependence, and Nonstationarity of Electroencephalographic Recordings from Epilepsy Patients. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2012, 86, 046206. [Google Scholar] [CrossRef]
- Swami, P.; Gandhi, T.; Panigrahi, B.K.; Tripathi, M.; Anand, S. A novel robust diagnostic model to detect seizures in electroencephalography. Expert Syst. Appl. 2016, 56, 116–130. [Google Scholar] [CrossRef]
- Shah, V.; von Weltin, E.; Lopez, S.; McHugh, J.R.; Veloso, L.; Golmohammadi, M.; Obeid, I.; Picone, J. The Temple University Hospital Seizure Detection Corpus. Front. Neuroinform. 2018, 12, 83. [Google Scholar] [CrossRef]
- Stevenson, N.; Tapani, K.; Lauronen, L.; Vanhatalo, S. A Dataset of Neonatal EEG Recordings with Seizures Annotations. Sci. Data 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Detti, P.; Vatti, G.; de Lara, G. EEG Synchronization Analysis for Seizure Prediction: A Study on Data of Noninvasive Recordings. Processes 2020, 8, 846. [Google Scholar] [CrossRef]
- Al-Aweel, I.C.; Krishnamurthy, K.B.; Hausdorff, J.M.; Mietus, J.E.; Ives, J.R.; Blum, A.S.; Schomer, D.L.; Goldberger, A.L. Postictal Heart Rate Oscillations in Partial Epilepsy. Neurology 1999, 53, 1590–1592. [Google Scholar] [CrossRef]
- Chatzichristos, C.; Claro Bhagubai, M. SeizeIT1, KU Leuven RDR: Lovaina, Bélgica, 2023. Available online: https://rdr.kuleuven.be/dataset.xhtml?persistentId=doi:10.48804/P5Q0OJ (accessed on 30 July 2024).
- Ryvlin, P.; Alonso, D.A.; Benini, L.; Frossard, P. PEDESITE: Personalized Detection of Epileptic Seizure in the Internet of Things (IoT) Era. Available online: https://data.snf.ch/grants/grant/193813 (accessed on 30 July 2024).
- Empatica Embrace Store|Embrace2|Seizure & Epilepsy Watch|Empatica. Available online: https://www.empatica.com/store/embrace2/ (accessed on 31 July 2024).
- Epihunter, N.V. Professionals—Epihunter. Available online: https://www.epihunter.com/professionals (accessed on 31 July 2024).
- Byteflies Neurology. Available online: https://byteflies.com/neurology#OurService (accessed on 31 July 2024).
- Wu, A.; Liu, G. Continuous Ambulatory Epilepsy Detection System Incorporating Feature Engineering. Theor. Nat. Sci. 2024, 32, 93–101. [Google Scholar] [CrossRef]
- Xiong, W.; Stirling, R.E.; Payne, D.E.; Nurse, E.S.; Kameneva, T.; Cook, M.J.; Viana, P.F.; Richardson, M.P.; Brinkmann, B.H.; Freestone, D.R.; et al. Forecasting Seizure Likelihood from Cycles of Self-Reported Events and Heart Rate: A Prospective Pilot Study. EBioMedicine 2023, 93, 104656. [Google Scholar] [CrossRef] [PubMed]
- Stirling, R.E.; Grayden, D.B.; D’Souza, W.; Cook, M.J.; Nurse, E.; Freestone, D.R.; Payne, D.E.; Brinkmann, B.H.; Pal Attia, T.; Viana, P.F.; et al. Forecasting Seizure Likelihood with Wearable Technology. Front. Neurol. 2021, 12, 704060. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, M.; Pal Attia, T.; Joseph, B.; Gregg, N.M.; Nurse, E.S.; Viana, P.F.; Worrell, G.; Dümpelmann, M.; Richardson, M.P.; Freestone, D.R.; et al. Ambulatory Seizure Forecasting with a Wrist-Worn Device Using Long-Short Term Memory Deep Learning. Sci. Rep. 2021, 11, 21935. [Google Scholar] [CrossRef]
- Meisel, C.; El Atrache, R.; Jackson, M.; Schubach, S.; Ufongene, C.; Loddenkemper, T. Machine Learning from Wristband Sensor Data for Wearable, Noninvasive Seizure Forecasting. Epilepsia 2020, 61, 2653–2666. [Google Scholar] [CrossRef]
- Mishra, P.; Biancolillo, A.; Roger, J.M.; Marini, F.; Rutledge, D.N. New Data Preprocessing Trends Based on Ensemble of Multiple Preprocessing Techniques. TrAC Trends Anal. Chem. 2020, 132, 116045. [Google Scholar] [CrossRef]
- Krishnamurthi, R.; Gopinathan, D.; Kumar, A. Chapter 10—Using Wavelet Transformation for Acoustic Signal Processing in Heavy Vehicle Detection and Classification. In Autonomous and Connected Heavy Vehicle Technology; Krishnamurthi, R., Kumar, A., Gill, S.S., Eds.; Academic Press: New York, NY, USA, 2022; pp. 199–209. ISBN 978-0-323-90592-3. [Google Scholar]
- Sharmila, A.; Geethanjali, P. Evaluation of Time Domain Features on Detection of Epileptic Seizure from EEG Signals. Health Technol. 2020, 10, 711–722. [Google Scholar] [CrossRef]
- Dastgoshadeh, M.; Rabiei, Z. Detection of Epileptic Seizures through EEG Signals Using Entropy Features and Ensemble Learning. Front. Hum. Neurosci. 2023, 16, 1084061. [Google Scholar] [CrossRef]
- Ma, D.; Zheng, J.; Peng, L. Performance Evaluation of Epileptic Seizure Prediction Using Time, Frequency, and Time–Frequency Domain Measures. Processes 2021, 9, 682. [Google Scholar] [CrossRef]
- Acharya, U.R.; Hagiwara, Y.; Adeli, H. Automated Seizure Prediction. Epilepsy Behav. 2018, 88, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Yousif, M.A.A.; Öztürk, M. Epileptic Seizure Detection using Time-Domain and Wavelet-Domain Features. In Proceedings of the International Symposium of Scientific Research and Innovative Studies (ISSRIS’21), Bursa, Turkey, 31 January–3 February 2022; pp. 1–15. [Google Scholar]
- Tamanna, T.; Rahman, M.A.; Sultana, S.; Haque, M.H.; Parvez, M.Z. Predicting Seizure Onset Based on Time-Frequency Analysis of EEG Signals. Chaos Solitons Fractals 2021, 145, 110796. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Shu, M. Epileptic Seizure Prediction Based on Region Correlation of EEG Signal. In Proceedings of the 2020 IEEE 33rd International Symposium on Computer-Based Medical Systems (CBMS), Rochester, MN, USA, 28–30 July 2020; pp. 120–125. [Google Scholar]
- Hernández, D.E.; Trujillo, L.; Z-Flores, E.; Villanueva, O.M.; Romo-Fewell, O. Detecting Epilepsy in EEG Signals Using Time, Frequency and Time-Frequency Domain Features. In Computer Science and Engineering—Theory and Applications; Sanchez, M.A., Aguilar, L., Castañón-Puga, M., Rodríguez-Díaz, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 167–182. ISBN 978-3-319-74060-7. [Google Scholar]
- Malarvili, M.B.; Howe, T.A.; Ramanathan, S.; Alexie, M.; Singh, O.P. Chapter Five—Analysis of Capnogram Using Signal Processing Techniques. In Systems and Signal Processing of Capnography as a Diagnostic Tool for Asthma Assessment; Malarvili, M.B., Howe, T.A., Ramanathan, S., Alexie, M., Singh, O.P., Eds.; Academic Press: New York, NY, USA, 2023; pp. 101–129. ISBN 978-0-323-85747-5. [Google Scholar]
- Ribeiro, P.; Marques, J.A.L.; Pordeus, D.; Zacarias, L.; Leite, C.F.; Sobreira-Neto, M.A.; Peixoto, A.A.; de Oliveira, A.; do Vale Madeiro, J.P.; Rodrigues, P.M. Machine Learning-Based Cardiac Activity Non-Linear Analysis for Discriminating COVID-19 Patients with Different Degrees of Severity. Biomed. Signal Process. Control 2024, 87, 105558. [Google Scholar] [CrossRef]
- Shukla, P.K.; Maheshwary, P.; Kundu, S.; Mondal, D.; Kumar, A.; Joshi, S.; Pareek, P.K. Analyzing Physical Activity Impact Based on Ubiquitous Nonlinear Dynamics and Electroencephalography Data. Technol. Health Care 2024, 32, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Lehnertz, K. Epilepsy and Nonlinear Dynamics. J. Biol. Phys. 2008, 34, 253–266. [Google Scholar] [CrossRef]
- Chaddad, A.; Wu, Y.; Kateb, R.; Bouridane, A. Electroencephalography Signal Processing: A Comprehensive Review and Analysis of Methods and Techniques. Sensors 2023, 23, 6434. [Google Scholar] [CrossRef] [PubMed]
- Bou Assi, E.; Gagliano, L.; Rihana, S.; Nguyen, D.K.; Sawan, M. Bispectrum Features and Multilayer Perceptron Classifier to Enhance Seizure Prediction. Sci. Rep. 2018, 8, 15491. [Google Scholar] [CrossRef]
- Chua, K.C.; Chandran, V.; Acharya, U.R.; Lim, C.M. Application of Higher Order Statistics/Spectra in Biomedical Signals—A Review. Med. Eng. Phys. 2010, 32, 679–689. [Google Scholar] [CrossRef]
- Omar, A.; Abd El-Hafeez, T. Optimizing Epileptic Seizure Recognition Performance with Feature Scaling and Dropout Layers. Neural Comput. Appl. 2024, 36, 2835–2852. [Google Scholar] [CrossRef]
- Dash, D.P.; Kolekar, M.; Chakraborty, C.; Khosravi, M.R. Review of Machine and Deep Learning Techniques in Epileptic Seizure Detection Using Physiological Signals and Sentiment Analysis. ACM Trans. Asian Low-Resour. Lang. Inf. Process. 2024, 23, 1–29. [Google Scholar] [CrossRef]
- Gulati, V.; Raheja, N. PCSVD: A Hybrid Feature Extraction Technique Based on Principal Component Analysis and Singular Value Decomposition. J. Auton. Intell. 2023, 5, 1–14. [Google Scholar] [CrossRef]
- Artoni, F.; Delorme, A.; Makeig, S. Applying Dimension Reduction to EEG Data by Principal Component Analysis Reduces the Quality of Its Subsequent Independent Component Decomposition. Neuroimage 2018, 175, 176–187. [Google Scholar] [CrossRef]
- Singh, N.; Jain, M.; Kamal, M.M.; Bodhi, R.; Gupta, B. Technological Paradoxes and Artificial Intelligence Implementation in Healthcare. An Application of Paradox Theory. Technol. Forecast. Soc. Chang. 2024, 198, 122967. [Google Scholar] [CrossRef]
- Taye, M.M. Understanding of Machine Learning with Deep Learning: Architectures, Workflow, Applications and Future Directions. Computers 2023, 12, 91. [Google Scholar] [CrossRef]
- Cascarano, A.; Mur-Petit, J.; Hernández-González, J.; Camacho, M.; de Toro Eadie, N.; Gkontra, P.; Chadeau-Hyam, M.; Vitrià, J.; Lekadir, K. Machine and Deep Learning for Longitudinal Biomedical Data: A Review of Methods and Applications. Artif. Intell. Rev. 2023, 56, 1711–1771. [Google Scholar] [CrossRef]
- Moguerza, J.M.; Muñoz, A. Support Vector Machines with Applications. Stat. Sci. 2006, 21, 322–336. [Google Scholar] [CrossRef]
- Akhbardeh, F.; Vasefi, F.; MacKinnon, N.; Amini, M.; Akhbardeh, A.; Tavakolian, K. Classification and Assessment of Hand Arthritis Stage Using Support Vector Machine. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; Volume 2019, pp. 4080–4083. [Google Scholar] [CrossRef]
- Huang, J.S.; Chen, B.Q.; Zeng, N.Y.; Cao, X.C.; Li, Y. Accurate Classification of ECG Arrhythmia Using MOWPT Enhanced Fast Compression Deep Learning Networks. J. Ambient. Intell. Humaniz. Comput. 2023, 14, 5703–5720. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, J.; Hu, W.; Kummert, A. Epileptic Signal Classification with Deep EEG Features by Stacked CNNs. IEEE Trans. Cogn. Dev. Syst. 2020, 12, 709–722. [Google Scholar] [CrossRef]
- Truong, N.D.; Nguyen, A.D.; Kuhlmann, L.; Bonyadi, M.R.; Yang, J.; Ippolito, S.; Kavehei, O. Convolutional Neural Networks for Seizure Prediction Using Intracranial and Scalp Electroencephalogram. Neural Netw. 2018, 105, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, S.; Jafarnia, N.; Motie, A.; Dourado, A. Prediction of Epileptic Seizures Based on Heart Rate Variability. Technol. Health Care 2016, 24, 795–810. [Google Scholar] [CrossRef]
- Jayalalitha, S.; Sussan, D.; Kumari, S.; Archana, B. K-Nearest Neighbour Method of Analysing the ECG Signal (To Find out the Different Disordrs Related to Heart). J. Appl. Sci. 2014, 14, 1628–1632. [Google Scholar] [CrossRef][Green Version]
- Araújo, T.; Nunes, N.; Gamboa, H.; Fred, A. Generic Biometry Algorithm Based on Signal Morphology Information: Application in the Electrocardiogram Signal. Pattern Recognit. Appl. Methods 2015, 318, 301–310. [Google Scholar] [CrossRef]
- Shang, P.; Li, X.; Kamae, S. Chaotic Analysis of Traffic Time Series. Chaos Solitons Fractals 2005, 25, 121–128. [Google Scholar] [CrossRef]
- Ardhapure, O.; Patil, G.; Udan, D.; Jetha, K. Comparative study of classification algorithm for text based categorization. Int. J. Res. Eng. Technol. 2016, 05, 217–220. [Google Scholar] [CrossRef]
- Shobha, G.; Rangaswamy, S. Machine Learning. Handb. Stat. 2018, 38, 197–228. [Google Scholar] [CrossRef]
- Jukic, S.; Saracevic, M.; Subasi, A.; Kevric, J. Comparison of Ensemble Machine Learning Methods for Automated Classification of Focal and Non-Focal Epileptic EEG Signals. Mathematics 2020, 8, 1481. [Google Scholar] [CrossRef]
- Pichler, M.; Hartig, F. Machine Learning and Deep Learning—A Review for Ecologists. Methods Ecol. Evol. 2023, 14, 994–1016. [Google Scholar] [CrossRef]
- Liu, H.; Lang, B. Machine Learning and Deep Learning Methods for Intrusion Detection Systems: A Survey. Appl. Sci. 2019, 9, 4396. [Google Scholar] [CrossRef]
- Sarker, I.H. Deep Learning: A Comprehensive Overview on Techniques, Taxonomy, Applications and Research Directions. SN Comput. Sci. 2021, 2, 420. [Google Scholar] [CrossRef] [PubMed]
- Caballé, N.C.; Castillo-Sequera, J.L.; Gómez-Pulido, J.A.; Gómez-Pulido, J.M.; Polo-Luque, M.L. Machine Learning Applied to Diagnosis of Human Diseases: A Systematic Review. Appl. Sci. 2020, 10, 5135. [Google Scholar] [CrossRef]
- Yao, G.; Lei, T.; Zhong, J. A Review of Convolutional-Neural-Network-Based Action Recognition. Pattern Recognit. Lett. 2019, 118, 14–22. [Google Scholar] [CrossRef]
- Ahmedt-Aristizabal, D.; Armin, M.A.; Hayder, Z.; Garcia-Cairasco, N.; Petersson, L.; Fookes, C.; Denman, S.; McGonigal, A. Deep Learning Approaches for Seizure Video Analysis: A Review. Epilepsy Behav. 2024, 154, 109735. [Google Scholar] [CrossRef]
- Bernaś, M.; Płaczek, B.; Lewandowski, M. Ensemble of RNN Classifiers for Activity Detection Using a Smartphone and Supporting Nodes. Sensors 2022, 22, 9451. [Google Scholar] [CrossRef] [PubMed]
- Murad, A.; Pyun, J.Y. Deep Recurrent Neural Networks for Human Activity Recognition. Sensors 2017, 17, 2556. [Google Scholar] [CrossRef]
- Gillioz, A.; Casas, J.; Mugellini, E.; Khaled, O.A. Overview of the Transformer-Based Models for NLP Tasks. In Proceedings of the 2020 15th Conference on Computer Science and Information Systems (FedCSIS), Sofia, Bulgaria, 6–9 September 2020; pp. 179–183. [Google Scholar]
- Yousif, M.Z.; Zhang, M.; Yu, L.; Vinuesa, R.; Lim, H. A Transformer-Based Synthetic-Inflow Generator for Spatially Developing Turbulent Boundary Layers. J. Fluid Mech. 2023, 957, A6. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, Ł.; Polosukhin, I. Attention Is All You Need. Adv. Neural Inf. Process. Syst. 2017, 30. [Google Scholar] [CrossRef]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine Learning and Deep Learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Alkhrijah, Y.; Khalid, S.; Usman, S.M.; Jameel, A.; Zubair, M.; Aldossary, H.; Anwar, A.; Arif, S. Feature Fusion Ensemble Classification Approach for Epileptic Seizure Prediction Using Electroencephalographic Bio-Signals. Front. Med. 2025, 12, 1566870. [Google Scholar] [CrossRef] [PubMed]
- Kalousios, S.; Müller, J.; Yang, H.; Eberlein, M.; Uckermann, O.; Schackert, G.; Polanski, W.H.; Leonhardt, G. ECG-based Epileptic Seizure Prediction: Challenges of Current Data-driven Models. Epilepsia Open 2025, 10, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Karasmanoglou, A.; Giannakakis, G.; Vorgia, P.; Antonakakis, M.; Zervakis, M. Semi-Supervised Anomaly Detection for the Prediction and Detection of Pediatric Focal Epileptic Seizures on Fused EEG and ECG Data. Biomed. Signal Process. Control 2025, 101, 107083. [Google Scholar] [CrossRef]
- Saadoon, Y.A.; Khalil, M.; Battikh, D. Predicting Epileptic Seizures Using EfficientNet-B0 and SVMs: A Deep Learning Methodology for EEG Analysis. Bioengineering 2025, 12, 109. [Google Scholar] [CrossRef]
- Ding, T.Y.; Gagliano, L.; Jahani, A.; Toffa, D.H.; Nguyen, D.K.; Bou Assi, E. Epileptic Seizure Forecasting with Wearable-based Nocturnal Sleep Features. Epilepsia Open 2024, 9, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilpour, A.; Tabarestani, S.S.; Niazi, A. Deep Learning-based Seizure Prediction Using EEG Signals: A Comparative Analysis of Classification Methods on the CHB-MIT Dataset. Eng. Rep. 2024, 6, e12918. [Google Scholar] [CrossRef]
- Qureshi, M.M.; Kaleem, M. EEG-Based Seizure Prediction with Machine Learning. Signal Image Video Process 2023, 17, 1543–1554. [Google Scholar] [CrossRef]
- Karasmanoglou, A. ECG-Based Semi-Supervised Anomaly Detection for Early Detection and Monitoring of Epileptic Seizures. Int. J. Environ. Res. Public Health 2023, 20, 5000. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Malhotra, J. Two-Layer LSTM Network-Based Prediction of Epileptic Seizures Using EEG Spectral Features. Complex Intell. Syst. 2022, 8, 2405–2418. [Google Scholar] [CrossRef]
- Kill, J.B.; Ciarelli, P.M.; Côco, K.F. Analysis of EEG Microstates to Predict Epileptic Seizures in an Online Approach. Res. Biomed. Eng. 2022, 38, 409–421. [Google Scholar] [CrossRef]
- Muhammad Usman, S.; Khalid, S.; Bashir, S. A Deep Learning Based Ensemble Learning Method for Epileptic Seizure Prediction. Comput. Biol. Med. 2021, 136, 104710. [Google Scholar] [CrossRef]
- Savadkoohi, M.; Oladunni, T.; Thompson, L. A Machine Learning Approach to Epileptic Seizure Prediction Using Electroencephalogram (EEG) Signal. Biocybern. Biomed. Eng. 2020, 40, 1328–1341. [Google Scholar] [CrossRef]
- Guevara, E.; Flores-Castro, J.-A.; Peng, K.; Nguyen, D.K.; Lesage, F.; Pouliot, P.; Rosas-Romero, R. Prediction of Epileptic Seizures Using FNIRS and Machine Learning. J. Intell. Fuzzy Syst. 2020, 38, 2055–2068. [Google Scholar] [CrossRef]
- Albalawi, F.; Alshehri, S.; Chahid, A.; Laleg-Kirati, T.-M. Voxel Weight Matrix-Based Feature Extraction for Biomedical Applications. IEEE Access 2020, 8, 121451–121459. [Google Scholar] [CrossRef]
- Muhammad Usman, S.; Khalid, S.; Aslam, M.H. Epileptic Seizures Prediction Using Deep Learning Techniques. IEEE Access 2020, 8, 39998–40007. [Google Scholar] [CrossRef]
- Giannakakis, G.; Tsiknakis, M.; Vorgia, P. Focal Epileptic Seizures Anticipation Based on Patterns of Heart Rate Variability Parameters. Comput. Methods Programs Biomed. 2019, 178, 123–133. [Google Scholar] [CrossRef]
- Perez-Sanchez, A.V.; Valtierra-Rodriguez, M.; Perez-Ramirez, C.A.; De-Santiago-Perez, J.J.; Amezquita-Sanchez, J.P. Epileptic Seizure Prediction Using Wavelet Transform, Fractal Dimension, Support Vector Machine, and EEG Signals. Fractals 2022, 30, 2250154. [Google Scholar] [CrossRef]
- Altaf, Z.; Unar, M.A.; Narejo, S.; Zaki, M.A. Naseer-u-Din Generalized Epileptic Seizure Prediction Using Machine Learning Method. Int. J. Adv. Comput. Sci. Appl. 2023, 14, 502–510. [Google Scholar] [CrossRef]
- Perez-Sanchez, A.V.; Amezquita-Sanchez, J.P.; Valtierra-Rodriguez, M.; Adeli, H. A New Epileptic Seizure Prediction Model Based on Maximal Overlap Discrete Wavelet Packet Transform, Homogeneity Index, and Machine Learning Using ECG Signals. Biomed. Signal Process. Control 2024, 88, 105659. [Google Scholar] [CrossRef]
- Shaik Gadda, A.A.; Vedantham, D.; Thomas, J.; Rajamanickam, Y.; Menon, R.N.; Agastinose Ronickom, J.F. Optimization of Pre-Ictal Interval Time Period for Epileptic Seizure Prediction Using Temporal and Frequency Features. In Caring Is Sharing–Exploiting the Value in Data for Health and Innovation; IOS Press: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Jiang, X.; Liu, X.; Liu, Y.; Wang, Q.; Li, B.; Zhang, L. Epileptic Seizures Detection and the Analysis of Optimal Seizure Prediction Horizon Based on Frequency and Phase Analysis. Front. Neurosci. 2023, 17, 1191683. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Nagpal, B.; Jain, P.K.; Abraham, A.; Gabralla, L.A. Epileptic Seizure Prediction Based on Hybrid Seek Optimization Tuned Ensemble Classifier Using EEG Signals. Sensors 2023, 23, 423. [Google Scholar] [CrossRef]
- Budde, B.; Maksimenko, V.; Sarink, K.; Seidenbecher, T.; van Luijtelaar, G.; Hahn, T.; Pape, H.-C.; Lüttjohann, A. Seizure Prediction in Genetic Rat Models of Absence Epilepsy: Improved Performance through Multiple-Site Cortico-Thalamic Recordings Combined with Machine Learning. eNeuro 2022, 9, ENEURO.0160-21.2021. [Google Scholar] [CrossRef]
- Abbaszadeh, B.; Teixeira, C.A.D.; Yagoub, M.C.E. Online Seizure Prediction System: A Novel Probabilistic Approach for Efficient Prediction of Epileptic Seizure with IEEG Signal. Open Biomed. Eng. J. 2022, 16, e187412072208300. [Google Scholar] [CrossRef]
- Xu, X.; Lin, M.; Xu, T. Epilepsy Seizures Prediction Based on Nonlinear Features of EEG Signal and Gradient Boosting Decision Tree. Int. J. Environ. Res. Public Health 2022, 19, 11326. [Google Scholar] [CrossRef]
- Chen, H.-H.; Cherkassky, V. Performance Metrics for Online Seizure Prediction. Neural Netw. 2020, 128, 22–32. [Google Scholar] [CrossRef]
- Saboo, K.V.; Cao, Y.; Kremen, V.; Sladky, V.; Gregg, N.M.; Arnold, P.M.; Karoly, P.J.; Freestone, D.R.; Cook, M.J.; Worrell, G.A.; et al. Individualized Seizure Cluster Prediction Using Machine Learning and Chronic Ambulatory Intracranial EEG. IEEE Trans. Nanobiosci. 2023, 22, 818–827. [Google Scholar] [CrossRef]
- Coşgun, E.; Çelebi, A. FPGA Based Real-Time Epileptic Seizure Prediction System. Biocybern. Biomed. Eng. 2021, 41, 278–292. [Google Scholar] [CrossRef]
- Afrin, K.; Dusi, R.; Zhong, Y.; Reddy, D.S.; Bukkapatnam, S.T.S. Prognosis of Epileptic Seizure Event Onsets Using Random Survival Forests. IISE Trans. Healthc. Syst. Eng. 2022, 12, 221–231. [Google Scholar] [CrossRef]
- Foody, G.M. Challenges in the Real World Use of Classification Accuracy Metrics: From Recall and Precision to the Matthews Correlation Coefficient. PLoS ONE 2023, 18, e0291908. [Google Scholar] [CrossRef] [PubMed]
- Ben Mbarek, M.; Assali, I.; Hamdi, S.; Ben Abdallah, A.; David, O.; Aissi, M.; Carrere, M.; Bedoui, M.H. Automatic and Manual Prediction of Epileptic Seizures Based on ECG. Signal Image Video Process 2024, 18, 4175–4190. [Google Scholar] [CrossRef]
- Ghaempour, M.; Hassanli, K.; Abiri, E. An Approach to Detect and Predict Epileptic Seizures with High Accuracy Using Convolutional Neural Networks and Single-Lead-ECG Signal. Biomed. Phys. Eng. Express 2024, 10, 025041. [Google Scholar] [CrossRef]
- Shafiezadeh, S.; Duma, G.M.; Mento, G.; Danieli, A.; Antoniazzi, L.; Del Popolo Cristaldi, F.; Bonanni, P.; Testolin, A. Calibrating Deep Learning Classifiers for Patient-Independent Electroencephalogram Seizure Forecasting. Sensors 2024, 24, 2863. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Lv, J.-J.; Rui, L.-G.; Yang, Y.-X.; Chen, Y.-G.; Ma, C.; Gao, Z.-K. Seizure Prediction in Scalp EEG Based Channel Attention Dual-Input Convolutional Neural Network. Phys. A Stat. Mech. Its Appl. 2021, 584, 126376. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Y.; Yang, P.; Chen, W.; Lo, B. Epilepsy Seizure Prediction on EEG Using Common Spatial Pattern and Convolutional Neural Network. IEEE J. Biomed. Health Inform. 2020, 24, 465–474. [Google Scholar] [CrossRef]
- Hu, S.; Liu, J.; Yang, R.; Wang, Y.; Wang, A.; Li, K.; Liu, W.; Yang, C. Exploring the Applicability of Transfer Learning and Feature Engineering in Epilepsy Prediction Using Hybrid Transformer Model. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1321–1332. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Lou, X.; Kong, H.; Li, X.; Li, Z.; Zhong, L. Epileptic Seizure Prediction Based on EEG Using Pseudo-Three-Dimensional CNN. Front. Neuroinform. 2024, 18, 1354436. [Google Scholar] [CrossRef] [PubMed]
- Toraman, S. Preictal and Interictal Recognition for Epileptic Seizure Prediction Using Pre-Trained 2D-CNN Models. Trait. Signal 2020, 37, 1045–1054. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhu, M.; Liu, Z.; Shabaz, M.; Ullah, I.; Tong, M.C.F.; Sambas, A.; Men, L.; Chen, Y.; Chen, S. Multi-Feature Fusion-Based Convolutional Neural Networks for EEG Epileptic Seizure Prediction in Consumer Internet of Things. IEEE Trans. Consum. Electron. 2024, 70, 5631–5643. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Z.; Zhang, M.; Wang, Y.; Yang, L.; Lin, J.; Kärkkäinen, T.; Cong, F. Combination of Channel Reordering Strategy and Dual CNN-LSTM for Epileptic Seizure Prediction Using Three IEEG Datasets. IEEE J. Biomed. Health Inform. 2024, 28, 6557–6567. [Google Scholar] [CrossRef]
- Usman, S.M.; Khalid, S.; Bashir, Z. Epileptic Seizure Prediction Using Scalp Electroencephalogram Signals. Biocybern. Biomed. Eng. 2021, 41, 211–220. [Google Scholar] [CrossRef]
- Xu, T.; Wu, Y.; Tang, Y.; Zhang, W.; Cui, Z. Dynamic Functional Connectivity Neural Network for Epileptic Seizure Prediction Using Multi-Channel EEG Signal. IEEE Signal Process. Lett. 2024, 31, 1499–1503. [Google Scholar] [CrossRef]
- Shu, K.; Wu, L.; Zhao, Y.; Liu, A.; Qian, R.; Chen, X. Data Augmentation for Seizure Prediction with Generative Diffusion Model. IEEE Trans. Cogn. Dev. Syst. 2025, 17, 577–591. [Google Scholar] [CrossRef]
- Ryu, S.; Joe, I. A Hybrid DenseNet-LSTM Model for Epileptic Seizure Prediction. Appl. Sci. 2021, 11, 7661. [Google Scholar] [CrossRef]
- Nazari, J.; Motie Nasrabadi, A.; Menhaj, M.B.; Raiesdana, S. Epilepsy Seizure Prediction with Few-Shot Learning Method. Brain Inform. 2022, 9, 21. [Google Scholar] [CrossRef]
- Quadri, Z.F.; Saqib Akhoon, M.; Loan, S.A. Epileptic Seizure Prediction Using Stacked CNN-BiLSTM: A Novel Approach. IEEE Trans. Artif. Intell. 2024, 5, 5553–5560. [Google Scholar] [CrossRef]
- Jana, R.; Mukherjee, I. Deep Learning Based Efficient Epileptic Seizure Prediction with EEG Channel Optimization. Biomed. Signal Process. Control 2021, 68, 102767. [Google Scholar] [CrossRef]
- Assali, I.; Ghazi Blaiech, A.; Ben Abdallah, A.; Ben Khalifa, K.; Carrère, M.; Hédi Bedoui, M. CNN-Based Classification of Epileptic States for Seizure Prediction Using Combined Temporal and Spectral Features. Biomed. Signal Process. Control 2023, 82, 104519. [Google Scholar] [CrossRef]
- Ramesh, J.V.N.; Misba, M.; Balaji, S.; Kumar, K.K.; Muniyandy, E.; El-Ebiary, Y.A.B.; Bala, B.K.; Elbasir, R.A.M. Hybrid Attention-Based Transformers-CNN Model for Seizure Prediction Through Electronic Health Records. Int. J. Adv. Comput. Sci. Appl. 2025, 16, 1111–1120. [Google Scholar] [CrossRef]
- Polat, H.; Aluçlu, M.U.; Özerdem, M.S. Evaluation of Potential Auras in Generalized Epilepsy from EEG Signals Using Deep Convolutional Neural Networks and Time-Frequency Representation. Biomed. Eng./Biomed. Tech. 2020, 65, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, M.A.; Cura, O.K.; Akan, A. Epileptic EEG Classification by Using Time-Frequency Images for Deep Learning. Int. J. Neural Syst. 2021, 31, 2150026. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wen, A.; Sun, L.; Wang, H.; Guo, Y.; Ren, Y. An Epileptic Seizure Prediction Method Based on CBAM-3D CNN-LSTM Model. IEEE J. Transl. Eng. Health Med. 2023, 11, 417–423. [Google Scholar] [CrossRef]
- Priya Prathaban, B.; Balasubramanian, R. Dynamic Learning Framework for Epileptic Seizure Prediction Using Sparsity Based EEG Reconstruction with Optimized CNN Classifier. Expert Syst. Appl. 2021, 170, 114533. [Google Scholar] [CrossRef]
- Choi, W.; Kim, M.-J.; Yum, M.-S.; Jeong, D.-H. Deep Convolutional Gated Recurrent Unit Combined with Attention Mechanism to Classify Pre-Ictal from Interictal EEG with Minimized Number of Channels. J. Pers. Med. 2022, 12, 763. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, J.; Wu, H.; Zhu, J.; Sawan, M. Power Efficient Refined Seizure Prediction Algorithm Based on an Enhanced Benchmarking. Sci. Rep. 2021, 11, 23498. [Google Scholar] [CrossRef]
- Lian, Q.; Qi, Y.; Pan, G.; Wang, Y. Learning Graph in Graph Convolutional Neural Networks for Robust Seizure Prediction. J. Neural Eng. 2020, 17, 035004. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Wang, Y.; Yang, L.; Liang, Z.; Cong, F. One-Dimensional Convolutional Neural Networks Combined with Channel Selection Strategy for Seizure Prediction Using Long-Term Intracranial EEG. Int. J. Neural Syst. 2022, 32, 2150048. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Nie, W.; Zhou, W.; Xu, F.; Yuan, S.; Leng, Y.; Yuan, Q. Epileptic Seizure Prediction Based on Local Mean Decomposition and Deep Convolutional Neural Network. J. Supercomput. 2020, 76, 3462–3476. [Google Scholar] [CrossRef]
- Chung, Y.G.; Jeon, Y.; Choi, S.A.; Cho, A.; Kim, H.; Hwang, H.; Kim, K.J. Deep Convolutional Neural Network Based Interictal-Preictal Electroencephalography Prediction: Application to Focal Cortical Dysplasia Type-II. Front. Neurol 2020, 11, 594679. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yang, F.; Wen, P.; Song, B.; Li, Y. A Real-Time Epilepsy Seizure Detection Approach Based on EEG Using Short-Time Fourier Transform and Google-Net Convolutional Neural Network. Heliyon 2024, 10, e31827. [Google Scholar] [CrossRef]
- Mohankumar, N.; Kumar, G.; Rajalakshmi, P.; Sridevi, S.; Fathima, S.K.; Koti Reddy, M.; Padma Lata, M.K. An Optimized Hybrid CNN-LSTM Model for Epileptic Seizure Detection and Prediction. Eng. Technol. Appl. Sci. Res. 2025, 15, 26085–26090. [Google Scholar] [CrossRef]
- Chambers, J.D.; Cook, M.J.; Burkitt, A.N.; Grayden, D.B. Using Long Short-Term Memory (LSTM) Recurrent Neural Networks to Classify Unprocessed EEG for Seizure Prediction. Front. Neurosci. 2024, 18, 1472747. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Li, Z.; Xia, Y.; Fan, Z.; Zhou, J. Semi-Supervised Seizure Prediction Model Combining Generative Adversarial Networks and Long Short-Term Memory Networks. Appl. Sci. 2023, 13, 11631. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, H.; Xiao, J.; Sun, J. Multi-Scale Spatio-Temporal Attention Network for Epileptic Seizure Prediction. IEEE J. Biomed. Health Inform. 2025, 29, 4784–4795. [Google Scholar] [CrossRef]
- Kim, J.; Amorim, E.; Rao, V.R.; Glass, H.C.; Bernardo, D. Short-Horizon Neonatal Seizure Prediction Using EEG-Based Deep Learning. PLOS Digit. Health 2025, 4, e0000890. [Google Scholar] [CrossRef]
- Li, H.; Liao, J.; Wang, H.; Zhan, C.A.; Yang, F. EEG Power Spectra Parameterization and Adaptive Channel Selection towards Semi-Supervised Seizure Prediction. Comput. Biol. Med. 2024, 175, 108510. [Google Scholar] [CrossRef]
- Lopes, F.; Pinto, M.F.; Dourado, A.; Schulze-Bonhage, A.; Dümpelmann, M.; Teixeira, C. Addressing Data Limitations in Seizure Prediction through Transfer Learning. Sci. Rep. 2024, 14, 14169. [Google Scholar] [CrossRef]
- Kalita, D.; Dash, S.; Mirza, K.B. EpiNET: AN Optimized, Resource Efficient Deep Gru-Lstm Network for Epileptic Seizure Prediction. Biomed. Eng. 2024, 36, 2450021. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Zhang, R.; Xu, T. Patient-Specific Method for Predicting Epileptic Seizures Based on DRSN-GRU. Biomed. Signal Process. Control 2023, 81, 104449. [Google Scholar] [CrossRef]
- Singh, Y.P.; Lobiyal, D.K. Automatic Prediction of Epileptic Seizure Using Hybrid Deep ResNet-LSTM Model. AI Commun. 2023, 36, 57–72. [Google Scholar] [CrossRef]
- Prakash, V.; Kumar, D. A Modified Gated Recurrent Unit Approach for Epileptic Electroencephalography Classification. J. Inf. Commun. Technol. 2023, 22, 587–617. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Z.; Zhang, T.; Zhang, L.; Qiao, L. An End-to-End Seizure Prediction Approach Using Long Short-Term Memory Network. Front. Hum. Neurosci. 2023, 17, 1187794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ding, J.; Kong, W.; Liu, Y.; Wang, Q.; Jiang, T. Biomedical Signal Processing and Control Epilepsy Prediction through Optimized Multidimensional Sample Entropy and Bi-LSTM. Biomed. Signal Process. Control 2021, 64, 102293. [Google Scholar] [CrossRef]
- Cheng, C.; You, B.; Liu, Y.; Dai, Y. Patient-Specific Method of Sleep Electroencephalography Using Wavelet Packet Transform and Bi-LSTM for Epileptic Seizure Prediction. Biomed. Signal Process. Control 2021, 70, 102963. [Google Scholar] [CrossRef]
- Varnosfaderani, S.M.; McNulty, I.; Sarhan, N.J.; Abood, W.; Alhawari, M. An Efficient Epilepsy Prediction Model on European Dataset with Model Evaluation Considering Seizure Types. IEEE J. Biomed. Health Inform. 2024, 28, 5842–5854. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, S.; Chen, W.; Du, G.; Fu, Q.; Jiang, H. A Scheme Combining Feature Fusion and Hybrid Deep Learning Models for Epileptic Seizure Detection and Prediction. Sci. Rep. 2024, 14, 16916. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, B.; Kim, T.; Joe, I.; Chong, J.; Min, K.; Jung, K. A ResNet-LSTM Hybrid Model for Predicting Epileptic Seizures Using a Pretrained Model with Supervised Contrastive Learning. Sci. Rep. 2024, 14, 1319. [Google Scholar] [CrossRef]
- Sidaoui, B. Predicting states of epilepsy patients using deep learning models. Appl. Comput. Sci. 2024, 20, 109–125. [Google Scholar] [CrossRef]
- Chen, R.; Parhi, K.K. Seizure Prediction Using Convolutional Neural Networks and Sequence Transformer Networks. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; IEEE: New York, NY, USA, 2021; pp. 6483–6486. [Google Scholar]
- Yan, J.; Li, J.; Xu, H.; Yu, Y.; Xu, T. Seizure Prediction Based on Transformer Using Scalp Electroencephalogram. Appl. Sci. 2022, 12, 4158. [Google Scholar] [CrossRef]
- Xiang, J.; Li, Y.; Wu, X.; Dong, Y.; Wen, X.; Niu, Y. Synchronization-Based Graph Spatio-Temporal Attention Network for Seizure Prediction. Sci. Rep. 2025, 15, 4080. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Z.; Zhuang, J.; Liu, F. Dual-Modality Transformer with Time Series Imaging for Robust Epileptic Seizure Prediction. Appl. Sci. 2025, 15, 1538. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, S.; Wu, D.; Wu, X. Self-Supervised Learning with Adaptive Frequency-Time Attention Transformer for Seizure Prediction and Classification. Brain Sci. 2025, 15, 382. [Google Scholar] [CrossRef]
- Qiao, W.; Bi, X.; Han, L.; Zhang, Y. Epilepsy Prediction and Detection Using Attention-CssCDBN with Dual-Task Learning. Sensors 2024, 25, 51. [Google Scholar] [CrossRef]
- Dong, X.; He, L.; Li, H.; Liu, Z.; Shang, W.; Zhou, W. Deep Learning Based Automatic Seizure Prediction with EEG Time-Frequency Representation. Biomed. Signal Process. Control 2024, 95, 106447. [Google Scholar] [CrossRef]
- Ma, H.; Wu, Y.; Tang, Y.; Chen, R.; Xu, T.; Zhang, W. Parallel Dual-Branch Fusion Network for Epileptic Seizure Prediction. Comput. Biol. Med. 2024, 176, 108565. [Google Scholar] [CrossRef]
- Shi, S.; Liu, W. B2-ViT Net: Broad Vision Transformer Network with Broad Attention for Seizure Prediction. IEEE Trans. Neural Syst. Rehabil. Eng. 2024, 32, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yan, K.; Wang, S.; Liu, J.-X.; Wang, J. EEG-Based Seizure Prediction Using Hybrid DenseNet–ViT Network with Attention Fusion. Brain Sci. 2024, 14, 839. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; He, Z.; Chen, Z.; Zhou, Y. Combining Temporal and Spatial Attention for Seizure Prediction. Health Inf. Sci. Syst. 2023, 11, 38. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, A.; Cui, X.; Qian, R.; Chen, X. A General Sample-Weighted Framework for Epileptic Seizure Prediction. Comput. Biol. Med. 2022, 150, 106169. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Zhang, L.; Qiao, L. Epileptic Seizure Prediction Using Successive Variational Mode Decomposition and Transformers Deep Learning Network. Front. Neurosci. 2022, 16, 982541. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Sun, Q.; Lu, J.; Ma, X. An Effective Dual Self-Attention Residual Network for Seizure Prediction. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1604–1613. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Cheng, Y.; He, Z.; Wei, X.; Chen, Z.; Zhou, Y. A Spatiotemporal Graph Attention Network Based on Synchronization for Epileptic Seizure Prediction. IEEE J. Biomed. Health Inform. 2023, 27, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.; Lee, S.; Ward, R. Multi-Channel Vision Transformer for Epileptic Seizure Prediction. Biomedicines 2022, 10, 1551. [Google Scholar] [CrossRef]
- Saeizadeh, A.; Schonholtz, D.; Uvaydov, D.; Guida, R.; Demirors, E.; Johari, P.; Jimenez, J.M.; Neimat, J.S.; Melodia, T. SeizNet: An AI-Enabled Implantable Sensor Network System for Seizure Prediction. In Proceedings of the 2024 19th Wireless On-Demand Network Systems and Services Conference (WONS), Chamonix, France, 29–31 January 2024. [Google Scholar]
- Lih, O.S.; Jahmunah, V.; Palmer, E.E.; Barua, P.D.; Dogan, S.; Tuncer, T.; García, S.; Molinari, F.; Acharya, U.R. EpilepsyNet: Novel Automated Detection of Epilepsy Using Transformer Model with EEG Signals from 121 Patient Population. Comput. Biol. Med. 2023, 164, 107312. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, J.; Xu, M.; Liu, Y.; Li, J.; Nie, W.; Yuan, Q. Combining Meta and Ensemble Learning to Classify EEG for Seizure Detection. Sci. Rep. 2025, 15, 10755. [Google Scholar] [CrossRef]
- Axenie, C.; Halilov, E.; Main, J.; Weiss, D. Edge Neuro-Statistical Learning for Event-Based Visual Motion Detection and Tracking in Roadside Safety Systems. Neuromorphic Comput. Eng. 2025, 5, 024003. [Google Scholar] [CrossRef]
- Lin, T.H.; Chang, C.T.; Zhuang, T.H.; Putranto, A. Real-Time Hollow Defect Detection in Tiles Using on-Device Tiny Machine Learning. Meas. Sci. Technol. 2024, 35, 056006. [Google Scholar] [CrossRef]
- Oyejide, A.; Stroppa, F.; Sarac, M. Miniaturized Soft Growing Robots for Minimally Invasive Surgeries: Challenges and Opportunities. Prog. Biomed. Eng. 2025, 7, 033001. [Google Scholar] [CrossRef] [PubMed]
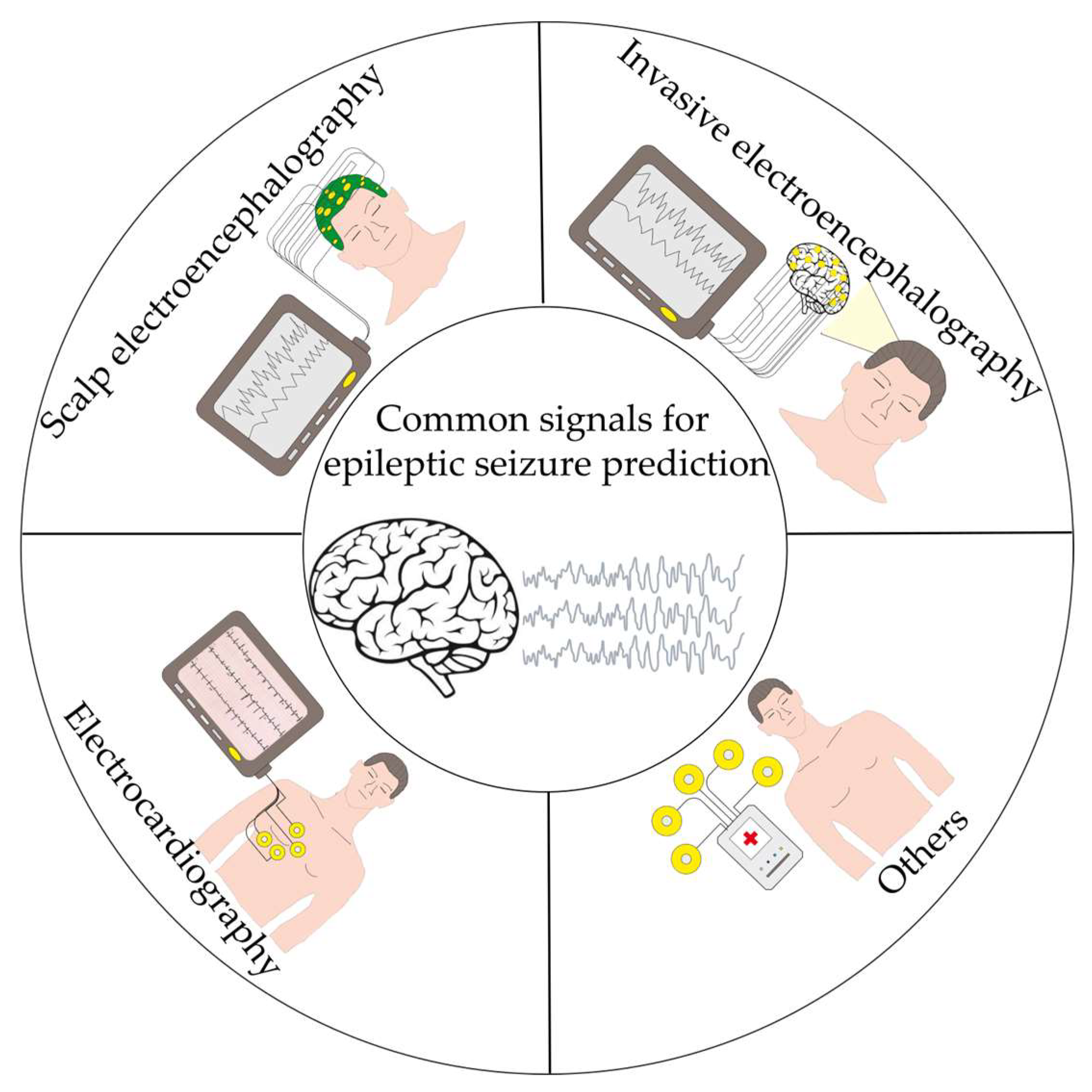
| Characteristics | iEEG | sEEG | ECG |
|---|---|---|---|
| Origin | Brain neuron activity. | Brain neuron activity. | Action potentials of heart muscle cells. |
| Frequency range (Hz) | 0.1–100 | 0.1–100. | 0.5–100 |
| Amplitude (µV) | 5–1000 | 1–100 | 100–3000 |
| Invasive? | Yes (intracortical electrode) | No (surface electrode) | No (surface electrode) |
| Is it affected by power line interference noise? | Yes, it is caused by electrical wiring, which results in frequency interference in the range of 50 to 60 Hz, leading to amplitude distortion; this is less prone to environmental noise than sEEG due to its placement inside the skull. The iEEG can reach a signal–noise ratio of around 30–50 dB, depending on the setup and conditions. | Yes, it is caused by electrical wiring, which results in frequency interference in the range of 50 to 60 Hz, leading to amplitude distortion. Depending on the setup and conditions, the sEEG can reach signal noise ratio of around 10–20 dB. | Yes, it is caused by electrical wiring, which introduces interference with a frequency in the range of 50 to 60 Hz and an amplitude of up to 5 mV, distorting the waveform and potentially masking important features such as the T-wave, QRS complex, and P-wave. The ECG can reach signal–noise ratio of around 10–30 dB. |
| Is it affected by baseline wander noise? | Yes, but less than sEEG because the electrodes are placed directly within brain tissue, minimizing interference from scalp, muscle, and environmental noise. The result in iEEG is 5–10 times lower baseline than sEEG. | Yes, it is caused by head movement, electrode contact, and sweat on the scalp. These lead to alterations in frequencies below 1 Hz with amplitudes of 200 to 300 mV, resulting in an sEEG signal–noise ratio of around 10–20 dB. | Yes, it results from body movements and inadequate electrode contact, and high impedance between the electrode and skin. This results in alterations in frequencies from 0.05 to 1 Hz, causing distortion in QRS complex and ST segment and low-frequency components, resulting in an ECG signal noise ratio of around 5–10 dB. |
| Database | No. of Patients | Signal Type | No. of Channels | Data Continuity | Recording per Segment (s) | Balance Class | Sampling Frequency (Hz) |
|---|---|---|---|---|---|---|---|
| Melbourne-neuroVista seizure trial [29] | 15 | iEEG | 16 | Noncontinuous | Average of 107 | No | 400 |
| Kaggle-Melbourne-University AES-MathWorks-NIH [30] | 3 | iEEG | 16 | Noncontinuous | 600 | No | 400 |
| Freiburg [31] | 21 | iEEG | 128 | Short-term continuous | Average of 3000 | No | 256 |
| Bern Barcelona [32] | 5 | iEEG | More or less than 64 | No data | 20 | No data | 512 |
| CHB-MIT Scalp EEG [1] | 22 | sEEG | 23–26 | Continuous | Average of 36,000 | No | 256 |
| Neurology and Sleep Centre Hauz Khas [33] | 10 | sEEG | 1 | Noncontinuous | 5.12 | Yes | 200 |
| TUH EEG Epilepsy Corpus (TUSZ) [34] | 200 | sEEG | 23–31 | Short-term continuous | 3600 | No | Least 250 |
| Helsinki University Hospital EEG [35] | 79 | sEEG | 19 | Short-term continuous | Average of 4440 | No | 256 |
| Siena Scalp EEG [36] | 14 | sEEG | 20–29 | Short-term continuous | Differing | No | 512 |
| Postictal Heart Rate Oscillations in Partial Epilepsy [37] | 5 | ECG | 1 | Short-term continuous | Differing | No | 200 |
| SeizelT1 [38] | 82 | sEEG/ECG | 25/1 | Continuous | Average of 36,000 | No | 250 |
| PEDESITE: Personalized Detection of Epileptic Seizure on the Internet of Things (IoT) Era [39] | 1200 | sEEG, ECG, PPG, SPO2, EDA, 3D-ACC and asEMG | ----- | ----- | ----- | ----- | ----- |
| ML Classifier | Advantages | Disadvantages |
|---|---|---|
| SVM | Effective in high-dimensional spaces. Can handle nonlinear data (i.e., sEEG, iEEG, ECG, etc.), employing kernel. functions. High accuracy, especially for small to medium datasets with clear class separation. | Computationally intensive for large data (i.e., large databases). Requires careful turning of kernel functions and hyperparameters. Not easily scalable for large datasets due to memory and computation requirements. Difficult to interpret, especially with nonlinear kernels. |
| NB | Easy to interpret due to its probabilistic nature. Fast computation even with large data. Highly scalable; performs well with large datasets. Low computational cost; very fast training and prediction. Moderate accuracy performs well with categorical data. | Assumes strong feature independence (this is supposed to be a problem in EEG data that may not always hold). Sensitive to rare events in data (may perform poorly with highly correlated features or noisy data. Characteristics that are commonly in bio signals). |
| KNN | Easy to understand and implement. No training phase required. Can adapt to new data in an online setting. | Requires careful turning of k and hyperparameters. Computationally expensive for large datasets. Sensitive to noisy data and irrelevant features. Low scalability: computational cost increases significantly with dataset size. |
| DT | Minimal data processing required. Easy to interpret and visualize. Can handle with categorical and continuous data. Easily scalable. | Prone to overfitting with noisy data. Requires pruning to improve generalization. Small changes in data can lead to significant model variations. Depth and size can become issues with large datasets. |
| DL Algorithm | Advantages | Disadvantages |
|---|---|---|
| CNNs | Excellent for spatial features extraction (i.e., sEEG and iEEG). Effective in large-scale datasets. High accuracy in image-based and spatial pattern recognition tasks. Highly scalable using GPUs and parallel processing. | Requires large, labeled datasets for training. Struggles to capture temporal dependencies. Computationally expensive. Low interpretability due to the complexity of layers and parameters. |
| RNNs | Suitable for sequential and time-series data. Can capture long-term temporal dependencies in data. Effective for time series data. Good accuracy for sequential and temporal data. Moderate interpretability. | Prone to disappearing or exploding gradient issues over long sequences. Slow training times and resource-intensive. Requires large amounts of labeled data. High computational cost, especially with long sequences due to vanishing gradients. Limited scalability for long sequences. |
| TBMs | Handles long range dependencies are better than RNNs and CNNs. High accuracy for sequential tasks. Highly flexible for capturing complex patterns in data. Scalable and parallelizable. | Requires considerable computational resources and memory. A training in a large amount of data is required. Complex model tuning and hyperparameter optimization. Low interpretability due to complex architecture makes it challenging to understand. |
| ML-Based Algorithms | Proposal Advantages | Opportunities of Research | Time Prediction | Application |
|---|---|---|---|---|
| SVM | The application reduces noise and isolates critical features, preserving essential frequency components associated with seizure activity. Feature selection and dimensionality reduction streamline the classification process, enabling the model to handle complex, high-dimensional data while maintaining computational efficiency. The classifier employed is robust and offers strong generalization capabilities. | The application is computationally demanding, posing challenges for real-time seizure prediction. The reliance on extensive pre-processing and feature extraction steps introduces the potential for overfitting. | 23 min y 36 seg | Altaf et al. [114] |
| KNN | This application allows for a detailed examination of time and frequency characteristics, providing a comprehensive understanding of the underlying patterns preceding a seizure. The method used for feature selection ensures that the most relevant and statistically significant features are retained, enhancing the model’s ability to identify seizure precursors accurately. Additionally, the classification method employed is well-suited for recognizing patterns in the data, enabling reliable seizure prediction that is essential for timely intervention. | This approach is computationally demanding, potentially limiting its application in real-time scenarios where quick processing is essential. While useful, the focus on specific features may lead to the omission of other relevant patterns, reducing the model’s overall robustness. Additionally, the approach may be sensitive to parameter selection and could struggle with handling imbalanced datasets, impacting the accuracy and reliability of seizure predictions. Reliance on distance measures in the classification process can also present challenges. | 20 min | Perez-Sanchez et al. [115] |
| Automatic threshold | This application effectively isolates relevant features. The feature selection process is robust, focusing on statistically significant features that enhance the model’s predictive accuracy. The classification strategy is straightforward and efficient, allowing for quick and reliable seizure prediction. | This may be limited by its reliance on specific features that might not fully capture the complexity of the pre-seizure state, potentially reducing predictive robustness. The application may be vulnerable to noise and artifacts in the data, which could impact the accuracy of its predictions. While the simplicity of the classification strategy is beneficial for efficiency, it may result in a less nuanced analysis, potentially leading to a higher rate of false positives or missed seizures. | Variant up to 60 maximum minutes | Mbarek et al. [127] |
| DT | This application effectively isolates relevant frequency bands from data. The feature selection process is rigorous, ensuring that only the most statistically significant features are included, which helps reduce the data’s dimensionality while maintaining predictive accuracy. The classification strategy is interpretable and straightforward, allowing for transparent decision making, which is critical in a clinical setting. | The approach may be limited by its sensitivity to noise and artifacts in the data, which could impact the reliability of the predictions. Although the classification strategy is easily interpretable, it might not fully represent the complexity of seizure precursors, leading to a higher risk of false positives or missed predictions. | 8 h | Saboo et al. [123] |
| Classifier | Advantages | Opportunity of Research | Time Prediction | Application |
|---|---|---|---|---|
| Pseudo-3D CNN–BiLSTM 3D, Attention3D | This application captures the signal’s complexity and irregularity through advanced entropy measures and fractal analysis, providing a rich set of highly informative features for seizure prediction. The feature selection process ensures that the most relevant and least redundant features are retained, optimizing the model’s predictive power. The classification model combines spatial and temporal information with attention mechanisms. This complex architecture is particularly well-suited for capturing the nuanced dynamics of seizure development, offering high predictive accuracy and robustness. | The approach is computationally intensive, particularly in the feature extraction and classification stages, which may pose challenges for real-time applications. While powerful, the complexity of the model increases the risk of overfitting, particularly if not carefully tuned and validated across diverse patient datasets. The model’s sensitivity to variations in the input data could lead to a higher incidence of false positives or missed predictions. | 15 min | Liu et al. [158] |
| BiLSTM | This application offers a comprehensive data analysis by combining linear and nonlinear features, capturing a wide range of signal characteristics relevant to seizure prediction. Advanced feature fusion techniques enhance the representation of spatial features, leading to a more robust model that can accurately identify pre-seizure patterns. Integrating an attention mechanism further refines the feature selection process, enabling the model to focus on the most critical aspects of the data, thereby improving predictive accuracy. The classification model is well-suited for handling temporal dependencies in the signals. | This approach’s complexity may pose challenges regarding computational demands, particularly during the feature extraction and classification stages, which could limit its applicability in real-time scenarios. While enhancing predictive performance, advanced fusion techniques and attention mechanisms can lead to overfitting, especially with limited data, if not properly managed. Additionally, the intricate nature of the model reduces interpretability. | 40 min | Ahmad et al. [135] |
| 1-D CNN | This application effectively enhances the model’s stability and convergence by normalizing the input data during pre-processing, which leads to improved training efficiency and predictive accuracy. The classification model employs a one-dimensional CNN. Combining techniques allows for precise and reliable detection of relevant signal features, enabling accurate predictions. The model’s ability to handle large amounts of data and detect subtle changes in the signal. | The approach may face challenges related to computational resource requirements, particularly during the DL model’s training phase, which could limit real-time applicability. Additionally, while batch normalization improves training stability, it may not fully address the variability and noise inherent in data, potentially affecting the model’s performance. Though effective for sequential data, the one-dimensional CNN might struggle with capturing more complex temporal patterns and interactions in the signals, which could lead to reduced predictive performance. | 60 min | Saeizadeh et al. [159] |
| Parallel Dual-Branch Fusion Network | This application leverages sophisticated feature extraction and classification methods to provide a detailed analysis of signals. Using an advanced fusion network for feature selection and classification enhances the model’s ability to integrate and analyze multiple aspects of the data simultaneously, leading to improved predictive accuracy. The parallel architecture allows for the efficient processing of complex signals, making it well-suited for handling large datasets and diverse patient profiles, thereby increasing the model’s robustness and generalizability. | The approach’s complexity and reliance in advanced neural network architectures can lead to significant computational demands, potentially limiting its feasibility in real-time or resource-constrained environments. The intricate nature of the model also increases the risk of overfitting, mainly if the training data are not sufficiently diverse or abundant. | 60 min | Ma et al. [184] |
| Transformer deep model | This application used correlation-based feature extraction, allowing for the identification of strong, relevant signal patterns indicative of pre-seizure states. The integration of positional encoding enhances the model’s ability to capture temporal dependencies and contextual information within the data. With its advanced architecture, the classification model can learn complex patterns and long-range dependencies in the data, resulting in high predictive accuracy. | The approach involves significant computational complexity, particularly with DL models and large-scale data processing, which can pose challenges for real-time implementation. The model’s complexity also increases the risk of overfitting, especially if the training data is not sufficiently comprehensive or diverse. The advanced architecture may also reduce interpretability. | 60 min | Lih et al. [191] |
| CNN | The application effectively streamlines the data by pre-processing it to focus on the most relevant information, enhancing the model’s ability to detect pre-seizure patterns with higher accuracy. The use of DL classification models allows for extracting intricate features and patterns from the processed data, which is crucial for identifying subtle changes indicative of impending seizures. The reduced data volume after pre-processing helps accelerate the training process and improves computational efficiency. | In this approach, the pre-processing steps may result in the loss of potentially important information, such as filtering, down-sampling, and undersampling can omit significant signal details critical for accurate seizure prediction. Additionally, the DL model’s complexity may lead to high computational demands, potentially hindering real-time applications. While effective at feature extraction, the CNN model may struggle with generalizing across diverse patient data or varying seizure types, potentially leading to reduced performance in different scenarios. | 15 min | Saeizadeh et al. [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Sanchez, A.V.; Valtierra-Rodriguez, M.; De-Santiago-Perez, J.J.; Perez-Ramirez, C.A.; Garcia-Perez, A.; Amezquita-Sanchez, J.P. Artificial Intelligence-Based Epileptic Seizure Prediction Strategies: A Review. AI 2025, 6, 274. https://doi.org/10.3390/ai6100274
Perez-Sanchez AV, Valtierra-Rodriguez M, De-Santiago-Perez JJ, Perez-Ramirez CA, Garcia-Perez A, Amezquita-Sanchez JP. Artificial Intelligence-Based Epileptic Seizure Prediction Strategies: A Review. AI. 2025; 6(10):274. https://doi.org/10.3390/ai6100274
Chicago/Turabian StylePerez-Sanchez, Andrea V., Martin Valtierra-Rodriguez, J. Jesus De-Santiago-Perez, Carlos A. Perez-Ramirez, Arturo Garcia-Perez, and Juan P. Amezquita-Sanchez. 2025. "Artificial Intelligence-Based Epileptic Seizure Prediction Strategies: A Review" AI 6, no. 10: 274. https://doi.org/10.3390/ai6100274
APA StylePerez-Sanchez, A. V., Valtierra-Rodriguez, M., De-Santiago-Perez, J. J., Perez-Ramirez, C. A., Garcia-Perez, A., & Amezquita-Sanchez, J. P. (2025). Artificial Intelligence-Based Epileptic Seizure Prediction Strategies: A Review. AI, 6(10), 274. https://doi.org/10.3390/ai6100274








