Abstract
The shells of dead Pinna nobilis individuals are important habitats in sedimentary coastal ecosystems, yet their ecological role is poorly understood. This study investigated macrofaunal communities associated with 80 P. nobilis shells from Soline Bay and Valovine Bay, northern Adriatic, analyzing variations in species abundance and biodiversity between shell orientations (vertical and horizontal) and across seasons. Shell dimensions were recorded, with larger shells and higher faunal abundance observed in Soline Bay compared to Valovine Bay. A total of 2225 individuals representing 183 species across 19 taxonomic groups were identified, with Malacostraca, Bivalvia, and Polychaeta being the most abundant. Vertically positioned shells hosted significantly more organisms than horizontally positioned ones, likely due to greater available surface area for settlement. Seasonal changes influenced organism abundance, with peaks in winter for Valovine Bay and spring for Soline Bay, correlating with environmental factors such as eutrophication. The most frequent species associated with the shells of dead individuals were the polychaete Sabella spallanzanii and the bivalve Rocellaria dubia, which can impact shell degradation. Despite their temporary nature, the shells of dead P. nobilis provide vital refuge and enhance biodiversity. The findings underscore the ecological importance of P. nobilis shells as biodiversity hotspots and highlight the need for their conservation and further study.
1. Introduction
Habitat-forming species play a critical role in biodiversity, ecosystem processes, functions, and services. With increasing anthropogenic impacts on marine ecosystems, studying and protecting these key species is necessary [1]. Among them is the noble pen shell (Pinna nobilis Linnaeus, 1758), the largest bivalve in the Mediterranean [2]. P. nobilis can grow up to 120 cm and is typically buried in sediment, with about one-third of the shell anchored upright by byssal threads [3]. Under favorable conditions, this species has a lifespan of around 50 years and inhabits depths ranging from 0.5 to 60 m [2]. It occupies sandy and muddy bottoms and is frequently found in seagrass meadows of Posidonia oceanica (Linnaeus) Delile, 1813, and Cymodocea nodosa (Ucria) Ascherson, 1870 [4].
In addition to its role as a filter feeder, which contributes to water clarity by purifying significant amounts of detritus daily, the portion of the shell above the sediment serves as a potential habitat for epibiotic species [5]. The size and density of P. nobilis populations enable the formation of three-dimensional microecosystems, where organisms utilize the rough surfaces of the shells to create entire communities. These ecological characteristics make P. nobilis a priority for research and conservation to ensure its continued role as an ecosystem engineer in marine environments [6]. However, various anthropogenic impacts—such as illegal collection, trawl fishing, anchoring, and pollution leading to eutrophication—have contributed to the species’ endangered status [4,7,8]. P. nobilis is protected under Annex II of the Barcelona Convention (SPA/BD Protocol 1995), the EU Habitats Directive (92/43/EEC), and Croatia’s Regulation on Strictly Protected Species (NN 144/2013, NN 73/2016), which have helped slow down its decline. Unfortunately, in 2016, a mass mortality event (MME) began, further exacerbating the species’ decline [2], thus deeply concerning the scientific community. Until now, nearly 100% of Mediterranean populations have collapsed, leading to P. nobilis being classified as Critically Endangered on the IUCN Red List [9,10,11]. In response, significant efforts have been made to determine the causes of this catastrophic decline. Recent large-scale analyses indicate that the protozoan Haplosporidium pinnae is more strongly associated with MMEs than mycobacteria or other pathogens [2,12,13,14]. There are even indications of a possible complete extinction of its populations even in areas considered last refuges, where the presence of live individuals can still be confirmed [15]. The decline of this foundation species and its “reefs” also indicates habitat degradation. In this context, gaining insight into the beneficial interactions between foundation species and their associated communities is essential for effective conservation and restoration efforts [16,17].
The first MME, of the same previously mentioned origin, in the Adriatic Sea was recorded in the southern part of the basin in the spring of 2019, and by the autumn of the same year, it had spread to the central Adriatic [18]. By May 2020, the northern Adriatic populations of noble pen shells had also been affected [19], including the entire area of the Istrian Peninsula [8]. This widespread mortality left behind numerous “meadows” of open shells of dead individuals. While the degradation of these shells began immediately after the death of the individuals—at varying rates depending on environmental factors—these shells could now serve as temporary ecological niches, possibly offering new opportunities for other life forms to settle and develop [20].
In general, pen shells provide an important habitat by offering shelter for many species and a hard substrate for settling sessile species and egg-laying fish. Arthropods, such as amphipods, crabs, and isopods, occur on the shells of dead individuals at high densities, with their distribution ranging from clumped to random patterns, indicating varying spatial scales of growth and dispersion [21].
As seen in previous studies, the relationship between a similar Pinnidae species (Atrina zelandica J. E. Gray, 1835) and benthic macrofauna is complex and varies across spatial scales [22]. A comparable dynamic may apply to P. nobilis, where interactions with associated macrofauna are likely shaped by spatial distribution, habitat structure, and broader environmental factors rather than just species density. Given that A. zelandica has demonstrated spatial variability in its ecological role [22], it is reasonable to expect that P. nobilis exhibits a similar pattern, with its function as a habitat provider influenced by factors such as hydrodynamics, substrate type, and seagrass cover—even when the specimen is no longer alive. This underscores the need to integrate multi-scale statistical modeling and landscape-scale patch dynamics into research on P. nobilis rather than relying solely on density-based comparisons to evaluate its ecological role.
A study conducted in the northern Adriatic (Gulf of Trieste) identified numerous mollusk taxa within the shells of dead P. nobilis, emphasizing their continued role as habitat for epibenthic communities [6]. The findings revealed significant taxonomic and functional diversity within the mollusk community, demonstrating that these shells still serve important ecological functions. Although P. nobilis populations have declined, their empty shells remain valuable microhabitats, providing shelter and supporting diverse species in both hard- and soft-bottom environments.
The shells of dead P. nobilis are expected to gradually erode and disappear, leading to the loss of this biogenic hard substrate. However, in the protected and partially protected bays of southern Istria, many shells remain present even three years post-MME, with a significant number, both vertically and horizontally positioned. In contrast, in the infralittoral zones of bays in the central and southern Adriatic, particularly in the open sea around major southern Adriatic islands such as Mljet, Vis, and Lastovo, most shells have undergone significant degradation and are now found much less frequently (Iveša, N., personal observation).
This disparity is possibly influenced by differences in wave exposure between the northern and southern Adriatic. The northern Adriatic, including the Istrian Peninsula, is characterized by a relatively shallow and semi-enclosed environment with lower wave energy, which helps preserve P. nobilis shells for a longer period. In contrast, the southern Adriatic, particularly in more exposed offshore areas, experiences stronger hydrodynamic forces due to deeper waters and greater wave action. These conditions accelerate the degradation and dispersal of shells. Additionally, wave exposure regulates the intensity of drag forces acting on individuals [23]. Consequently, upright individuals with intact shells are notably rare in these regions compared to the Istrian Peninsula (Iveša, N., personal observation). Although MME in the northern Adriatic began a year later than in the central and southern Adriatic, the prolonged persistence of the shells of dead P. nobilis in Istrian waters suggests that they may function as a transitional ecosystem following mass mortality. Notably, in the bays of southern Istria, these shells remain present in the habitat years after the initial mortality. While the shells gradually lose their structural integrity and ecological quality over time, they may continue to play a role in the habitat during this transitional period, influencing local ecosystem dynamics. Analyzing the macrofaunal composition associated with these temporary structures can provide valuable insights for potential habitat bioremediation efforts following the loss of P. nobilis.
Therefore, this study aims to evaluate the transitional ecological role of the shells of dead P. nobilis as ecosystem engineers by structurally and functionally analyzing the overall associated macrofaunal community. For the first time, the study will investigate the complete macrofauna associated with deceased P. nobilis specimens. The focus will be on a qualitative and quantitative assessment of the entire macrofaunal assemblage, extending beyond mollusks to include other invertebrates and fish. This approach will provide a broader functional perspective on this shifting habitat, offering valuable insights into the ecological role of P. nobilis shells even after the death of the species.
2. Materials and Methods
2.1. Research Area
The sampling sites were selected based on known populations of P. nobilis in southern Istria. Following preliminary surveys aimed at determining the density of shellfish for a sustainable year-round study, two locations were chosen: Valovine Bay, Stoja (44°51′41.1″ N 13°48′41.4″ E), and Soline Bay, Vinkuran (44°49′43.9″ N 13°51′36.7″ E) (Figure 1). Both locations are near urban centers and have historically been used for local fishing. In recent years, their role has expanded to include nautical and recreational tourism. As a result, the seabed of both bays contains remnants of outdated nautical infrastructure, such as deteriorated piers, concrete blocks, discarded ropes, and chains. However, due to differences in size and exposure to open waters, Soline Bay experiences significantly greater anthropogenic impact, particularly from nautical activities. Valovine Bay is the smaller of the two study sites, with an entrance approximately 290 m wide and extending roughly 500 m inland. The bay is relatively exposed to wave and wind impacts due to its open southwestern orientation and wide entrance, which increases water current speed and enhances water exchange within the bay. This hydrodynamic environment plays a significant role in shaping the ecological conditions. The sampling area in Valovine Bay is also under significant anthropogenic influence due to its proximity to beaches, tourist facilities (e.g., ski-lift), hospitality establishments, and fishing activities. Fishing pressure is the most pronounced, particularly in areas used for mooring small fishing boats. In terms of habitat, the shells of dead P. nobilis were found in biocenoses of muddy sands on protected shorelines at depths ranging from 1.3 to 4.6 m. Other notable habitats in this bay include Zostera noltei (Hornemann, 1832) meadows, dense infralittoral algal biocenoses, P. oceanica meadows, and biocenoses of fine surface sands.
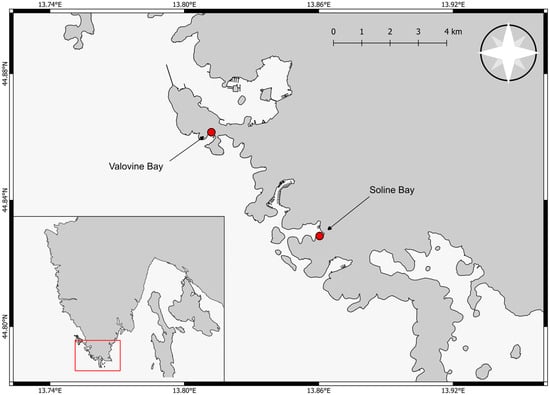
Figure 1.
Sampling locations in southern Istria.
Soline Bay, in contrast, is less affected by abiotic factors, primarily due to its geographical location. The bay extends approximately 1400 m inland, with an entrance about 400 m wide. The presence of Veruda Island in front further shields the bay, reducing current speed and water exchange. These conditions result in a smaller sediment fraction and slower decomposition of P. nobilis shells compared to Valovine Bay. Soline Bay, being relatively sheltered from wave-induced hydrodynamic forces, serves as an anchorage site. However, during the tourist season, many boats exceed the prescribed capacity and anchor illegally. The sampling area in Soline Bay is located in front of the beach and near hospitality establishments. The shells of dead P. nobilis were found in the biocenoses of muddy sands at depths ranging from 1.2 to 3.2 m.
2.2. Empty P. nobilis Shells Sampling, Morphometric Measurements, and Hydrographic Data
From March 2023 to March 2024, under permission granted by the Ministry of Environment Protection and Green Transition of the Republic of Croatia, P. nobilis shells were collected and analyzed across four seasons. Ten shells of dead individuals were sampled per location during each season, with the requirement that all specimens be returned to their original positions after analysis. The density of shells was estimated by visual census along three random transects (50 m long, 2 m wide) per sampling location. Data were standardized to densities per 1 m2. Due to various factors, including anthropogenic influences, abiotic conditions, and marine hydrodynamics, some P. nobilis shells were found lying flat (horizontally) on the seafloor rather than upright (vertically) in the sediment. To ensure a balanced study, five vertically and five horizontally positioned specimens were analyzed from each site. Sampling was repeated with new shells each season at both locations (Table 1). Specimens were randomly selected using free-diving techniques, ensuring that no live specimens were collected. During each dive, sampling was conducted at a greater distance from the previous location to minimize the risk of resampling identical shells and to ensure the collection of new specimens during each sampling period. After collection, the shells were measured using a measuring tape with an accuracy of ±1.10 mm over 10 m length to record dorsoventral length (from the umbo to the most distal part of the shell), width, and height (Figure 2). Sampled shells were carefully handled throughout to preserve their integrity. Sea temperature, salinity, and pH were measured in situ with a HANNA Instruments meter (model HI98194) during each sampling period. When such an instrument was unavailable, the sea temperature was recorded using a Nepto diving watch (Cressi, SKU: KS841098).

Table 1.
Morphometric values of vertically and horizontally positioned P. nobilis shells from both research locations (SD—standard deviation).
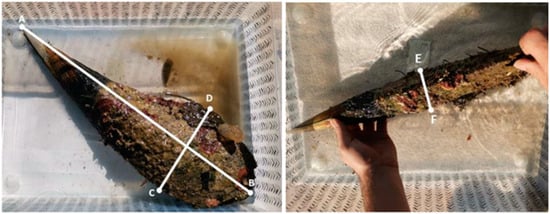
Figure 2.
Morphometric measurements of collected shells: length (distance from A to B), width (distance from C to D), and height (distance from E to F).
2.3. Taxonomic Identification and Functional Classification
To prevent the loss and/or escape of macrofaunal invertebrates and fish during handling and transport to the shore, each deceased Pinna nobilis was enclosed in a cylindrical net with a mesh size of 1 mm before being extracted from the sediment. Only once the shells were fully enclosed in the net were they removed from the sediment and transported ashore. Then, the shells were immediately measured, and analysis began promptly to minimize exposure to external conditions and to facilitate the safe return of organisms to the sea. The external and internal surfaces of the shells were thoroughly rinsed with seawater to accurately collect vagile fauna. The rinsed material, along with sediment from inside the shells, was sieved through a mesh with a pore size of 1 mm. The remaining sediment was then carefully inspected to detect any additional vagile organisms. After the inspection, the shells were returned to the sea in their original orientation. Only living individuals were recorded. Most organisms were identified in situ using available identification keys [24,25,26,27,28,29,30,31,32], while those that could not be immediately identified were collected for further analysis in the laboratory. This especially applied to small, cryptic, or unidentified species that were transported to the laboratory in glass containers filled with seawater for further analysis. In the laboratory, these specimens were examined under the microscope (Olympus CX21), allowing for detailed identification to the lowest possible taxonomic level.
Species were categorized based on mobility (motile vs. sessile) and substrate preference (hard-bottom, soft-bottom, or both) using existing ecological classifications from the literature and field observations. Abundance data were compiled by counting the number of individuals per species across all sampling stations. Species richness was calculated as the total number of unique species within each ecological category. Data were then grouped and analyzed to determine relative proportions by mobility and substrate affinity.
2.4. Statistical Analyses
The approximate surface area of all shells was calculated through 3D modeling of a single specimen in MaxSurf Pro 22 software, with parametric variation applied to adjust dimensions. This calculation included the total area of both the inner and outer surfaces of the two valves. Statistica 9.0 software was used for comparisons of areas, seasons and shell positions with the non-parametric Kruskal–Wallis test, followed by the post hoc Mann–Whitney U test to identify specific differences. Statistical data analyses explained further in the text were performed in R version 4.4.2 [33]. Univariate analyses were performed for total abundance, species richness, and Shannon–Wiener diversity, computed in the vegan package [34]. The effects of shell position and season on these diversity measures were assessed by means of generalized linear mixed models (GLMM). In order to control for individual shell variability and locations (non-independence of data points), random intercepts considering the individual shell nested within location were included in the models. Adding this random term was also important to eliminate overdispersion issues. Overdispersion was tested for models with and without the random intercepts. Poisson distribution of errors was used for abundance and richness, whereas Gaussian distribution was used for Shannon–Wiener diversity. GLMMs were fitted with the lme4 package [35]. Tukey post hoc pairwise comparisons were performed using the emmeans package (estimated marginal means, also known as least-squares means) [36]. To assess differences in assemblages (compositional differences) several multivariate methods were performed. A subset of 16 most abundant species (with relative abundance >1%) was analyzed as a contingency table. A correspondence analysis was carried out to assess relationships between species (columns) and samples (rows, samples belonging to different seasons and positions). A chi-square test was computed to test the statistical differences of these relationships. This analysis was performed using the FactoMineR package [37]. Finally, a non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarity was performed using the complete set of species, followed by a two-way (Season x Position) Permutational Analysis of Variance (PERMANOVA) based on the same distance matrix using the vegan package [34]. Homogeneity of multivariate dispersion (PERMANOVA assumption) was assessed for main factors (season and position), as well as for interaction. Furthermore, the design of this study was balanced, and PERMANOVA has been noted to be robust in the face of heterogeneity for balanced designs [38].
3. Results
3.1. Seawater Conditions and Biodiversity Comparison
Hydrographic variables were compared with the number of individuals and species per season and location (Figure 3 and Figure 4). Salinity and pH levels, as expected, did not show notable variation throughout the year nor between locations, ranging from 34.17 to 36.20 and from 8.06 to 8.33, respectively. Temperature values were similar at both locations.
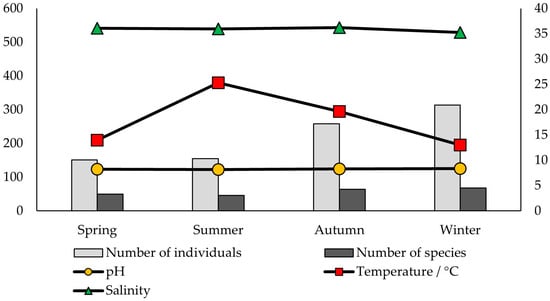
Figure 3.
Seawater variables (pH, temperature, and salinity), the number of individuals, and the number of species detected on the shells of dead P. nobilis throughout the seasons in Valovine Bay.
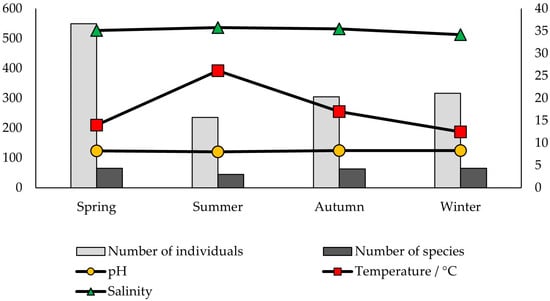
Figure 4.
Seawater variables (pH, temperature, and salinity), the number of individuals, and the number of species detected on the shells of dead P. nobilis throughout the seasons in Soline Bay.
3.2. Morphometric Parameters and Density of the Noble Pen Shells
A total of 80 noble pen shells were sampled from both locations, 40 in a vertical position and 40 in a horizontal position. During this study, a single living specimen of the noble pen shell P. nobilis was found in Soline Bay. The length of the shells of dead individuals ranged from 36 cm to 67 cm, the width from 13 cm to 21 cm, and the height from 5 cm to 12 cm. The average length was 51.70 ± 6.38 cm, width 16.93 ± 1.68 cm, and height 7.64 ± 1.34 cm (Figure 5). Vertically positioned shells were larger on average, as shown in Table 1. Additionally, the average size of P. nobilis shells was greater in Soline Bay compared to the other locations. The average density of noble pen shells estimated in both sampled areas was 0.10 shells of dead individuals m−2. Our personal data collected prior to these mortality events show densities of 0.25 individuals m−2.
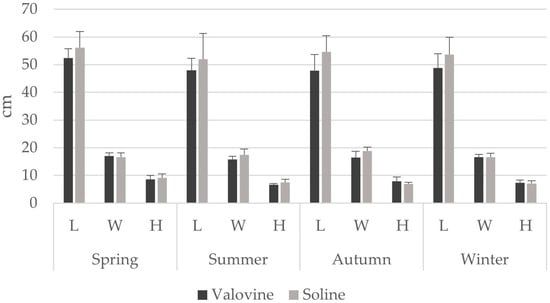
Figure 5.
Seasonal variations of shell dimensions (means ± st. dev.) for both sampling locations (L—length, W—width, H—height).
The approximate surface area of the shells of dead P. nobilis ranged between 1260 and 3260 cm2. The number of individuals in each shell of dead P. nobilis was compared with its surface area, but no correlation was found (Figure 6).
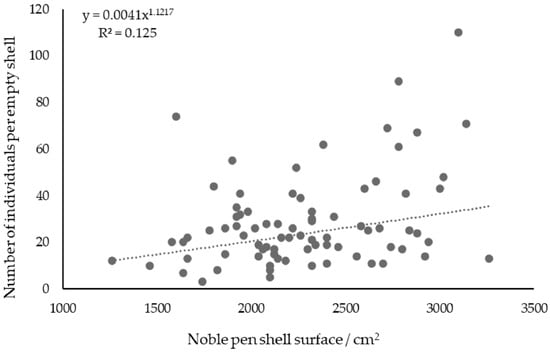
Figure 6.
The relationship between the number of individuals per shell of dead noble pen and its surface (cm2).
3.3. Diversity in the Macrofauna of the Noble Pen Shells
A total of 2225 different individuals belonging to 183 animal species were determined through 19 taxonomic classes (Table 2). Out of 183 species, 69 were found in both locations, while 53 species were exclusive to Valovine Bay and 62 to Soline Bay. The presence of colonial organisms, such as certain tunicates and bryozoans, was recorded, but their individual numbers could not be counted.

Table 2.
Seasonal variation of identified species that inhabited shells of dead P. nobilis (presence of colonial organisms is marked with a plus sign, Sp—Spring, Su—Summer, A—Autumn, W—Winter).
Most of the specimens found on P. nobilis shell surfaces belonged to Malacostraca (758 individuals, 34%). Furthermore, a large number of organisms from the classes Bivalvia (542 individuals, 24%), Polychaeta (353 individuals, 16%), and Gastropoda (212 individuals, 9%) were recorded. The highest number of species belonged to malacostracan crustaceans (43 species, 23%), followed by the bivalves (32 species, 17%), gastropods (31 species, 17%), and polychaetes (26 species, 16%) (Figure 7). The most abundant species of malacostracan crustaceans was Pisidia bluteli (39%), while for polychaetes, it was Sabella spallanzanii (72%). The most numerous bivalves and gastropods species were Rocellaria dubia (32%) and Cerithium vulgatum (22%), respectively (Table 2).
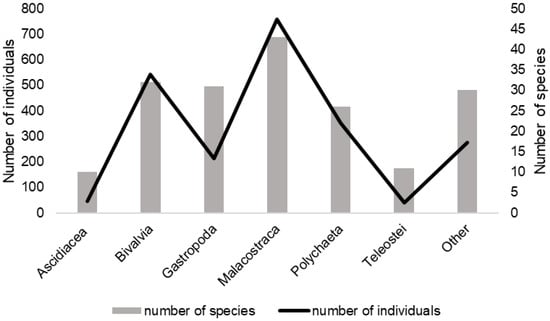
Figure 7.
Variations in the number of individuals and number of species of the most abundant animal classes observed in the shells of dead P. nobilis across both locations during the entire research period.
Abundance by category showed motile species contributing with 1138 individuals (51%), while sessile species accounted for 1087 individuals (49%), out of a total of 2225 individuals. Substrate preferences indicated hard-bottom species with 1366 individuals (62%), soft-bottom species with 408 individuals (18%), and species that can inhabit both substrate types with 451 individuals (20%). Species richness by category included 119 motile species (65%) and 64 sessile species (35%), with hard-bottom species numbering 83 (45%), soft-bottom species 40 (22%), and mixed species 60 (33%), out of a total of 183 species (Figure 8).
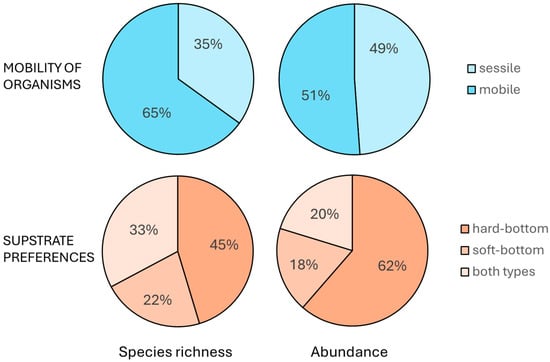
Figure 8.
Distribution of macrofaunal abundance and diversity by mobility and substrate preference associated with shells of dead P. nobilis.
Across all sampling locations and seasons, an average of 28.58 ± 19.87 individuals was found on the 80 analyzed shells of dead P. nobilis. The lowest abundance was recorded during summer, with just 3 individuals observed on a horizontally positioned shell in Valovine Bay, while in spring, the highest number was recorded, with 110 individuals on a vertically positioned shell in Soline Bay. Across seasons and orientations, horizontally positioned shells in Valovine Bay during summer had the lowest average abundance (10.6 ± 11.0), in contrast to vertically positioned shells in Soline Bay during spring, which exhibited the highest average abundance (72.2 ± 31.2) (Figure 9).
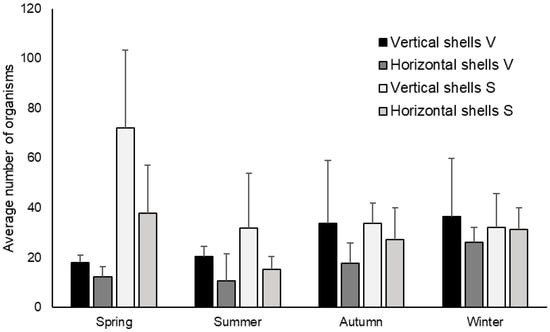
Figure 9.
Seasonal variation of the average number of organisms per shell of dead P. nobilis based on their position and location (mean value + standard deviation). Abbreviations of bays: Valovine Bay (V) and Soline Bay (S).
A comparison of the average number of organisms per shell between the two locations throughout the entire research period indicated a greater number of individuals inhabiting empty shells in Soline Bay (35.2 ± 21.9) compared to Valovine Bay (21.95 ± 15.2) (Figure 10). The non-parametric Mann–Whitney U test revealed a statistically significant difference in the number of organisms per empty noble pen shell between the two investigated locations (p = 0.001).
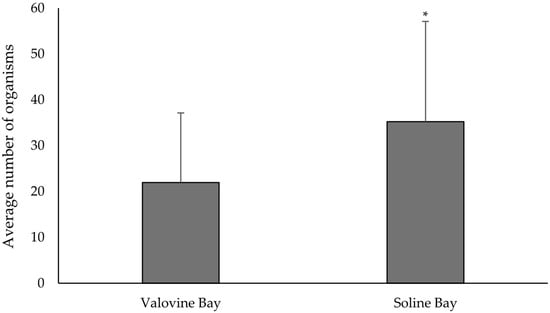
Figure 10.
Comparison of the number of organisms (mean value + standard deviation) per shell of dead P. nobilis at both locations during the entire research period. The asterisk indicates a statistically significant difference (* Mann–Whitney U test, p = 0.001).
Furthermore, Soline Bay had a higher average number of organisms per shell of dead individuals compared to the shells of dead individuals collected in Valovine Bay throughout all four sampling seasons (Figure 11). The non-parametric Mann–Whitney U test revealed a statistically significant difference between the investigated locations in spring (p = 0.001). Seasons had a greater influence in Soline Bay (Kruskal–Wallis test: H, p = 0.007) compared to Valovine Bay (Kruskal–Wallis test: H, p = 0.02) when the number of individuals was compared by season. At Soline Bay, a statistically significant difference was found in the number of individuals inhabiting noble pen shells in spring and summer (Mann–Whitney test, p = 0.004), while at Valovine Bay, a difference was found in spring and winter (Mann–Whitney test, p = 0.037). Additionally, the number of organisms in both bays increased from summer to winter, and in winter reached the average maximum abundance in Valovine Bay (31.40 ± 17.0), while the maximum in Soline Bay was reached in spring (55.00 ± 30.4). The lowest average abundance of organisms in Valovine Bay was recorded in spring (15.10 ± 4.5), while in Soline Bay, the minimum was recorded in summer (23.60 ± 17.4). The Shannon–Wiener index throughout the entire survey for Valovine Bay was 3.89 and for Soline Bay, 3.47.
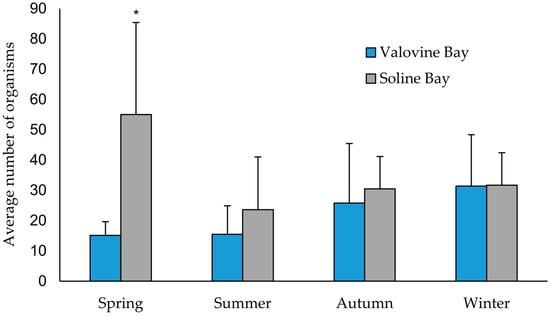
Figure 11.
Comparison of the number of organisms (mean value + standard deviation) per shell of dead P. nobilis at both locations for each sampling season. The asterisk indicates a statistically significant difference (* Mann–Whitney U test, p = 0.001).
Generalized linear mixed models (GLMM) for abundance showed a significant main effect of season (χ2 = 12.19, p = 0.006) and position (χ2 = 14.21, p < 0.001) but not for the interaction (χ2 = 3.70, p = 0.29). Vertical shells tended to have a greater abundance (Figure 12). Regarding season, the post hoc test indicated significant differences only between spring and summer (z = 2.87, p = 0.02) as well as between summer and winter (z = −3.29, p = 0.005). During summer, total abundance tended to be lower in comparison to winter and spring (Figure 13).
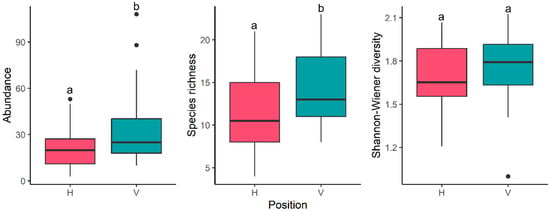
Figure 12.
Boxplots by position: abundance, richness, and diversity (Shannon–Wiener diversity). Different letters indicate significant differences according to the GLMMs.
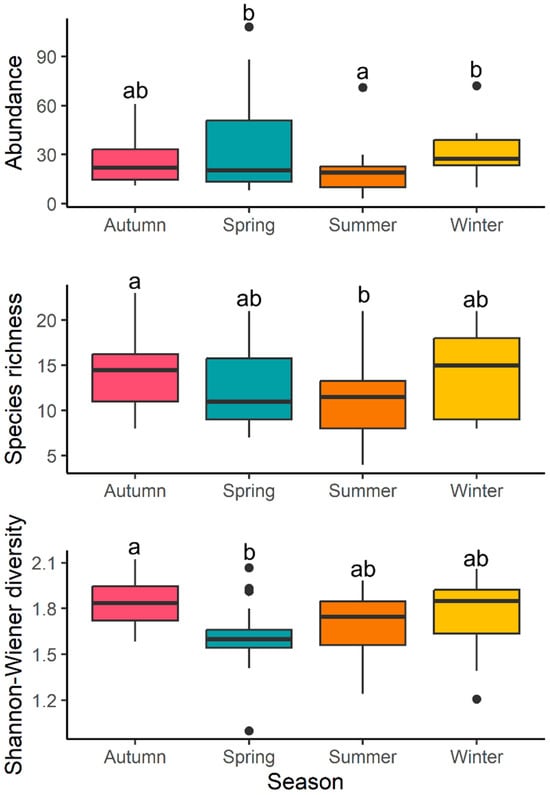
Figure 13.
Boxplots by season: abundance, richness, and diversity (Shannon–Wiener diversity). Different letters indicate significant differences according to Tukey post hoc pairwise comparisons.
Results for richness were similar to the ones obtained for abundance. Analysis showed a significant effect of season (χ2 = 9.59, p = 0.02) and position (χ2 = 9.07, p = 0.03), but not for the interaction (χ2 = 1.53, p = 0.67). Vertical shells tended to have a higher species richness than horizontal ones (Figure 10). Seasonal differences were found only between autumn and summer (z = 2.79, p = 0.03), with a tendency for a lower number of species during summer (Figure 13).
Regarding diversity, significant effects were found for season (F = 4.26, p = 0.008) but not for position (F = 2.07, p = 0.15) or the interaction (F = 0.31, p = 0.06). Significant differences in diversity were found only between autumn and spring (t = 3.39, p = 0.006), with diversity being higher during autumn in comparison to spring (Figure 13).
Correspondence analysis results are depicted in Figure 14. No clear pattern of ordination by season and position was found. However, a few samples were remarkable. Some samples of vertical shells analyzed in spring showed a clear association with S. spallanzanii. A couple of autumn and spring vertical shells seem to be associated with C. erythropus abundance. The chi-square test indicated a significant association between species and samples (χ2 = 5334, p < 0.001). The first two CA axes accounted for 35% of variability in the data set.
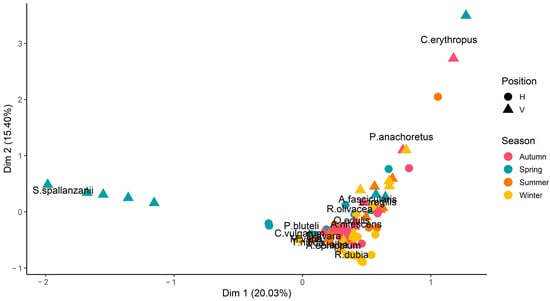
Figure 14.
Correspondence analysis biplot showing species and sample associations.
The NMDS with all species is shown in Figure 15. Clear separation between vertical and horizontal shells was observed only in spring. Another clear separation was observed between winter and summer, which was more conspicuous between summer vertical and winter horizontal shells. These results were supported by PERMANOVA results, which showed a significant effect of the interaction season x position (F = 1.43, p = 0.01), as well as for main effects of season (F = 4.10, p < 0.001) and position (F = 2.10, p = 0.002). Polychaetae assemblages differed between vertical and horizontal shells depending on the season. Some differences in seasons were dependent on the shells’ position.
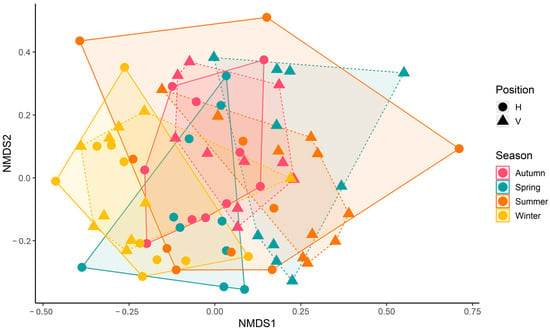
Figure 15.
NMDS scatter plot. Solid lines indicate spread of horizontal shell data points. Dashed lines indicate the spread of vertical shell data points. Analysis was run with three dimensions; for simplicity, only the first two dimensions are displayed. Stress = 0.2.
4. Discussion
Understanding biodiversity and abundance dynamics of macrofauna associated with the shells of dead P. nobilis is valuable for ecological research and conservation efforts. Shells of dead individuals serve as essential habitats for various marine organisms, offering both refuge and substrate for settlement in sedimentary environments. Pinnidae, including P. nobilis, are recognized as ecosystem engineers due to their ability to modify habitats by providing structural complexity in otherwise homogeneous sedimentary bottoms, enhancing biodiversity, and supporting epifaunal and infaunal communities [39,40,41]. This role is well documented in studies of related species like Pinna spp. and Atrina spp., where shell structures increased local species richness by offering attachment surfaces and shelter [21,42]. An in situ experiment transplanting empty P. nobilis shells into a bare soft-bottomed area revealed a significant engineering effect, with species richness, abundance, and diversity increasing compared to the relatively stable surrounding soft-sediment communities [43]. This supports the role of P. nobilis as an ecosystem engineer, enhancing habitat complexity and biodiversity, consistent with our findings of elevated macrofaunal abundance and diversity associated with the shells of dead individuals. A manipulative experiment in an urban estuary showed that artificial Pinna bicolor shell mimics, both open and closed, quickly increased epifaunal biodiversity and provided settlement surfaces for epiphytes within 12 weeks [44]. While sediment and infaunal composition remained largely unchanged, these results support the role of pinnid structures in enhancing aboveground ecological communities, aligning with our findings on P. nobilis shells.
The seasonal increase in the number of organisms and species richness followed the decrease in temperature from summer to winter. Knott et al. [45] compared species richness with temperature change and found a positive proportionality. The authors elaborated in their study that the link stems from seasonal variation in food availability, which is closely related to temperature and phytoplankton blooms. Our results show an inverse proportionality between the abundance and species richness in relation to temperature changes, except during spring in Soline Bay, likely due to eutrophication processes occurring during that season, when the sea starts to warm up and the first phytoplankton bloom appears. The previously mentioned inverse relationship between organism abundance or species richness and temperature changes can be attributed to the global warming of sea temperatures due to climate change. Higher temperatures during warmer winter months can shift the reproduction periods of marine invertebrates and thus cause a seasonal change in the distribution of species [46]. In coastal marine ecosystems, dense populations of suspension feeders represent a significant component both in terms of biomass and abundance [47]. The most dominant species was Sabella spallanzanii (72% of all recorded individuals), a filter feeder typical of eutrophic environments, where it can reach high densities, up to 800 individuals per square meter [48]. The size and abundance of this polychaete in spring align with its seasonal reproductive shift, a phenomenon driven by climate change, specifically the rise in sea temperatures, which can lead to earlier or extended reproductive cycles in marine invertebrates [49]. In soft as well as hard bottoms, polychaetes often represent the most dominant macrofaunal taxa, both in abundance as well as in the number of species and biomass [50,51,52], which is in accordance with the results of this study. In addition to Sabella spallanzanii, one of the most abundant polychaete species in this study was Nereis rava, typically associated with sciaphilous algal or coralligenous habitats [53], which are similar to the conditions found within P. nobilis shells.
The size of shells of dead P. nobilis showed no correlation with the number of individuals, consistent with findings from studies on mollusk communities associated with the shells of dead P. nobilis [6]. The positive correlation of species richness and abundance of organisms with habitat area is a generally accepted hypothesis, but it can be influenced by many factors [54]. Research conducted on the fouling and epifauna of living P. nobilis organisms confirmed this hypothesis [55,56]. Deviation of the results by Iannucci et al. [6] from the stated hypothesis, as well as those of this study, could be attributed to the sampling of shells of similar sizes. Giacobbe [55] studied the mollusk community on live P. nobilis and came to similar conclusions in samples with noticeable cracks on the shells. Thus, one can expect that abundance and diversity are dependent on the area available, confirming the original hypothesis. The differences in the average sizes of vertically and horizontally positioned shells of dead P. nobilis in this study can be explained by three hypotheses. The first is the greater resistance of larger shells to sea currents and perturbations in the environment due to the larger surface area of the shells buried in the sediment, which strengthens the shells. The second hypothesis considers the faster decomposition of the horizontal shells, which touch the chemically active sedimentary bottom with a larger surface area than the vertically positioned shells. And lastly, horizontal shells will be more easily buried in the sediment. Soline Bay is tucked into the land, and the influence of waves and water currents is less pronounced than in Valovine Bay, which results in a lower intensity of abrasion and, consequently, greater preservation of the shells. Another possibility could be the increased anthropogenic influence on the shells of the dead P. nobilis population in Valovine Bay, where tourism and snorkeling can be the cause of damage.
It is important to point out the species R. dubia and its influence on the degradation of the shells of dead P. nobilis. This bivalve can live inside the shells of other bivalves that it bores through mechanical and chemical erosion [57]. The number of these bivalves could reduce the temporal availability of the habitat of the shells of dead P. nobilis. The polychaete Sabella spallanzanii can influence macrofaunal assemblages by altering habitat structure and hydrodynamic conditions. Its physical presence can reduce the abundance of surface-dwelling crustaceans, likely due to changes in sediment stability, oxygen levels, and organic matter availability. Additionally, it may impact larval supply by filtering larvae from the water column and shading the substrate, affecting recruitment patterns [58]. Furthermore, climate change-driven shifts in ocean currents and storm intensity may exacerbate these hydrodynamic effects, accelerating habitat destabilization and altering the long-term viability of species that rely on P. nobilis shells for shelter and settlement. Increased frequency of extreme weather events, coupled with ocean acidification, may also accelerate shell degradation, reducing the availability of these critical microhabitats for benthic communities [59].
The shells of dead P. nobilis proved to be home to a vast number of macrofauna. The average abundance of 21.95 organisms per sample in Valovine Bay and 35.20 in Soline Bay indicate the great role of shells of dead P. nobilis in the biocenoses of silty sands of protected coves. Sandy and muddy bottoms are generally places with fewer organisms than associated habitats such as seagrass meadows and rocky bottoms, which provide sheltered, stable, and productive habitats for the development of different stages of organisms, especially juvenile fish [60,61]. However, sedimentary bottoms receive less research attention compared to habitats prioritized for protection despite being the most widespread in coastal areas worldwide. This is partly due to their sheer abundance and the undervaluation of invertebrate biodiversity and ecosystem functions in these habitats, which are often overlooked in marine spatial planning [62]. The importance of P. nobilis on sedimentary beds was recognized during the animal’s lifetime, but this work adds importance to their shells even after death. Whether they serve sessile organisms as a substrate for growth or provide protection for small organisms from predators, the shells of dead P. nobilis have become a place of accumulation for a variety of animals.
The shells of dead P. nobilis provided shelter for a significantly higher number of individuals in Soline Bay compared to Valovine Bay. The explanation for this lies in the specific characteristics of these two bays. The previously mentioned eutrophication of Soline Bay results in a higher amount of available food, which may be one reason for the greater number of organisms. While larger shells theoretically offer a greater surface area for settlement, our results indicate no statistically significant correlation between shell size and macrofaunal abundance. Increased anthropogenic influence in Valovine Bay is one of the factors that could negatively affect the community of P. nobilis shells. Despite these factors, the overall Shannon–Wiener biodiversity index showed a lower value in Soline Bay. This is a consequence of the unevenness within the community, with a large dominance of a few species: S. spallanzanii, Anomia ephippium, R. dubia, and P. bluteli. The latter two species are also present in large numbers in Valovine Bay, where they do not show a dramatically higher abundance compared to other species. Besides the significant difference between the sampling locations, there is also a notable difference in the abundance of organisms among the shells of dead P. nobilis found in different positions (vertically or horizontally located on the sea floor). The lower average abundance of organisms in the horizontally positioned shells of dead P. nobilis could be due to the fact that the large portion of the shell facing the sediment is unavailable for settlement by sessile organisms. However, during sampling, a large number of fish from the family Gobiidae were observed using the space between the shell and the sediment as shelter. Moreover, the two most abundant species of sampled fish, Zebrus zebrus (25%) and Gobius niger (20%), used to prefer sedimentary habitats with stones and vegetation, which were present in the horizontal dead noble pen shells [63,64]. Indeed, the sampling method made it difficult to collect samples from beneath the horizontally positioned P. nobilis shells, allowing some fish to escape.
It is important to mention that the two crustacean species, Nepinnotheres pinnotheres and Pontonia pinnophylax [65], which inhabit living pen shells, were not observed. Corriero and Pronzato [56] identified 35 species from the phylum Porifera on 14 live P. nobilis individuals from the Tyrrhenian Sea, while only nine species were found in this study. However, conclusions should not be drawn from this comparison due to differences in sampling location and depth (10–20 m), as well as the fact that the aforementioned study was conducted by sponge specialists. The same applies to a study on the mollusk community of live P. nobilis along the coast of Sicily, which recorded 101 species of gastropods and 18 species of bivalves [55]. However, the most relevant comparison is with a study conducted on dead noble pen shells from the Gulf of Trieste by Iannucci et al. [6]. This study recorded 57 species of gastropods, 38 species of bivalves, and four species of polyplacophorans, which are proportionally similar to the numbers found in the present study. The dominance of the same bivalve species (R. dubia) was observed, along with similar abundances of Rhyssoplax olivacea, A. ephippium, and Tritia incrassata. Further comparisons will require the development of models of functional diversity and trophic composition of the sampled community, which would provide greater insight into the significance of this habitat.
This study underscores how seasonal changes and habitat orientation influence ecological communities associated with shells, providing valuable insights into coastal ecosystem dynamics. Both season and shell position were identified as major drivers of organism abundance and species richness, with vertical shells consistently supporting more individuals and greater species diversity than horizontal ones, likely due to improved shelter or resource availability. Abundance and richness declined noticeably in summer, likely due to environmental stress such as heat or desiccation. Conversely, autumn showed a peak in diversity, suggesting a recovery phase with a more balanced community structure. In contrast to abundance and richness, diversity showed no clear relationship to shell position, indicating that while orientation affects numbers and variety, it does not necessarily influence species evenness. Community composition varied with both season and shell position, with some seasons highlighting more pronounced differences between vertical and horizontal shells and specific species associations emerging under certain conditions, such as certain taxa thriving in spring vertical habitats. These findings are consistent with broader ecological research, such as studies showing seasonal declines in stressed benthic communities [66] or habitat complexity promoting species richness [67], though our lack of combined season–position effects contrasts with the synergistic influences observed in other studies [68]. Overall, these results highlight how temporal and spatial factors independently shape community structure, with important implications for resilience and habitat roles in dynamic marine ecosystems.
The results also highlight motile species slightly outnumbering sessile ones in abundance and showing greater diversity, while hard-bottom species dominate in both abundance and richness compared to soft-bottom species and species that can inhabit both substrate types. This motile dominance, driven by taxa like Pisidia bluteli, suggests that shells serve as refuges or foraging hubs for mobile predators and scavengers, possibly due to their structural complexity offering protection in a soft-bottom environment. Meanwhile, the strong presence of sessile hard-bottom species, such as Rocellaria dubia and Anomia ephippium, alongside soft-bottom species like Loripinus fragilis, reflects the shells’ dual role as a habitat—offering hard exterior surfaces for attachment and boring organisms, and sediment-filled interiors for burrowing fauna, as noted for other pen shells species [21]. The notable abundance of hard-bottom species, combined with high diversity across Mollusca, Crustacea, and Polychaeta, emphasizes the shells’ importance as a biodiversity hotspot, likely enhancing ecological interactions in otherwise uniform seabed environments. This mirrors findings by Hewitt et al. [40], who noted that spatial configuration of biogenic structures, like different sizes of shells debris patches, drives community variability. Shells of dead P. nobilis play an important role in supporting diverse macrofauna, with significant variations in species composition and abundance influenced by environmental factors. Our findings emphasize the ecological importance of such habitats, particularly in sedimentary coastal areas, and highlight the need for further research into their role in marine biodiversity.
5. Conclusions
Despite being a transient habitat, the shells of dead P. nobilis play a valuable role in maintaining marine biodiversity and ecosystem structure. Their rigid, three-dimensional structure offers a rare hard substrate in predominantly soft-bottom environments, facilitating the settlement of sessile organisms and providing shelter for motile macrofauna. This function is particularly important in sandy or muddy coastal ecosystems found along the northern Adriatic. The presence of diverse taxa, including commercially and ecologically significant species, suggests that the remains of P. nobilis provide local hotspots of biodiversity, trophic dynamics, and habitat complexity lasting several years. Additionally, the role of P. nobilis shells as nursery grounds for juvenile fish and invertebrates highlights their importance in sustaining population connectivity and ecosystem resilience even after the organism’s demise. Given the widespread decline of P. nobilis populations, understanding the ecological functions of the shells of dead individuals is essential for conservation and management strategies. Future studies should explore the functional diversity and trophic interactions within these temporary habitats, as well as the role of associated flora in shaping the community composition. Considering the inevitable complete degradation of P. nobilis shells in the years to come, it is necessary to assess whether artificial structures or habitat restoration efforts could compensate for their loss. Finally, raising public awareness—especially among coastal stakeholders and tourists—about the ecological importance of P. nobilis and its post-mortem contributions to marine biodiversity is important for fostering conservation initiatives and promoting the protection of soft-bottom habitats.
Author Contributions
Conceptualization, P.B., N.I. and M.B.; Formal Analysis, P.B., A.B., I.K., A.J., G.M., P.P., E.P. and T.M.; Investigation, P.B., N.I., A.B., A.Ž., I.M., A.J., P.P. and M.B.; Methodology, N.I., A.B., I.M., G.M., P.P., T.M. and M.B.; Resources, P.B., N.I., G.M., E.P. and M.B.; Supervision, P.B., N.I., E.P. and M.B.; Visualization, P.B., A.B. and M.B.; Writing—Original Draft Preparation, P.B. and A.B.; Writing—Review and Editing, N.I., A.Ž., I.K., A.J. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Ministry of Environment Protection and Green Transition of the Republic of Croatia (CLASS: UP/I-352-04/23-08/84, REG. NO: 517-10-1-2-23-4, date of approval: 29 March 2023 and CLASS: UP/I-352-04/24-08/28, REG. NO: 517-10-1-2-23-2, date of approval: 7 February 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors can provide the data if needed.
Acknowledgments
Thanks are also extended to the students of the Faculty of Natural Sciences in Pula, Jakov Jurman, and Aleksandar Mijojlić, who contributed by assisting with fieldwork.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mayer-Pinto, M.; Ledet, J.; Crowe, T.P.; Johnston, E.L. Sublethal Effects of Contaminants on Marine Habitat-Forming Species: A Review and Meta-Analysis. Biol. Rev. 2020, 95, 1554–1573. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple Non-Species-Specific Pathogens Possibly Triggered the Mass Mortality in Pinna nobilis. Life 2020, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Čižmek, H.; Čolić, B.; Gračan, R.; Grau, A.; Catanese, G. An Emergency Situation for Pen Shells in the Mediterranean: The Adriatic Sea, One of the Last Pinna nobilis Shelters, Is Now Affected by a Mass Mortality Event. J. Invertebr. Pathol. 2020, 173, 107388. [Google Scholar] [CrossRef]
- Addis, P.; Secci, M.; Brundu, G.; Manunza, A.; Corrias, S.; Cau, A. Density, Size Structure, Shell Orientation and Epibiontic Colonization of the Fan Mussel Pinna nobilis L. 1758 (Mollusca: Bivalvia) in Three Contrasting Habitats in an Estuarine Area of Sardinia (W Mediterranean). Sci. Mar. 2009, 73, 143–152. [Google Scholar] [CrossRef]
- Cortés-Melendreras, E.; Gomariz-Castillo, F.; Alonso-Sarría, F.; Giménez Martín, F.J.; Murcia, J.; Canales-Cáceres, R.; Ramos Esplá, A.A.; Barberá, C.; Giménez-Casalduero, F. The Relict Population of Pinna nobilis in the Mar Menor Is Facing an Uncertain Future. Mar. Pollut. Bull. 2022, 185, 114376. [Google Scholar] [CrossRef]
- Iannucci, S.; Auriemma, R.; Davanzo, A.; Ciriaco, S.; Segarich, M.; Del Negro, P. Can the Empty Shells of Pinna nobilis Maintain the Ecological Role of the Species? A Structural and Functional Analysis of the Associated Mollusc Fauna. Diversity 2023, 15, 956. [Google Scholar] [CrossRef]
- Martinović, R.; Joksimović, D.; García-March, J.R.; Vicente, N.; Gačić, Z. Evaluation of Physiological State of Pen Shell Pinna nobilis (Linnaeus, 1758) by a Non-Invasive Heart Rate Recording under Short-Term Hyposalinity Test. Micromachines 2022, 13, 1549. [Google Scholar] [CrossRef] [PubMed]
- Bakran-Petricioli, T.; Kujundžić, D.; Naranđa, M.; Petricioli, D.; Petricioli, L.; Kipson, S. Fouling Community on Pinna nobilis Larval Collectors in the Adriatic—Impact of Invasive Species. J. Mar. Sci. Eng. 2023, 11, 618. [Google Scholar] [CrossRef]
- Kersting, D.; Mouloud, B.; Cizmek, H.; Grau, A.; Jimenez, C.; Katsanevakis, S.; Oztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna nobilis. The IUCN Red List of Threatened Species 2019; IUCN: Gland, Switzerland, 2019. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Álvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jiménez, S.; Kersting, D.; Moreno, D.; Pérez, M.; et al. S.O.S. Pinna nobilis: A Mass Mortality Event in Western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 220. [Google Scholar] [CrossRef]
- García-March, J.R.; Tena, J.; Henandis, S.; Vázquez-Luis, M.; López, D.; Téllez, C.; Prado, P.; Navas, J.I.; Bernal, J.; Catanese, G.; et al. Can We Save a Marine Species Affected by a Highly Infective, Highly Lethal, Waterborne Disease from Extinction? Biol. Conserv. 2020, 243, 108498. [Google Scholar] [CrossRef]
- Künili, İ.E.; Ertürk Gürkan, S.; Aksu, A.; Turgay, E.; Çakir, F.; Gürkan, M.; Altinağaç, U. Mass Mortality in Endangered Fan Mussels Pinna nobilis (Linnaeus 1758) Caused by Co-Infection of Haplosporidium Pinnae and Multiple Vibrio Infection in Çanakkale Strait, Turkey. Biomarkers 2021, 26, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Grau, A.; Villalba, A.; Navas, J.I.; Hansjosten, B.; Valencia, J.M.; García-March, J.R.; Prado, P.; Follana-Berná, G.; Morage, T.; Vázquez-Luis, M.; et al. Wide-Geographic and Long-Term Analysis of the Role of Pathogens in the Decline of Pinna nobilis to Critically Endangered Species. Front. Mar. Sci. 2022, 9, 666640. [Google Scholar] [CrossRef]
- Carella, F.; Palić, D.; Šarić, T.; Župan, I.; Gorgoglione, B.; Prado, P.; Andree, K.B.; Giantsis, I.A.; Michaelidis, B.; Lattos, A.; et al. Multipathogen Infections and Multifactorial Pathogenesis Involved in Noble Pen Shell (Pinna nobilis) Mass Mortality Events: Background and Current Pathologic Approaches. Vet. Pathol. 2023, 60, 560–577. [Google Scholar] [CrossRef]
- Nebot-Colomer, E.; Hernandis, S.; Mourre, B.; Fraile-Nuez, E.; Álvarez, E.; Deudero, S.; Albentosa, M.; Vázquez-Luis, M. No Recruits for an Ageing Population: First Signs of Probable Population Extinction in One of the Last Reservoirs of the Critically Endangered Species Pinna nobilis. J. Nat. Conserv. 2024, 79, 126600. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Thrush, S.F.; Dayton, P.K. Disturbance to Marine Benthic Habitats by Trawling and Dredging: Implications for Marine Biodiversity. Annu. Rev. Ecol. Evol. Syst. 2002, 33, 449–473. [Google Scholar] [CrossRef]
- Šarić, T.; Župan, I.; Aceto, S.; Villari, G.; Palić, D.; De Vico, G.; Carella, F. Epidemiology of Noble Pen Shell (Pinna nobilis L. 1758) Mass Mortality Events in Adriatic Sea Is Characterised with Rapid Spreading and Acute Disease Progression. Pathogens 2020, 9, 776. [Google Scholar] [CrossRef]
- Mihaljević, Ž.; Pavlinec, Ž.; Zupičić, I.G.; Oraić, D.; Popijač, A.; Pećar, O.; Sršen, I.; Benić, M.; Habrun, B.; Zrnčić, S. Noble Pen Shell (Pinna nobilis) Mortalities along the Eastern Adriatic Coast with a Study of the Spreading Velocity. J. Mar. Sci. Eng. 2021, 9, 764. [Google Scholar] [CrossRef]
- Rubino, F.; Fanelli, G.; Denti, G. The Queen Is Dead, Long Live the Queen: The Vanishing of Pinna nobilis and the Onset of the Congeneric P. Rudis (Mollusca: Bivalvia). Diversity 2024, 16, 341. [Google Scholar] [CrossRef]
- Munguia, P. Spatial Structure of Communities on Dead Pen Shells (Atrina Rigida) in Sea Grass Beds. Mar. Biol. 2007, 152, 149–156. [Google Scholar] [CrossRef]
- Hewitt, J.E.; Thrush, S.F.; Legendre, P.; Cummings, V.J.; Norkko, A. Integrating Heterogeneity across Spatial Scales: Interactions between Atrina Zelandica and Benthic Macrofauna. Mar. Ecol. Prog. Ser. 2002, 239, 115–128. [Google Scholar] [CrossRef]
- García-March, J.R.; García-Carrascosa, A.M.; Peña Cantero, A.L.; Wang, Y.-G. Population Structure, Mortality and Growth of Pinna nobilis Linnaeus, 1758 (Mollusca, Bivalvia) at Different Depths in Moraira Bay (Alicante, Western Mediterranean). Mar. Biol. 2007, 150, 861–871. [Google Scholar] [CrossRef]
- Melzer, R.; Cesena, F.; Buršić, M.; Lehman, T.; Mayer, R.; Mavrič, B.; Makovec, T.; Pfannkuchen, M.; McHenry, J.; Hess, M. Knights, Ballerinas and Invisibles: The Decapod Crustaceans of the Brijuni Marine Protected Area; Javna Ustanova Nacionalni Park Brijuni: Pula, Croatia, 2019; ISBN 978-953-7264-11-6. [Google Scholar]
- Gofas, S.; Moreno, D.; Salas, C. Moluscos Marinos de Andalucía, 1st ed.; Universidad de Málaga: Málaga, Spain, 2011; ISBN 978-84-9747-356-9. [Google Scholar]
- Poppe, G.T.; Goto, Y. European Seashells. Scaphopoda, Bivalvia, Cephalopoda; Verlag Christa Hemmen: Wiesbaden, Germany, 1993; Volume 2, ISBN 3-925919-10-4. [Google Scholar]
- Jardas, I. Jadranska Ihtiofauna; Školska Knjiga: Zagreb, Croatia, 1996; ISBN 953-0-61501-9. [Google Scholar]
- Riedl, R. Fauna e Flora del Mediterraneo—Dalle Alghe ai Mammiferi: Una Guida Sistematica alle Specie che Vivono Nel Mar Mediterraneo; Franco Muzzio Editore: Padova, Italy, 2010; ISBN 978-88-7413-224-9. [Google Scholar]
- Parapar, J.; Moreira, J.; Núñez, J.; Barnich, R.; del Carmen Brito, M.; Fiege, D.; Capaccioni-Azzati, R.; El-Haddad, M. Fauna Ibérica—Annelida: Polychaeta IV; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2015; Volume 41, ISBN 978-84-00-08294-9. [Google Scholar]
- Parapar, J.; Alós, C.; Núñez, J.; Moreira, J.; López, E.; Aguirrezabalaga, F.; Besteiro, C.; Martínez, A. Fauna Ibérica—Annelida: Polychaeta III; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científica: Madrid, Spain, 2012; Volume 36, ISBN 978-84-00-08294-9. [Google Scholar]
- Vieitez, J.M. Fauna Ibérica—Annelida: Polychaeta I; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2004; Volume 25, ISBN 978-84-00-07010-6. [Google Scholar]
- San Martin, G. Fauna Ibérica—Annelida: Polychaeta II; Museo Nacional de Ciencias Naturales, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2003; Volume 21, ISBN 978-84-00-08294-9. [Google Scholar]
- R Core Team, R. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 21 February 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; O’Hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package. R Package Version 2.5–6. 2019. Community Ecol. 2020, 8, 732–740. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lenth, R.V.; Banfai, B.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; et al. Emmeans: Estimated Marginal Means, aka Least-Squares Means 2025. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 20 February 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel Test in the Face of Heterogeneous Dispersions: What Null Hypothesis Are You Testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Cummings, V.J.; Thrush, S.F.; Hewitt, J.E.; Turner, S.J. The Influence of the Pinnid Bivalve Atrina Zelandica (Gray) on Benthic Macroinvertebrate Communities in Soft-Sediment Habitats. J. Exp. Mar. Biol. Ecol. 1998, 228, 227–240. [Google Scholar] [CrossRef]
- Hewitt, J.E.; Thrush, S.F.; Halliday, J.; Duffy, C. The Importance of Small-Scale Habitat Structure for Maintaining Beta Diversity. Ecology 2005, 86, 1619–1626. [Google Scholar] [CrossRef]
- Lohrer, A.M.; Rodil, I.F.; Townsend, M.; Chiaroni, L.D.; Hewitt, J.E.; Thrush, S.F. Biogenic Habitat Transitions Influence Facilitation in a Marine Soft-Sediment Ecosystem. Ecology 2013, 94, 136–145. [Google Scholar] [CrossRef]
- Lopes, E.; Monteiro, N.; Santos, A. Epibiotic Assemblages on the Pen Shell Pinna Rudis (Bivalvia, Pinnidae) at Matiota Beach, São Vicente Island, Cabo Verde. Afr. J. Mar. Sci. 2020, 42, 13–21. [Google Scholar] [CrossRef]
- Rabaoui, L.; Belgacem, W.; Ben Ismail, D.; Mansour, L.; Tlig-Zouari, S. Engineering Effect of Pinna nobilis Shells on Benthic Communities. Oceanologia 2015, 57, 271–279. [Google Scholar] [CrossRef]
- Tiller, G.; Martin, B.; Baring, R. Razor Clam (Pinna Bicolor) Structural Mimics as Drivers of Epibenthic Biodiversity; a Manipulative Experiment. Mar. Environ. Res. 2024, 200, 106658. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.E.; Thonig, A.; Heiskanen, S.; Hansen, B.W.; Banta, G.T. Seasonal Variation in Diversity of Marine Benthic Invertebrates Leads to a Positive Species-Genetic Diversity Correlation (SGDC). Mar. Ecol. Prog. Ser. 2018, 592, 129–140. [Google Scholar] [CrossRef]
- Lawrence, A.J.; Soame, J.M. The Effects of Climate Change on the Reproduction of Coastal Invertebrates. Ibis 2004, 146, 29–39. [Google Scholar] [CrossRef]
- Coma, R.; Ribes, M.; Gili, J.-M.; Zabala, M. Seasonality in Coastal Benthic Ecosystems. Trends Ecol. Evol. 2000, 15, 448–453. [Google Scholar] [CrossRef]
- Giangrande, A.; Cavallo, A.; Licciano, M.; Mola, E.; Pierri, C.; Trianni, L. Utilization of the Filter Feeder Polychaete Sabella. Aquacult Int. 2005, 13, 129–136. [Google Scholar] [CrossRef]
- Giangrande, A.; Licciano, M.; Musco, L.; Stabili, L. Shift in Sabella Spallanzanii (Polychaeta, Sabellidae) Spawning Period in the Central Mediterranean Sea: A Consequence of Climate Change? Mediterr. Mar. Sci. 2010, 11, 373–380. [Google Scholar] [CrossRef]
- Knox, G.A. The Role of Polychaetes in Benthic Soft-Bottom Communities. In Essays on Polychaetous Annelids in Memory of Olga Hartman; University of Southern California, Allan Hancock Press: Santa Maria, CA, USA, 1977; pp. 547–604. [Google Scholar]
- Fauchald, K.; Jumars, P.A. The Diet of Worms: A Study of Polychaete Feeding Guilds. Oceanogr. Mar. Biol. Ann. Rev. 1979, 17, 193–284. [Google Scholar]
- Hutchings, P. Biodiversity and Functioning of Polychaetes in Benthic Sediments. Biodivers. Conserv. 1998, 7, 1133–1145. [Google Scholar] [CrossRef]
- Giangrande, A. Polychaete Zonation and Its Relation to Algal Distribution down a Vertical Cliff in the Western Mediterranean (Italy): A Structural Analysis. J. Exp. Mar. Biol. Ecol. 1988, 120, 263–276. [Google Scholar] [CrossRef]
- Connor, E.F.; McCoy, E.D. The Statistics and Biology of the Species-Area Relationship. Am. Nat. 1979, 113, 791–833. [Google Scholar] [CrossRef]
- Giacobbe, S. Epibiontic Mollusc Communities on Pinna nobilis L. (Bivalvia, Mollusca). J. Nat. Hist. 2002, 36, 1385–1396. [Google Scholar] [CrossRef]
- Corriero, G.; Pronzato, R. Epibiontic Sponges on the Bivalve Pinna nobilis. Mar. Ecol. Prog. Ser. 1987, 35, 75–82. [Google Scholar] [CrossRef]
- Morton, B.; Peharda, M.; Petrić, M. Functional Morphology of Rocellaria Dubia (Bivalvia: Gastrochaenidae) with New Interpretations of Crypt Formation and Adventitious Tube Construction, and a Discussion of Evolution within the Family. Biol. J. Linn. Soc. 2011, 104, 786–804. [Google Scholar] [CrossRef]
- O’Brien, A.L.; Ross, D.J.; Keough, M.J. Effects of Sabella Spallanzanii Physical Structure on Soft Sediment Macrofaunal Assemblages. Mar. Freshwater Res. 2006, 57, 363–371. [Google Scholar] [CrossRef]
- Wang, B.; Hua, L.; Mei, H.; Wu, X.; Kang, Y.; Zhao, N. Impact of Climate Change on the Dynamic Processes of Marine Environment and Feedback Mechanisms: An Overview. Arch. Computat. Methods Eng. 2024, 31, 3377–3408. [Google Scholar] [CrossRef]
- Coelho, R.; Monteiro, P.; Abecasis, D.; Blot, J.Y.; Gonçalves, J.M.S. Macrofauna Assemblages in a XVIIth Century Shipwreck: Comparison with Those on Natural Reefs and Sandy Bottoms. Braz. J. Oceanogr. 2012, 60, 447–462. [Google Scholar] [CrossRef]
- Cecapolli, E.; Calò, A.; Giakoumi, S.; Di Lorenzo, M.; Greco, S.; Fanelli, E.; Milisenda, G.; Di Franco, A. Sandy Bottoms Have Limited Species Richness but Substantially Contribute to the Regional Coastal Fish β-Diversity: A Case Study of the Central Mediterranean Sea. Mar. Environ. Res. 2024, 201, 106701. [Google Scholar] [CrossRef]
- Harper, L.M.; Lefcheck, J.S.; Whippo, R.; Jones, M.S.; Foltz, Z.; Duffy, J.E. Blinded by the Bright: How Species-Poor Habitats Contribute to Regional Biodiversity across a Tropical Seascape. Divers. Distrib. 2022, 28, 2272–2285. [Google Scholar] [CrossRef]
- Wiederholm, A.-M. Habitat Selection and Interactions between Three Marine Fish Species (Gobiidae). Oikos 1987, 48, 28–32. [Google Scholar] [CrossRef]
- Patzner, R.A. Habitat Utilization and Depth Distribution of Small Cryptobenthic Fishes (Blenniidae, Gobiesocidae, Gobiidae, Tripterygiidae) in Ibiza (Western Mediterranean Sea). Environ. Biol. Fishes 1999, 55, 207–214. [Google Scholar] [CrossRef]
- Basso, L.; Vázquez-Luis, M.; García-March, J.R.; Deudero, S.; Alvarez, E.; Vicente, N.; Duarte, C.M.; Hendriks, I.E. The Pen Shell, Pinna nobilis. In Advances in Marine Biology; Curry, B.E., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 71, pp. 109–160. [Google Scholar]
- Dafforn, K.A.; Kelaher, B.P.; Simpson, S.L.; Coleman, M.A.; Hutchings, P.A.; Clark, G.F.; Knott, N.A.; Doblin, M.A.; Johnston, E.L. Polychaete Richness and Abundance Enhanced in Anthropogenically Modified Estuaries Despite High Concentrations of Toxic Contaminants. PLoS ONE 2013, 8, e77018. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Wilson, B.J.; Tobin, M.L.; Hill, A.S.; Hawkins, S.J. Biologically Generated Habitat Provision and Diversity of Rocky Shore Organisms at a Hierarchy of Spatial Scales. J. Exp. Mar. Biol. Ecol. 1996, 202, 73–84. [Google Scholar] [CrossRef]
- Olsgard, F.; Brattegard, T.; Holthe, T. Polychaetes as Surrogates for Marine Biodiversity: Lower Taxonomic Resolution and Indicator Groups. Biodivers. Conserv. 2003, 12, 1033–1049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).