Explainable AI-Driven 1D-CNN with Efficient Wireless Communication System Integration for Multimodal Diabetes Prediction
Abstract
1. Introduction
- Integrates essential preprocessing methods, including feature encoding and normalization.
- Develops an accurate diabetes prediction tool and reliable communication that can help in early detection and intervention. This can be achieved through finding the optimum required hyperparameters for the 1D-CNN using Bayesian optimization while implementing XAI.
- Implements XAI in the proposed model to enhance the transparency and the accuracy of the proposed model. A multi-method explainable AI (XAI) framework combining SHAP (SHapley Additive exPlanations), permutation feature importance, and LIME (Local Interpretable Model-agnostic Explanations) is implemented.
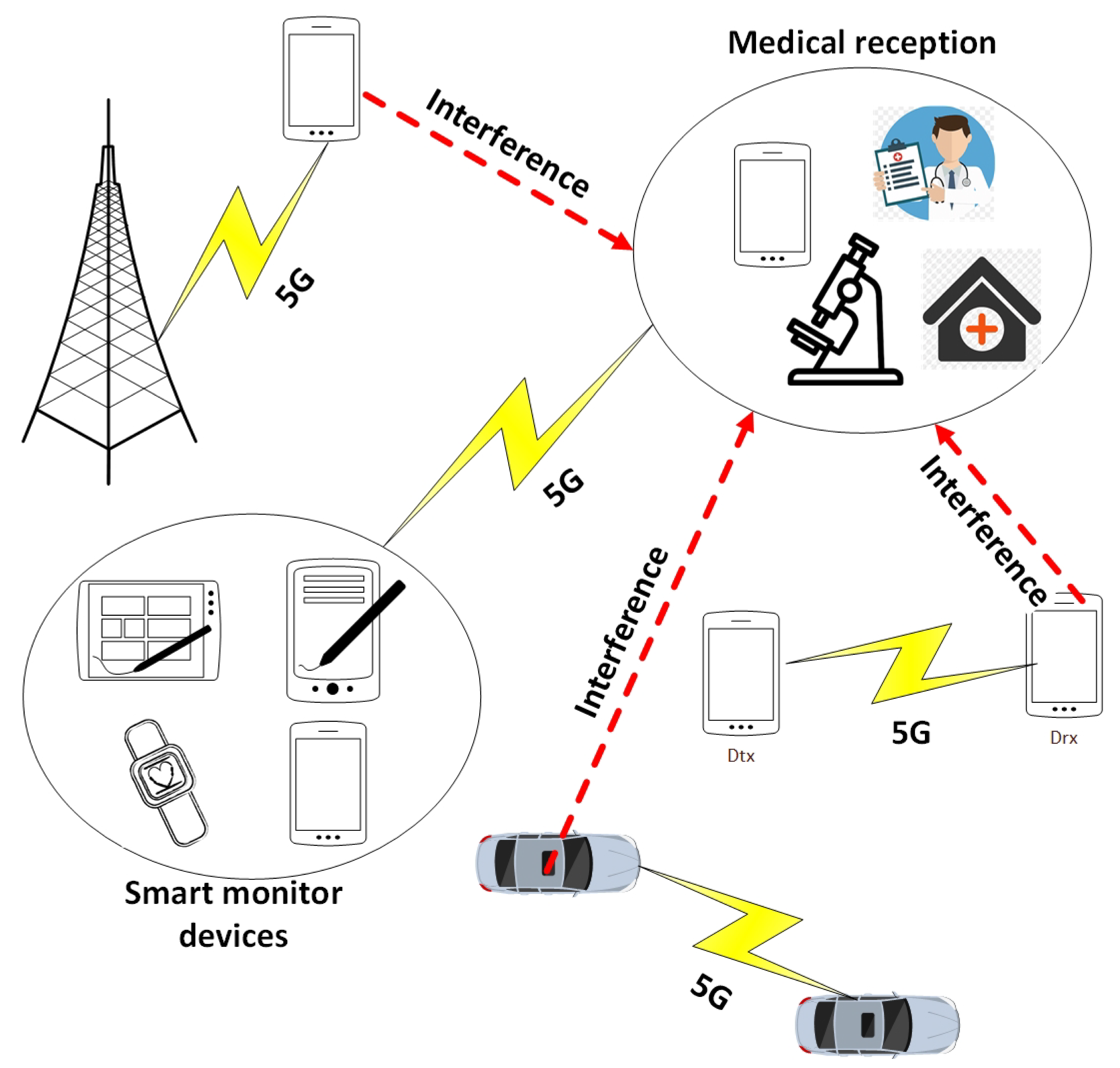
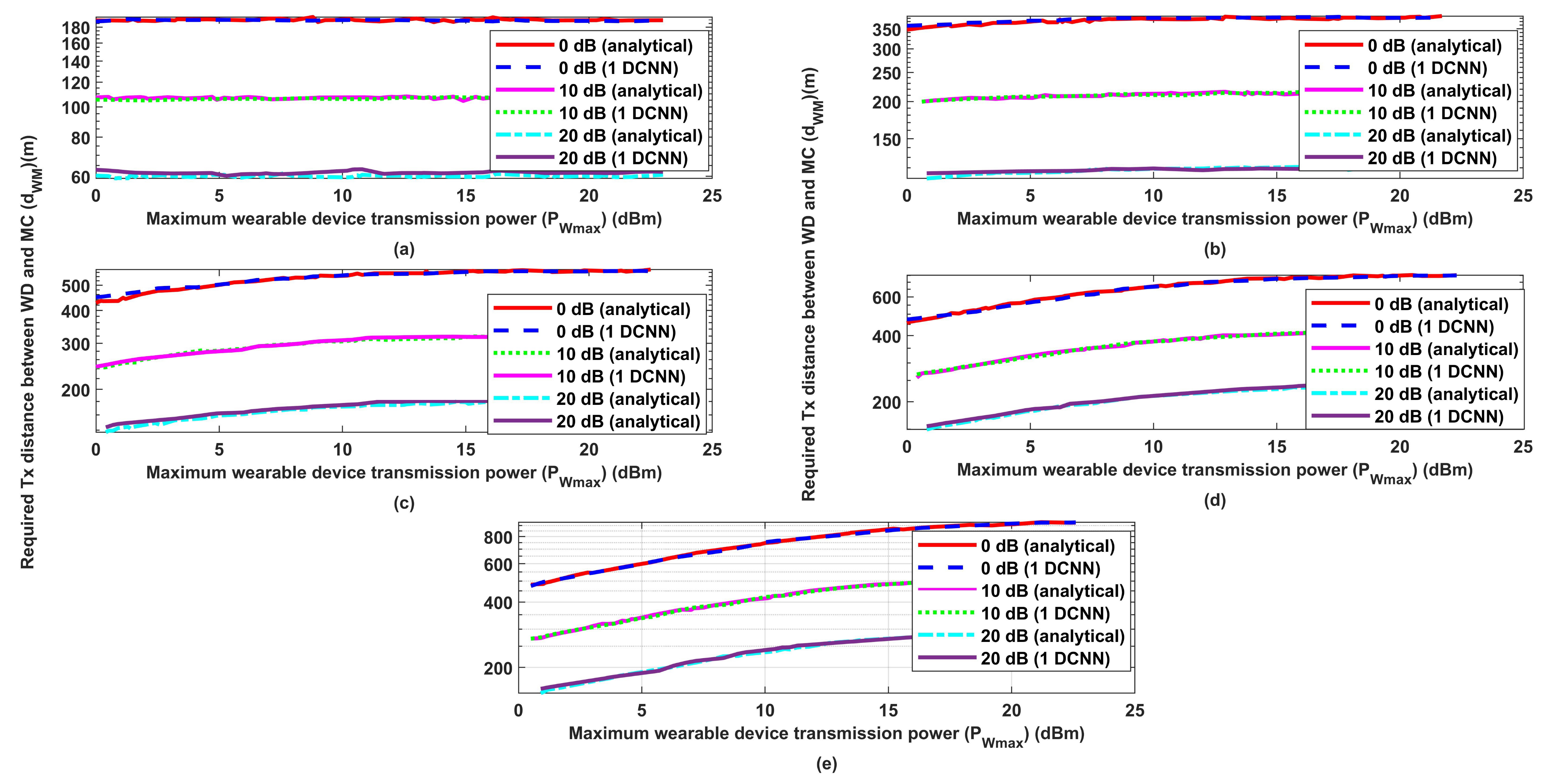
- Uses Lagrange optimization to find the optimum required transmission distance between the medical centers and the wearable devices to effectively send the patient information.
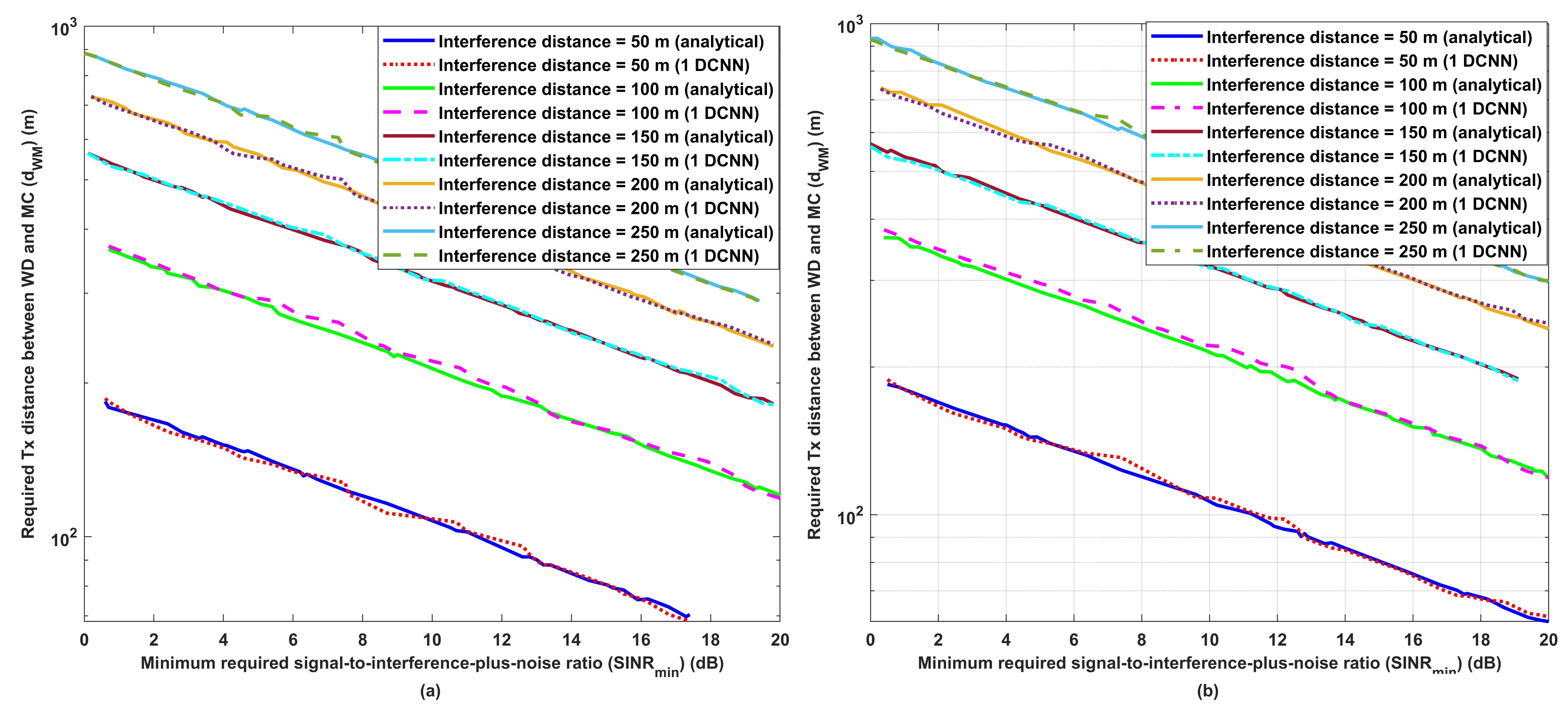
- Uses the obtained results as a dataset for a 1D-CNN, which will be able to predict the optimum required distance under different environmental conditions such as required signal-to-interference-plus-noise (SINR), transmission power, interference transmission power, path loss, and interference transmission distances.
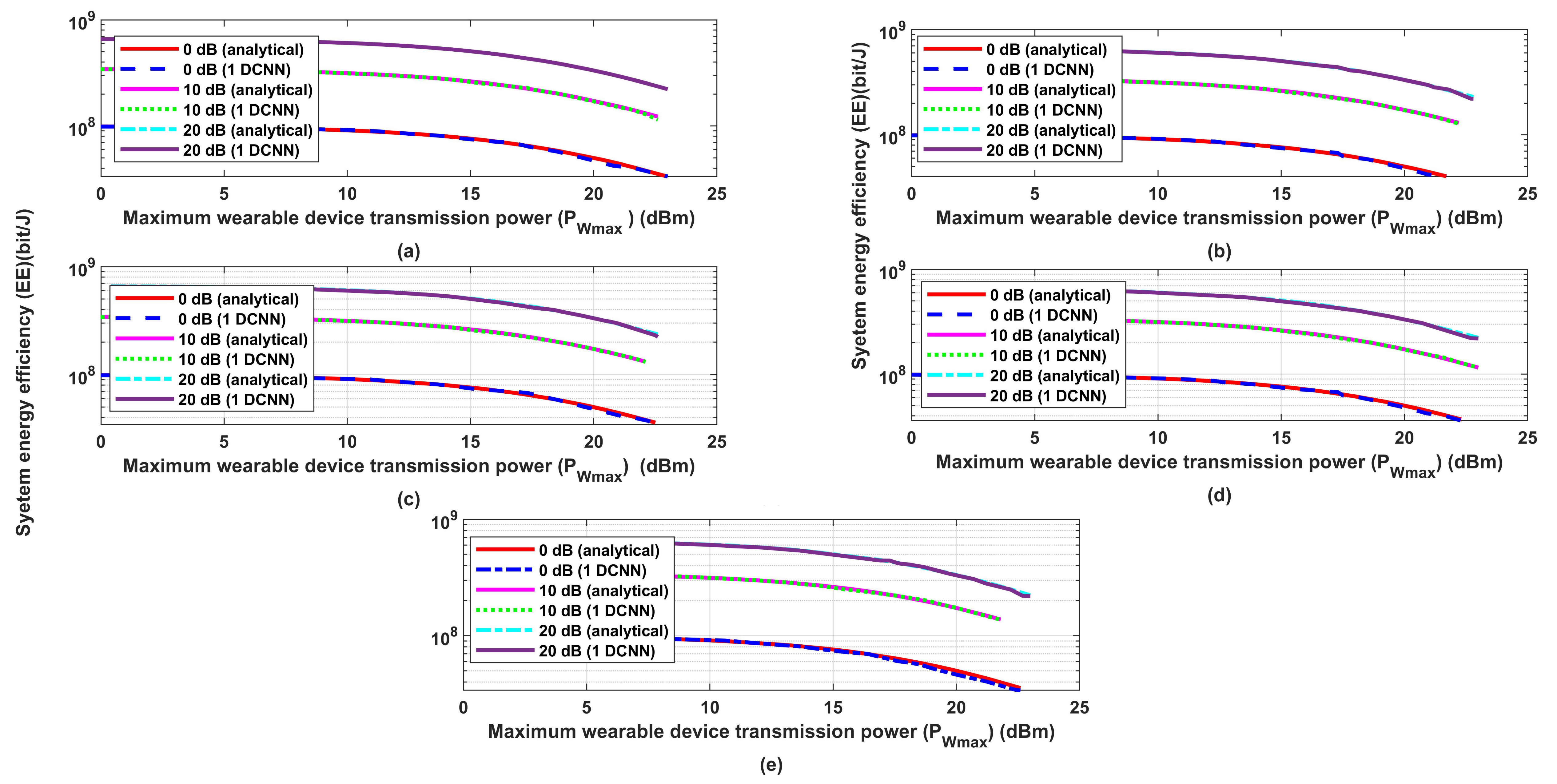
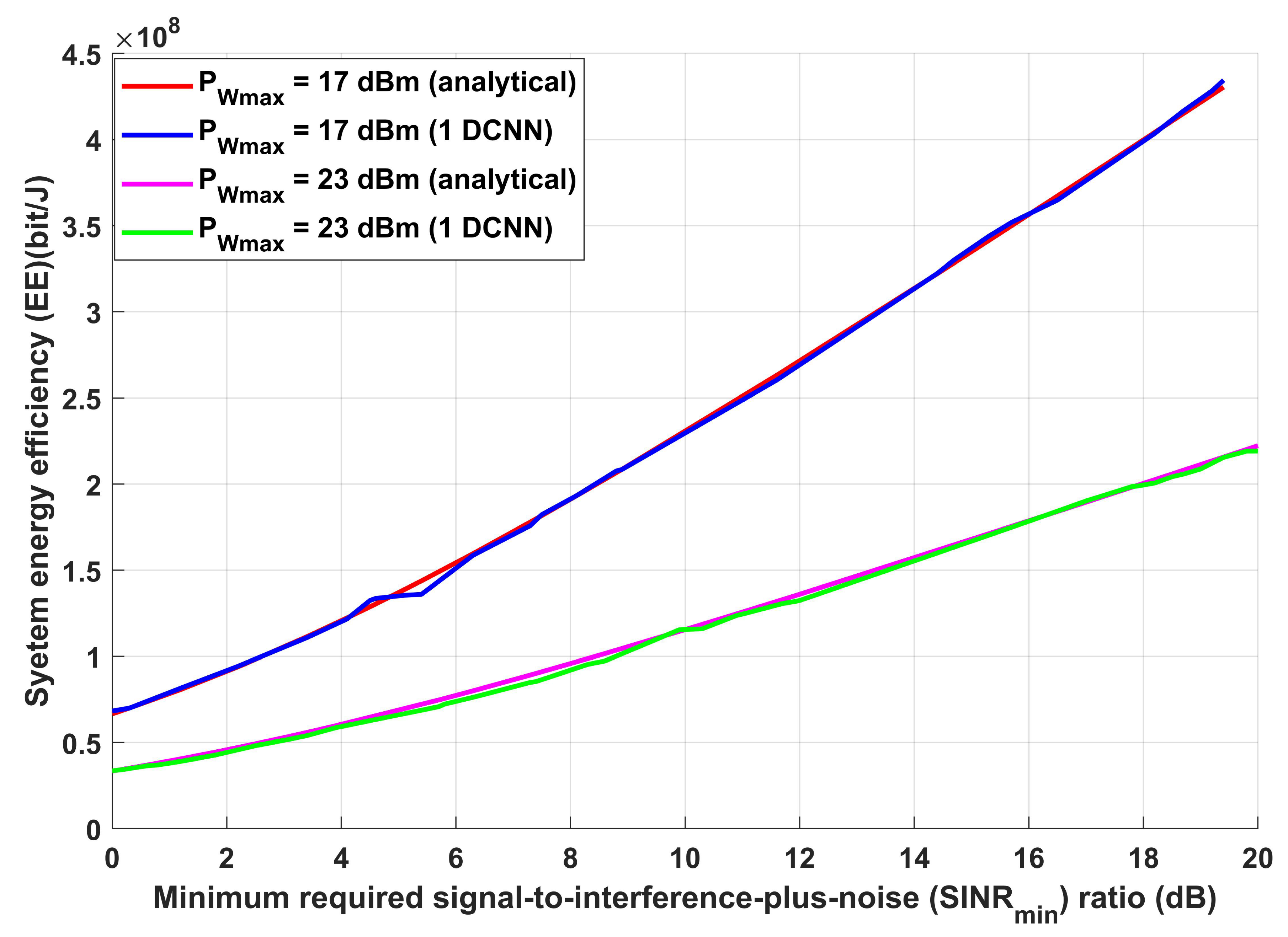
- Implements Bayesian optimization as well for the 1D-CNN used for predicting the required transmission distance to enhance the system performance in terms of energy efficiency.
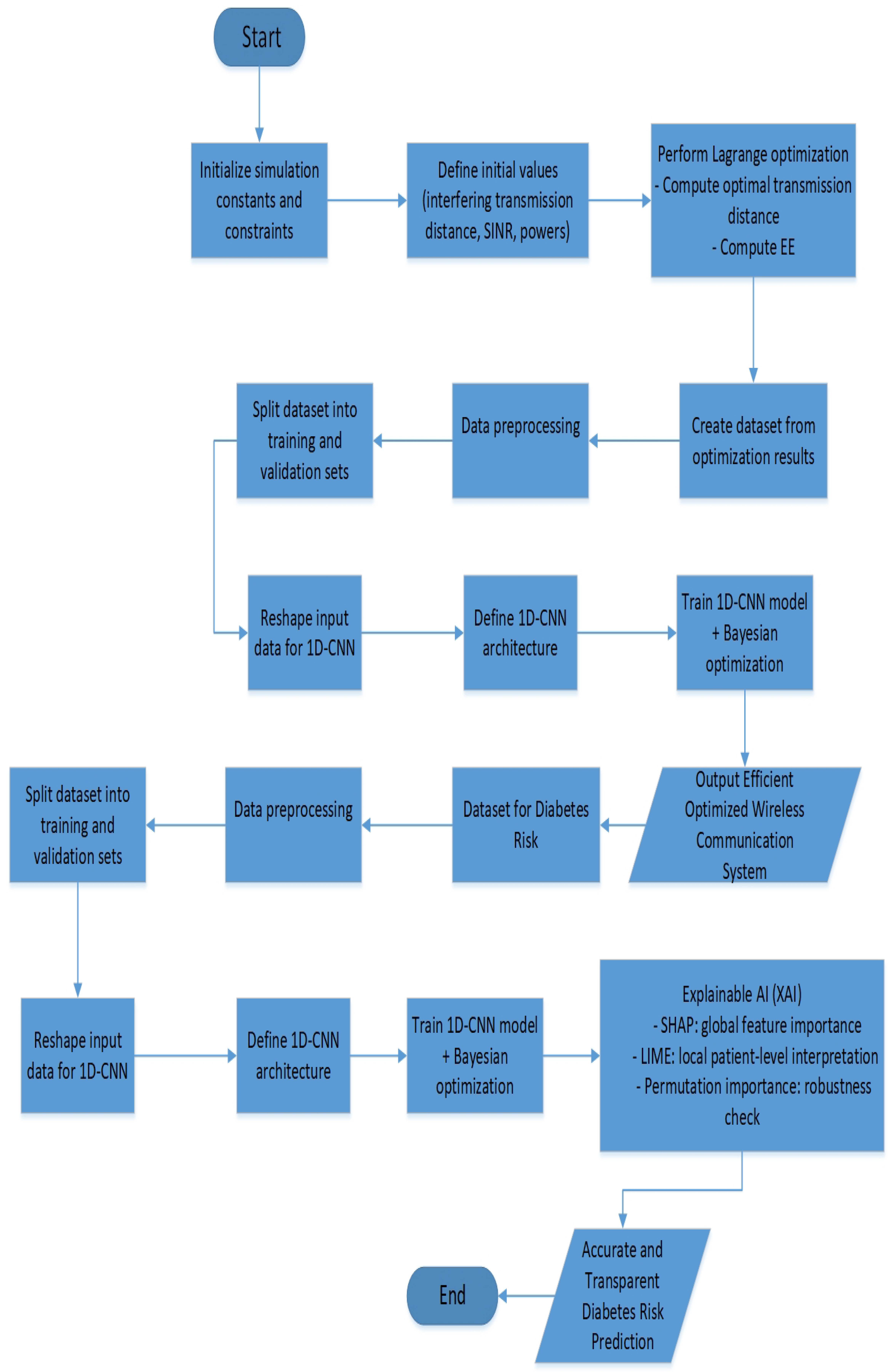
2. Materials and Methods
2.1. Proposed Wireless Communication Model
2.1.1. Data Generation
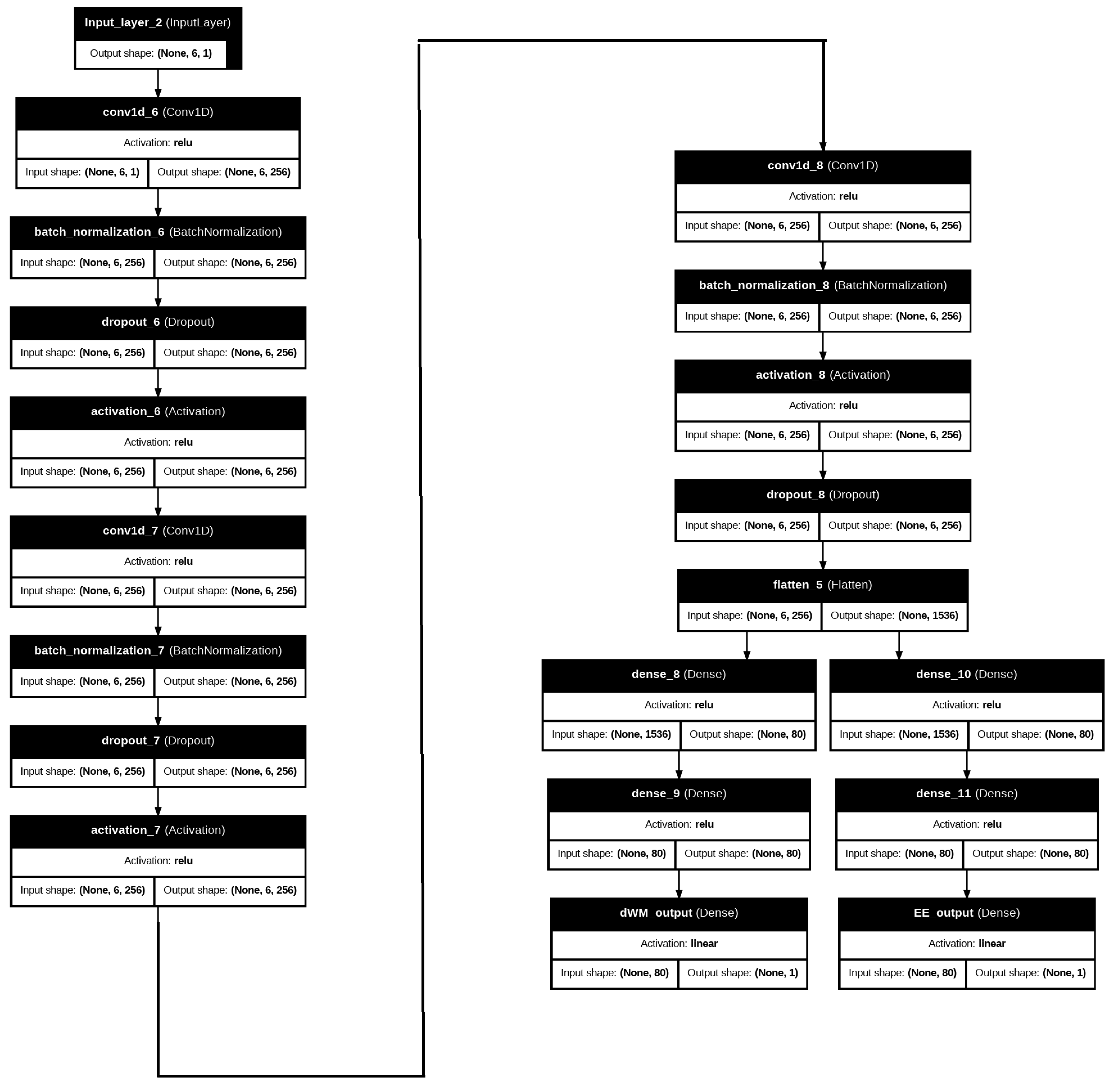
2.1.2. Proposed Wireless Communication Deep Learning Model
2.2. Proposed Diabetes Risk Prediction
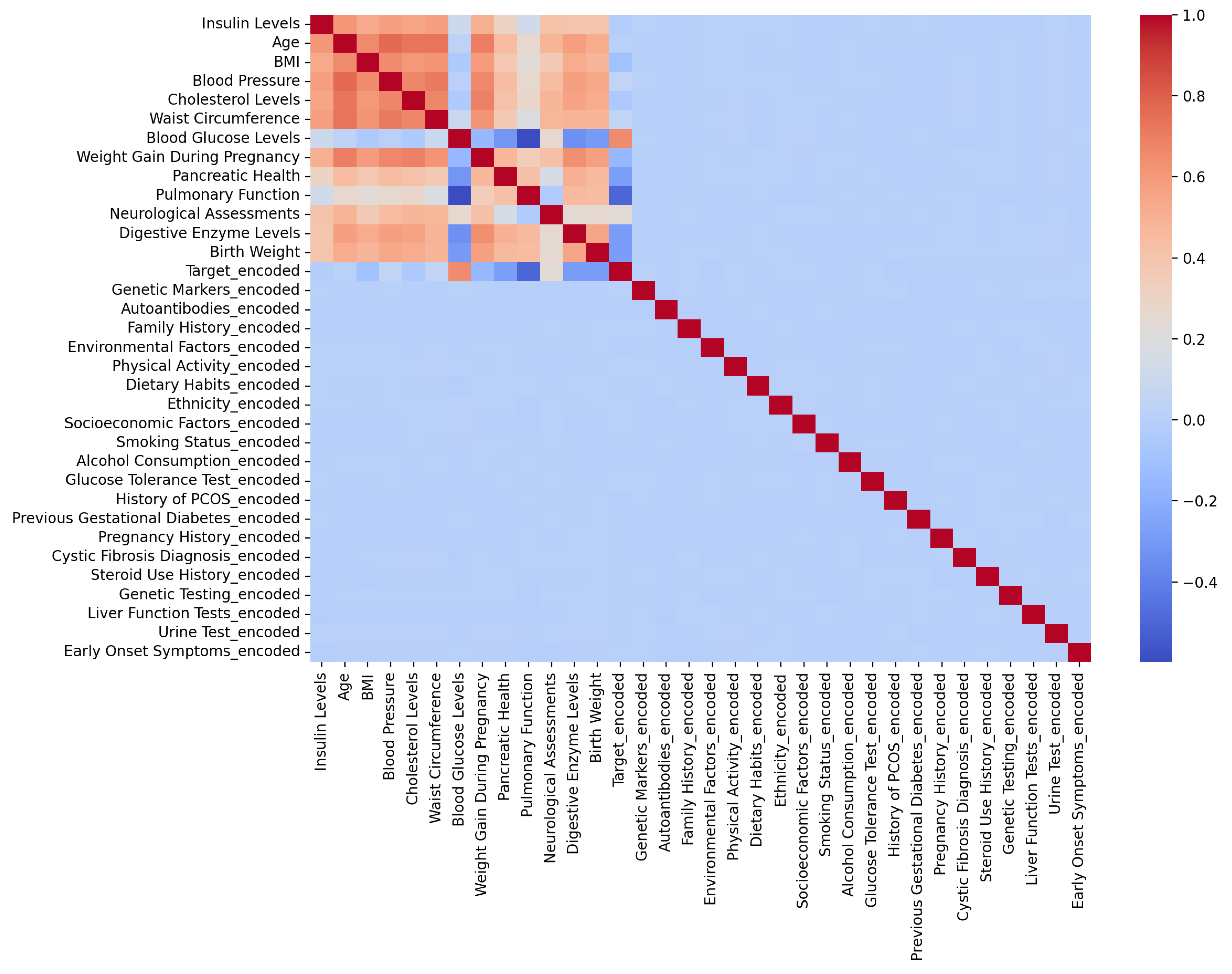
2.2.1. Dataset Description
- Genetic Markers: diabetes genetic signs that allow the model to take family risk factors into consideration.
- Body Mass Index, or BMI: a commonly used metric that evaluates body fat based on height and weight and is strongly linked to metabolic diseases.
- Blood Glucose Levels: a very important marker to treat and diagnose diabetes, such as postprandial and fasting glucose levels.
- Cholesterol Levels: contains measurements of total cholesterol, HDL (high-density lipoprotein), and LDL (low-density lipoprotein); all these are important indicators of metabolic and cardiovascular health.
- Lifestyle Factors: characteristics of people’s habits that can be changed to reduce the risk of diabetes, such as levels of physical activity, eating patterns, smoking status, and alcohol intake.
2.2.2. Data Preprocessing
- Handling Missing Data: In real-world datasets, missing values are a very common issue that may affect model performance. The imputation approach is the implemented method for handling the missing values in this dataset based on the distribution of each feature. The mean and median are used to impute continuous variables such as blood glucose, cholesterol, and BMI. The data distribution should be taken into account when choosing between mean and median imputation. However, only the median is used for skewed distributions to avoid bias from extreme findings. To maintain consistency across instances, the mode is used to impute missing values for categorical variables. This approach ensured little information loss while maintaining the integrity of the dataset.
- Normalization: The normalization of continuous features is essential to guarantee that the model can effectively learn from the data without being impacted by the magnitude of any one characteristic. Variables like blood glucose, cholesterol, and BMI should be subjected to min–max normalization. This method improves the numerical stability during training by scaling the data to a range between 0 and 1 to guarantee that the input values fall within a consistent scale. Given that significant differences in feature values can impede learning and slow convergence, this transformation is particularly crucial for neural networks. We guarantee that every feature makes an equal contribution to the model’s learning process by normalizing the data.
- Categorical Encoding: The dataset contains a number of categorical variables that cannot be used in machine learning models because they are not numeric values, such as alcohol consumption, physical activity, and smoking status. One-hot and label encoding were used to transform these variables into a machine-readable format. Therefore, label encoding is used to transfer the non-numeric categorical variables (such as smoking status: yes/no) to numeric values by giving a distinct integer value for each variable. One-hot encoding is used for the multi-class categorical variables such as physical activity levels or target. This change maintains the data’s fundamental structure while enabling the model to handle each category separately.
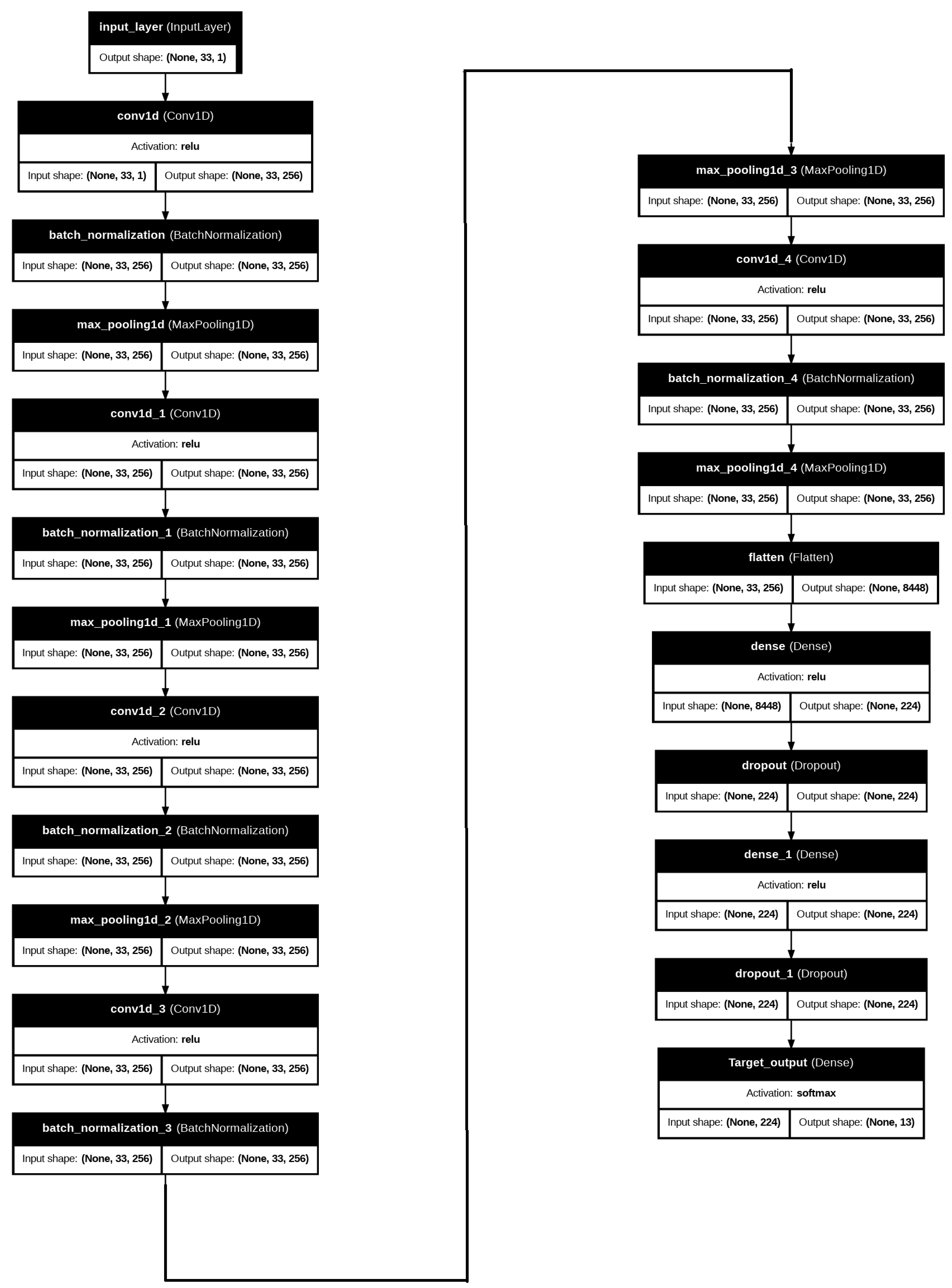
2.2.3. Proposed Diabetes Prediction Deep Learning Model
- The input layer takes in a dataset that has 34 normalized characteristics per instance, each of which represents a different genetic, lifestyle, clinical, and environmental component associated with diabetes. With each element representing a normalized value of one of the 34 qualities, these features are organized as a vector. In order to improve the stability and effectiveness of the learning process, the dataset has been preprocessed and normalized to guarantee that all values fall within a consistent range.
- Convolutional Layers: At the heart of the CNN design, the convolutional layers are in charge of extracting high-level feature representations and local patterns from the input data. To capture local relationships and hierarchical patterns between adjacent features, each convolutional layer employs filters (kernels) that move over the data while carrying out a convolution operation. The CNN layer has the ability to uncover intricate relationships between the input features when the dataset contains interactions between several variables.
- Activation Functions: The Rectified Linear Unit (ReLU) activation function is implemented after each convolutional layer. The ReLU function helps the model discover more complex patterns that a straightforward linear model is unable to grasp. The ReLU function allows the features to handle both negative and positive values in the data. This can improve the capacity of the model to discover complex relationships and decision boundaries between the features.
- Dense Layers: The data pass through fully connected (dense) layers, where every neuron is coupled to every other neuron in the preceding layer, following the convolutional layers. Dense layers aid in preparing the final output by integrating the learned information from the convolutional layers. These layers enable the model to integrate data from many features and identify more complex patterns that are essential for precise diabetes risk assessment.
- Output Layer: The forecasts based on diabetes risk scores are produced by the output layer. This output layer, which usually consists of a single neuron for regression tasks, generates a continuous value that is equivalent to the anticipated diabetes risk score. The likelihood that each person in the sample will acquire diabetes may then be estimated from this score.
- Optimizer and Loss Function: The optimizer used in this proposed model is the most well-known optimization, the Adam optimizer. Adam, known for its effectiveness and resilience in deep learning model training, is used to optimize the model. During training, Adam has the ability to modify the learning rate, facilitating a quicker and more efficient convergence of the model.
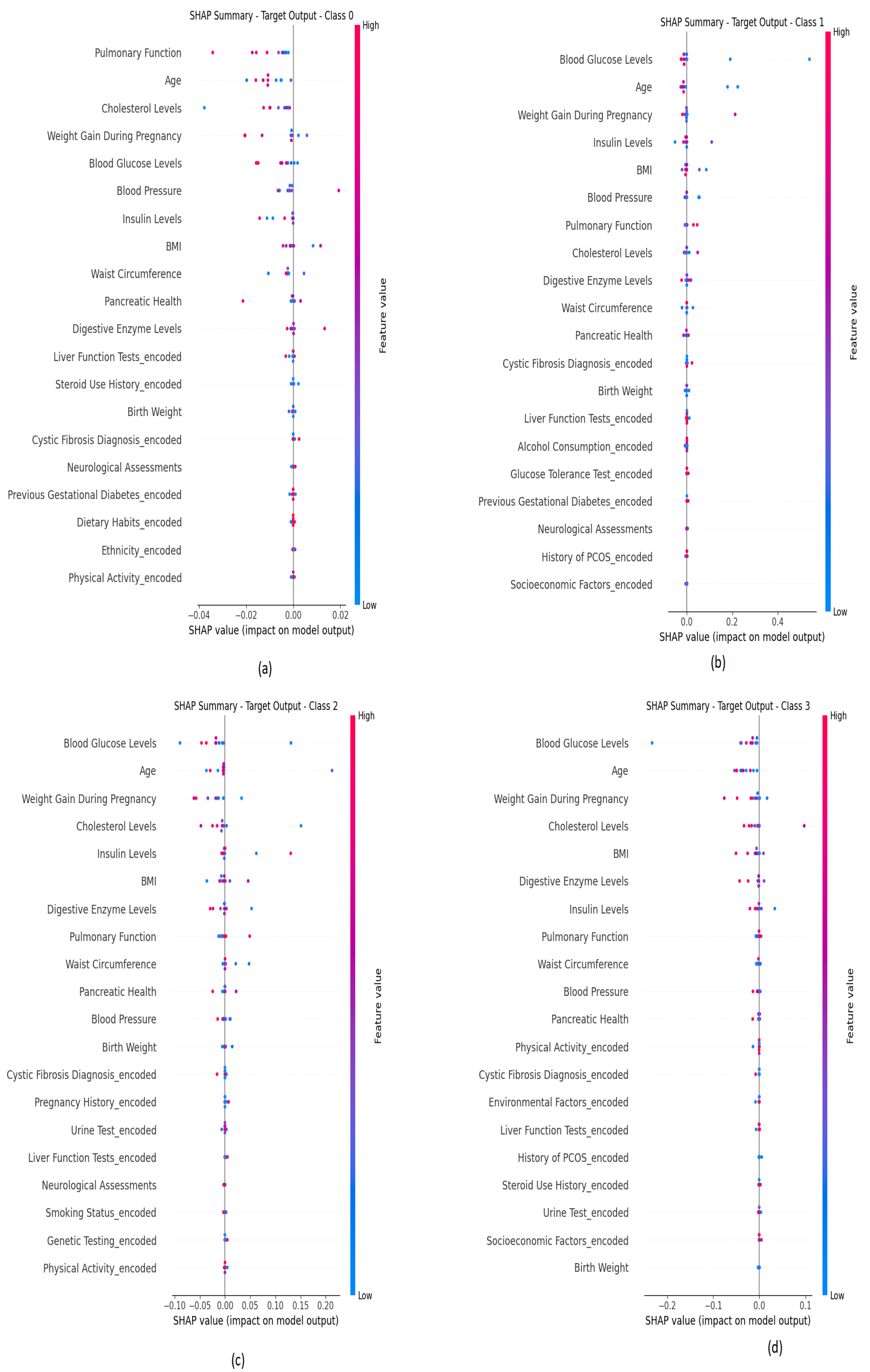
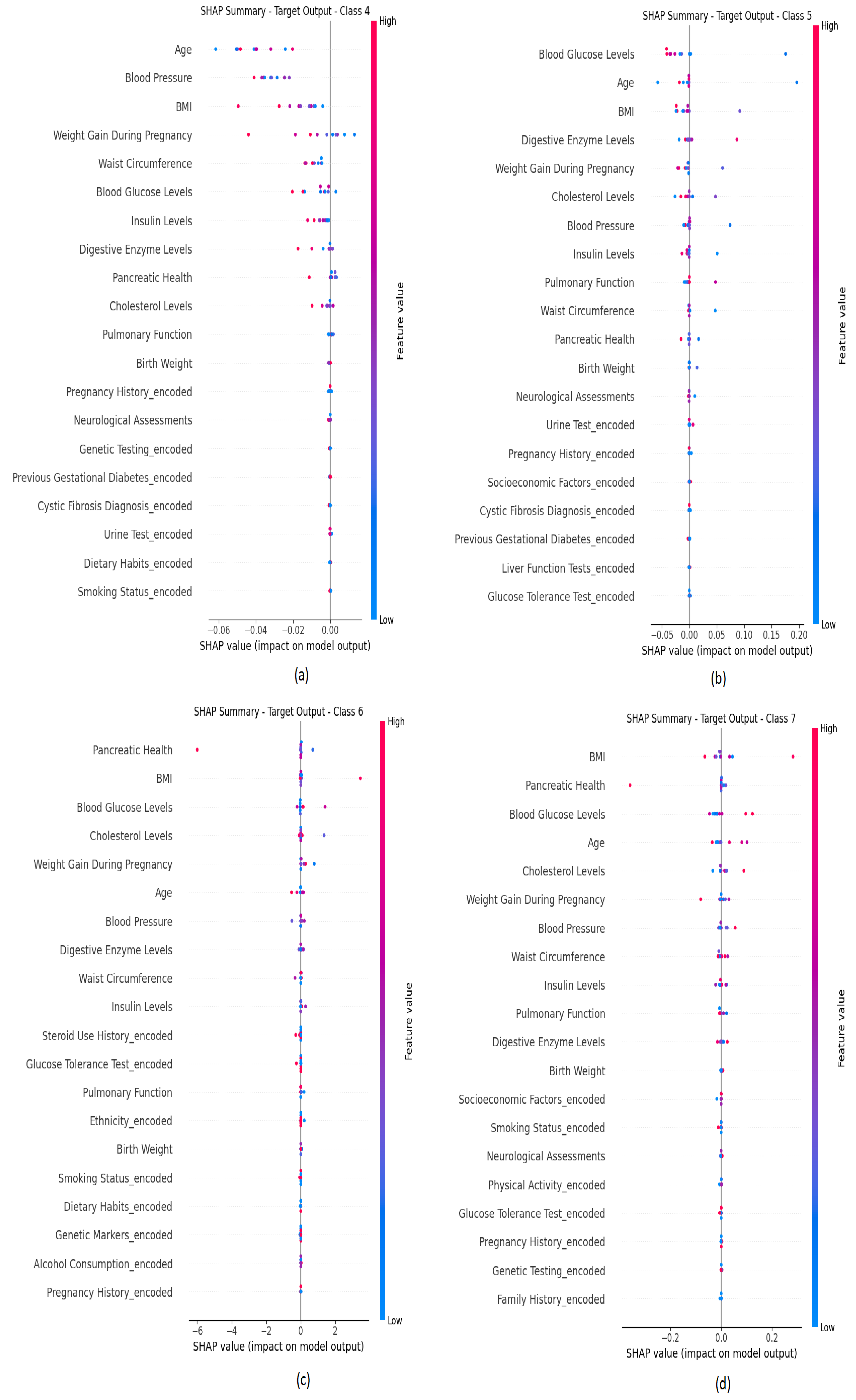
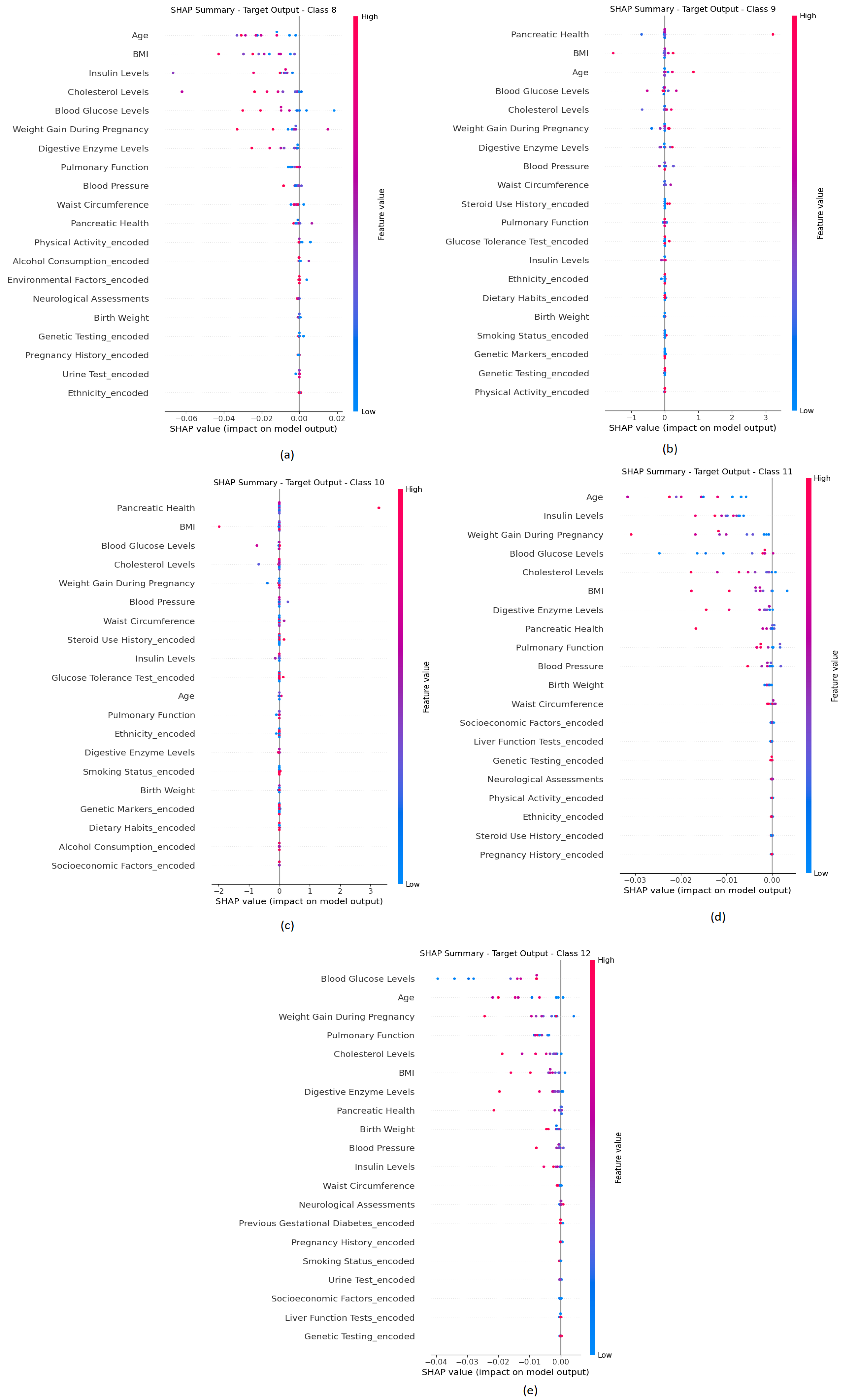
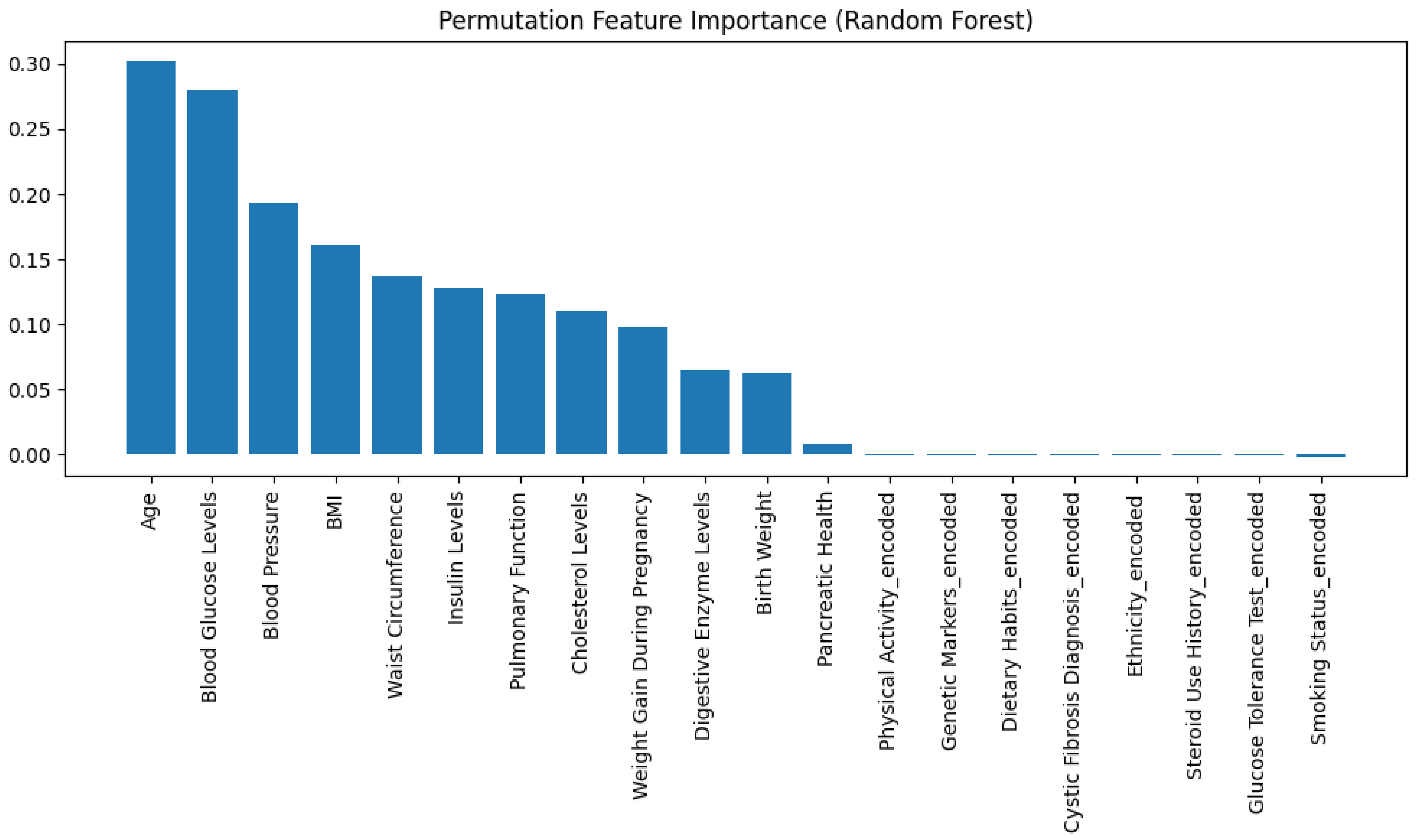
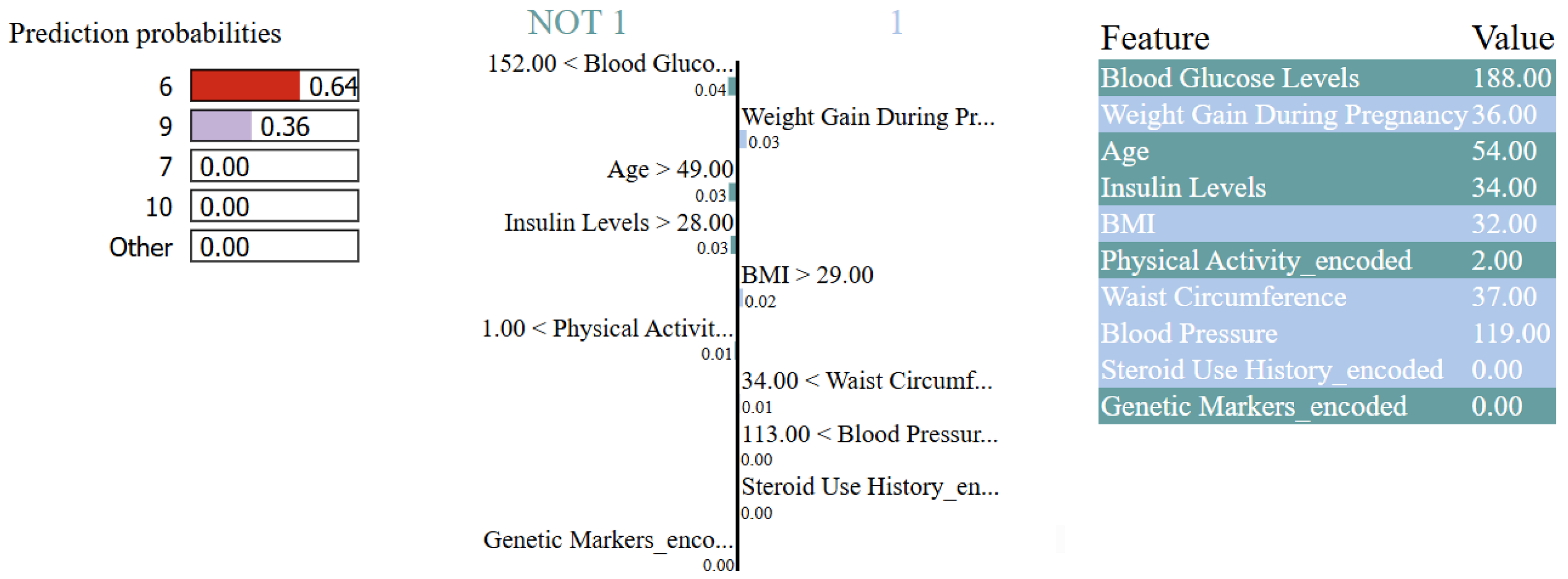
- XAI With SHAP and LIME: To enhance the transparency and the accuracy of the proposed deep learning model, a multi-method explainable AI (XAI) framework combining SHAP (SHapley Additive exPlanations), permutation feature importance, and LIME (Local Interpretable Model-agnostic Explanations) is implemented. SHAP is used to compute both global and local feature attributions, identifying the most important input variables for each output target class across the entire dataset. The top-ranked features, based on mean absolute SHAP values, were then selected for retraining the model to reduce complexity while maintaining predictive performance. Permutation feature importance is applied using a surrogate Random Forest model trained on these selected features, identifying the impact of each feature on model accuracy when shuffled. Additionally, LIME is implemented to provide a clean explanation for individual predictions, showing the contribution of each feature for a specific test instance.
2.2.4. Evaluation Metrics for the Proposed Diabetes Risk Prediction Model
2.2.5. Computational Environment
3. Results
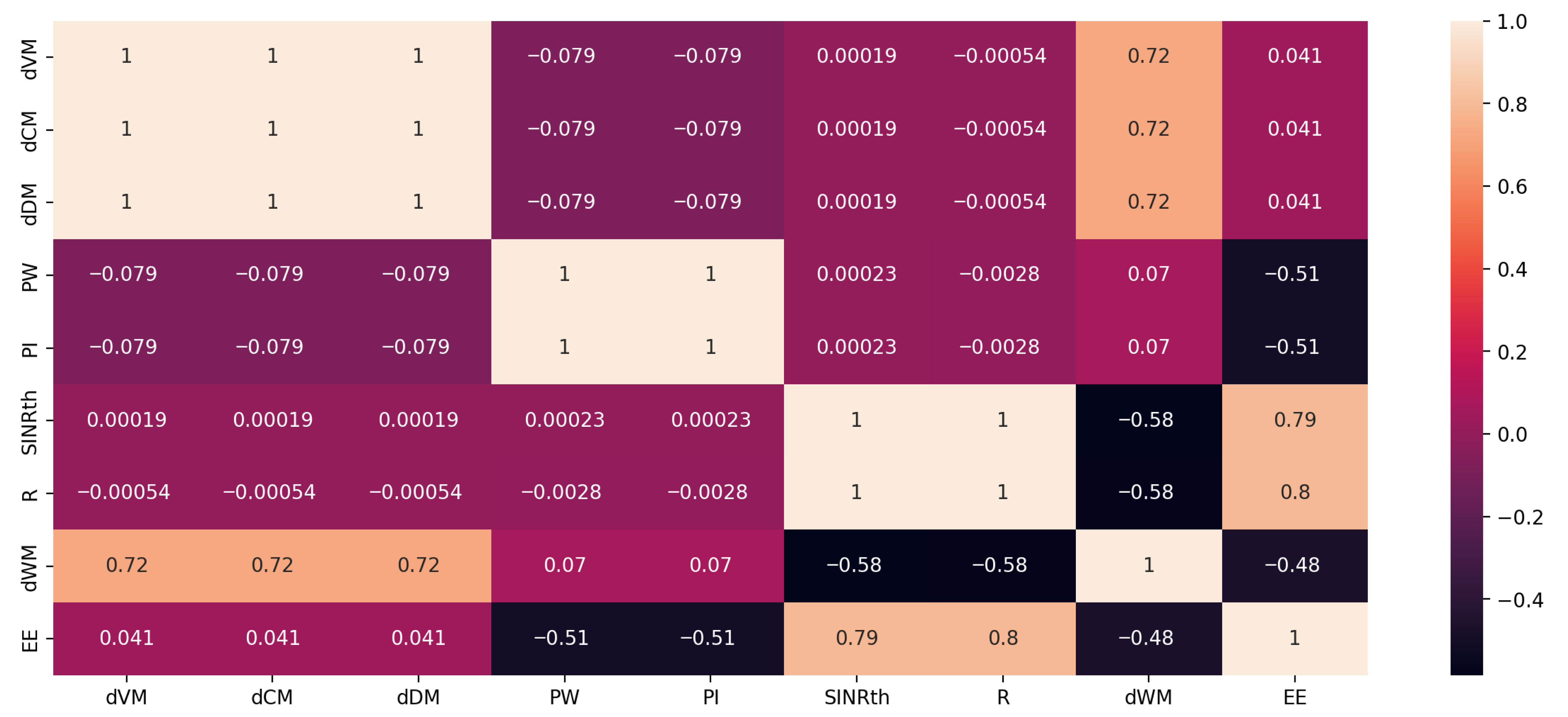
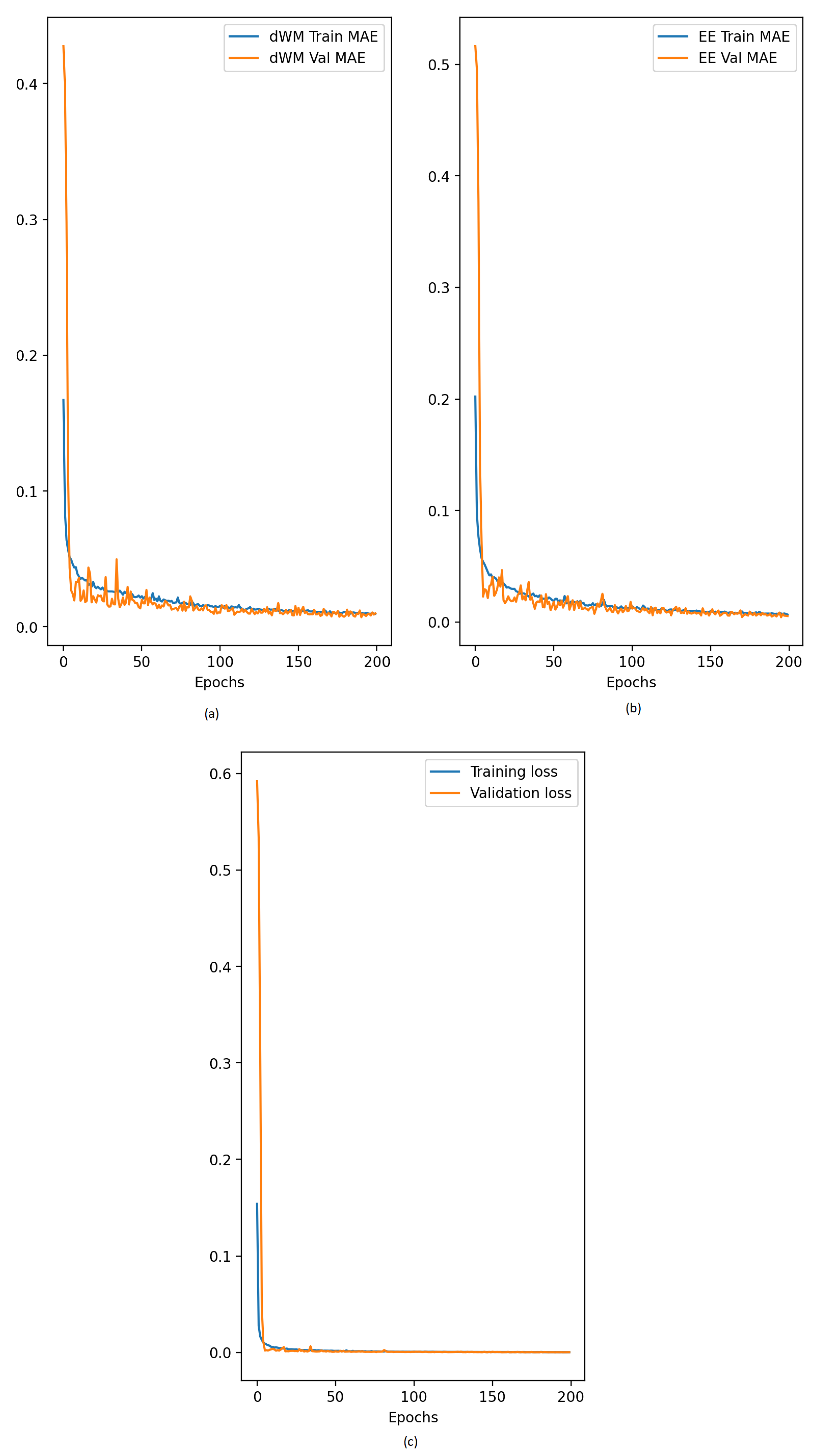
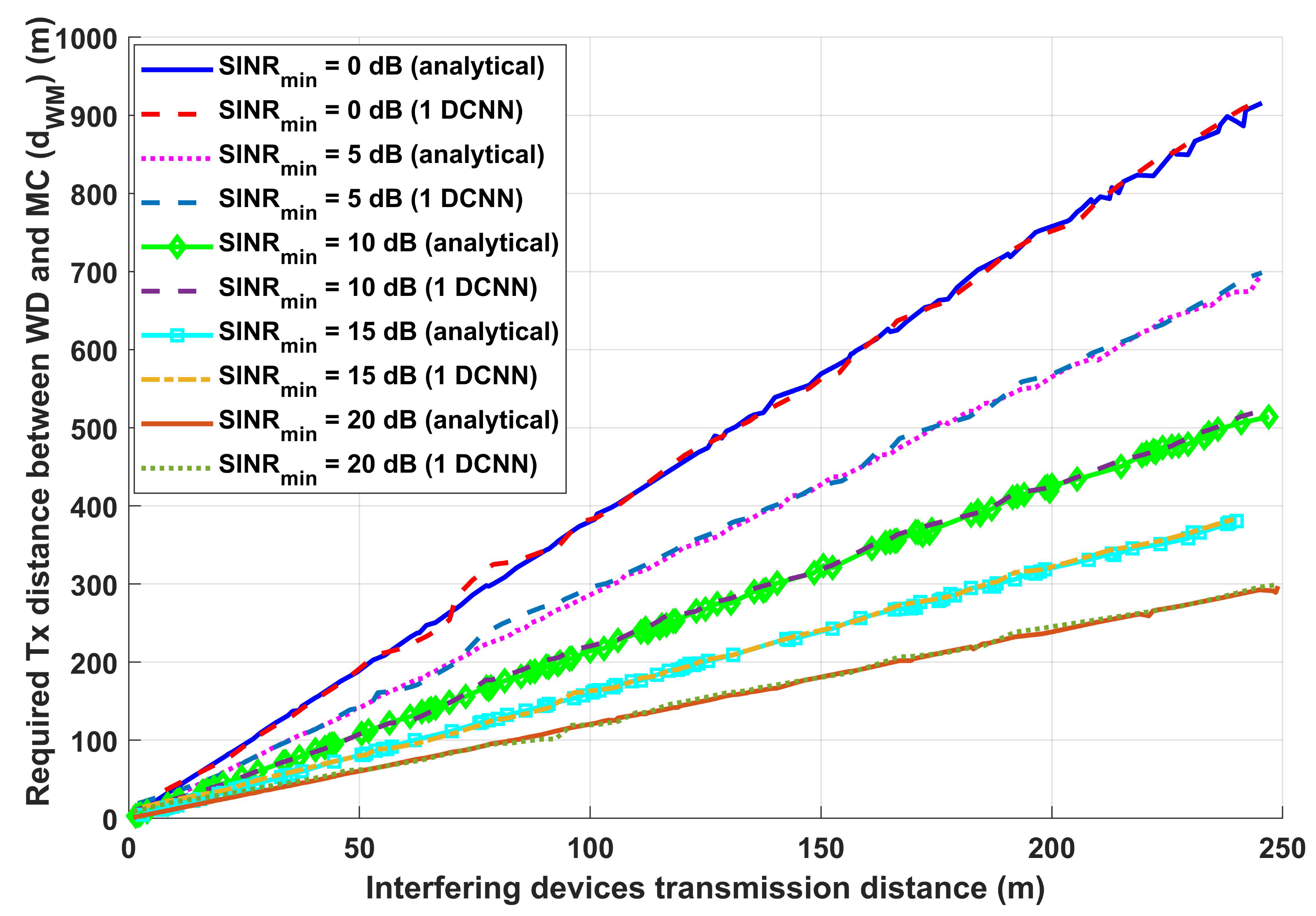
3.1. Performance Evaluation for the Proposed Wireless Communication Model
3.2. Performance Evaluation for the Proposed Diabetes Prediction Risk
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Parameter | Definition |
| Wearable device transmission power | |
| Maximum wearable device transmission power | |
| Interference transmission power | |
| Maximum interference transmission power | |
| Dtx transmission power | |
| CUE transmission power | |
| Vtx transmission power | |
| Internal circuitry power consumption | |
| EE | Energy efficiency |
| 1 D-CNN | 1D Convolutional Neural Network |
| LSTM | Long Short-Term Memory |
| GRU | Gated Recurrent Unit |
| SHAP | SHapley Additive exPlanations |
| LIME | Local Interpretable Model Agnostic Explanation |
| AI | Artificial intelligence |
| ML | Machine learning |
| NLP | Natural Language Processing |
| RPA | Robotic Process Automation |
| EHR | Electronic Health Record |
| XAI | Explainable AI |
| LPDS | Liver Patients Detection Strategy |
| IB2OA | Improved Binary Butterfly Optimization Algorithm |
| PHY | Physical layer |
| ECG | Electrocardiogram |
| BLE-5-based | Bluetooth Low Energy version 5 |
| WBAN | Wireless Body Area Network |
| SINR | Signal-to-interference-plus-noise |
| Minimum signal-to-interference-plus-noise | |
| CUE | Cellular user equipments |
| D2D | Device-to-device |
| V2V | Vehicle-to-vehicle |
| WD | Wearable device |
| MC | Medical centers |
| n | The additive white Gaussian noise |
| The noise power | |
| I | Interference |
| Direct channel gain from CUE–MC | |
| Direct channel gain from Drx–MC | |
| Direct channel gain from Vtx–MC | |
| Transmission distance CUE–MC | |
| Transmission distance Dtx–MC | |
| Transmission distance Vtx–MC | |
| Transmission distance WD–MC | |
| AWGN | Additive white Gaussian noise |
| NOMA | Non-Orthogonal Multiple Access |
| , and | The non-negative Lagrange multiplier |
| MAE | Mean Absolute Error |
| MSE | Mean Square Error |
| TP | The number of True Positives |
| TN | The number of True Negatives |
| FP | The number of False Positives |
| FN | The number of False Negatives |
| RMSE | Root Mean Square Error |
| Coefficient of Determination | |
| QoS | Quality-of-service |
| ReLU | Rectified Linear Unit |
| PReLU | Parametric Rectified Linear Unit |
References
- Setyawati, R.; Astuti, A.; Utami, T.P.; Adiwijaya, S.; Hasyim, D.M. The importance of early detection in disease management. J. World Future Med. Health Nurs. 2024, 2, 51–63. [Google Scholar]
- Pennisi, F.; Gentile, L.; Borlini, S.; Gianfredi, V.; Signorelli, C. Direct and indirect healthcare costs of ocular diseases in Italy: A literature review on glaucoma, diabetic retinopathy, and macular degeneration. Ann. Ig. 2025, 10, 532–555. [Google Scholar]
- Kovács, N.; Shahin, B.; Andrade, C.A.S.; Mahrouseh, N.; Varga, O. Lifestyle and metabolic risk factors, and diabetes mellitus prevalence in European countries from three waves of the European Health Interview Survey. Sci. Rep. 2024, 14, 11623. [Google Scholar] [CrossRef]
- Górczyńska-Kosiorz, S.; Kosiorz, M.; Dzięgielewska-Gęsiak, S. Exploring the interplay of genetics and nutrition in the rising epidemic of obesity and metabolic diseases. Nutrients 2024, 16, 3562. [Google Scholar] [CrossRef]
- Siddiqui, M.N.I. Global epidemic of diabetes, growing burden of young-onset diabetes, and regional perspective. J. Assoc. Clin. Endocrinol. Diabetol. Bangladesh 2025, 4, 1–3. [Google Scholar] [CrossRef]
- Al-Busafi, S.A.; Alwassief, A. Global perspectives on the hepatitis B vaccination: Challenges, achievements, and the road to elimination by 2030. Vaccines 2024, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Alubaie, M.A.; Sayed, M.Y.; Alnakhli, R.E.; Alshaia, F.I.N.; Aldossary, S.B.; Alsubaie, N.M.; Mubarek, S.A.A.; Raeedi, W.A.; Alhamdan, A.M.; Hassani, A.H.; et al. The efficiency and accuracy gains of real-time health data integration in healthcare management: A comprehensive review of current practices and future directions. Egypt. J. Chem. 2024, 67, 1725–1729. [Google Scholar] [CrossRef]
- Alharbe, N.; Almalki, M. IoT-enabled healthcare transformation leveraging deep learning for advanced patient monitoring and diagnosis. Multimed. Tools Appl. 2025, 84, 21331–21344. [Google Scholar] [CrossRef]
- Asif, S.; Wenhui, Y.; ur-Rehman, S.; ul-Ain, Q.; Amjad, K.; Yueyang, Y.; Awais, M. Advancements and prospects of machine learning in medical diagnostics: Unveiling the future of diagnostic precision. Arch. Comput. Methods Eng. 2025, 32, 853–883. [Google Scholar] [CrossRef]
- Faiyazuddin, M.; Rahman, S.J.Q.; Anand, G.; Siddiqui, R.K.; Mehta, R.; Khatib, M.N.; Gaidhane, S.; Zahiruddin, Q.S.; Hussain, A.; Sah, R. The impact of artificial intelligence on healthcare: A comprehensive. Health Sci. Rep. 2025, 8, e70312. [Google Scholar] [CrossRef]
- El-Kenawy, E.-S.M.; Alhussan, A.A.; Khodadadi, N.; Mirjalili, S.; Eid, M.M. Predicting potato crop yield with machine learning and deep learning for sustainable agriculture. Potato Res. 2025, 68, 759–792. [Google Scholar] [CrossRef]
- Zhou, X.; Leung, C.K.; Kevin, I.; Wang, K.; Fortino, G. Editorial deep learning-empowered big data analytics in biomedical applications and digital healthcare. IEEE/ACM Trans. Comput. Biol. Bioinform. 2024, 21, 516–520. [Google Scholar] [CrossRef]
- Osman, R.A. Internet of Medical Things (IoMT) optimization for healthcare: A deep learning-based interference avoidance model. Comput. Netw. 2024, 248, 110491. [Google Scholar] [CrossRef]
- Kothinti, R.R. Deep learning in healthcare: Transforming disease diagnosis, personalized treatment, and clinical decision-making through AI-driven innovations. World J. Adv. Res. Rev. 2024, 24, 2841–2856. [Google Scholar] [CrossRef]
- Van Roosmale, S.; Hellinckx, P.; Meysman, J.; Verbeke, S.; Audenaert, A. Building automation and control systems for office buildings: Technical insights for effective facility management—A literature review. J. Build. Eng. 2024, 97, 110943. [Google Scholar] [CrossRef]
- Osman, R.A. Energy-efficient IoT-based tracking and management system for patients with cognitive diseases. J. Netw. Comput. Appl. 2025, 243, 104289. [Google Scholar] [CrossRef]
- Osman, R.A. Energy-efficient communication between IoMT devices and emergency vehicles for improved patient care. PLoS ONE 2025, 20, e0330695. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.U.R.; Saba, T.; Khan, I.; Mahmood, T.; Khan, A.R.; Singh, S.K.; Daradkeh, Y.I.; Ullah, I. Proactive and data-centric internet of things-based fog computing architecture for effective. Comput. Electr. Eng. 2025, 123, p.110030. [Google Scholar] [CrossRef]
- Wang, S.C.; Nickel, G.; Venkatesh, K.P.; Raza, M.M.; Kvedar, J.C. AI-based diabetes care: Risk prediction models and implementation concerns. NPJ Digit. Med. 2024, 7, 36. [Google Scholar] [CrossRef]
- Angelucci, F.; Ai, A.R.; Piendel, L.; Cerman, J.; Hort, J. Integrating AI in fighting advancing Alzheimer: Diagnosis, prevention, treatment, monitoring, mechanisms, and clinical trials. Curr. Opin. Struct. Biol. 2024, 87, 102857. [Google Scholar] [CrossRef]
- Tian, F.; Liu, D.; Wei, N.; Fu, Q.; Sun, L.; Liu, W.; Sui, X.; Tian, K.; Nemeth, G.; Feng, J. Prediction of tumor origin in cancers of unknown primary origin with cytology-based deep learning. Nat. Med. 2024, 30, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Tanim, S.A.; Shrestha, T.E.; Emon, M.R.I.; Mridha, M.F.; Miah, M.S.U. Explainable deep learning for diabetes diagnosis with DeepNetX2. Biomed. Signal Process. Control 2025, 99, 106902. [Google Scholar] [CrossRef]
- Kalra, N.; Verma, P.; Verma, S. Advancements in AI based healthcare techniques with focus on diagnostic techniques. Comput. Biol. Med. 2024, 179, 108917. [Google Scholar] [CrossRef]
- Jabeen, K.; Khan, M.A.; Damaševičius, R.; Alsenan, S.; Baili, J.; Zhang, Y.-D.; Verma, A. An intelligent healthcare framework for breast cancer diagnosis based on the information fusion of novel deep learning architectures and improved optimization algorithm. Eng. Appl. Artif. Intell. 2024, 137, 109152. [Google Scholar] [CrossRef]
- Shaban, W.M. Early diagnosis of liver disease using improved binary butterfly optimization and machine learning algorithms. Multimed. Tools Appl. 2024, 83, 30867–30895. [Google Scholar] [CrossRef]
- Elbedwehy, S.; Hassan, E.; Saber, A.; Elmonier, R. Integrating neural networks with advanced optimization techniques for accurate kidney disease diagnosis. Sci. Rep. 2024, 14, 21740. [Google Scholar] [CrossRef]
- Ashwini, A.; Chirchi, V.; Balasubramaniam, S.; Shah, M.A. Bio inspired optimization techniques for disease detection in deep learning systems. Sci. Rep. 2025, 15, 18202. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Gautam, S.; Bal, N.S.; Kumar, S.; Saeed, N. IoT-enabled energy-efficient and long-range solution for remote patient monitoring using Bluetooth low energy 5.x. IEEE J. Radio Freq. Identif. 2025, 9, 527–541. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, M.; Kwon, K.W.; Kim, J.S.; Jung, J.M.; Lee, H.M. An energy-efficient real-time wearable ECG acquisition system with near-data pre-diagnosis for mobile applications. IEEE Trans. Instrum. Meas. 2025, 74, 2003711. [Google Scholar] [CrossRef]
- Alatawi, M.N. Optimizing security and energy efficiency in IoT-based health monitoring systems for wireless body area networks. Sci. Rep. 2025, 15, 24921. [Google Scholar] [CrossRef]
- Meftah, E.H.; Benmahmoud, S.; Rabehi, A. Innovative wireless solutions for real-time ECG monitoring: Leveraging BLE, Wi-Fi, and LoRa technologies. In Proceedings of the 2024 International Conference on Telecommunications and Intelligent Systems (ICTIS), Djelfa, Algeria, 14–15 December 2024; pp. 1–6. [Google Scholar]
- Tesema, W.; Jimma, W.; Khan, M.I.; Stiens, J.; da Silva, B. A taxonomy of low-power techniques in wearable medical devices for healthcare applications. Electronics 2024, 13, 3097. [Google Scholar] [CrossRef]
- Ankitbatra1210. Diabetes Dataset. Kaggle. 2025. Available online: https://www.kaggle.com/datasets/ankitbatra1210/diabetes-dataset/data (accessed on 21 March 2025).
- Abuajwa, O.; Muhammad, S.; Ambak, Z.; Mitani, S.; Tiang, J.-J. Energy efficiency techniques in non-orthogonal multiple access (NOMA) in 5G network: An overview and outlook. Wirel. Pers. Commun. 2025, 140, 971–1013. [Google Scholar] [CrossRef]
- Kakavand, M.; Hassannejad Bibalan, M.; Baghani, M. Capacity analysis over fractional order Rayleigh fading channel under additive white generalized Gaussian noise. IET Commun. 2024, 18, 1391–1402. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Wu, X.; Tang, Y.; Liu, W.; Sun, D. Energy-Efficient Resource Allocation for V2X Communications. IEEE Internet Things J. 2024, 11, 30014–30026. [Google Scholar] [CrossRef]
- Cao, X.; Wang, S.; Ren, X. IRS-Enhanced V2X Communication and Computation Systems: Resource Allocation and Performance Optimization. IEEE Internet Things J. 2024, 12, 9180–9194. [Google Scholar] [CrossRef]
- Alkhanbouli, R.; Almadhaani, H.M.A.; Alhosani, F.; Simsekler, M.C.E. The role of explainable artificial intelligence in disease prediction: A systematic literature review and future research directions. BMC Med. Inform. Decis. Mak. 2025, 25, 110. [Google Scholar] [CrossRef] [PubMed]
- El-Sofany, H.; El-Seoud, S.A.; Karam, O.H.; Abd El-Latif, Y.M.; Taj-Eddin, I.A. A proposed technique using machine learning for the prediction of diabetes disease through a mobile app. Int. J. Intell. Syst. 2024, 2024, 6688934. [Google Scholar] [CrossRef]
| Parameter | Value |
|---|---|
| B | 10 Mbit/s [36] |
| N | −174 dBm/Hz [36] |
| 0.1 W | |
| 4 | |
| 17–23 dBm [37] | |
| 20 dB | |
| dB [37] | |
| dB [37] | |
| dB [37] |
| Layer Type | Hyperparameter | Value |
|---|---|---|
| Input Layer | Input Shape | (None, 33, 1) |
| Conv1D | Filters | 256 |
| Kernel Size | 3 | |
| Dropout | 0.4 | |
| Activation Function | ReLU | |
| Batch Normalization | - | - |
| Activation | Activation Function | ReLU |
| Dense | Units | 224 |
| Activation Function | ReLU | |
| Output Layer (Target) | Units | 1 |
| Adam | ||
| Learning Rate | 0.001 | |
| Batch Size | 50 | |
| Epochs | 200 | |
| Loss Function | sparse categorical crossentropy | |
| Validation Split | 0.2 | |
| Random Forest Regressor | ||
| n_estimators | 100 | |
| max_depth | 10 | |
| random_state | 42 |
| Algorithm | MSE | RMSE | MAE | MSE | RMSE | MAE | ||
|---|---|---|---|---|---|---|---|---|
| Parameter | EE | |||||||
| GRU | 0.00128 | 0.03574 | 0.0278 | 0.992 | 0.00115 | 0.034 | 0.02647 | 0.995 |
| LSTM | 0.00154 | 0.04 | 0.031 | 0.9914 | 0.00143 | 0.038 | 0.03123 | 0.9945 |
| 1D-CNN | 0.054 | 0.2321 | 0.1934 | 0.666 | 0.1008 | 0.3175 | 0.2665 | 0.615 |
| Proposed 1D-CNN | 0.000372 | 0.0193 | 0.01453 | 0.999 | 0.00013 | 0.011404 | 0.0081 | 0.9995 |
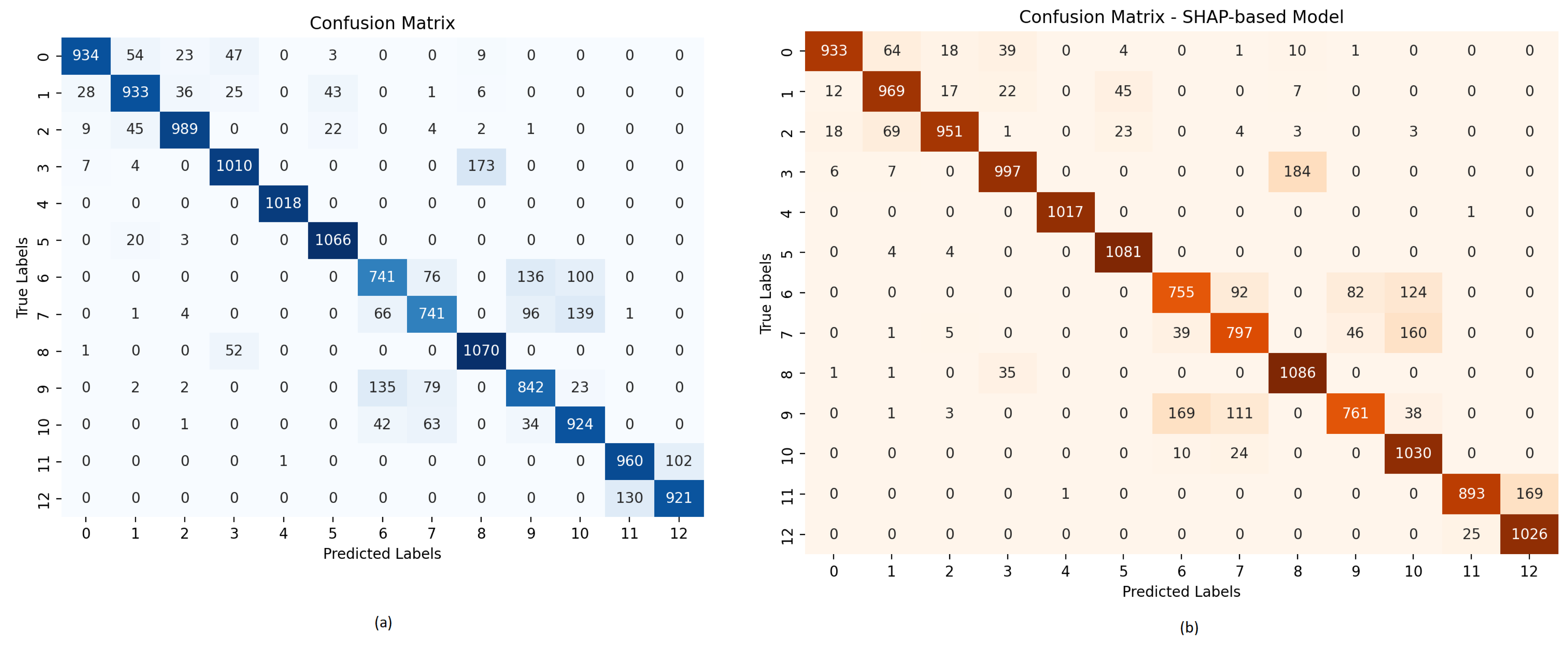
| Algorithm | Accuracy | Precision | Recall | F-1 Score | ROC-AUC | Matthews Correlation Matrix |
|---|---|---|---|---|---|---|
| GRU | 0.7746 | 0.7945 | 0.7739 | 0.7727 | 0.983 | 0.7572 |
| LSTM | 0.6917 | 0.7007 | 0.6914 | 0.6671 | 0.9726 | 0.6708 |
| 1D-CNN | 0.8487 | 0.8577 | 0.8487 | 0.8478 | 0.9918 | 0.8370 |
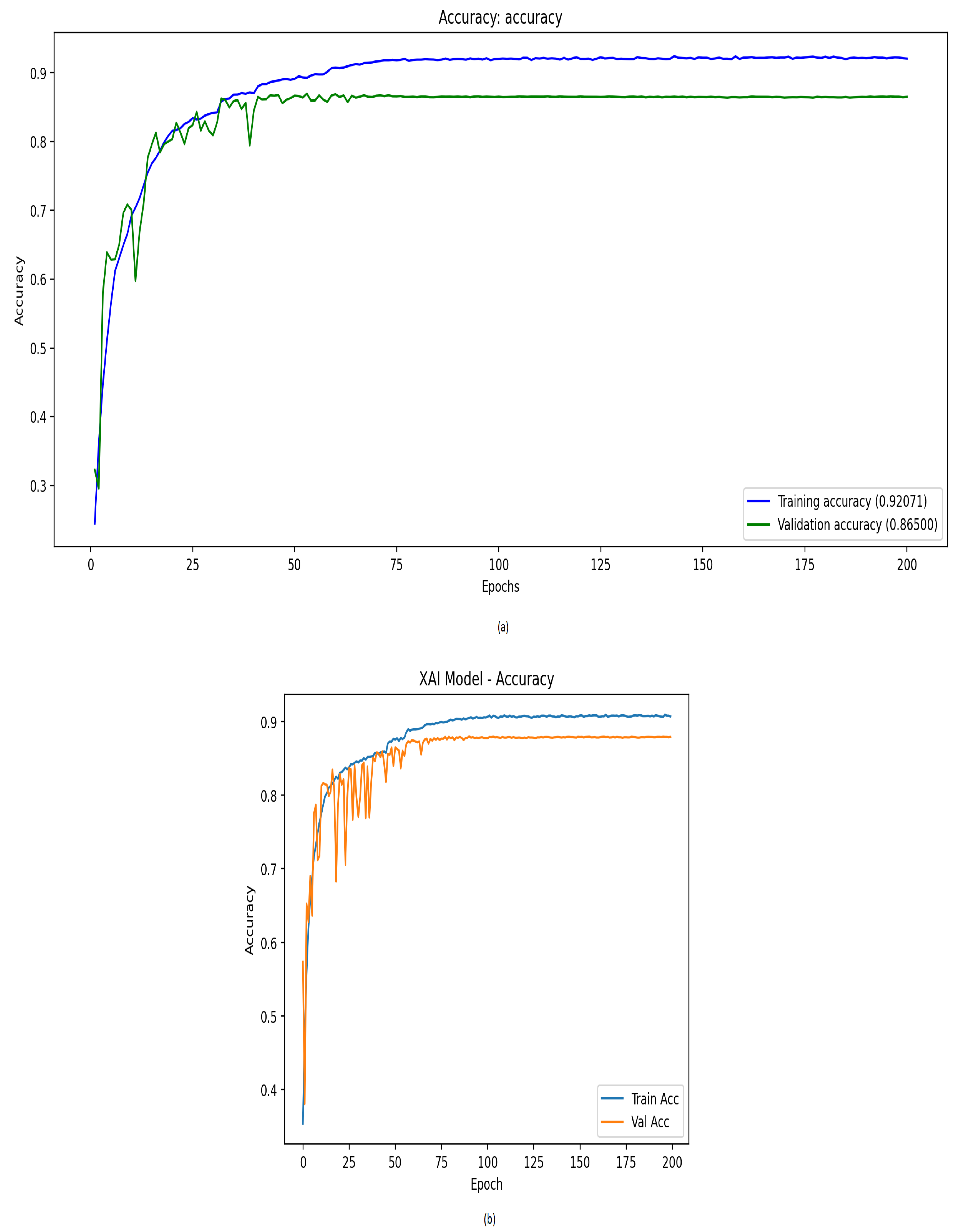
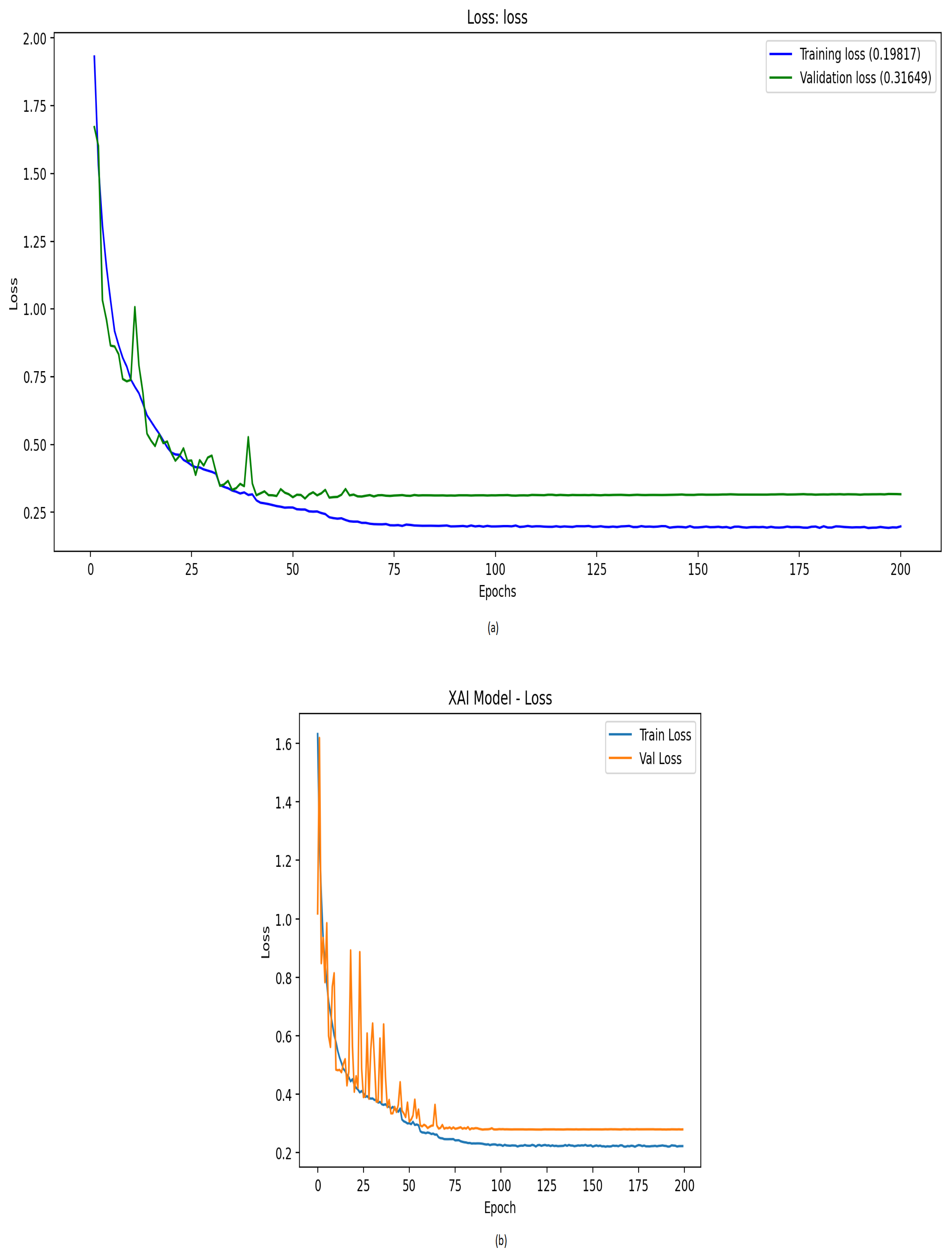
| Optimized 1D-CNN | 0.8678 | 0.8684 | 0.8667 | 0.8672 | 0.9941 | 0.8569 |
| Optimized 1D-CNN with XAI | 0.8783 | 0.8822 | 0.8768 | 0.8773 | 0.9945 | 0.8687 |
| Algorithm | Accuracy | Precision | Recall | F-1 Score |
|---|---|---|---|---|
| Logistic Regression | 0.7700 | 0.6300 | 0.7000 | 0.6600 |
| Random Forest | 0.7500 | 0.6000 | 0.6600 | 0.6300 |
| KNN | 0.6400 | 0.4900 | 0.7300 | 0.5900 |
| Decision Tree | 0.7200 | 0.7900 | 0.7700 | 0.7800 |
| Bagging | 0.7500 | 0.8100 | 0.8100 | 0.8100 |
| AdaBoost | 0.7300 | 0.8000 | 0.7700 | 0.7800 |
| XGBoost | 0.8310 | 0.7000 | 0.8400 | 0.7600 |
| Voting | 0.7500 | 0.8300 | 0.7600 | 0.7900 |
| SVM | 0.7700 | 0.8700 | 0.7700 | 0.8200 |
| Naïve Bayes | 0.8120 | 0.7300 | 0.7100 | 0.7200 |
| Optimized 1D-CNN + XAI | 0.8783 | 0.8822 | 0.8768 | 0.8773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, R.A. Explainable AI-Driven 1D-CNN with Efficient Wireless Communication System Integration for Multimodal Diabetes Prediction. AI 2025, 6, 243. https://doi.org/10.3390/ai6100243
Osman RA. Explainable AI-Driven 1D-CNN with Efficient Wireless Communication System Integration for Multimodal Diabetes Prediction. AI. 2025; 6(10):243. https://doi.org/10.3390/ai6100243
Chicago/Turabian StyleOsman, Radwa Ahmed. 2025. "Explainable AI-Driven 1D-CNN with Efficient Wireless Communication System Integration for Multimodal Diabetes Prediction" AI 6, no. 10: 243. https://doi.org/10.3390/ai6100243
APA StyleOsman, R. A. (2025). Explainable AI-Driven 1D-CNN with Efficient Wireless Communication System Integration for Multimodal Diabetes Prediction. AI, 6(10), 243. https://doi.org/10.3390/ai6100243






