Acropora spp. Coral Gardening Using Fragmentation and Direct Transplantation: A Feasibility Study at Boundary Island
Abstract
1. Introduction
2. Materials and Methods
2.1. Restoration Site
2.2. Coral Reef Survey
2.3. Coral Species Identification
2.4. Studied Species
2.5. Construction of Coral Nurseries
2.6. Coral Fragmentation
2.7. Coral Transplantation and In Situ Nursery
2.8. Costing Exercise
2.9. Data Analysis
3. Results
3.1. Coral Reef Survey Results
3.2. Ex Situ Nursery
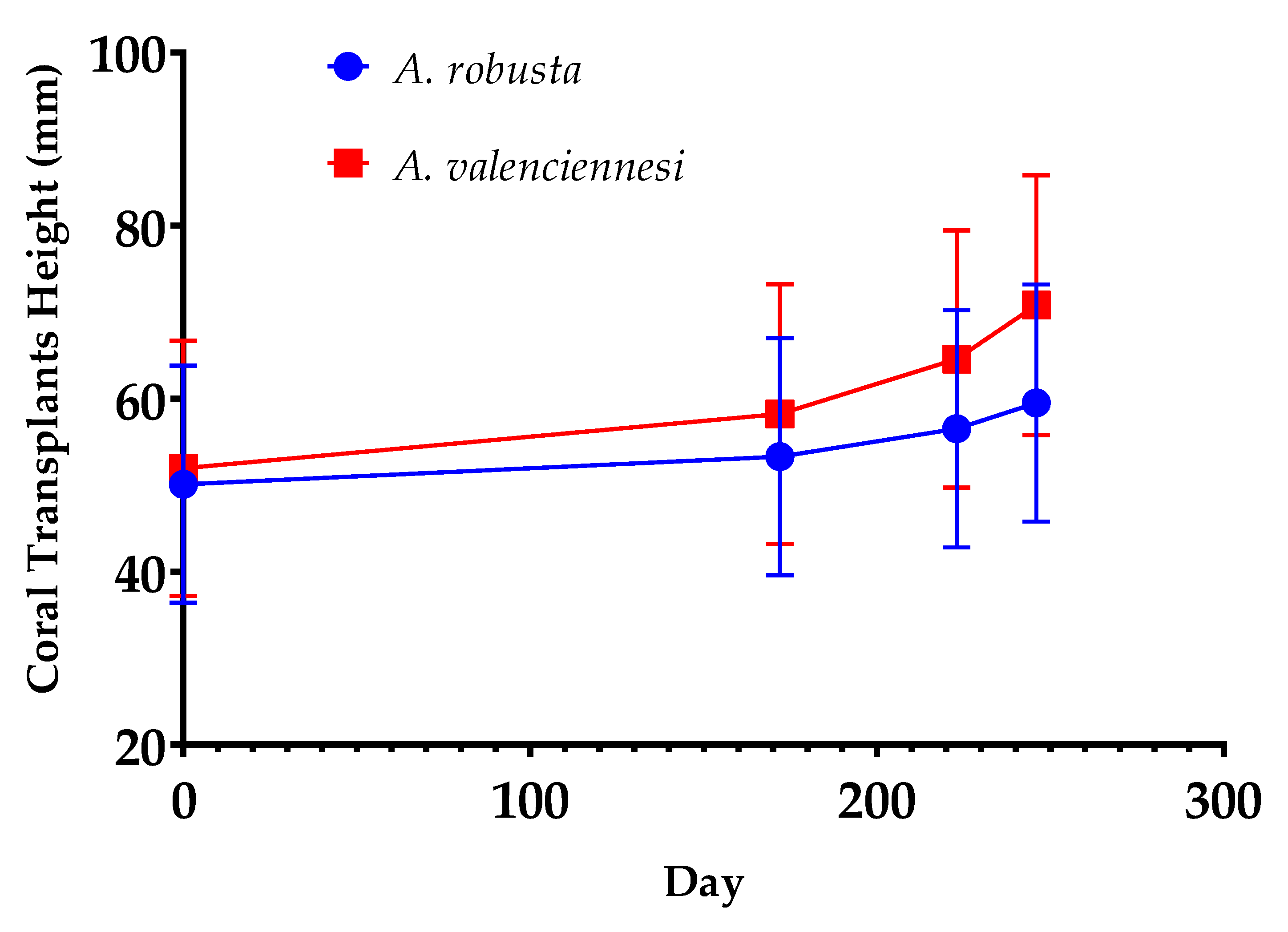
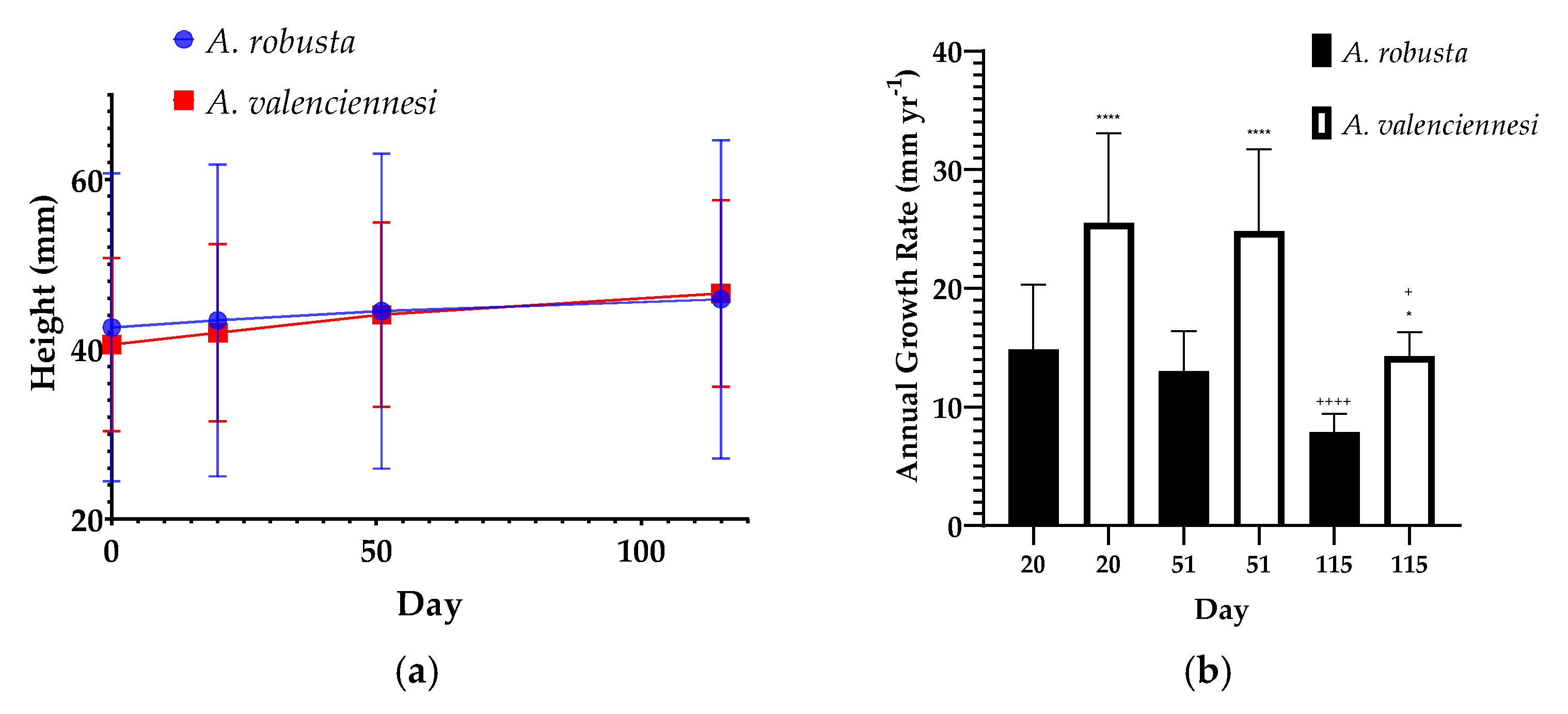
3.3. Coral Transplantation, Survival and Growth
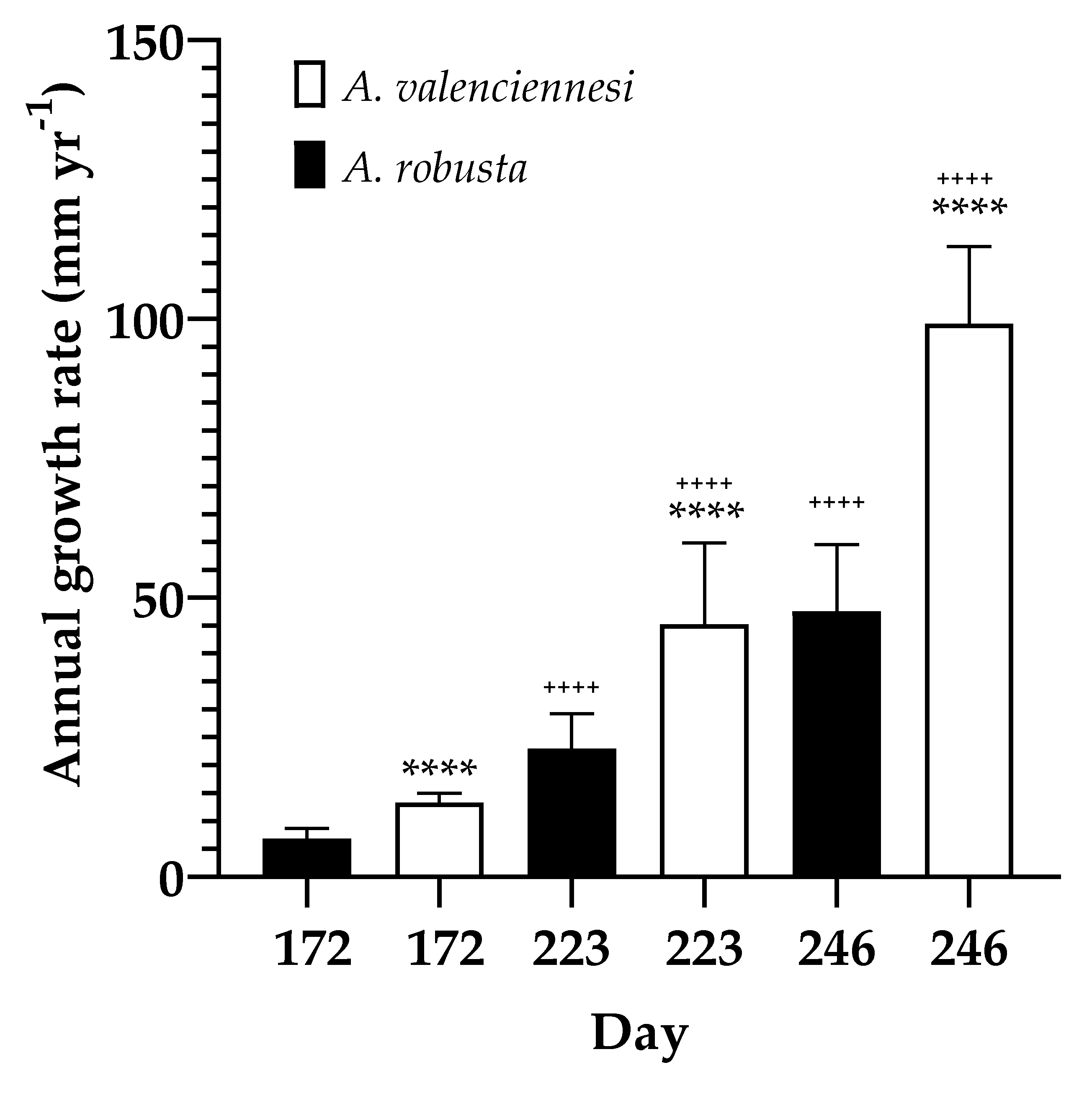
3.4. Species Impact on Growth Rate
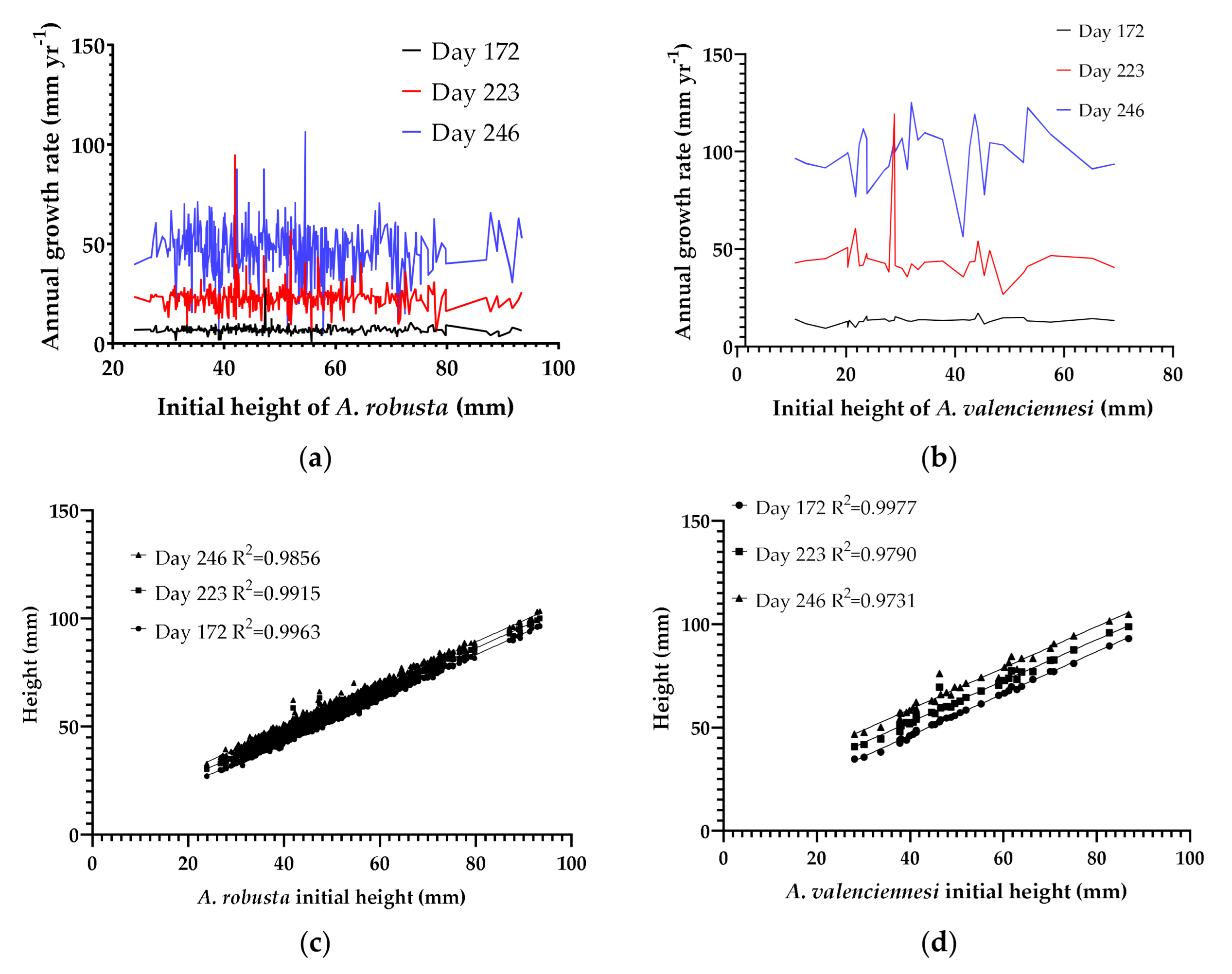
3.5. Fragment Size Impact on Growth Rate over Time
3.6. Growth of Coral Fragments in Individual Plugs
3.7. Estimation of Financial Cost
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gramling, C.; Ogasa, N. Climate Change Made 2023 the Hottest Year on Record. Available online: https://www.sciencenews.org/article/climate-heat-hottest-year-record-2023 (accessed on 16 April 2024).
- Leslie, J.; Rodgers, K. NOAA Confirms 4th Global Coral Bleaching Event. National Oceanic and Atmospheric Administration. Available online: https://www.noaa.gov/news-release/noaa-confirms-4th-global-coral-bleaching-event (accessed on 16 April 2024).
- Mcguirk, R. Most Great Barrier Reef coral studied this year was bleached. AP News. 11 May 2022. Available online: https://apnews.com/article/climate-science-australia-united-nations-corals-0bbcbcf4eaed841a5a72b4d4f3d40587 (accessed on 16 April 2024).
- Souter, D.; Planes, S.; Wicquart, J.; Logan, M.; Obura, D.; Staub, F. Status of Coral Reefs of the World: 2020; Souter, D., Planes, S., Wicquart, J., Logan, M., Obura, D., Staub, F., Eds.; Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI): San Francisco, CA, USA, 2021. [Google Scholar] [CrossRef]
- Eddy, T.D.; Lam, V.W.Y.; Reygondeau, G.; Cisneros-Montemayor, A.M.; Greer, K.; Palomares, M.L.D.; Bruno, J.F.; Ota, Y.; Cheung, W.W.L. Global Decline in Capacity of Coral Reefs to Provide Ecosystem Services. One Earth 2021, 4, 1278–1285. [Google Scholar] [CrossRef]
- Ainsworth, T.D.; Heron, S.F.; Ortiz, J.C.; Mumby, P.J.; Grech, A.; Ogawa, D.; Eakin, C.M.; Leggat, W. Climate Change Disables Coral Bleaching Protection on the Great Barrier Reef. Science 2016, 352, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, K.; Daraghmeh, N.; Lozano-Cortés, D.; Benzoni, F.; Berumen, M.L.; Carvalho, S. Differential Spatio-temporal Responses of Red Sea Coral Reef Benthic Communities to a Mass Bleaching Event. Sci. Rep. 2024, 14, 24229. [Google Scholar] [CrossRef]
- Schmidt-Roach, S.; Duarte, C.M.; Hauser, C.A.E.; Aranda, M. Beyond Reef Restoration: Next-Generation Techniques for Coral Gardening, Landscaping, and Outreach. Front. Mar. Sci. 2020, 7, 539330. [Google Scholar] [CrossRef]
- Buerger, P.; Alvarez-Roa, C.; Coppin, C.W.; Pearce, S.L.; Chakravarti, L.J.; Oakeshott, J.G.; Edwards, O.R.; van Oppen, M.J.H. Heat-Evolved Microalgal Symbionts Increase Coral Bleaching Tolerance. Sci. Adv. 2020, 6, eaba2498. [Google Scholar] [CrossRef]
- Humanes, A.; Lachs, L.; Beauchamp, E.; Bukurou, L.; Buzzoni, D.; Bythell, J.; Craggs, J.R.K.; de la Torre Cerro, R.; Edwards, A.J.; Golbuu, Y.; et al. Selective Breeding Enhances Coral Heat Tolerance to Marine Heatwaves. Nat. Commun. 2024, 15, 8703. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, R.P.; Djari, A.A.; Rahman, A.; Kabul, A.; Sagala, H.A. Analysis of Transplanted Coral Growth Using the Rock Pile Method in Karimunjawa National Park, Central Java, Indonesia. AACL Bioflux 2023, 16, 546–554. [Google Scholar]
- Reyes, M.Z.; Raymundo, D.J.C.; Rizwan, S.J.; Licuanan, W.Y.; Shields, B.A. Coral Gardening: Issues and Challenges. DLSU-AKI Policy Brief 2017, XI, 1–3. [Google Scholar]
- Mostrales, T.P.I.; Rollon, R.N.; Licuanan, W.Y. Evaluation of the Performance and Cost-Effectiveness of Coral Microfragments in Covering Artificial Habitats. Ecol. Eng. 2022, 184, 106770. [Google Scholar] [CrossRef]
- Lirman, D.; Schopmeyer, S. Ecological Solutions to Reef Degradation: Optimizing Coral Reef Restoration in the Caribbean and Western Atlantic. PeerJ 2016, 4, e2597. [Google Scholar] [CrossRef]
- Storlazzi, C.D.; Reguero, B.G.; Alkins, K.C.; Shope, J.B.; Gaido-Lassarre, C.; Viehman, T.S.; Beck, M.W. Hybrid Coral Reef Restoration Can Be a Cost-Effective Nature-Based Solution to Provide Protection to Vulnerable Coastal Populations. Sci. Adv. 2025, 11, eadn4004. [Google Scholar] [CrossRef] [PubMed]
- Knapp, I.S.S.; Forsman, Z.H.; Greene, A.; Johnston, E.C.; Bardin, C.E.; Chan, N.; Wolke, C.; Gulko, D.; Toonen, R.J. Coral Micro-Fragmentation Assays for Optimizing Active Reef Restoration Efforts. PeerJ 2022, 10, e13653. [Google Scholar] [CrossRef] [PubMed]
- Horoszowski-Fridman, Y.B.; Izhaki, I.; Katz, S.M.; Barkan, R.; Rinkevich, B. Shifting Reef Restoration Focus from Coral Survivorship to Biodiversity Using Reef Carpets. Commun. Biol. 2024, 7, 141. [Google Scholar] [CrossRef]
- Hughes, T.P.; Baird, A.H.; Morrison, T.H.; Torda, G. Principles for Coral Reef Restoration in the Anthropo-cene. One Earth 2023, 6, 656–665. [Google Scholar] [CrossRef]
- Omori, M. Degradation and Restoration of Coral Reefs: Experience in Okinawa, Japan. Mar. Biol. Res. 2011, 7, 3–12. [Google Scholar] [CrossRef]
- Guest, J.R.; Baria, M.V.; Gomez, E.D.; Heyward, A.J.; Edwards, A.J. Closing the Circle: Is It Feasible to Rehabilitate Reefs with Sexually Propagated Corals? Coral Reefs 2014, 33, 45–55. [Google Scholar] [CrossRef]
- Dehnert, I.; Saponari, L.; Galli, P.; Montano, S. Comparing Different Farming Habitats for Mid-Water Rope Nurseries to Advance Coral Restoration Efforts in the Maldives. PeerJ 2022, 10, e12874. [Google Scholar] [CrossRef]
- Wee, S.Y.C.; Sam, S.Q.; Sim, W.T.; Ng, C.S.L.; Taira, D.; Afiq-Rosli, L.; Kikuzawa, Y.P.; Toh, T.C.; Chou, L.M. The Role of in Situ Coral Nurseries in Supporting Mobile Invertebrate Epifauna. J. Nat. Conserv. 2019, 50, 125710. [Google Scholar] [CrossRef]
- Frias-Torres, S.; Reveret, C.; Henri, K.; Shah, N.; Maya, P.H.M. A Low-Tech Method for Monitoring Survival and Growth of Coral Transplants at a Boutique Restoration Site. PeerJ 2023, 11, e15062. [Google Scholar] [CrossRef]
- Mahmoud, M.A.M.; Dar, M.A.; Hussein, H.N.M.; El-Metwally, M.E.A.; Maaty, M.M.; Omar, M.Y.; Seraj, M.R.; Mohammed, T.A.A. Survivorship and Growth Rates for Some Transplanted Coral Reef Building Species and Their Potential for Coral Reef Rehabilitation in the Red Sea. Egypt. J. Aquat. Biol. Fish. 2019, 23, 183–193. [Google Scholar] [CrossRef]
- Toth, L.T.; Storlazzi, C.D.; Kuffner, I.B.; Quataert, E.; Reyns, J.; McCall, R.; Stathakopoulos, A.; Hillis-Starr, Z.; Holloway, N.H.; Ewen, K.A.; et al. The Potential for Coral Reef Restoration to Mitigate Coastal Flooding as Sea Levels Rise. Nat. Commun. 2023, 14, 2313. [Google Scholar] [CrossRef] [PubMed]
- Tortolero-Langarica, J.J.A.; Rodríguez-Troncoso, A.P.; Cupul-Magaña, A.L.; Rinkevich, B. Mi-cro-Fragmentation as an Effective and Applied Tool to Restore Remote Reefs in the Eastern Tropical Pacific. Int. J. Environ. Res. Public Health 2020, 17, 6574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Yang, H.; He, W.; Li, B.; Zhu, X.; Liu, S.; Jia, S.; Li, R.; Tang, K.H.D. Leaching of Chemicals from Microplastics: A Review of Chemical Types, Leaching Mechanisms and Influencing Factors. Sci. Total Environ. 2024, 906, 167666. [Google Scholar] [CrossRef]
- Egea, L.G.; Brun, F.G.; Jiménez-Ramos, R. Dissolved Organic Carbon Leaching from Microplastics and Bioa-vailability in Coastal Ecosystems. Sci. Total Environ. 2024, 909, 168673. [Google Scholar] [CrossRef] [PubMed]
- Forrester, G.E.; Maynard, A.; Schofield, S.; Taylor, K. Evaluating Causes of Transplant Stress in Fragments of Acropora palmata Used for Coral Reef Restoration. Bull. Mar. Sci. 2012, 88, 1099–1113. [Google Scholar] [CrossRef]
- dela Cruz, D.W.; Rinkevich, B.; Gomez, E.D.; Yap, H.T. Assessing an Abridged Nursery Phase for Slow Growing Corals Used in Coral Restoration. Ecol. Eng. 2015, 84, 408–415. [Google Scholar] [CrossRef]
- Rinkevich, B. Restoration Strategies for Coral Reefs Damaged by Recreational Activities: The Use of Sexual and Asexual Recruits. Restor. Ecol. 1995, 3, 241–251. [Google Scholar] [CrossRef]
- Page, C.A.; Muller, E.M.; Vaughan, D.E. Microfragmenting for the Successful Restoration of Slow Growing Massive Corals. Ecol. Eng. 2018, 123, 86–94. [Google Scholar] [CrossRef]
- Yeemin, T.; Chaithanavisut, N.; Aunkhongthong, W.; Chamchoy, C.; Pengsakun, S.; Klinthong, W.; Limpichat, J.; Chuabsak, P.; Sutthacheep, M. Survival and Growth Rate of Coral Micro-Fragments for Coral Reef Restoration in Chonburi Province, the Upper Gulf of Thailand. Ramkhamhaeng Int. J. Sci. Technol. 2024, 7, 38–48. [Google Scholar]
- Kamal Muzaki, F.; Saptarini, D.; Rahma Azizah, I.; Kartika Sari, I.; Tian Edo Pramono, A. Survival and Growth of Acropora millepora Coral Fragments Transplanted in Turbid Water of Sepulu, Bangkalan-Madura. Ecol. Environ. Conserv. 2020, 26, S26–S31. [Google Scholar]
- Grace, R.; Dizon, H.; Cayetano, W.V. Survival and Growth Rate of Acropora Species in Coral Nursery Units. Southeast Asian J. Sci. Technol. 2020, 5. [Google Scholar]
- Sangpaiboon, P.; Kongjandtre, N. Growth and Survival Rate of Three Transplanted Coral Taxa (Acropora robusta, Pocillopora damicornis and Platygyra daedalea) on Coral Reefs in Eastern Thailand. Burapha Sci. J. 2022, 1, 605–628. [Google Scholar]
- Crabbe, M.J.C.; Smith, D.J. Sediment Impacts on Growth Rates of Acropora and Porites Corals from Fringing Reefs of Sulawesi, Indonesia. Coral Reefs 2005, 24, 437–441. [Google Scholar] [CrossRef]
- Geng, X.; Wu, C.; Yang, Z.; Zhu, J.; Tang, K.; Lin, J.; Liu, Y.; Zhang, Y.; An, M.; Zhao, W.; et al. Spatiotemporal Variations and Impact Factors of Nutrients in the Sanya Bay, Northern South China Sea. Environ. Sci. Pollut. Res. 2023, 30, 76784–76797. [Google Scholar] [CrossRef]
- Yang, D. Cool Water Brought by Upwelling in the Sanya Bay Benefits Corals in the Background of Global Warming. Acta Oceanol. Sin. 2017, 36, 14–19. [Google Scholar] [CrossRef]
- Lizcano-Sandoval, L.D.; Londoño-Cruz, E.; Zapata, F.A. Growth and Survival of Pocillopora damicornis (Scleractinia: Pocilloporidae) Coral Fragments and Their Potential for Coral Reef Restoration in the Tropical Eastern Pacific. Mar. Biol. Res. 2018, 14, 887–897. [Google Scholar] [CrossRef]
- Sam, S.Q.; Ng, C.S.L.; Kikuzawa, Y.P.; Toh, T.C.; Sim, W.T.; Chou, L.M. Influence of Fragment Size on Post Transplantation Growth and Survival of Domed Scleractinian Corals. Mar. Biol. Res. 2021, 17, 327–340. [Google Scholar] [CrossRef]
- Forsman, Z.H.; Page, C.A.; Toonen, R.J.; Vaughan, D. Growing Coral Larger and Faster: Micro-Colony-Fusion as a Strategy for Accelerating Coral Cover. PeerJ 2015, 3, e1313. [Google Scholar] [CrossRef]
- Lirman, D.; Schopmeyer, S.; Galvan, V.; Drury, C.; Baker, A.C.; Baums, I.B. Growth Dynamics of the Threatened Caribbean Staghorn Coral Acropora cervicornis: Influence of Host Genotype, Symbiont Identity, Colony Size, and Environmental Setting. PLoS ONE 2014, 9, e107253. [Google Scholar] [CrossRef]
- Ladd, M.C.; Shantz, A.A.; Harrell, C.; Hayes, N.K.; Gilliam, D.S.; Muller, E.M.; O’Neil, K.L.; Reckenbeil, B.; Craig, Z.; Lirman, D. Acclimation and Size Influence Predation, Growth, and Survival of Sexually Produced Diploria labyrinthiformis Used in Restoration. Sci. Rep. 2024, 14, 26362. [Google Scholar] [CrossRef]
- Lange, I.D.; Perry, C.T. A Quick, Easy and Non-Invasive Method to Quantify Coral Growth Rates Using Pho-togrammetry and 3D Model Comparisons. Methods Ecol. Evol. 2020, 11, 714–726. [Google Scholar] [CrossRef]
- Million, W.C.; O’Donnell, S.; Bartels, E.; Kenkel, C.D. Colony-Level 3D Photogrammetry Reveals That Total Linear Extension and Initial Growth Do Not Scale with Complex Morphological Growth in the Branching Coral, Acropora cervicornis. Front. Mar. Sci. 2021, 8, 646475. [Google Scholar] [CrossRef]
- Lirman, D.; D’Alessandro, M. The Role of Outplant Density on Coral Survivorship, Growth, Predation Impacts, and Disease Spread; Florida Department of Environmental Protection: Miami, FL, USA, 2024. [Google Scholar]
- Bayraktarov, E.; Saunders, M.I.; Abdullah, S.; Mills, M.; Beher, J.; Possingham, H.P.; Mumby, P.J.; Lovelock, C.E. The Cost and Feasibility of Marine Coastal Restoration. Ecol. Appl. 2016, 26, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sik Ok, Y.; Bank, M.S.; Sonne, C. Macro- and Microplastics as Complex Threats to Coral Reef Ecosystems. Environ. Int. 2023, 174, 107914. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.M.D.; Botana, M.T.; Elias, A.d.C.; Nomura, C.S.; Saldanha-Corrêa, F.; Espósito, B.P. Effect of Iron Speciation on Growth and Heat Resistance of Symbiodiniaceae. Ocean. Coast. Res. 2022, 70, e22016. [Google Scholar] [CrossRef]
- Dellisanti, W.; Zhang, Q.; Ferrier-Pagès, C.; Kühl, M. Contrasting Effects of Increasing Dissolved Iron on Photosynthesis and O2 Availability in the Gastric Cavity of Two Mediterranean Corals. PeerJ 2024, 12, e17259. [Google Scholar] [CrossRef]
- Peixoto, R.S.; Voolstra, C.R.; Baums, I.B.; Camp, E.F.; Guest, J.; Harrison, P.L.; Montoya-Maya, P.H.; Pollock, F.J.; Smith, D.J.; Wangpraseurt, D.; et al. The Critical Role of Coral Reef Restoration in a Changing World. Nat. Clim. Change 2024, 14, 1219–1222. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse, S.C.A.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; et al. Coral Restoration—A Systematic Review of Current Methods, Successes, Failures and Future Directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef]
- Mulà, C.; Bradshaw, C.J.A.; Cabeza, M.; Manca, F.; Montano, S.; Strona, G. Restoration Cannot Be Scaled up Globally to Save Reefs from Loss and Degradation. Nat. Ecol. Evol. 2025, 9, 822–832. [Google Scholar] [CrossRef]
- Mission: Iconic Reefs Shares Strategic Priorities for 2022–2025. NOAA Fisheries. Available online: https://www.fisheries.noaa.gov/feature-story/mission-iconic-reefs-shares-strategic-priorities-2022-2025 (accessed on 7 May 2025).
- The Big Build: A History-Making Coral Reef Restoration Event. Mars, Incorporated. Available online: https://www.mars.com/en-gb/news-and-stories/articles/the-big-build-history-making-coral-reef-restoration-event (accessed on 7 May 2025).
- Shumway, N.; Foster, R.; Fidelman, P. The Governance of Marine and Coral Reef Restoration, Lessons and Paths Forward for Novel Interventions. Environ. Sci Policy 2025, 164, 103999. [Google Scholar] [CrossRef]

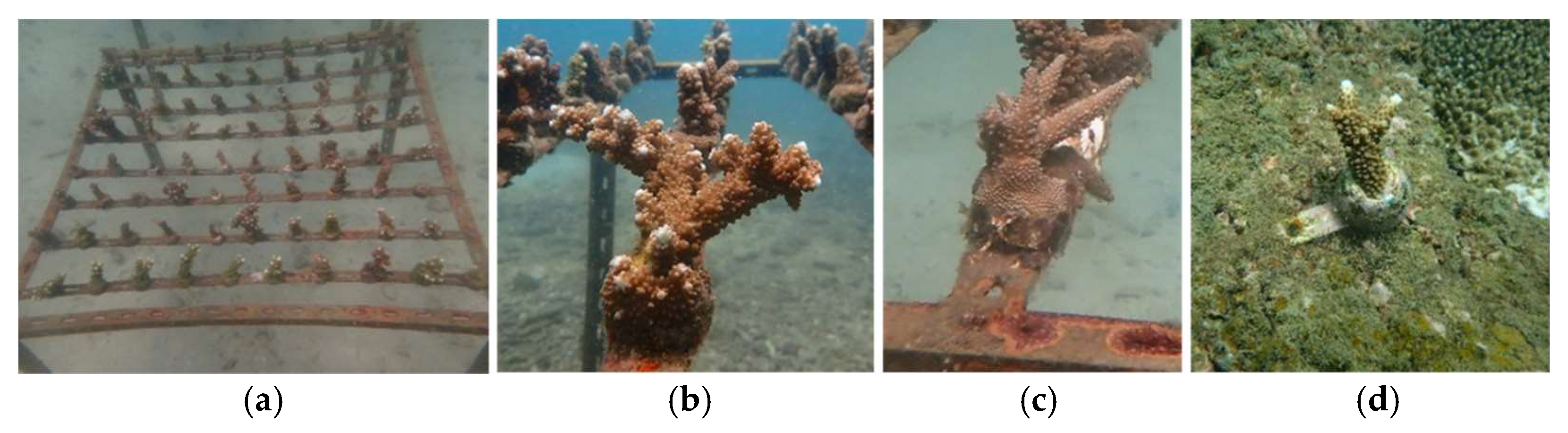
| Species | Transplantation Method | Number of Transplants | Survived Transplants | Survival Rate (%) |
|---|---|---|---|---|
| Acropora robusta | Grid assay | 443 | 419 | 94.58 |
| Acropora valenciennesi | Grid assay | 33 | 33 | 100 |
| Acropora robusta | Individual plug | 21 | 21 | 100 |
| Acropora valenciennesi | Individual plug | 6 | 6 | 100 |
| Description | Cost/Unit (USD) | Quantity | Total (USD) |
|---|---|---|---|
| 1 m of perforated 304-grade flat stainless-steel bar | 4 | 240 | 960 |
| Stainless steel propagation plug | 0.3 | 1920 | 576 |
| 1 m of perforated 304-grade L-shaped stainless-steel bar | 5.8 | 240 | 1392 |
| Nuts, bolts and screws | 0.02 | 3000 | 60 |
| Adhesive | 50 | 1 | 50 |
| Researcher | 120 | 2 | 240 |
| Diver | 170 | 2 | 340 |
| Boat crew | 65 | 2 | 130 |
| Trained manual labor/graduate students | 35 | 4 | 140 |
| Boat rental | 800 | 1 | 800 |
| Dive equipment | 20 | 2 | 40 |
| Food | 5 | 16 | 80 |
| Total (USD) | 4808 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Lee, D.; Xiong, X.; Zhu, L.; Wang, A.; Wan, W.; Chin, Y.; Wang, P. Acropora spp. Coral Gardening Using Fragmentation and Direct Transplantation: A Feasibility Study at Boundary Island. Oceans 2025, 6, 42. https://doi.org/10.3390/oceans6030042
Li M, Lee D, Xiong X, Zhu L, Wang A, Wan W, Chin Y, Wang P. Acropora spp. Coral Gardening Using Fragmentation and Direct Transplantation: A Feasibility Study at Boundary Island. Oceans. 2025; 6(3):42. https://doi.org/10.3390/oceans6030042
Chicago/Turabian StyleLi, Min, Dechuan Lee, Xiaofei Xiong, Le Zhu, Aimin Wang, Wubo Wan, Yaoxian Chin, and Peizheng Wang. 2025. "Acropora spp. Coral Gardening Using Fragmentation and Direct Transplantation: A Feasibility Study at Boundary Island" Oceans 6, no. 3: 42. https://doi.org/10.3390/oceans6030042
APA StyleLi, M., Lee, D., Xiong, X., Zhu, L., Wang, A., Wan, W., Chin, Y., & Wang, P. (2025). Acropora spp. Coral Gardening Using Fragmentation and Direct Transplantation: A Feasibility Study at Boundary Island. Oceans, 6(3), 42. https://doi.org/10.3390/oceans6030042






