Environmental Variables Influencing the Distribution of Penaeus Shrimp (Decapoda: Dendrobranchiata: Penaeidae) in a Subtropical Estuary of the Gulf of Mexico
Abstract
1. Introduction
2. Materials and Methods
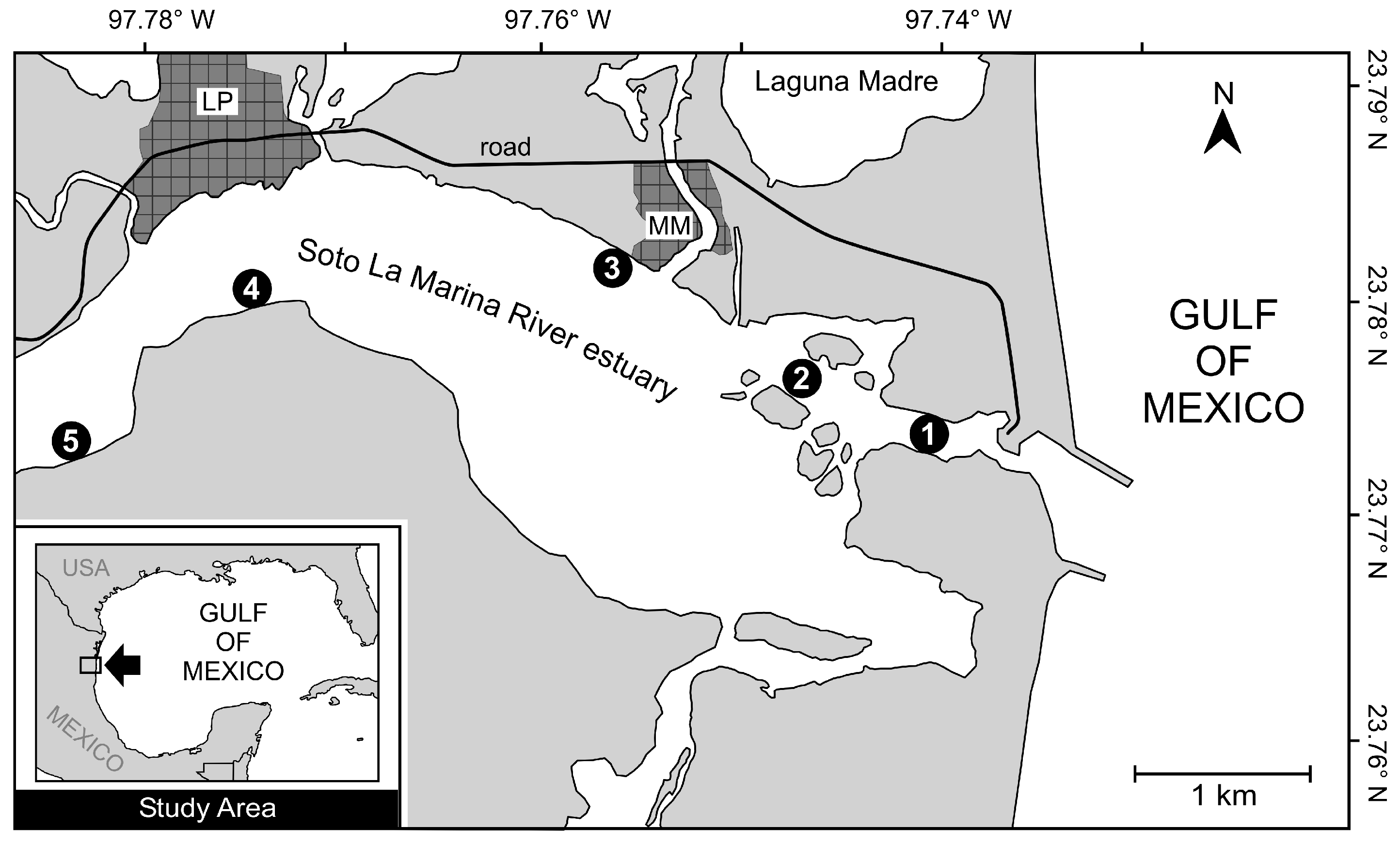
2.1. Study Area
2.2. Sampling and Laboratory Procedures
2.3. Data Analysis
3. Results
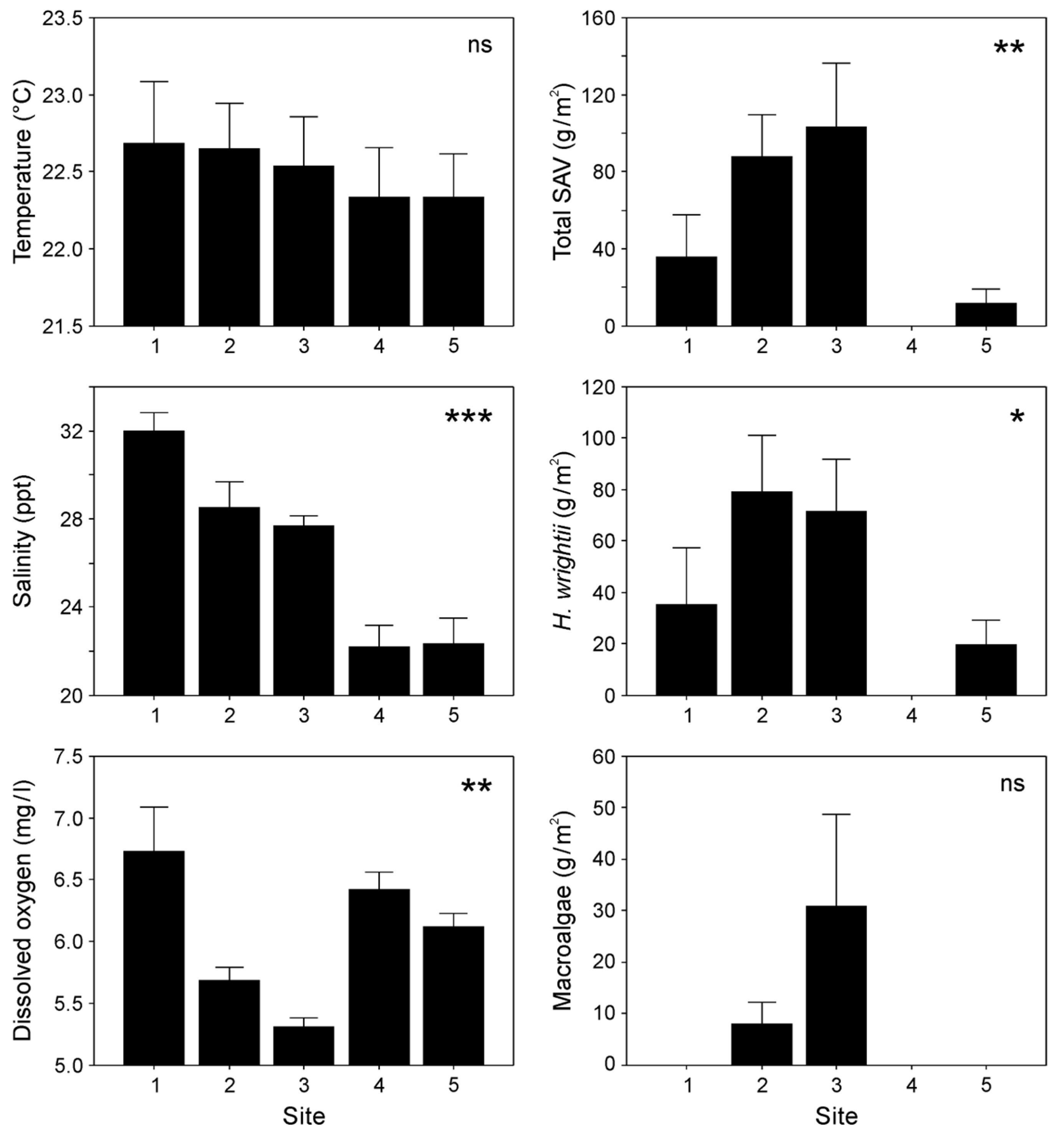
3.1. Environmental Variables
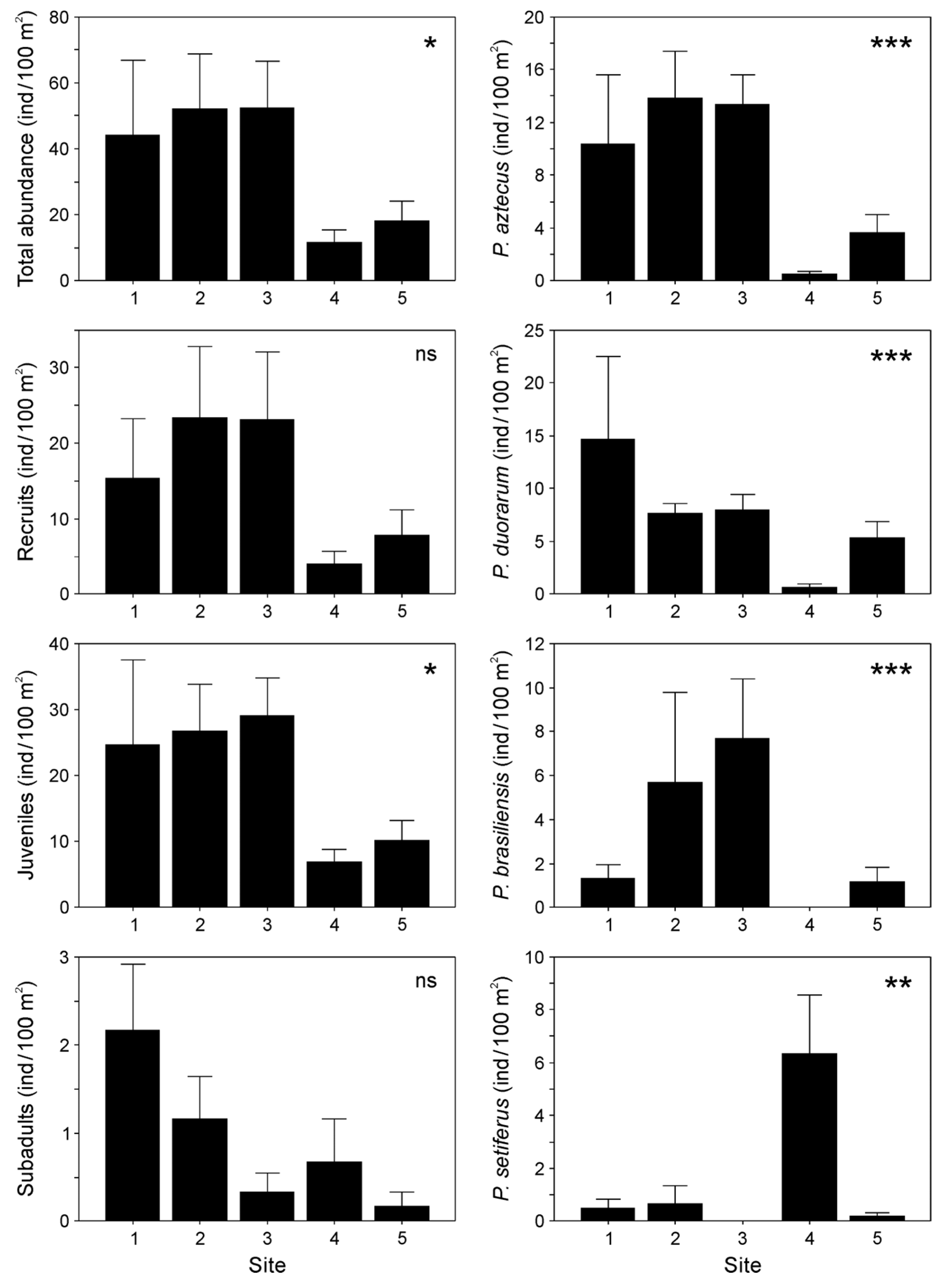
3.2. Shrimp Populations
4. Discussion
4.1. Habitat Environmental Factors
4.2. Distribution of Penaeid Shrimps Along the Estuary
4.3. Influence of Environmental Factors on Shrimp Abundance
4.4. Utilization of Subtropical Estuaries by Penaeids in Other Regions of the World
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderon-Aguilera, L.E.; Marinone, S.G.; Aragón-Noriega, E.A. Influence of oceanographic processes on the early life stages of the blue shrimp (Litopenaeus stylirostris) in the Upper Gulf of California. J. Mar. Syst. 2003, 39, 117–128. [Google Scholar] [CrossRef]
- Manzano-Sarabia, M.M.; Aragón-Noriega, E.A.; Salinas-Zavala, C.A.; Lluch-Cota, D.B. Distribution and abundance of penaeid shrimps in a hypersaline lagoon in northwestern Mexico, emphasizing the brown shrimp Farfantepenaeus californiensis life cycle. Mar. Biol. 2007, 152, 1021–1029. [Google Scholar] [CrossRef]
- Cházaro-Olvera, S.; Winfield, I.; Coria-Olvera, V. Transport of Farfantepenaeus aztecus postlarvae in three lagoon-system inlets in the Southwestern Gulf of Mexico. Crustaceana 2009, 82, 425–437. [Google Scholar] [CrossRef]
- Browder, J.A.; Zein-Eldin, Z.; Criales, M.M.; Robblee, M.B.; Wong, S.; Jackson, T.L.; Johnson, D. Dynamics of pink shrimp (Farfantepenaeus duorarum) recruitment potential in relation to salinity and temperature in Florida Bay. Estuaries 2002, 25, 1355–1371. [Google Scholar] [CrossRef]
- Pérez-Castañeda, R.; Blanco-Martínez, Z.; Sánchez-Martínez, J.G.; Rábago-Castro, J.L.; Aguirre-Guzmán, G.; Vázquez-Sauceda, M.L. Distribution of Farfantepenaeus aztecus and F. duorarum on submerged aquatic vegetation habitats along a subtropical coastal lagoon (Laguna Madre, Mexico). J. Mar. Biol. Assoc. UK 2010, 90, 445–452. [Google Scholar] [CrossRef]
- Day, J.W.; Crump, B.C.; Kemp, W.M.; Yáñez-Arancibia, A. Estuarine Ecology, 2nd ed.; Wiley-Blackwell Press: Hoboken, NJ, USA, 2013; pp. 1–550. [Google Scholar]
- Ruas, V.M.; Rufener, M.-C.; D’Incao, F. Distribution and abundance of post-larvae and juvenile pink shrimp Farfantepenaeus paulensis (Pérez Farfante, 1967) in a subtropical estuary. J. Mar. Biol. Assoc. UK 2019, 99, 923–932. [Google Scholar] [CrossRef]
- Kimball, M.E.; Eash-Loucks, W.E. Estuarine nekton assemblages along a marsh-mangrove ecotone. Estuaries Coasts 2021, 44, 1508–1520. [Google Scholar] [CrossRef]
- Pérez-Castañeda, R.; Defeo, O. Population variability of four sympatric penaeid shrimps (Farfantepenaeus spp.) in a tropical coastal lagoon of Mexico. Estuar. Coast. Shelf Sci. 2001, 52, 631–641. [Google Scholar] [CrossRef]
- Pérez-Castañeda, R.; Defeo, O. Spatial distribution and structure along ecological gradients: Penaeid shrimps in a tropical estuarine habitat of Mexico. Mar. Ecol. Prog. Ser. 2004, 273, 173–185. [Google Scholar] [CrossRef]
- Lüchmann, K.H.; Freire, A.S.; Ferreira, N.C.; Daura-Jorge, F.G.; Marques, M.R.F. Spatial and temporal variations in abundance and biomass of penaeid shrimps in the subtropical Conceição Lagoon, southern Brazil. J. Mar. Biol. Assoc. UK 2008, 88, 293–299. [Google Scholar] [CrossRef]
- Ferreira, N.C.; Freire, A.S. Spatio-temporal variation of the pink shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae) associated to the seasonal overture of the sandbar in a subtropical lagoon. Iheringia Sé. Zool. 2009, 99, 390–396. [Google Scholar] [CrossRef]
- Barbier, E.; Hacker, S.D.; Kennedy, C.; Koch, E.W.; Stier, A.C.; Silliman, B.R. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011, 81, 169–193. [Google Scholar] [CrossRef]
- Gallegos-Martínez, M.E.; Hernández-Cárdenas, G. Pastos marinos. In Atlas de Línea Base Ambiental del Golfo de México; Herzka, S.Z., Zaragoza-Álvarez, R.A., Peters, E.M., Hernández-Cárdenas, G., Eds.; CIGOM; CICESE; Universidad Autónoma Metropolitana: Ensenada, Mexico, 2020; pp. 1–104. [Google Scholar]
- Beck, M.W.; Heck, K.L., Jr.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J.; et al. The identification, conservation and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 2001, 51, 633–641. [Google Scholar] [CrossRef]
- Jackson, E.L.; Rowden, A.A.; Attrill, M.J.; Bossey, S.J.; Jones, M.B. The importance of seagrass beds as a habitat for fishery species. In Oceanography and Marine Biology. An Annual Review; Gibson, R.N., Barnes, M., Atkinson, R.J.A., Eds.; Taylor & Francis: New York, NY, USA, 2001; Volume 39, pp. 269–303. [Google Scholar]
- Snedden, G.A.; Cable, J.E.; Kjerfve, B. Estuarine geomorphology and coastal hydrology. In Estuarine Ecology, 2nd ed.; Day, J.W., Crump, B.C., Kemp, W.M., Yáñez-Arancibia, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 19–38. [Google Scholar]
- Ramírez-Rodríguez, M.; Santos-Ortega, Y.; Navarrete-Del-Proo, A. Juvenile pink shrimp, Farfantepenaeus duorarum (Burkenroad, 1939): Length composition in three nursery areas in Campeche Sound, Gulf of Mexico. Crustaceana 2004, 77, 1107–1116. [Google Scholar]
- Baker, R.; Minello, T.J. Growth and mortality of juvenile white shrimp Litopenaeus setiferus in a marsh pond. Mar. Ecol. Prog. Ser. 2010, 413, 95–104. [Google Scholar] [CrossRef]
- Brito, R.; Gelabert, R.; Amador del Ángel, L.E.; Alderete, Á.; Guevara, E. Diel variation in the catch of the shrimp Farfantepenaeus duorarum (Decapoda, Penaeidae) and length-weight relationship, in a nursery area of the Terminos Lagoon, Mexico. Rev. Biol. Trop. 2017, 65, 65–75. [Google Scholar] [CrossRef]
- Zink, I.C.; Browder, J.A.; Lirman, D.; Serafy, J.E. Pink shrimp Farfantepenaeus duorarum spatiotemporal abundance trends along an urban, subtropical shoreline slated for restoration. PLoS ONE 2018, 13, e0198539. [Google Scholar] [CrossRef]
- Glover, K.M.; Kimball, M.E.; Pfirrmann, B.W.; Pelton, M.M.; Dunn, R.P. Juvenile brown shrimp (Farfantepenaeus aztecus) use of salt marsh intertidal creeks as nursery habitat. Estuaries Coasts 2023, 46, 1895–1906. [Google Scholar] [CrossRef]
- Howe, J.C.; Wallace, R.K.; Rikard, F.S. Habitat utilization by postlarval and juvenile penaeid shrimps in Mobile Bay, Alabama. Estuaries 1999, 22, 971–979. [Google Scholar] [CrossRef]
- Zimmerman, R.J.; Minello, T.J. Densities of Penaeus aztecus, Penaeus setiferus, and other natant macrofauna in a Texas salt marsh. Estuaries 1984, 7, 421–433. [Google Scholar] [CrossRef]
- Pérez-Castañeda, R.; Robles-Hernández, C.A.; Sánchez-Martínez, J.G. Interspecific variations in population structure of penaeids from an artisanal shrimp fishery in a hypersaline coastal lagoon of Mexico. J. Coastal Res. 2012, 28, 187–192. [Google Scholar] [CrossRef]
- Blanco-Martínez, Z.; Pérez-Castañeda, R. Does the relative value of submerged aquatic vegetation for penaeid shrimp vary with proximity to a tidal inlet? Mar. Freshwater Res. 2017, 68, 581–591. [Google Scholar] [CrossRef]
- Pérez-Farfante, I. Diagnostic Characters of Juveniles of the Shrimps Penaeus aztecus aztecus, P. duorarum duorarum, and P. brasiliensis (Crustacea, Decapoda, Penaeidae). Special Scientific Report-Fisheries No. 599; U.S. Fish and Wildlife Service: Washington, DC, USA, 1970; pp. 1–26.
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall Press: Upper Saddle River, NJ, USA, 1999; pp. 1–663. [Google Scholar]
- McLusky, D.S.; Elliot, M. The Estuarine Ecosystem: Ecology, Threats, and Management, 3rd ed.; Oxford University Press: New York, NY, USA, 2004; pp. 1–214. [Google Scholar]
- Rasmusson, L.M.; Lauritano, C.; Procaccini, G.; Gullström, M.; Buapet, P.; Björk, M. Respiratory oxygen consumption in the seagrass Zostera marina varies on a diel basis and is partly affected by light. Mar. Biol. 2017, 164, 140. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Martínez, Z.; Pérez-Castañeda, R.; Sánchez-Martínez, J.G.; Benavides-González, F.; Rábago-Castro, J.L.; Vázquez-Sauceda, M.L.; Garrido-Olvera, L. Density-dependent condition of juvenile penaeid shrimps in seagrass-dominated aquatic vegetation beds located at different distance from a tidal inlet. PeerJ 2020, 8, e10496. [Google Scholar] [CrossRef]
- Bartenfelder, A.; Kenworthy, W.J.; Puckett, B.; Deaton, C.; Jarvis, J.C. The abundance and persistence of temperate and tropical seagrasses at their edge-of-range in the Western Atlantic Ocean. Front. Mar. Sci. 2022, 9, 917237. [Google Scholar] [CrossRef]
- Morris, L.J.; Hall, L.M.; Jacoby, C.A.; Chamberlain, R.H.; Hanisak, M.D.; Miller, J.D.; Virnstein, R.W. Seagrass in a changing estuary, the Indian River Lagoon, Florida, United States. Front. Mar. Sci. 2022, 8, 789818. [Google Scholar] [CrossRef]
- Dall, W.; Hill, B.J.; Rothlisberg, P.C.; Sharples, D.J. The biology of the Penaeidae. In Advances in Marine Biology; Blaxter, J.H.S., Southward, A.J., Eds.; Academic Press: London, UK, 1990; Volume 27, pp. 1–489. [Google Scholar]
- Ruas, V.M.; Rodrigues, M.A.; Dumont, L.F.C.; D’Incao, F. Habitat selection of the pink shrimp Farfantepenaeus paulensis and the blue crab Callinectes sapidus in an estuary in southern Brazil: Influence of salinity and submerged seagrass meadows. Nauplius 2014, 22, 113–125. [Google Scholar] [CrossRef]
- May-Kú, M.A.; Ordóñez-López, U. Spatial patterns of density and size structure of penaeid shrimps Farfantepenaeus brasiliensis and Farfantepenaeus notialis in a hypersaline lagoon in the Yucatan Peninsula, Mexico. Bull. Mar. Sci. 2006, 79, 259–271. [Google Scholar]
- Brito, R.; Chimal, M.E.; Rosas, C. Effect of salinity in survival, growth, and osmotic capacity of early juveniles of Farfantepenaeus brasiliensis (decapoda: Penaeidae). J. Exp. Mar. Biol. Ecol. 2000, 244, 253–263. [Google Scholar] [CrossRef]
- Zink, I.C.; Browder, J.A.; Lirman, D.; Serafy, J.E. Review of salinity effects on abundance, growth, and survival of nearshore life stages of pink shrimp (Farfantepenaeus duorarum). Ecol. Indic. 2017, 81, 1–17. [Google Scholar] [CrossRef]
- Castille, F.L.; Lawrence, A.L. The effect of salinity on the osmotic, sodium and chloride concentrations in the hemolymph of euryhaline shrimp of the genus Penaeus. Comp. Biochem. Physiol. Physiol. 1981, 68A, 75–80. [Google Scholar] [CrossRef]
- Valdez, G.; Díaz, F.; Denisse Re, A.; Sierra, E. Effect of salinity on physiological energetics of white shrimp Litopenaeus vannamei (Boone). Hidrobiológica 2008, 18, 105–115. [Google Scholar]
- May-Kú, M.A.; Criales, M.M.; Montero-Muñoz, J.L.; Ardisson, P.L. Differential use of Thalassia testudinum habitats by sympatric penaeids in a nursery ground of the Southern Gulf of Mexico. J. Crustacean Biol. 2014, 34, 144–156. [Google Scholar] [CrossRef]
- Loneragan, N.R.; Haywood, M.D.E.; Heales, D.S.; Kenyon, R.A.; Pendrey, R.P.; Vance, D.J. Estimating the influence of prawn stocking density and seagrass type on the growth of juvenile tiger prawns (Penaeus semisulcatus): Results from field experiments in small enclosures. Mar. Biol. 2001, 139, 343–354. [Google Scholar]
- Pérez-Castañeda, R.; Defeo, O. Growth and mortality of transient shrimp populations (Farfantepenaeus spp.) in a coastal lagoon of Mexico: Role of the environment and density-dependence. ICES J. Mar. Sci. 2005, 62, 14–24. [Google Scholar] [CrossRef]
- Hewitt, D.E.; Smith, T.M.; Raoult, V.; Taylor, M.D.; Gaston, T.F. Stable isotopes reveal the importance of saltmarsh-derived nutrition for two exploited penaeid prawn species in a seagrass dominated system. Estuar. Coast. Shelf Sci. 2020, 236, 106622. [Google Scholar] [CrossRef]
- Minello, T.J. Chronographic tethering: A technique for measuring prey survival time and testing predation pressure in aquatic habitats. Mar. Ecol. Prog. Ser. 1993, 101, 99–104. [Google Scholar] [CrossRef]
- Peterson, B.J.; Thompson, K.R.; Cowan, J.H., Jr.; Heck, K.L., Jr. Comparison of predation pressure in temperate and subtropical seagrass habitats based on chronographic tethering. Mar. Ecol. Prog. Ser. 2001, 224, 77–85. [Google Scholar] [CrossRef]
- Barcelona, A.; Serra, T.; Colomer, J.; Infantes, E. Shrimp habitat selection dependence on flow within Zostera marina canopies. Estuar. Coast. Shelf Sci. 2024, 305, 108858. [Google Scholar] [CrossRef]
- Meager, J.J.; Vance, D.J.; Loneragan, N.R.; Williamson, I. Seasonal variation and environmental influences on juvenile banana prawn (Penaeus merguiensis) abundance in a subtropical estuary (Logan River) of eastern Australia. Estuar. Coast. Shelf Sci. 2003, 57, 569–576. [Google Scholar] [CrossRef]
- Poh, B.; Tweedley, J.R.; Chaplin, J.A.; Trayler, K.M.; Crisp, J.A.; Loneragan, N.R. Influence of physico-chemical and biotic factors on the distribution of a penaeid in a temperate estuary. Estuar. Coast. Shelf Sci. 2019, 218, 70–85. [Google Scholar] [CrossRef]
- Potter, I.C.; Manning, R.J.G.; Loneragan, N.R. Size, movements, distribution and gonadal stage of the western king prawn (Penaeus latisulcatus) in a temperate estuary and local marine waters. J. Zool. 1991, 223, 419–445. [Google Scholar] [CrossRef]

| Total Shrimp | Recruits | Juveniles | Subadults | P. aztecus | P. duorarum | P. brasiliensis | |
|---|---|---|---|---|---|---|---|
| Intercept | −49.79 (31.77) | 8.63 (3.63) * | −24.09 (15.48) | −3.03 (1.07) * | −15.56 (6.03) * | −20.79 (9.39) * | 1.95 (1.07) |
| Halodule wrightii (g/m2) | 0.27 (0.12) * | 0.15 (0.06) * | 0.15 (0.06) * | ns | 0.09 (0.02) ** | ns | ns |
| Macroalgae (g/m2) | ns | ns | ns | −0.03 (0.01) * | ns | ns | 0.16 (0.06) * |
| Salinity (ppt) | 2.80 (1.24) * | ns | 1.40 (0.60) * | 0.16 (0.04) ** | 0.76 (0.23) ** | 1.05 (0.35) ** | ns |
| R2 adjusted | 0.49 ** | 0.25 * | 0.55 ** | 0.57 ** | 0.73 *** | 0.37 ** | 0.26 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Cruz, A.M.; Pérez-Castañeda, R.; Blanco-Martínez, Z.; Sánchez-Martínez, J.G.; Vázquez-Sauceda, M.d.l.L.; Benavides-González, F.; Rábago-Castro, J.L. Environmental Variables Influencing the Distribution of Penaeus Shrimp (Decapoda: Dendrobranchiata: Penaeidae) in a Subtropical Estuary of the Gulf of Mexico. Oceans 2025, 6, 16. https://doi.org/10.3390/oceans6010016
Ayala-Cruz AM, Pérez-Castañeda R, Blanco-Martínez Z, Sánchez-Martínez JG, Vázquez-Sauceda MdlL, Benavides-González F, Rábago-Castro JL. Environmental Variables Influencing the Distribution of Penaeus Shrimp (Decapoda: Dendrobranchiata: Penaeidae) in a Subtropical Estuary of the Gulf of Mexico. Oceans. 2025; 6(1):16. https://doi.org/10.3390/oceans6010016
Chicago/Turabian StyleAyala-Cruz, Ayla Marisol, Roberto Pérez-Castañeda, Zeferino Blanco-Martínez, Jesús Genaro Sánchez-Martínez, María de la Luz Vázquez-Sauceda, Flaviano Benavides-González, and Jaime Luis Rábago-Castro. 2025. "Environmental Variables Influencing the Distribution of Penaeus Shrimp (Decapoda: Dendrobranchiata: Penaeidae) in a Subtropical Estuary of the Gulf of Mexico" Oceans 6, no. 1: 16. https://doi.org/10.3390/oceans6010016
APA StyleAyala-Cruz, A. M., Pérez-Castañeda, R., Blanco-Martínez, Z., Sánchez-Martínez, J. G., Vázquez-Sauceda, M. d. l. L., Benavides-González, F., & Rábago-Castro, J. L. (2025). Environmental Variables Influencing the Distribution of Penaeus Shrimp (Decapoda: Dendrobranchiata: Penaeidae) in a Subtropical Estuary of the Gulf of Mexico. Oceans, 6(1), 16. https://doi.org/10.3390/oceans6010016






