Abstract
Cnidome morphology appears to be a valuable tool for anthozoan (Octocarallia, Ceriantharia, and Hexacorallia) taxonomy. Ceriantharian cnidomes consist of b-mastigophores, isorhizas, spirocysts, and ptychocysts, including different subtypes. The b-mastigophores are the most valuable ceriantharian cnidae for species identification. The Ceriantharian b-mastigophore terminology is congruent to the b-mastigophores of Carlgren, b-rhabdoids, including the “faltstück” of Schmidt, spirulae, and also potentially the penicilli of den Hartog. The apical tightly folded, inverted b-mastigophore shafts, the “faltstück”, are important species-specific characteristics due to their various patterns. The mesenterial structures known as craspedonemes, cnidorages, and acontioids also have high taxonomic value. Drop-shaped b-mastigophores might be characteristic nematocysts for mesenteries. The occurrence of isorhizas and striations on the inner ptychocyst capsule walls are other species’ characteristics. The morphological similarities of spirulae and penicilli to b-mastigophores within Hexacorallia are highlighted by naming spirulae and penicilli as b-mastigophores/spirulae and b-mastigophores/penicilli subtypes, respectively. The slight morphological distinction between spirulae and penicilli will doubtfully justify the suborders Spirularia and Penicillaria. The spirocysts presence in Ceriantharia and Hexacorallia indicates a closer relationship between Ceriantharia and Hexacorallia than between Ceriantharia and Octocorallia. Octocorallia are the only anthozoans without spirocysts. This work underlines the importance of cnidome morphological analysis for taxonomic identification and classification.
1. Introduction
Ceriantharia, Octocorallia, and Hexacorallia represent the three subclasses of Anthozoa [1,2,3,4]. Ceriantharian species, the so-called tube anemones, are solitary anemone-like anthozoans found in all oceans from the intertidal zone to abyssal depths [5]. Adult Ceriantharians are benthic and usually inhabit long clay or gravel tubes [6]. The tubes are built from a cnida type termed ptychocysts, a special cnida type found exclusively in Ceriantharia [7]. Discharged ptychocyst tubules produce a thin membrane around the anemone column (i.e., column membrane), which is later incrusted by the surrounding substrate particles and forms a thick internal membrane, termed the tube membrane [6]. Ceriantharia is represented by three tube anemone families, the Cerianthidae, the Botrucnidiferidae, and the Arachnactidae.
Knowledge of ceriantharian cnidae has been neglected over time and, indirectly, considered as having low taxonomic value. Nowadays, despite their complicated terminology, the cnida morphology is generally accepted as having high taxonomic value within the main cnidarian groups. The term “cnidome” refers to complete cnida variations in a species or an animal’s structure. Cnidae, also called cnidocysts, include three different categories: nematocysts, spirocysts, and ptychocysts [7,8]. Among the Anthozoa, nematocysts are classified into four different types, namely (i) b-mastigophores, (ii) p-mastigophores, (iii) p-amastigophores, and (iv) isorhizas, while spirocysts and ptychocysts do not exist in various types. The cnidome of the Ceriantharia, in addition to spirocysts and ptychocysts, consists only of the two nematocyst types: b-mastigophores and isorhizas [7,8].
Carlgren (1940) provided a thorough description of cnida types within the Anthozoa, in which some ceriantharians were included [8]. The penetrating nematocysts, so-called mastigophores [9], were differentiated by Carlgren (1940) into b-mastigophores and p-mastigophores displaying a distal tubule, and p-amastigophores lacking the distal tubule [8]. The b-mastigophores present a gradual transition from shaft to distal tubule diameter, while p-mastigophores/p-amastigophores have a sharp transition between the broad shaft and the narrow distal tubule. The inverted shaft-end of the p-mastigophores/p-amastigophores form a deep funnel-shaped opening, the v-notch, from which the inverted tubule emerges. This important cnida description created by Carlgren (1940) greatly improved the earlier classification of Weill (1934) [8,9]. For Ceriantharia, Carlgren (1940) recorded the cnida types: microbasic b-mastigophores, holotrichs (later called isorhizas), spirocysts and atrichs (later called ptychocysts), but no p-mastigophores/p-amastigophores [8]. Despite Carlgren’s progress in knowledge of ceriantharian cnidae, the cnida types were not ascribed as taxonomic tools for Ceriantharia identification. Later, the deep funnel-shaped opening, the v-notch, was shown to be formed by the invagination of the inverted shaft end during the late p-mastigophore/p-amastigophore development [10,11].
More than 20 years later, Schmidt [10,12,13] discarded the descriptive terms b-mastigophores and p-mastigophores/p-amastigophores [8,9] and replaced them with the alphanumeric coded b- and p-rhabdoides, respectively. As with the p-mastigophores/p-amastigophores, the p-rhabdoides were characterized by a distal funnel-shaped opening, the v-notch, at the inverted shaft end. In the sea anemone Metridium senile, Schmidt divided the shaft of some b-rhabdoids and p-rhabdoids into “faltstück” and “hauptstück”. “Faltstück” stands for the apical shaft region, which is tightly folded, then inverted, due to the lack of spines or loosely-set small spines, often randomly arranged. The remaining shaft, the “main shaft”, or “hauptstück”, is heavily armed with long, closely-set spines, and compared with the “faltstück” is less densely twisted and folded. Later on, Schmidt (1972, 1974) reported the presence of “faltstück” in Ceriantharia [12,13].
Shafts including “faltstück” can greatly vary in length when unfolded due to differences in the lengths of the tightly folded, inverted apical shaft part. Three shaft types have been depicted: (i) discharged shafts, less than one and a half times the capsule length, considered as microbasic, (ii) mesobasic shafts, which are more than one and a half times, but less than four times the capsule length, and (iii) macrobasic shafts, with a minimum length equal to or more than four times the capsule length [11,14,15,16,17].
Schmidt (1972, 1974) highlighted the need to combine the cnida types with specific morphological traits to improve Ceriantharia taxonomy [12,13]. For Anthozoa, Schmidt suggested two subclasses: Alcyonaria (current Octocorallia) and Zoantharia (current Hexacorallia), in which Alcyonaria (Octocorallia) has the most primitive cnida type. Based on the morphology of holotrichs (isorhizas) from Cerianthus membranaceus, Schmidt placed Ceriantharia as the most primitive group within the Zoantharia (Hexacorallia). Today, Ceriantharia is regarded as a sister group of Hexacorallia [1,2,3,4]. England (1991) commented on the b- and p-mastigophores/amastigophores created by Carlgren (1940) and on the terminology of b- and p-rhabdoides of Schmidt (1972, 1974) [8,12,13,16].
A few years later, den Hartog (1977) improved the ceriantharian taxonomy by adding nematocysts as basic morphological features [18]. Instead of the terms b- and p-mastigophores/amastigophores and b- and p-rhabdoides, den Hartog adopted the terminology spirulae and penicilli proposed by Stephenson (1928) [19]. Spirulae, representing b-mastigophores, are defined as nematocysts with a prominent shaft, whose diameter gradually tapers towards the distal tubule. Similar to spirulae, penicilli are nematocysts with a prominent shaft with a diameter that gradually tapers, but presents a tiny v-shaped notch at the end of the shaft, formed by the last shaft spines. Den Hartog created the suborders Spirularia and Penicillaria, of which Spirularia includes ceriantharians with numerous spirulae in addition to the presence of homotrichs (isorhizas), but with the absence of the penicilli nematocysts with the tiny v-notch. Penicillaria includes ceriantharian species with numerous penicilli, less occurrence of spirulae, and no homotrichs (isorhizas).
Forero Mejia et al. (2020), in a recent Ceriantharia molecular phylogeny, questioned the validity of the suborder Spirularia, recommending its revision [1]. Spirularia was found to be polyphyletic, whereas Penicillaria was regarded as monophyletic. The slight morphological differences between the nematocysts spirulae and penicilli do not justify the classification of the two suborders or their names as Spirularia and Penicillaria. The question is how to replace or rename the suborders. Forero Mejia et al. (2020) placed Ceriantharia as sister group to the rest of the Anthozoa [1]. Other recent studies support this Ceriantharia phylogenetic position as the first hexacorallian lineage [2,3,4,20].
This article aims to revise the different cnida types occurring within the Ceriantharia, illustrate their morphological variation, and evaluate the potential of cnidae as taxonomic and phylogenetic tools. Cnidae in species from the three currently accepted Ceriantharia families Cerianthidae, Botrucnidiferidae, and Arachnactidae are examined. The three cnida categories: nematocysts, spirocysts, and ptychocysts occur in all examined species. Based on their morphological variation, the various encountered cnidae are divided into subtypes, enabling a detailed description. The specific cnida structures occurring in the mesenteries, named craspedonemes, cnidorages, and acontioids [21], display high taxonomic value, as they include specific cnida subtypes and are present in different families. Moreover, throughout this work, a workable, consensus terminology for the ceriantharian cnidae is proposed. A glossary in the Supplementary Materials is provided to clarify the terminology of the different cnidae and the cnida structures.
Current cnida descriptions are primarily compared and discussed with the cnida descriptions made by Carlgren (1940), Schmidt (1974), and den Hartog (1977) [8,13,18]. In addition, Arai (1965) wrote a synonym list of cnida terminology [22]. Recent works are also studied to validate cnida similarities and differences within Ceriantharia species [23,24,25,26,27].
This survey highlights the importance of a detailed examination of cnidae and their subtypes as taxonomic tools. Indeed, species-specific or genus-specific cnida subtypes, their morphologies, and their occurrence in different structures are powerful for ceriantharian species identification. Dominating cnida subtypes in certain structures can represent high taxonomic value, whereas the less represented cnida subtypes, occurring in all species and structures, are less valuable for species identification.
2. Materials and Methods
2.1. Investigated Species and Tissue Preparations
Cnida type assessments were performed on (i) Cerianthidae: Cerianthus membranaceus (Gmelin, 1791), Cerianthus sp. 1, Pachycerianthus multiplicatus (Carlgren, 1912), Pachycerianthus torreyi (Arai, 1965), and Pachycerianthus dohrni (van Beneden, 1923); (ii) Botrucnidiferidae: Botrucnidifer sp. 1 and Botruanthus benedeni (Torrey & Kleeberger, 1909); and (iii) Arachnactidae: Isarachnanthus maderensis (Johnson, 1861) (e.g., Figure S1). Their sampling locations are provided in Table S1. The species were collected during the years 2014 to 2023. To illustrate common cnida types from other anthozoan and staurozoan groups, species of (i) Octocorallia: Funiculina quadrangularis (Pallas, 1766); (ii) Hexacorallia: Metridium senile (Linnaeus, 1761), Lophelia pertusa (Pallas, 1766), and Caryophyllia smithii (Stroke & Broderip, 1828); and (iii) Staurozoa: Haliclystus auricula (Rathke, 1806) were collected from the Swedish west coast during the years 2006–2018. Living P. multiplicatus specimens from the Gullmars Fjord were kept at Kristineberg Marine Research Station (Fiskebäckskil, University of Gothenburg, Sweden) in aquaria with fresh seabed clay and running seawater. The tube anemones were fed weekly with shrimps.
Squashed cnidae preparations were examined whenever possible on fresh material, otherwise on preserved polyps, in 4% paraformaldehyde in a 0.2 M sodium cacodylate buffer with 0.3 M sucrose. Preparations and measurements of cnida were carried out according to Strömberg & Östman (2016) [28]. Some preparations were stained with eosin or blue household colour to improve the visibility of the armature of the shaft and tubule of undischarged and discharged cnidae. On slightly dried, very thin squashed preparations, the spine and tubule pattern of undischarged and discharged cnidae may appear distinct.
Cnida types, based on their capsule size and shape, shaft, and tubule pattern, were arranged in different subtypes. Based on the capsule length, we ranked the cnidae from P. multiplicatus into approximate size groups: small (up to ≤25 µm), medium (from 25 to 50 µm), large (from 50 to 80 µm), and very large (larger than ≥80 µm). For the other examined species, the limit between medium and large cnidae was placed at 40 µm, otherwise, the range of the size groups was similar.
Through access to live specimens, cnida measurements of P. multiplicatus, the type species of Pachycerianthus, were more detailed. Cnidae were measured from at least three living specimens. Cnida squashed preparations, performed for measurements from marginal and labial tentacles, and the column membrane, were prepared from living anemones without harming the animal (Figure S1). To perform sampling of cnidae from the actinopharynx and column, living tube anemones were lightly anesthetized with MgCl2 (Figure 1d). Then, after cnida sampling, specimens were transferred to fresh seawater aquaria to recover. Finally, to obtain cnida preparation from the mesenterial filaments, including the specific cnida structures the craspedonemes and cnidoglandular tract, tube anemone specimens were anesthetized in MgCl2 and dissected (Figure S1). When possible, at least 20 length/width measurements were obtained from each dominant or common cnida subtype from each studied structure on at least three specimens. For the less common, rare, or seldom occurring cnida subtypes, fewer measurements were taken, down to single ones. Owing to the easy access to tentacles and column membranes, the numbers of cnida measurements from these structures were higher than those from the other tube anemone structures.
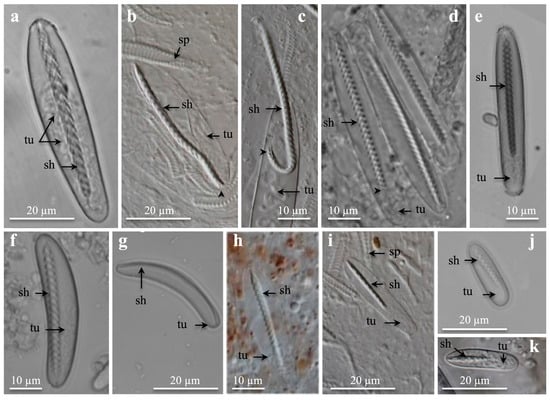
Figure 1.
Light microscopies of undischarged microbasic b-mastigophores showing inverted shaft and tubule. Note upside-down v-shaped spine-patterns on shaft. (a) Large, broad oval to drop-shaped b-mastigophores from cnidorages. (b–d) Large, broad, parallel capsule b-mastigophores from marginal tentacles. Arrowhead points at last spines at shaft end. (e) From labial tentacle and (f,g) from mesenterial filament and marginal tentacle, respectively. (e,f) Medium to large b-mastigophores and (g) medium bow-shaped b-mastigophores. (h) From labial tentacle, and (i) from marginal tentacles. (h,i) Medium, broad b-mastogophores. (j) Small, oval broad b-mastigophore from mesenterial filament, and (k) small, broad b-mastigophore from marginal tentacles. (a) Botruanthus benedeni. (b,c,h,i,k) Pachycerianthus torreyi. (d) P. multiplicatus. (e) P. dorhni. (f,g,j) Cerianthus sp. 1. Abbreviations: sh, shaft; sp, spirocyst; tu, tubule. Arrowhead points at last spines at shaft-end.
Compared with P. multiplicatus, overall, fewer specimens and fewer cnida measurements were obtained from the other studied Ceriantharia species due to the shortness of material and available time. Mostly, these cnida measurements were made on fixed tube anemones. Cnida differences and similarities between species and structures were analysed by comparing the cnida abundance and mean range of capsule length with the mean range of capsule width and mean length/width measurements.
2.2. Light Microscopic (LM) Examination
Tube anemone structures were observed under a Leica M205C (Leica Microsystems, Wetzlar, Germany) stereomicroscope. For cnidocyst studies a Leitz DMRBE Epifluorescence LM microscope (Leica Microsystems, Wetzlar, Germany) equipped with interference-contrast optics 100×/1.30 PL was used. Before September 2020, the stereomicroscope Leica M205C and the light microscope Leitz DMRBE equipped with Leica cameras (M205C with Leica DFC420C and DMRBE with Leica DFC295) were both controlled by the Leica Application Suite software (LAS V3.8). From September 2020, the stereomicroscope Leica M205C and the light microscope Leitz DMRBE were equipped with Zeiss cameras (M205C with Axiocam 305 color and DMRBE with Axiocam 705 color; Zeiss, Oberkochen, Germany) and both controlled by the Zeiss ZEN microscope software version 3.7 (ZEN desk, Carl Zeiss Microscopy GmbH, Jena, Germany). For a short period, an Olympus BX43 microscope with differential interference contrast (DIC) optics with objectives of 40×/0.75, 60×/0.90, 100×/1.30, connected to an Olympus UC50 digital camera, was used.
2.3. Scanning Electron Microscopic (SEM) Examination
Spirocysts from marginal tentacles and ptychocysts from the tube membranes of C. membranaceus, Cerianthus sp. 1, and P. dohrni were studied using a scanning microscope (FEI Magellan 400 XHR-SEM, FEI, Hillsboro, OR, USA). The SEM preparations were fixed overnight in 2–2.5% glutaraldehyde in 0.1 M cacodylate buffer at 4 °C, washed 3 times, 15 min each, in 0.1 M cacodylate buffer at pH 7.2–7.4, and post-fixed in darkness the for one hour in 1% osmium tetroxide in 0.1 M cacodylate buffer. A second washing was performed 3 times, 15 min each in enough distilled H2O; thereafter, the sample was dehydrated in ethanol series, transferred from 100% ethanol into a 1:2 solution of hexamethyldisilazane (HMDS) and 100% ethanol for 20 min, then into a fresh solution of 2:1 HMDS and ethanol for 20 min, and finally into 100% HMDS solution for 20 min, 2 times. The samples were covered or loosely capped and left in a fume hood to dry overnight in the 100% HMDS solution and then coated with 3 nm platinum/palladium for SEM imaging.
2.4. Transmission Electron Microscopic (TEM) Examination
Spirocysts and one b-mastigophore from marginal tentacles of Cerianthus sp. 1 were studied using a transmission microscope TEM (Titan Cryo Twin, FEI Company, Hillsboro, OR, USA). The examined tissues were fixed in 2.5% glutaraldehyde in cacodylate buffer 0.1 M, pH 7.4, for a minimum of 48 h. Osmification was performed with reduced osmium (a 1:1 mixture of 2% osmium tetroxide and 3% potassium Ferro cyanide). After pre-embedding in 1% agar, samples were dehydrated in ethanol series and embedded in epoxy resin. Thin sections (100–150 nm thick) were placed on/attached to copper grids and contrasted with lead citrate. Images were acquired with the TEM operating at 300 kV equipped with a 4 k CCD camera (Gatan Inc., Pleasanton, CA, USA).
2.5. Cnida Types among Anthozoa
To visualize the distribution of the different cnida types among the main anthozoan groups (i.e., Octocorallia, Ceriantharia, and Hexacorallia), a diagram based on the recent molecular phylogeny from Forero Mejia et al. [1] was used. Cnida types were identified according to the classification system of Weill [9] modified by Carlgren [8,29], Schmidt [10], Mariscal [15], Mariscal et al. [7,30], Östman [17], and Östman et al. [11]. The nematocysts terminology followed Watson and Wood [31].
3. Results
3.1. Cnida Types and Distribution
The three cnida categories, the nematocysts, spirocysts, and ptychocysts, appeared in all examined Ceriantharia species representing the Cerianthidae, Botrucnidiferidae, and Arachnactidae. The b-mastigophores and isorhizas were the only two main nematocyst types identified in Ceriantharia (Figure 1, Figure 2 and Figure 3).
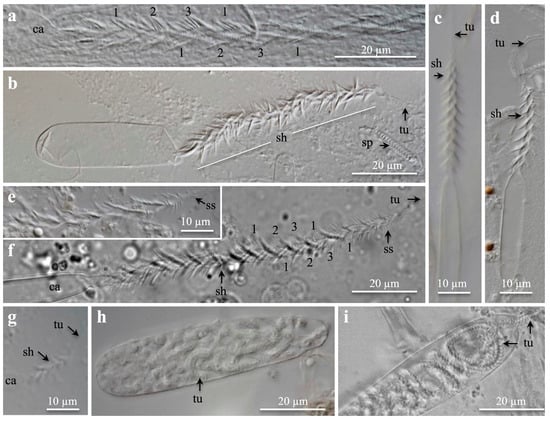
Figure 2.
Light microscopies pictures of (a–g) discharged microbasic b-mastigophores and (h,i) isorhizas. (a–g) Capsule, shaft, and tubule, (a–e) from tentacles, (f) from craspedonemes, and (h,i) from column membrane. (a) Shaft showing helical spine rows (1, 2, 3, 1). (b) Medium to large broad b-mastigophore. (c,d) Medium to large narrow b-mastigophore. (e,f) Shafts of large b-mastigophores showing helical spine rows. Note small spines at shaft end. (g) Small, broad b-mastigophore. (h,i) Large, broad isorhizas, (h) undischarged, and (i) partly discharged. Note tubule pattern. (a–e,g,h) Pachycerianthus torreyi. (f,i) P. multiplicatus. Abbreviations: ca, capsule; sh, shaft; sp, spirocyst; ss, small spines; tu, tubule.
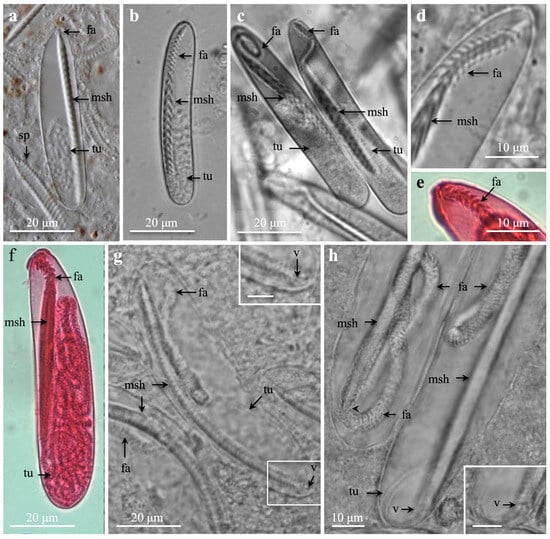
Figure 3.
Light microscopies of large, broad b-mastigophores showing apical, tightly folded, inverted shaft ”faltstück” and main shaft. (a) b-mastigophore with a short ”faltstück” from labial tentacle. (b–d) b-mastigophore from marginal tentacles, (b) straight ”faltstück”, (c) coiled and undulating ”faltstück”, and (d) enlargement of undulating ”faltstück”. (e,f) b-mastigophore with a hook-shaped ”faltstück” and mesobasic shaft from marginal tentacle, (e) enlargement of “faltstück”. (g,h) b-mastigophore with irregular, long, coiled ”faltstück” from acontioids, arrowhead points at transition between main shaft and ”faltstück”. Note, inserts in (g) (scale bar: 5 µm) and in (h) (scale bar: 10 µm) show enlarged basal shaft with interpreted putative tiny ‘penicilli v-notch’, formed by last shaft spines. (a) Pachycerianthus torreyi. (b–d) P. multiplicatus. (e,f) Botrucnidifer sp. 1. (g,h) Isarachnanthus maderensis. Abbreviations; fa, ”faltstück”; msh, main shaft; sp, spirocyst; tu, tubule; v, ‘penicilli’ v-notch.
3.1.1. b-Mastigophores
The ceriantharian b-mastigophores were identified by their broad, generally straight, rod-shaped shaft, which tapered in broadness slightly and gradually from the capsule tip towards the shaft end (Figure 1a). The upside-down v-shaped spine-pattern or the twisted spine-pattern was visible (Figure 1a–d), especially on slightly dried preparations and early discharging shafts. The nematocyst capsules varied in shape, from oval–broad (Figure 1a) to broad elongate (Figure 1b,c) and narrow elongate with parallel sides (Figure 1d,e). The b-mastigophore capsules appeared in several size groups from large (Figure 1a–d), medium to large (Figure 1e), medium (Figure 1f–i), and small (Figure 1j,k) whose presence/absence varied within the different species and structures (Figure 1, Figure 2 and Figure 3; Table 1, Table 2 and Table 3); some overlapping occurred. Large and medium, broad and narrow b-mastigophores with parallel capsules were most abundant in the tentacles (Figure 1d,e,h; Table 1 and Table 2) and were more common than mesenterial oval- to drop-shaped b-mastigophores representing craspedonemes, cnidorages, and the cnidoglandular tract (Figure 1a,j; Tables S2 and S3). Medium to large bow-formed capsules were seldom identified (Figure 1f,g). Small b-mastigophores varied in abundance from seldom to common (Figure 1j,k). The inverted shaft varied in length from long to short, especially in medium and small b-mastigophores (Figure 1h–k). Generally, the spine rows decreased in number with decreasing capsule sizes, from large b-mastigophores (Figure 1a–d, up to 30 spine-rows) to medium (Figure 1f–i) and small (Figure 1j,k), with around 8–9 spine-rows.

Table 1.
Cnidae from marginal tentacles of at least three Pachycerianthus multiplicatus specimens showing, for each cnida subtype, numbers (n) and abundances of measured cnidae, mean values of (range length) × mean values of (range width) and mean values of length/width in µm. Congruent cnidae recorded by Carlgren [8] and den Hartog [18] are noted.

Table 2.
Cnidae from labial tentacles of at least three Pachycerianthus multiplicatus specimens showing, for each cnida subtype, numbers (n) and abundances of measured cnidae, mean values of (range length) x mean values of (range width) and mean vales of length/width in µm. Congruent cnidae recorded by Carlgren [8] and den Hartog [18] are noted.

Table 3.
Cnidae from column membrane of at least three Pachycerianthus multiplicatus showing, for each cnida subtype, numbers (n) and abundance of measured cnidae, mean values of (range length) × mean values of (range width) and mean vales of length/width in µm. No information about cnidae from column membrane were given by Carlgren [8] and den Hartog [18].
The discharged shafts showed three helical rows of long spines (Figure 2a–g). The broadness of the capsules varied, which was most evident in empty, flattened capsules, when the squash preparation had dried (Figure 2b–d). In the fixed material, spines could clog together (Figure 2a–e), but in fresh material, the spine pattern was clearer (Figure 2f). The large, prominent spines gradually decreased in size towards the shaft end (Figure 2e,f). The decreasing number of spine rows is shown from large (Figure 2a,f) and medium (Figure 2c,d) to small b-mastigophores (Figure 2g). Immature capsules, late in development, were often bow-formed and slightly broader than mature ones.
The “faltstück” of Schmidt [10], congruent to the apical inverted, densely folded shaft part, present in certain large b-mastigophores, varied in length and pattern. b-mastigophores with very short or small “faltstück” (Figure 3a and Figure S2a) commonly occurred in labial tentacles (Table 2) and more sparsely in craspedonemes (Table S2) and columns (Table S4). Large b-mastigophores with straight (Figure 3b), undulating, and coiled (Figure 3c) “faltstück” from marginal tentacles in P. multiplicatus appeared so far to be species-specific. Hook-shaped “faltstück” from marginal tentacles of Botrucnidifer sp. 1 (Figure 3e,f) and long, irregularly coiled “faltstück” from acontioids of I. maderensis (Figure 3g,h), seemed so far to be species-specific.
The “faltstück” was either spineless or armed with loosely set, short spines (Figure S2c). Loosely set spines correspond to lose folds of the inverted “faltstück” wall (Figure 3b,d), whereas spineless “faltstück” were densely folded (Figure 3g,h). The wall of the discharged “faltstück” was often slightly wrinkled (Figure S2c). The “faltstück” significantly affected the length of the discharged shafts. Without “faltstück” or with very short “faltstück”, discharged b-mastigophore shafts were mainly microbasic (Figure 3a). Shafts with long, straight, undulating, or coiled “faltstück” were generally mesobasic in P. multiplicatus and Botrucnifer sp. 1 (Figure 3b–f and Figure S2c), while, in I. maderensis, the long, irregularly folded “faltstück” was macrobasic when everted (Figure 3g,h).
The remaining inverted shaft, the “main shaft” or “hauptstück” [7], was less densely twisted due to large, prominent spines. Similar to the shaft with no “faltstück”, the main shaft showed the regular, upside-down, v-shaped, spine row pattern and tapered in broadness slightly and gradually towards the shaft end (Figure 1a and Figures S2a and S3b). Shafts with short “faltstück” generally had more spine rows on the main shaft than the shafts with longer “faltstück” (Figure 3a–c and Figure S2a and S3b), except for the I. maderensis b-mastigophores with long “faltstück” (Figure 3g,h). The inverted last few spine rows in these I. maderensis b-mastigophores formed a hardly visible tiny v-shaped notch at the shaft end, from which the narrow, inverted distal tubule emerged. Generally, the inverted distal tubules created irregular coils, nearly reaching mid-capsule or slightly above it (Figure 1a,d–f and Figure S2a).
In the other investigated ceriantharian species (families Cerianthidae and Botrucnidiferidae), the tiny spine row pattern at the shaft end forming a tiny v-notch was hardly seen or mostly not visible in mature b-mastigophores (Figures S2a and S3b). In b-mastigophores, late in development, with the untwisted shaft, the decreasing size of the last spine rows at the shaft end was sometimes evident.
3.1.2. Isorhizas
Isorhizas appeared as broad and elongated, with a small protruding tip apically (Figure 2h,i and Figure S3c). The capsule did not vary in shape, but in size, from small, medium, and large to very large, where large ones, although not abundant, were most common. Isorhizas were not identified in all tube anemone structures. They were sparsely scattered in the column membrane and in the column (Table 3 and Tables S4 and S8), sometimes also present in the labial tentacles (Table 2 and Table S8), and seldomly identified in other structures (Tables S9 and S10). The inverted, helically twisted, broad tubule filled the capsule with close, slightly oblique, horizontal, irregular, large coils. Discharged isorhizas were seldom found. The broad isorhiza tubules of P. multiplicatus were armed with small dots, forming a helical pattern (Figure S2b).
3.1.3. Spirocysts
Spirocysts, the most abundant cnidae within the Ceriantharia, were particularly abundant in the marginal and labial tentacles (Table 1 and Table 2). They were densely parallel-oriented in the ectoderm, with the broad, apical end pointing outwards (Figure 4a,b). Medium elongate, large and broad, and large elongated spirocysts, all with rounded bases, dominated in tentacles (Figure 4c,d; Table 1 and Table 2). Except for the large, broad spirocysts, the various spirocyst size groups, with rounded or pointed capsule ends, were generally present in most structures (Figure 4e–g), although some were rare or infrequent (Table 1, Table 2 and Table 3 and Tables S2–S12). Small to medium spirocysts with dense, regular coils were also abundant in the tentacles and mostly rare in other structures (Figure 4f, Table 1, Table 2 and Table 3 and Tables S2–S12).
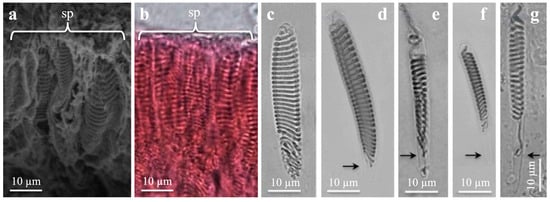
Figure 4.
Scanning electron microscopy (a) and light microscopies (b–g) of spirocysts from marginal tentacles. (a,b) General view from Cerianthus membranaceus (a) and Botruanthus benedeni (b). (c,e,g) Pachycerianthus multiplicatus. (d,f) P. dorhni. (c) Large, broad spirocyst. Note round capsule end. (d) Large elongate spirocyst with pointed capsule end. (e,f) Medium, narrow spirocysts. (g) Large, narrow spirocyst. Note long, narrow basal capsule end (arrow). (d–g) Note increasing length of narrow, pointed capsule end (arrows).
The inverted spirocyst tubule generally formed horizontal, close, regular coils along the capsule (Figure 4c–g and Figure S3d). Some irregular tubule coils may appear in the mid- and basal capsules. Mostly no tubule or a short, outstretched tubule end was present in the narrow capsule end (Figure 4e–g). Narrow capsules with narrow basal ends (Figure 4f,g) had generally fewer tubule coils than broader capsules with rounded bases. The tubule coils decreased in number with decreasing capsule size. Large, broad and large, elongate spirocysts presented up to 36 coils (Figure 4c,d and Figure S3d). Medium, elongate capsules with rounded capsule ends had generally 22–25 coils, and small spirocysts had circa 8–16 coils or fewer.
Spirocysts from marginal tentacles of Cerianthus sp. 1, studied via SEM (Figure 5a,d,g) and TEM (Figure 5b,c,e,f,h,i), showed a regularly folded tubule wall in pleats, a pattern also faintly visible in LM (Figure 4c and Figure S3d). Cross- and longitudinal TEM sections of spirocysts revealed a clear regular, longitudinal arrangement of microfibrillae/microtubuli in the central core/lumen of the inverted tubule (Figure 5c,e,f,h,i).
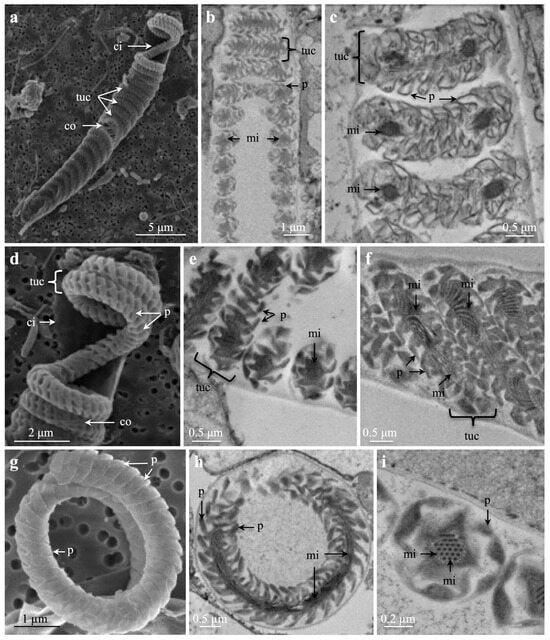
Figure 5.
Scanning electron microscopies (a,d,g) and transmission electron microscopies (b,c,e,f,h,i) of spirocysts from marginal tentacles of Cerianthus sp. 1. Note increasing magnifications (a–i). (a) Partly transparent capsule showing inner and outer capsule wall and regular tubule coils. (b,c) Parts of tubule coils. Note pleats on tubule wall and microfibrillae/mini-tubules inside tubule. (d) Enlarged upper capsule showing some tubule coils. Note inner and outer capsule wall and pleats on tubule wall. (e,f) Note regular pleats of tubule wall and microfibrillae in the centre/tubule core. (g) Part of a coiled tubule showing pleats on tubule wall. (h,i) Note pleats and microfibrillae, (h) cross-section of tubule coil, (i) cross-section of tubule. Abbreviations: ci, inner capsule wall; co, outer capsule wall; mi, microfibrillae/mini-tubule; p, pleat on tubule wall; tuc, tubule coil.
3.1.4. Ptychocysts
Ptychocysts, the specific Ceriantharia cnidae, are characterized by their spineless, flattened tubules, which are not helically twisted, unlike the nematocyst and spirocyst tubules. The ptychocyst tubule fills the capsule with tight, oblique folds, closely aligned with the long capsule axis (Figure 6a,c,d and Figure S2e). Pleated tubules with longitudinal folds in circumference were pictured both in LM (Figure S2g) and SEM (Figure 6h,i). Ptychocysts are arranged in a large number of subtypes due to their large morphological capsule variations, represented by tiny, small, medium, large, very large, elongate, broad, elongate narrow, drop-shaped, oval, and apically bent subtypes (Figure 6a–d,g and Figures S2d,e and S3e; Table 3). The abundance of these subtypes varied between different structures.
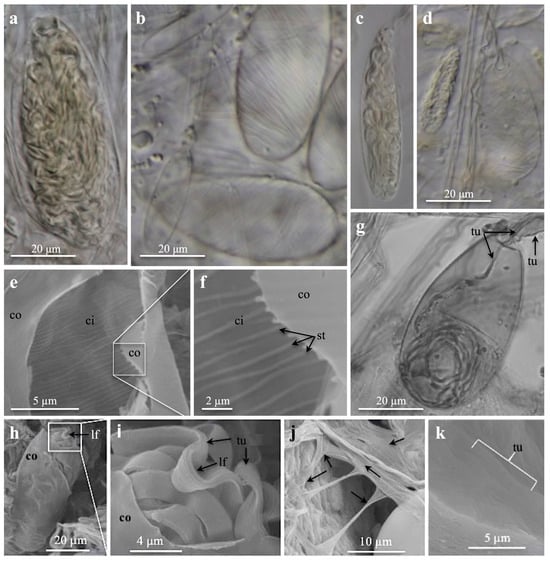
Figure 6.
Light microscopies (a–d,g) and scanning electron microscopies (e,f,h–k) of ptychocysts from tube membranes. (a) Very large, broad, drop-shaped ptychocysts, (b) large, broad, drop-shaped ptychocysts, (c) medium, elongate ptychocysts (magnification as in (a,b,d)), (d) small, elongate and broad, medium ptychocysts. (b,d) Note striations on capsule wall. (e,f) Inner capsule wall with striations, note outer capsule wall. (g) Large, broad, drop-shaped ptychocyst. Note absence of striations on capsule wall, and inverted tubule (arrows) inside everting tubule. (h–k) Inside tube. (h,i) Broken capsule (h) and enlargement (i) showing outer capsule wall, inverted, pleated tubule with longitudinal folds in circumference. (j,k) Parallel-oriented tubules forming the tube membrane, (j) note arrows pointing to tubules running in different directions. (a,e,f,h–k) Pachycerianthus dorhni. (b–d) P. torreyi. (g) Botrucnidifer sp. 1. Abbreviations: ci, inner capsule wall; co, outer capsule wall; lf, longitudinal tubule fold; tu, tubule; st, striation on capsule wall.
Discharged ptychocyst tubules build the column membrane and the tube membrane. The graceful, very thin, almost transparent column membrane, studied in LM (Figure S2f), showed discharged tubules in different directions, forming a network. By the broadness of their tubules, small, medium, and large ptychocysts were identified (Figure S2e–g). The robust tube membrane from inside the tube, examined in SEM, displayed a damaged capsule with an inverted tubule (Figure 6h,i). Several discharged ptychocyst tubules were observed running straight and parallel, oriented in various directions (Figure 6j,k).
A regular striation pattern on the inner capsule wall of ptychocysts is a new characteristic observed in the SEM of P. dohrni (Figure 6e,f). This striation pattern was also visible under LM in P. torreyi and P. dohrni (Figure 6b,d). The pattern was most evident on large, empty capsules (Figure 6b). On small capsules filled with tubules, the pattern was less recognizable, but could be seen at the capsule edges (Figure 6a,c,d).
3.2. Specific Cnida Structures
Craspedoneme structures (present in Cerianthidae), either branched or single, thread-like, flattened, were abundantly attached along the mesenteries of P. multiplicatus (Figure 7a). The thin gastrodermis (endothelia), surrounding the craspedoneme margin, contained abundant b-mastigophores and spirocysts of different size classes and a few ptychocyst subtypes (Figure 7b–g, Table S2). Medium, elongate spirocysts dominated (Figure 7b), followed by elongate broad to oval-shaped microbasic b-mastigophores of different sizes and with different shaft lengths (Figure 7c–e,g). Small spirocysts were common, while medium, elongate ptychocysts were the most abundant ptychocyst subtype (Figure 7f,g). Cnidae proliferation took place in the mesodermal tissues.
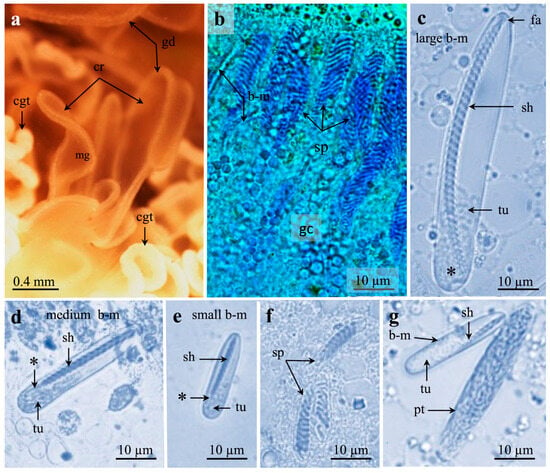
Figure 7.
Stereomicroscopy (a) and light microscopies (b–g) of mesenterial craspedonemes with cnidae of Pachycerianthus multiplicatus. (a) Craspedonemes with gastrodermis surrounding mesogloea. Note cnidoglandular tracts. (b) Gastrodermis with parallel-oriented spirocysts and microbasic b-mastigophores surrounded by granular gland cells. (c–e) Broad, microbasic b-mastigophores. Note tapering, narrow shaft end (*). (c) Large b-mastigophore, late in development, with very short “faltstück” and 31 clear spine rows. Note 4 very tiny spine rows (*). (d) Medium, broad b-mastigophore with 17 spine rows on long shaft. (e) Small b-mastigophore with 14 spine rows. (f) Small spirocysts, 9–10 tubule coils. (g) Medium, elongate ptychocyst and medium to small b-mastigophores with 10–11 spine rows on short shaft. Abbreviations: b-m, b-mastigophore; cgt, cnidoglandular tract; cr, craspedoneme; gd, gastrodermis; gc, granular gland cells; fa, “faltstück”; mg, mesogloea; pt, ptychocyst; sh, shaft; sp, spirocyst; tu, tubule. *, very tiny spines at shaft end.
Cnidorages (present in Botrucnidiferidae) occurred in grape-like structures in bunches, so-called botrucnids, on mesenteries in Botrucnidifer sp. 1 (Figure 8a). They contained medium to large, broad to oval-shaped b-mastigophores (Figure 8k), spirocysts, and mucus cells (Table S10). In developing Botruanthus benedeni, almost ball-shaped cnidorages were attached along the edge of modified craspedonemes (Figure 8b–e). Immature b-mastigophores and spirocysts, surrounded by gland cells, were oriented with their apical opening towards the free cnidorage side (Figure 8b,c). Cnidorages with mature cnidae and gland cells appeared in cavities inside marginal tentacles (Figure 8f, Table S11). Outside the lightly squashed marginal tentacles, mature ciliated cnidorages occurred (Figure 8h,i). In the cnidorage, cnidae were oriented with their apical opening towards the slightly flattened, basal cnidorage side corresponding to the free, unattached side of developing cnidorages. Their cnida bases pointed towards the rounded, apical cnidorage side, earlier attached to the craspedonemes. A partly mature, broad oval b-mastigophore with a non-twisted upper shaft showed the spine rows and helical spine pattern on the basal twisted shaft (Figure 8j).
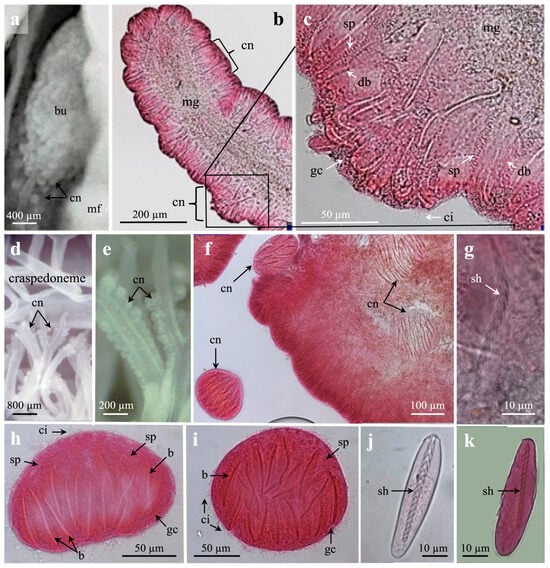
Figure 8.
Light microscopies of cnidorages from mesenterial filaments. (a) Cnidorages in botrucnids. (b–e) Developing cnidorages on each side of mesogloeal axis on modified craspedonemes. (b) Distal craspedoneme with developing cnidorages. (c) Enlargement from (b) of a developing cnidorage. Note developing b-mastigophores, spirocysts, granular gland cells, and cilia. (d,e) Craspedonemes with developing cnidorages. (e) Enlargement from (d). (f) Marginal tentacle with ball-shaped cnidorages inside and outside tentacle. (g) Immature b-mastigophore with non-twisted shaft, showing upside-down v-shaped spine pattern. (h,i) Ciliated, fully developed ball-shaped cnidorages with b-mastigophores, spirocysts, and granular gland cells. (h) Side view, basal side downwards. (i) From above, apical view. (j) Partly mature, broad oval-shaped b-mastigophores from cnidorage. Note non-twisted, upper shaft and helical pattern on remaining shaft. (k) Oval-shaped b-mastigophore. (a,k) Botrucnidifer sp. 1. (b,j) Botruanthus benedeni. Abbreviations: b, b-mastigophores; ci, cilia; cn, cnidorages; db, developing b-mastigophores; gc, granular gland cells; mf, mesenterial filament; mg, mesogloea; sh, shaft; sp, spirocysts.
Acontioids (present in Arachnactidae), occurring as short, elongate, thread-like structures, were present at the aboral end of mesenterial filaments in I. maderensis. Spirocysts of various sizes and b-mastigophores surrounded by mucus cells were abundant, of which large, broad, macrobasic b-mastigophores with long, coiled “faltstück” were species-specific to I. maderensis (Figure 3g,h; Table S12).
3.3. Cnida Distribution and Comparison between the Recorded Measurments
The examined species (Table S1) showed a closely similar distribution and abundance pattern of the three cnida categories. b-mastigophores, spirocysts, and ptychocysts were found in all examined tube anemone structures: (i) Spirocysts were the most abundant cnidae for all species and the dominant cnidae in marginal tentacles and labial tentacles (Table 1 and Table 2 and Tables S6–S12); (ii) b-mastigophores, including subtypes, were the second-most-abundant cnidae and were most common in tentacles and actinopharynx (Table 1 and Table 2 and Tables S5–S12); (iii) ptychocysts dominated in columns and column and tube membranes (Table 3 and Tables S4 and S6–S12); and (iv) isorhizas, the more rarely encountered nematocysts, were present in the column, although not abundant, and were rare or even absent from other structures (Tables S4 and S6–S12).
3.3.1. Spirocysts
Spirocysts were the most abundant cnidae in all examined structures of the studied species (Table 1, Table 2 and Table 3 and Table S2–S12). The largest spirocysts dominated in the marginal tentacles and craspedonemes in P. multiplicatus (Table 1 and Table S2). Medium and small spirocysts were generally more abundant in the other structures in all examined species. Small, narrow spirocysts were abundantly recorded in the labial tentacles and mesenterial filaments of P. torreyi (Table S8) and in the tentacles of P. dohrni (Table S9).
3.3.2. b-Mastigophores
The largest b-mastigophores were abundantly found in the tentacles (Table 1 and Table 2) and the actinopharynx (Table S5) of P. multiplicatus. They could also be sparsely found in all the other investigated structures (Table 3 and Tables S2–S4). Consistently, the same abundance pattern was retrieved for the same structures in all species investigated (Tables S6–S12). The largest recorded b-mastigophores were found in the marginal tentacle, mesenterial filament (acontioid), and tube membranes of I. maderensis, reaching up to 105, 98, and 94 µm in length, respectively (Table S12). In B. benedeni, very large b-mastigophores (79 mm in length) were also recorded in mesenterial filaments (cnidorage) (Table S11). Comparatively, the largest recorded b-mastigophores of Cerianthus sp. 1 only reached up to 54 µm (Table S7). The large b-mastigophores with straight, undulating, and coiled “faltstück” in P. multiplicatus reached up to 72 µm (Table 1). Large b-matigophores with hook-shaped “faltstück” occurred in marginal tentacles of Botrucnidifer sp. 1 (Table S10), while long, irregularly folded “faltstück” were found in acontioids of I. maderensis (Table S12). The large dominating b-mastigophores with tiny “faltstück” encountered in the labial tentacles of P. multiplicatus reaching up to 63 µm were also present in all the examined species (Table 2 and Tables S6–S12).
Small and medium b-mastigophores were present in all tube anemone structures, but were here more or less abundant (Table 1, Table 2 and Table 3 and Tables S2–S12). Broad oval to drop-shaped b-mastigophores with different shaft lengths are characteristic mesenterial b-mastigophores (Tables S2, S3 and S6–S12). They were found in the specific cnida structure craspedonemes (Cerianthidae) and the cnidoglandular tract of P. multiplicatus (Tables S2 and S3), and cnidorages (Botrucnidiferidae) of Botrucnidifer sp. 1 and B. benedeni (Tables S10 and S11). In the mesenterial acontioids (Arachnactidae) of I. maderensis, only the large macrobasic b-mastigophore with the long “faltstück” were recorded (Table S12).
3.3.3. Ptychocysts
The ptychocysts dominated in the column membranes and columns of P. multiplicatus (Table 3 and Table S4) and P. torreyi (Table S8). In the other species, they were at least present in those structures (Tables S6, S7 and S9–S12). Ptychocysts were represented by small, medium, large, very large, elongate, broad, drop-shaped, oval, and apically bent cnidae subtypes. In the P. multiplicatus column membrane, very large drop-shaped and very large elongate broad ptychocysts reached up to 106 and 102 µm, respectively (Table 3). Comparatively, very large, broad ptychocysts were recorded in P. torreyi (81 mm in length), P. dohrni (83 mm), and I. maderensis (94 µm) (Tables S8, S9 and S12). Among them, large, broad, drop-shaped and large, broad, apically bent elongated ptychocysts were the most abundantly recorded subtypes (Table 3 and Table S4). Medium elongated ptychocysts were present in marginal tentacles (Table 1). Small and medium ptychocysts were found scattered in most of the examined structures of all investigated species (Table 1, Table 2 and Table 3 and Tables S2–S12).
3.3.4. Isorhizas
In P. multiplicatus, isorhizas were recorded as medium–large–very large (44 to 90 µm in length), where large isorhizas were the most common. Isorhizas were the most abundant in the column and column membrane, although here they were seldomly recorded, as in labial tentacles (Table 2 and Table 3 and Table S4). Isorhizas were not found in the marginal tentacles, craspedonemes, cnidoglandular tract, and actinopharynx in P. multiplicatus. The other examined structures (Tables S6–S12) showed a very similar isorhizas pattern either absent, seldom, or present. Single to a few medium to large isorhizas were identified in the mesenterial filaments, column, and tube membrane in Cerianthus sp. 1, P. dorhni, and B. benedini (Tables S7, S9 and S11). In P. torreyi, however, small to medium isorhizas (14 to 40 µm in length) were identified in the marginal tentacles, while large to very large ones (up to 95 µm) were measured in the column and tube membrane (Table S8).
4. Discussion
4.1. Cnida Classification and Terminology
Over time, the cnida terminology has changed, causing inconvenience when cnidae are differently referred to and compared between studies (Table S13). In taxonomic works, an appropriate nomenclature is needed.
England [16] compiled a review of the classification systems of anthozoan nematocysts established by Weill [9] and modified by Carlgren [8], Cutress [14], Schmidt [10,12,13], and den Hartog [18] concerning the terms mastigophore, rhabdoid, faltstück, micro- and macrobasic, spirula, and penicillus. At the same time, England added the term mesobasic for the inverted shaft [16]. Schmidt [10,12,13] extensively revised the nematocyst classification initiated by Weill by discarding many of the descriptive nematocyst terms and replaced them with his codified alphanumeric rhabdoid system to show the nematocyst variations. Schmidt divided the rhabdoids into four categories (A, B, C, and D) according to their spine pattern, with category B having two subcategories (1, 2), each of which was again subdivided (a, b) (see [16]). For example, b-rhabdoids are equivalent to b-mastigophores, and p-rhabdoid B1b and p-rhabdoid B2a to p-mastigophores with or without distal tubule and faltstück [32]. This coded system did, however, not contain the very useful descriptive terminologies of Weill’s modified classification. England [16], however, proposed to combine the descriptive nematocyst terms initiated by Weill [9] and Carlgren [8] with the alphanumeric system of Schmidt [10,12,13], with letters and numbers presenting the morphological variation in the nematocysts.
Compared with the coded rhabdoid system of Schmidt [10,12,13], the descriptive modified classification of Weill [9] has lately been the most used system [26,27,33,34]. Gusmão et al. [35], in agreement with Sanamyan et al. [36], regarded that the terms “spirula” and “penicillus” [18,19] doubtless had priority over the “rhabdoid” of Schmidt [7,9,10] and “mastigophore” of Weill [9]. But, as mastigophore has, for a long time, been widely used and debated, and to preserve stability and consistence of nomenclature, they preferred to use the term “mastigophore” for these nematocysts. However, in agreement with England [16], Gusmão et al. [35] followed the terminology of Weill [9] and Carlgren [8], but captured the underlaying morphological cnida variation, showing, by letters and numbers, the morphologic cnida variations. In this work, we consider that descriptive morphological naming of the cnidocysts has a clearly higher priority than using letters and numbers to highlight the morphological cnidocyst variations.
Current b-mastigophores are congruent to b-mastigophors [8] and b-rhabdoids [10,12]. We regard spirulae [18] as a subtype of b-mastigophore (see below). The p-mastigophors/p-amastigophors [8] are congruent to p-rhabdoids [10,12], but are morphologically not quite similar to penicilli [18]. We regard penicilli as a subtype of b-mastigophores (see below). No p-mastigophores/amastigophores were identified in the present investigation.
Isorhizas are termed haploneme holotrichs/holotrichous, or homotrichs/homotrichous, and atrichs/atrichous (Table S13). Spirocyst, adopted by Weill [9], was thereafter used by Mariscal [15], Schmidt [13], den Hartog [18], and recent authors (Table S13). Atrichs/atrichous are suitable to use in comparisons with spined isorhiza tubules. Ptychocysts [7] were previously called atrichs [8,18]. Recent authors [26,27] still use atrichs for ptychocysts. We recommend that atrichs/atrichous should only be used for spineless isorhizas and not for ptychocysts. Spined ptychocysts do not exist.
4.2. Cnida Subtypes, Their Variations, and Taxonomic Value
Ceriantharian cnidae, arranged into different subtypes, reinforce similarities and differences between different tube anemones and their structures (Table 1, Table 2 and Table 3 and Tables S2–S12). Some subtypes, presented here, were also recorded and illustrated by Carlgren [8] and den Hartog [18] (Table 1 and Table 2 and Tables S2–S5), and recorded in recent Ceriantharia reports [23,24,25,26,27]. Apart from being less detailed and not presenting any “faltstück” at the b-mastigophores, the cnidomes, as described by den Hartog [18], are close to the cnidomes of P. multiplicatus presented here. Species-specific subtypes, such as the specific b-matigophores with “faltstück”, have high taxonomic value, whereas subtypes commonly occurring in most structures have less taxonomic value. Cnida subtypes specific to certain structures can be helpful for species identification.
Most ceriantharian cnidocyst types show great diversity of shapes and sizes, and the variations are considered useful for characterizing species or genera. However, it is also well known that cnidocysts may vary according to the environmental conditions to which the animal is subjected. They vary in size and shape during cnida development and in the size and conditions of specimens, and, in addition, to their variation in different structures. Zamponi and Acuña [37] recorded the size variations of nematocysts from the same species depending on the geographical and bathymetrical distribution of the specimens. During cnida development, they varied in size and shape [11,38,39,40]. They grow in size during cnidae migration along the tentacle from the base to the tip, and as the animal grows [40].
In accordance with Zamponi and Acuña [37], we inferred that nematocyst size should not be used as an absolute characteristic for the identification and diagnosis of species. Cnidocyst sizes have relative value for species identification. Some are species-specific or specific to genera, while some can only be used only as a complement for other characteristics to define the species or taxons. However, cnidocysts can be differentiated into different subtypes due to their size variations (see Table 3 and Table S4). Why the cnida variation is so large, for example, in ptychocysts (see Table 3 and Table S4), is not known. What functions do all these size and shape variations have?
For a better understanding of the variability and distribution of the cnidocysts and the cnidomes, Acuña et al. [32] and Garese et al. [33] presented descriptive and comparative statistical analyses including models, where the cnida distribution and capsule range in length were analysed. Such a quantitative description was not used in our work based on a comparative description and illustration of the variation in cnidomes, and the highlighting of species-specific cnidocysts from different structures (Figure 3 and Table 1). Multiple authors [41,42,43,44] recommended that at least 40 capsule measurements for each cnida type (when possible) of each individual structure in at least three individuals are needed to fully describe the cnidomes of a species and apprehend the variability and diversity of the cnidomes (even without underlying statistical analysis). Some of the different cnida types or subtypes found in the different structures are rare, and to find up to 40 capsules (here, at least 20 measurements were taken for each dominant or common cnida) to measure would be a lot of work. Moreover, some structures are more easily accessible than others. As the different types of mastigophores are difficult, if not impossible, to identify in undischarged capsules, measurements of the specific structures, such as the relative length of the shaft and of the “faltstück”, and the direction of the spines, need to be taken for discharged mastigophores. These measurements and representative pictures should be presented for each species description and redescription when possible.
4.2.1. b-Mastigophores
The two most common subtypes, the large and medium, broad, microbasic b-mastigophores, both with a prominent shaft in which the large ones are often found with a “faltstück” (Figure 1, Figure 2 and Figure 3 and Figure 7 and Figures S2 and S3), were also recorded by Carlgren [8] and appeared congruent to spirulae 2 of Pachycerianthus curacaoensis [18]. Our third-most-common subtype, the medium, narrow microbasic b-mastigophore with a narrow, short to long shaft (Figure 2), was recorded by Carlgren [8] and seemed congruent to spirulae 1 [18]. These three mentioned subtypes were also recorded in recent articles, but without mentioning “faltstück” [23,24,25].
In addition to the medium-sized “faltstück” patterns: coiled and undulating in large b-mastigophores from the marginal tentacle of P. multiplicatus reported by Carlgren [8], a straight “faltstück” was identified in the present work (Figure 3, Table 1). To date, these three “faltstück” patterns seemed genus-specific to P. multiplicatus and specific to marginal tentacles. The hook-shaped “faltstück” pattern present in the marginal tentacle in the large b-mastigophores of Botrucnidifer sp. 1 (Figure 3) is, for the first time, recorded. The large macrobasic b-mastigophores in the mesenterial acontioids from I. maderensis with the long, coiled “faltstück” (Figure 3) are, for the first time, illustrated with LM pictures. This observation is congruent with the schematic representation of penicillus 2 from the acontioids of I. nocturnus [18] and to p-rhabdoide IIb, illustrated from labial tentacles of Cerianthoides sp. [12]. To verify that macrocrobasic b-mastigophores are genus-specific in the acantioids of Isarachnanthus, more cnida studies are needed.
Broad oval or drop-shaped b-mastigophores in the craspedonemes (Table S2) and cnidoglandular tract (Table S3) of P. multiplicatus and in cnidorages of Botrucnidifer sp. 1 and Botruanthus benedeni (Figure 8) seemed to be specific mesenterial b-mastigophores, probably congruent to the drop-shaped spirulae 2 of P. curacaoensis [18], and match the drop-shaped mesenterial nematocysts from Ceriantharia species [23].
Bow-formed capsules have only been identified in Cerianthus sp. 1 and might be specific b-mastigophore subtypes (Figure 1). Note that immature b-mastigophores, during the development of the shaft, generally have bow-formed capsules [38]. These bow-formed capsules have, however, the typical twisted spine-row pattern of mature nematocysts (Figure 1). Other b-mastigophore subtypes, not found in the present investigation, were recorded in several Ceriantharia species (illustrations and pictures in [12,13,18,23,24,25,26,27]).
4.2.2. Isorhizas
Isorhizas with the least variable capsule and tubule pattern appeared as small, medium, large, and very large (Figure 2 and Figure S3; Table 2 and Table 3 and Tables S4, S7–S9 and S11). Owing to their rare presence in the column and column membrane, and even their lower frequency in other structures, their presence can be valuable for species identification. Large isorhizas were recorded in the column as homotrichs [18] and holotrichous isorhizas (Schmidt [12] as atrichs, atrichs type II [24], and holotrichs [23,25,26,27]). The isorhiza spine pattern is still uncertain. Upon the discharge of P. multiplicatus isorhizas, small dots formed three helices along the isorhiza tubules (Figure S2), similar to the typical three spine helices on distal tubules of b-mastigophores. den Hartog [18], however, illustrated in P. curacaoensis a discharged homotrich with a regular helical pattern of prominent spines. If the homotrichous tubule of P. curacaoensis [18] is correctly illustrated, certain ceriantharian isorhizas can be penetrators with spined distal tubules, like the b-mastigophore tubules. Everted isorhiza tubules from more Ceriantharia species need to be investigated to confirm the presence or absence of spines.
4.2.3. Spirocysts
Spirocysts (Figure 4 and Figure 5) are less useful for species identification. The same capsular variations and tubule pattern seemed similar in most Ceriantharia species (Table 1, Table 2 and Table 3 and Tables S2–S12). Large broad and large elongated, and medium broad and medium elongated spirocysts, with rounded basal capsule-ends, were most common in the tentacles (Figure 4 and Figure S3, Table 1 and Table 2), in which large broad spirocysts with rounded ends generally dominated (Figure 4) in marginal tentacles. Large elongated spirocysts with narrow pointed basal capsule ends were sometimes common (Figure 4). Medium and small elongate spirocysts with narrow pointed or rounded capsule ends, although rare, dominated in other structures (Figure 4 and Figure 7, Tables S2–S5). Similar to den Hartog [18], large and medium broad spirocysts with rounded bases had more tubule coils (ca. 29–32 and 18–24 tubule coils, respectively) than narrow spirocysts of the same length and with narrow bases.
LM, SEM, and TEM pictures of ceriantharian spirocysts (Figure 4 and Figure 5) and spirocysts from sea anemones [31,45,46] are very similar. The hexagonal microfibril pattern in the central tubule core in our TEM cross-section (Figure 5) seemed identical to the microfibril pattern of anthozoan spirocysts [30].
Rifkin [47] recorded microtubules (microfibrillae) in spirocysts of Cerianthus sp. from Australia, not only in the tubule core, but also inside the pleats of the tubule wall. Figure 6i did not show microfibrillae in the pleats. From discharged spirocysts in contact with the water and substrates, the microfibrillae formed an adhesive web [30]. Rifkin [47] suggested that microtubules, emanating in a helical fashion from discharged spirocyst tubules, might assist in the production of adhesive substances. Strömberg & Östman [28] showed a single helix of eosinophilic microfibrillae still attached to the everted spirocyst tubule of the cold-water coral Lophelia pertusa.
4.2.4. Ptychocysts
Although wide capsular variations in size and shape have given rise to a large number of morphological subtypes (Figure 6 and Figures S2 and S3, Table 1, Table 2 and Table 3 and Tables S2–S12), the taxonomic value of ptychocysts is thought to be low. The same subtypes generally appeared in the same structures in the examined species. In the different ptychocyst subtypes, the tubule-packing pattern, filling the whole capsule, was similar. The broadness of the discharged spineless tubule increased in width from small to large ptychocysts (Figure S2). The tubule broadness can thus sometimes reveal the presence and abundance of ptychocyst subtypes in different tube anemone structures. The longitudinal foldings of inverted ptychocyst tubules are clearly shown in SEM images of P. schlenzae, C. brasiliensis, I. nocturnus, and B. norvegicus [6]. This folding pattern is partly shown in LM in large ptychocysts from the column membrane of P. multiplicatus (Figure S2). The function of ptychocysts is tube building; consequently, the highest number of ptychocysts was found in columns and column and tube membranes, in which the large ptychocysts dominated (Figures S2 and S3; Table 3 and Tables S4 and S6–S12). The high abundance and function of medium and small ptychocysts in other tube anemone structures are, however, questioned. The presence of ptychocysts in the marginal tentacles of P. multiplicatus (Table 1) might be due to collection of clay material for tube building carried out by the tentacle. But how can the rich abundance of ptychocysts in labial tentacles (Table 1) and in actinopharynx (Table S5) be explained?
Carlgren [8], analysing the P. multiplicatus column, recorded the largest ptychocysts as atrichs, reaching 113 × 43 µm in length × width. In our analysed P. multiplicatus, very large, drop-shaped ptychocysts (106 × 46 µm) were identified in the column membrane (Table 3). Large column atrichs (ptychocysts) from Arachnanthus nocturnus (up to 96 × 22 µm) were recorded by den Hartog [18]. Molodtsova et al. [23], Lopes et al. [27], and Stampar et al. [26] recorded column ptychocysts from various species reaching 100 × 31, 94 × 35, and 77 × 35 µm, respectively. The same ptychocyst subtypes generally appeared in the same structures in examined species.
4.3. Specific Cnida Structures
In Ceriantharia, specific cnida structures (craspedonemes, cnidorages, and acontioids) are valuable mesenterial features for species identification. Multiple characteristics can be highlighted: (i) their presence is specific to certain ceriantharian families; (ii) they are comparable to other anthozoan structures; (iii) their morphology is specific and quite different; and (iv) their cnidomes show both similarities and differences.
Craspedonemes (Cerianthidae, suborder Spirularia), commonly found in Pachycerianthus spp. (Figure 7), bear no resemblance to any structure within the Hexacorallia. In its gastrodermis, spirocysts of various sizes surround b-mastigophores and ptychocysts (Figure 7), in which medium-sized spirocysts predominate. Large, microbasic b-mastigophores with tiny “faltstück” (Figure 7) might be closely congruent to spirulae 2 from craspedonemes of P. curacaoensis [18], except for the missing tiny “faltstück”. Small to medium, broad to oval b-mastigophores with long shafts seemed to be characteristic of craspedonemes (Figure 7).
Cnidorages, specific to Botrucnidiferidae (suborder Spirularia) (Figure 8), morphologically resemble nematosomes of the Nematostella vectensis anemone (Hexacorallia) [48,49]. The cnidorage origin, however, differs from that of the nematosomes [21,50]. Developing and mature cnidorages of B. benedeni, containing abundant large, reverse drop-shaped to oval-shaped b-mastigophores, spirocysts, and granular gland cells (Figure 8), are, for the first time, described in detail. Mature, ball-shaped cnidorages, free or inside marginal tentacles, are, for the first time, identified in B. benedeni (Figure 8). The B. benedeni b-mastigophores show a mature, helically twisted basal shaft and an apically immature spine row pattern. These nematocysts are not quite identical to the botrucnid b-mastigophores of Botrucnidifer sp. 1 (Figure 8) or to the drop-shaped–oval b-mastigophores of Botrucnidifer norvegicus [8]. Oval-shaped b-mastigophores were reported by den Hartog [18], Molodtsova et al. [23], and Stampar et al. [24].
Acontioids, specific to Arachnactidae (suborder Penicillaria), are, to some extent, similar to the acontia of sea anemones (Hexacorallia) [11]. Both tread-like structures are found at the mesentery bases and bear large penetrating nematocysts, with functions likely to kill captured prey in the gastral cavity. As mentioned, macrobasic acontioid b-mastigophores characteristic of I. maderensis seemed to be close in appearance to acontioid penicillus 2 from I. nocturnus [18].
The presence of broad oval to drop-shaped b-mastigophores may be characteristic of the mesenteries, including craspedonemes, cnidorages, acontioids, and cnidoglandular tract. Oval-shaped b-mastigophores were reported in mesenterial filaments of P. curacaoensis by den Hartog [18] and mesenteries of Pachycerianthus species [24]. Drop-shaped b-mastigophores have been reported in acontioids and mesenterial filament from Arachnanthus (Isarachnanthus) nocturnus [18] and in the cnidoglandular tract [23]. They might also be congruent to b-mastigophore type IV in the mesenteries of Cerianthomorphe species [27] and to b-mastigophores type M in the mesenteries of Ceriantheopsis microbotanicum and C. zelandiaensis of Stampar et al. [26]. The function of these nematocysts, in addition to killing prey, could be to digest food. Oval-shaped b-mastigophores were also reported from the labial tentacles of P. curacaoensis by den Hartog [18].
4.4. Spirularia–Spirula and Penicillaria–Penicillus, Terms to Be Retained or Deleted within the Ceriantharia Classification
The suborders Spirularia and Penicillaria [18] were recently accepted in ceriantharian taxonomy [16,23,26,51]. By combining morphology and genetics, Forero Mejia et al. [1] questioned the validity of the suborder Spirularia. The suborder Spirularia proved to be polyphyletic by including the families Cerianthidae and Botrucniferidae. The suborder Penicillaria, only including the Arachnactidae family, was considered monophyletic. Conversely, in DeBiasse et al. [4], Spirularia and Penicillaria were considered as monophyletic groups and conflicts between ceriantharian taxonomy and phylogeny were reported. The revision of the Ceriantharia at various taxonomic levels was thus recommended.
The morphological differences between spirulae and penicilli nematocysts are based on the presence or absence of the tiny penicilli v-notch [18]. In LM, the v-notch is hard to pinpoint and is, in our opinion, a very weak characteristic. All ceriantharian b-mastigophores have a tiny, more or less visible v-notch formed by the last spines at the shaft end. The last spines on the presumed ‘penicilli shaft end’ are slightly longer than the last shaft spines at the ‘spirula shaft end’. Thus, the ‘penicilli v-notch’ is more obvious than the ‘spirula v-notch’. In our opinion, the slight morphological differences between spirulae and penicilli nematocysts do not justify the two suborders and their names Spirularia and Penicillaria. The question is how to replace Spirularia and Penicillaria, both in terms of taxonomy and names.
Spirula nematocysts are, in our opinion, very similar to b-mastigophores, defined by Carlgren [8,29], and to b-rhabdoids, defined by Schmidt [10], in which the shaft tubule gradually tapers towards the diameter of the distal tubule. On the inverted ceriantharian shaft (Figure 2 and Figures S2 and S3), the v-shaped spine pattern is distinct due to the presence of prominent, long spines. Compared with the hexacorallian b-mastigophore shafts of sea anemones [11,39] and of cold-water corals [28], the inverted Ceriantharia shaft is broader and the v-shaped spine pattern is more distinct (Figure S3). Shafts with spines of the ceriantharian b-mastigophores are more similar to the broad shaft with large spines in the hexacorallian p-mastigophore/p-amastigophore shafts (Figure S3; [39]). To highlight the similarities to and differences from hexacorallian b-mastigophores, spirulae are here referred to b-mastigophores/spirulae subtype.
Penicilli nematocysts, identified by the small v-notch formed by the last spines at the inverted shaft end [18], do not fulfil the definition of the conspicuous, large, funnel-shaped v-notch in p-mastigophores/p-amastigophores [8] and in p-rhabdoids [10]. The tubule of the shaft end of p-mastigophores/p-amastigophores is broader compared with the narrow distal tubule. The large v-notch at the shaft end of the hexacorallian p-nematocysts (Figure S3) is formed by the invagination of the inverted shaft end, and not by the v-pattern of the last spine rows at the shaft end as in ceriantharian penicilli nematocysts. We consider it possible that, at the shaft end of the large b-mastigophore with the long coiled “faltstück” in I. maderensis (Figure 3), the last spines in the shaft may form a tiny ’penicilli v-notch’. Like spirulae, the penicilli shaft tapers gradually towards the shaft end. The ‘penicilli spines’ at the shaft end are slightly larger than the tiny ‘spirula spines’ (Figure S2) at the shaft end. Thus, the ‘penicilli v-notch’ is slightly more obvious, than the barely visible ‘spirula v-notch’ in LM (Figure S1). The small v-notch (Figure 3) at the shaft end on the large acontioid b-mastigophores with a long, coiled “faltstück” in I. maderensis might, as in I. nocturnus [18], be a possible ‘penicilli v-notch’. To highlight the similarities to the b-mastigophores, these penicilli nematocysts are here referred to as b-mastigophores/penicilli-subtype.
Regarding its cnidome, the possibly identified b-mastigophores/penicilli-subtype, I. maderensis is placed in the suborder Penicillaria. Stampar et al. [52] reported p-mastigophores in I. maderensis and I. nocturnus, but presented no pictures of p-mastigophores with a characteristic funnel-shaped v-notch. To verify the presence of the ‘tiny penicilli v-notch’, cnida studies from more specimens belonging to the Arachnactidae family are desirable. A prominent p-mastigophore/amastigophore v-notch has not been illustrated in the Ceriantharia.
The validity of the suborders Spirularia and Penicillaria, mainly based on the presence or absence of the v-notch, is weak. The polyphyletic Spirularia, including the families Cerianthidae and Botrucniferidae, is characterized by abundant spirulae, absence of penicilli, and presence of isorhizas, and the specific cnida structures craspedonemes (Cerianthidae, Table S2) and cnidorages (Botrucniferidae, Table S10). The monophyly of Penicillaria is evidenced by the presence of a single family, the Arachnactidae, with the presence of the specific cnida structure acontioids, in addition to morphological characteristics, such as the abundance of penicilli, few spirulae, and absence of isorhizas (Table S12). Multiple questions arose regarding the two suborders. Could the terms spirulae, penicilli, and the suborders Spirularia and Penicillaria be replaced or rejected? Could it be more beneficial to refer to the family level within Ceriantharia, rather than using the two suborders Spirularia and Penicillaria?
In agreement with Sanamyan et al. and Gusmão et al. [35,36], we consider that the term “mastigophore”, since it has been very widely used for a long time and to preserve stability and consistence of nomenclature, shall preferably be used instead of the terms “spirula” and “penicillus” [18,19]. But, to show that the spirulae and penicilli in Ceriantharia differ from the hexacorallian b- and p-mastigophores/amastigophores, we have referred to these nematocysts as b-mastigophore/spirula subtype and b-mastigophore/penicillus subtype.
4.5. Cnida Diversity and Evolution
Ceriantharia is the only cnidarian group possessing all three cnida categories (Figure 9). In addition to nematocysts [9], Ceriantharia has adhesive spirocysts [14,15,30] and tube-building ptychocysts [7]. The spineless spirocysts and ptychocysts are considered nearly monomorphic [1], even if the varieties of the ptychocyst capsules are great (Table 3 and Table S4).
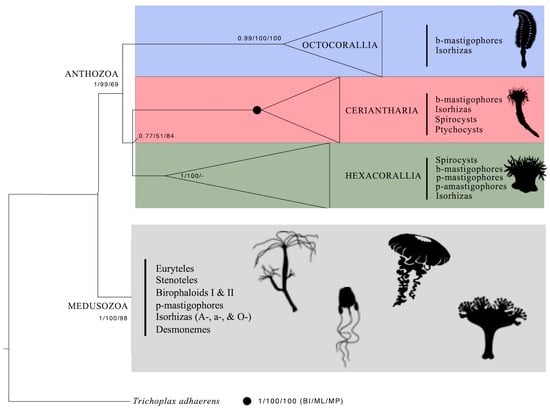
Figure 9.
Phylogenetic reconstruction of Anthozoa with supporting values for nodes following Forero Mejia et al., 2020 [1]. Cnida types are reported for each anthozoan group. Schematic pictures from https://www.phylopic.org/ (accessed on 23 November 2023).
In the Ceriantharia, there are only two main nematocyst types, the b-mastigophores and isorhizas. Compared with the Medusozoa, the variety of different nematocyst types is quite small within the Anthozoa, and especially in the Ceriantharia (Figure 9). Nematocysts are divided into haplonemes (isodiametric tubule as isorhizas) and heteronemes (tubule with basal enlargement as b- and p-mastigophores) (Figure S3) [9,15]. Isorhiza is the only nematocyst type that occurs in all cnidarian groups (Figure 9). Spines, if present in haploneme isorhizas, are generally of the same size. Schmidt [13] regarded haploneme isorhizas as the earliest developed and simplest nematocysts. Some isorhizas are small and harmless to humans, like those in Funiculina quadrangularis (Octocorallia) and Staurozoa species (Medusozoa) (Figure S3). Over time, isorhizas have, however, developed into highly specialized cnidae, representing a large diversity considering morphology, function, and toxicity [17,40].
Heteroneme nematocysts, with large basal spines on the shaft and smaller spines on the distal tubule, show great morphological variations and are considered highly developed. Medusozoa (including Staurozoa, Hydrozoa, Cubozoa, and Scyphozoa) has, in addition to b-mastigophores and several isorhiza types, stenoteles, euryteles, desmonemes, birophaloid I, and birophaloid II nematocysts [17,28,40], all playing an important role in prey capturing. On the other hand, Medusozoa lacks spirocysts and ptychocysts. To compensate for the small number of different cnida types, Ceriantharia might have evolved a large number of morphological cnida subtypes and size groups, perhaps with specialized functions.
Generally, the functions of both haplonemes and heteronemes are to capture and hold the prey and inject the toxin by penetrating. Compared with the most potent meduzoan stingers, members in Ceriantharia and most hexacorallian are harmless stingers, which may reflect the low diversity of penetrating nematocysts.
In the Ceriantharia, small and medium to very large isorhizas, with the same capsule shape and inverted tubule pattern were identified (Figure S3; Tables S2–S4) Ceriantharian isorhizas are very similar in capsule shape and inverted tubule pattern to the large homotrichous isorhizas of the stone coral Lophelia pertusa (Hexacorallia) (Figure S3) and to the large spineless isorhiza in the sea anemone Edwardsiella carnea (Hexacorallia) [38]. Homotrichous large spines were illustrated from large isorhizas of P. curacaoensis [18], suggesting that these isorhizas are penetrators. The large isorhizas of P. multiplicatus show only a pattern of small dots in helices on the tubules (Figure S2). Whether ceriantharian isorhizas have an entangling tubule with weak spines or a spined penetrating isorhiza tubule has not been verified here. Entangling isorhiza tubules with weak spines, wrapping around prey to prevent escape, is common [40]. In the Hexacorallia and Ceriantharia, the adherent spirocyst microfibrillae may partly have taken over the role of holding the prey.
Spirocysts, abundant in the Ceriantharia and in the Hexacorallia (Figure S3), differ in their tissue distributions [30]. In the Ceriantharia, spirocysts are more or less common in all structures studied (Table 1, Table 2 and Table 3 and Tables S2–S12), while in the Hexacorallia, spirocysts generally occur only in tentacles and in the actinopharynx [11], and less frequently in the column (e.g., [53]). The absence of spirocysts in the Octocorallia (Figure 9) distinguishes these anthozoans from the Ceriantharia [54,55] and Hexacorallia [30]. Since the adhesive ability of spirocysts to catch and hold prey is their main function, the largest spirocysts were found in the marginal tentacles of Ceriantharia (Figure S3; Table 1).
Ptychocysts, the key characteristics of Ceriantharia, are responsible for ceriantharian tube building. The ptychocyst tubules (Figure S1) differ in morphology and function from the helically twisted inverted nematocyst and spirocyst tubules. The longitudinal folding of the inverted tubules is shown in the SEM images (Figure 6) and Stampar et al. [24]. Folding also is partly seen in the LM (Figure S2). The long spineless, sticky ptychocyst tubules adhere to soft seafloor materials and gravels and construct the tube. In the column and tube and column membranes, large drop-shaped and large elongate or bent ptychocysts are the dominating cnidae (Table 3 and Table S4). But what specific functions do small and medium-sized ptychocysts of various capsule shapes, which also appear in the column and membranes and in the other tube anemone structures (Table 1 and Table 2 and Tables S2–S12), have? For what reason are medium and large elongate ptychocysts abundant in the actinopharynx (Table S8)? The high numbers of various ptychocyst subtypes might compensate for the monophyletic ptychocysts.
Specific morphologic capsule openings within the cnida categories include nematocysts, spirocysts, and ptychocysts, and are important phylogenetic features within the Cnidaria. Hydrozoan and scyphozoan nematocyst capsules are opened with an operculum/lid [40]. Three apical flaps open the nematocyst capsules in anthozoans [45,56], thus separating medusozoans from anthozoans [57]. Reft et al. [57] and Reft and Daly [58] analysed the ultrastructure of the capsule opening of cnidae from Anthozoa and Medusozoa species. Spirocysts and ptychocysts have neither an operculum nor three apical flaps. An apical thickened cap is present in spirocysts [45,47,59]. Our TEM of a ceriantharian b-mastigophore with a very short “faltstück” from Cerianthus sp. 1 might show an apical thickened capsular cap (Figure S4). In undischarged, large drop-shaped ptychocysts a thickened apical structure might be visible (Figure S3). More studies are needed to confirm a capsular cap in ceriantharian cnidae.
5. Conclusions
- The cnidae b-mastigophores, spirocysts, and ptychocysts are placed into different morphological subtypes and size groups, some of which are species-specific or specific to certain structures. Other subtypes are common in most structures and are of less taxonomic value. For species determination, detailed cnida surveys within Ceriantharia are valuable;
- The different patterns of the “faltstück” within b-mastigophores may be species-specific features;
- To highlight the similarities and differences between spirulae and penicilli [18] and hexacorallian b-mastigophores and p-mastigophores/p-amastigophores, we propose that spirulae and penicilli can be referred to as b-mastigophores/spirulae subtype and b-mastigophores/penicillin subtype, respectively, until the terms spirulae/Spirularia and penicilli/Penicillaria have been changed. To verify the validity of the penicilli-v-notch, cnida investigations of more Arachnactidae species are desirable;
- The following question is raised: Can the minor morphological differences between spirulae and penicilli nematocysts justify the names Spirularia and Penicillaria of the suborders?
- The occurrence/absence of the less common medium to very large isorhizas are valuable species characteristics;
- The pattern of the ptychocyst tubule is unique. Its function is tube-building. The inverted longitudinally and transversely folded tubules differ from the helically twisted and coiled nematocyst and spirocyst tubules;
- The occurrence of the regular striation pattern on the inner ptychocyst capsule wall in certain Ceriantharia species is a valuable species characteristic;
- Cnidomes combined with morphology and molecular data can be useful for phylogenetic analysis;
- The occurrence of an operculum, tripartite opercular flaps, or apical capsular cap shows possible evolutionary relationships and development within the Cnidaria.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/oceans5030029/s1, Figure S1: Overview of the species and structures analysed in Ceriantharia; Figure S2: Light microscopies of Pachycerianthus multiplicatus cnidae; Figure S3: Light microscopies of cnidocysts in Octocorallia, Ceriantharia, and Hexacorallia (Anthozoa), and Staurozoa (Medusozoa); Figure S4: Transmission electron microscopy of an apical capsule of what probably is a b-mastigophore with “Faltstück” from marginal tentacle of Cerianthus sp. 1; Table S1: Voucher specimens. List of ceriantharian species, analysed for their cnidomes, showing catalogue number, museum for deposit voucher specimens, sampling method, geographical locality, and country; Table S2: Cnidae from craspedonemes of three Pachycerianthus multiplicatus; Table S3: Cnidae from cnidoglandular tract of three Pachycerianthus multiplicatus; Table S4: Cnidae from column of at least three Pachycerianthus multiplicatus; Table S5: Cnidae from actinopharynx of three Pachycerianthus multiplicatus; Table S6: Cnidae from different structures of Cerianthus membranaceus; Table S7: Cnidae from different structures of Cerianthus sp. 1; Table S8: Cnidae from different structures of Pachycerianthus torreyi; Table S9: Cnidae from different structures of Pachycerianthus dorhni; Table S10: Cnidae from different structures of Boctrucnidifer sp. 1; Table S11: Cnidae from different structures of Botruanthus benedeni; Table S12: Cnidae from different structures of Isarachnanthus maderensis; Table S13: Cnidocyst terminology used in the present study and recorded differently in other studies; File S1: Glossary for cnidae terminology and for specific cnida structures, with associated literature.
Author Contributions
Conceptualization, A.C.F.-M. and C.Ö.; methodology, C.Ö.; software, A.C.F.-M., L.D., and C.Ö.; validation, A.C.F.-M. and C.Ö.; formal analysis, A.C.F.-M. and C.Ö.; investigation, A.C.F.-M. and C.Ö.; resources, A.C.F.-M. and C.Ö.; data curation, A.C.F.-M. and C.Ö.; writing—original draft preparation, A.C.F.-M., L.D., and C.Ö.; writing—review and editing, A.C.F.-M., L.D., and C.Ö.; visualization, C.Ö.; supervision, C.Ö.; project administration, C.Ö.; funding acquisition, C.Ö. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was obtained from Inez Johanssons and the zoological research foundations of the faculty of Science of Uppsala University, to C.Ö.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials; further inquiries can be directed to the corresponding author/s.
Acknowledgments
The research was partly carried out in Fiskebäckskil at Kristineberg Marine Research Station, University of Gothenburg, at the University of Uppsala, Sweden, and at King Abdullah University of Science and Technology (KAUST). The authors acknowledge the Integrative Systems Biology Laboratory (KAUST) and Biosciences Core Laboratory (KAUST) for their support and assistance. This research was undertaken in accordance with the policies and procedures of KAUST. Permissions relevant for KAUST to undertake the research were obtained from the applicable governmental agencies in the Kingdom of Saudi Arabia. We thank Giorgio Bavestrello from the Università Degli Studi di Genova for his invaluable support throughout the sampling of all the studied specimens here reported. We thank Frine Cardone from the Università Degli Studi di Bari for her support and effort during the collection of cerianthids in Taranto, Italy. We express our gratitude to the directors and staff for providing laboratory facilities and specimens.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Forero Mejia, A.; Molodtsova, T.; Östman, C.S.; Bavestrello, G.; Rouse, G.W. Molecular phylogeny of Ceriantharia (Anthozoa, Cnidaria) reveals non-monophyly of traditionally accepted families. Zool. J. Linn. Soc. 2020, 190, 397–416. [Google Scholar] [CrossRef]
- Quattrini, A.M.; Snyder, K.E.; Purow-Ruderman, R.; Seiblitz, I.G.L.; Hoang, J.; Floerke, N.; Ramos, N.I.; Wirshing, H.H.; Rodriguez, E.; McFadden, C.S. Mito-nuclear discordance within Anthozoa, with notes on unique properties of their mitochondrial genomes. Sci. Rep. 2023, 13, 7443. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, D.M.; Bessho-Uehara, M.; Haddock, S.H.D.; McFadden, C.S.; Quattrini, A.M. Evolution of bioluminescence in Anthozoa with emphasis on Octocorallia. Proc. R. Soc. B 2024, 291, 20232626. [Google Scholar] [CrossRef] [PubMed]
- DeBiasse, M.B.; Buckenmeyer, A.; Macrander, J.; Babonis, L.S.; Bentlage, B.; Cartwright, P.; Prada, C.; Reitzel, A.M.; Stampar, S.N.; Collins, A.G.; et al. A cnidarian phylogenomic tree fitted with hundreds of 18S leaves. Bull. Soc. Syst. Biol. 2024, 3, 2. [Google Scholar] [CrossRef]
- Molodtsova, T. Ceriantharia (Cnidaria) of the Gulf of Mexico. In Gulf of Mexico Origin, Waters, and Biota. Volume 1: Biodiversity; Felder, D.L., Camp, D.K., Eds.; Texas A&M University Press: College Station, TX, USA, 2009; pp. 365–367. [Google Scholar]
- Stampar, S.N.; Beneti, J.S.; Acuña, F.H.; Morandini, A.C. Ultrastructure and tube formation in Ceriantharia (Cnidaria, Anthozoa). Zool. Anz. 2015, 254, 67–71. [Google Scholar] [CrossRef]
- Mariscal, R.N.; Conklin, B.J.; Bigger, C.H. The ptychocyst, a major new category of cnida used in tube constraction by cerianthid anemone. Biol. Bull. 1977, 152, 392–405. [Google Scholar] [CrossRef]
- Calgren, O. A contribution to the knowledge of structure and distribution of cnidae in the Anthozoa. Acta Univ. Lundensis 1940, 36, 1–62. [Google Scholar]
- Weill, R. Contribution a l’étude des Cnidaires et leur nématocystes. I. Recherches sur les nématocystes. II. Valeur taxonomique du cnidom. In Travaux de la Station Zoologique de Wimereux; Biodiversity Heritage Library: Wimereux, France, 1934; Volume 10, pp. 1–701. [Google Scholar]
- Schmidt, H. Die Nesslkapseln der Aktinien und ihre differentialdiagnostische Bedeutung. Helgoländer Wiss. Meeresunters 1969, 19, 284–317. [Google Scholar] [CrossRef][Green Version]
- Östman, C.; Roat, C.; Roat Kultima, J.; Rundblom, K. Acontia and mesentery nematocysts of the sea anemone Metridium senile (Linnaeus, 1761) (Cnidaria, Anthozoa). Sci. Mar. 2010, 74, 483–497. [Google Scholar] [CrossRef]
- Schmidt, H. Die nesselkapseln der Anthozoen und irhe Bedeutung fur die phylogenetische Systematik. Helgoländer Wiss. Meeresunters 1972, 23, 422–458. [Google Scholar] [CrossRef]
- Schmidt, H. On evolution in the Anthozoa. In Proceedings of the Second International Coral Reef Symposium, Great Barrier Reef Committee, Queensland, Australasia, 22 June–2 July 1974; Volume 1, pp. 533–560. [Google Scholar]
- Cutress, C.E. An interpretation of the structure and distribution of cnidae in Anthozoa. Syst. Zool. 1955, 4, 120–137. [Google Scholar] [CrossRef]
- Mariscal, R.N. Nematocysts. In Coelenterate Biology: Reviews and New Perspectives; Muscatine, L., Lenhoff, H.M., Eds.; Academic Press: New York, NY, USA, 1974; pp. 129–178. [Google Scholar]
- England, K.W. Nematocysts of sea anemones (Actinaria, Ceriantharia and Corralimorpharia: Cnidaria): Nomenclature. In Coelenterate Biology: Recent Research on Cnidaria and Ctenophora. Developments in Hydrobiology; Williams, R.B., Cornelius, P.F.S., Hughes, R.G., Robson, E.A., Eds.; Springer: Dordrecht, The Netherlands, 1991; Volume 66, pp. 607–691. [Google Scholar] [CrossRef]
- Östman, C. A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Sci. Mar. 2000, 64, 31–46. [Google Scholar] [CrossRef]
- den Hartog, J.C. Description of two new Ceriantharia from the Caribbean region, Pachyceriantus curacaoensis n. sp. and Arachnanthus nocturnus n. sp. with discussion of the cnidom and of the classification of the Ceriantharia. Zool. Meded. 1977, 51, 211–242. [Google Scholar]
- Stephenson, T.A. The British Sea Anemones, Dulau & Co., Ltd: London, UK, 1928; Volume 1.
- Kayal, E.; Bastian, B.; Pankey, M.S.; Ohdeta, A.H.; Medina, M.; Placketzki, D.C.; Collins, A.G.; Ryan, J.F. Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol. Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Carlgren, O. Ceriantharia. Dan. Ingolf-Exped. 1912, 5, 1–79. [Google Scholar]
- Arai, M.N. A new species of Pachycerianthus, with a discussion of the genus and an appended glossary. Pac. Sci. 1965, 19, 205–218. [Google Scholar]
- Molodtsova, T.N.; Griffiths, C.L.; Acuña, F.H. A new species of shallow-water cerianthid (Cnidaria: Anthozoa) from South Africa, with remarks on the genus Ceriantheopsis. Afr. Nat. Hist. 2011, 7, 2–8. [Google Scholar]
- Stampar, S.N.; Morandini, A.C.; da Silveira, F.L. A new species of Pachycerianthus (Cnidaria, Anthozoa, Ceriantharia) from Tropical Southwestern Atlantic. Zootaxa 2014, 3827, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Stampar, S.N.; Scarabino, F.; Pastorino, F.; Morandini, A.C. A new species of tube-dwelling anemone (Cnidaria, Anthozoa, Cerinathaira, Ceriantheopsis) from the warm temperate South-western Atlantic. J. Mar. Biol. Assoc. U. K. 2015, 96, 1475–1481. [Google Scholar] [CrossRef]
- Stampar, S.N.; Sadie, V.; Keable, S. Ceriantharia (Cnidaria) from Australia, New Zealand and Antarctica with descriptions of four New Species. Rec. Aust. Mus. 2020, 72, 81–100. [Google Scholar] [CrossRef]
- Lopes, C.S.; Ceriello, H.; Morandini, A.C.; Stampar, N. Revision of the genus Ceriantheomorphe (Cnidaria, Anthozoa, Ceriantharia) with description of a new species form the Gulf of Mexico and northwestern Atlantic. Zookeys 2019, 874, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Strömberg, S.M.; Östman, C. The cnidome and internal morphology of Lophelia pertusa (Linnaeus, 1755) (Cnidaria, Anthozoa). Acta Zool. 2016, 98, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Carlgren, O. A survey of the ptychodactiaria, Corallimorpharia and Actiniaria. Kungl. Svenka Vetenskapsakademiens Handlingar 1949, 1, 1–121. [Google Scholar]
- Mariscal, R.N.; McLean, R.B.; Hand, C. The form and function of cnidarian spirocysts. 3. Ultrastructure of the thread and the function of spirocysts. Cell Tissue Res. 1977, 178, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Watson, G.M.; Wood, R.L. Colloquium on the terminology. In The Biology of Nematocysts; Heissinger, D.A., Lenhoff, H.M., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 21–23. [Google Scholar]
- Acuña, F.H.; Excoffon, A.C.; Zamponi, M.O.; Ricci, L. Importance of nematocysts in taxonomy of acontiarian sea anemones (Cnidaria, Actiniaria): A statistical comparative study. Zool. Anz. 2003, 242, 75–81. [Google Scholar] [CrossRef]
- Garese, A.; Correa, F.G.; Acuña, F.H.; Stampar, S.N. Cnidom in Ceriantharia (Cnidaria, Anthozoa): New findings in the composition and micrometric variations of Cnidocysts. PeerJ 2023, 11, e15549. [Google Scholar] [CrossRef] [PubMed]
- Molodtsova, T.N.; Moskalenko, V.N.; Lipukhin, E.V.; Antokhina, T.I.; Ananeva, M.S.; Simakova, U.V. Cerianthus lloydii (Ceriantharia: Anthozoa: Cnidaria): New status and new perspectives. Biology 2023, 12, 1167. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, L.C.; Grajales, A.; Rodríguez, E. Sea anemones through X-rays: Visualization of two species of Diadumene (Cnidaria, Actiniaria) using micro-CT. Am. Mus. Novit. 2018, 2018, 1–47. [Google Scholar] [CrossRef]
- Sanamyan, N.P.; Sanamyan, K.E.; Tabachnick, K.R. The first species of Actiniaria, Spongiactis japonica gen.n., sp.n. (Cnidaria: Anthozoa), an obligate symbiont of a glass sponge. Invert. Zool. 2012, 9, 127–141. [Google Scholar] [CrossRef]
- Zamponi, M.O.; Acuña, F.H. La variabilidad de los cnidocistos y su importancia en la determinación de clines. Physis 1991, 49, 7–18. [Google Scholar]
- Östman, C.; Møller, F.L. Gross morphology and cnidae of Edwardsiella anemones and larvae (Anthozoa, Edwardsiidae) from the Swedish West Coast. Acta Zool. 2021, 102, 467–482. [Google Scholar] [CrossRef]
- Östman, C.; Borg, F.; Roat, C.; Kultima, J.; Wong, S.Y. Cnidae in the sea anemone Sagartiogeton viduatus (Muller, 1776) (Cnidaria, Anthozoa); A comparison to cnidae in the sea anemone Metridium senile (linnaeus, 1761) (Cnidaria, Anthozoa). Acta Zool. 2012, 94, 392–409. [Google Scholar] [CrossRef]
- Östman, C.; Hydman, J. Nematocyst analysis of Cyanea capillata and Cyanea lamarckii (Scyphozoa, Cnidaria). Sci. Mar. 1997, 61, 313–344. [Google Scholar]
- Williams, R.B. Measurements of cnidae from sea anemones (Cnidaria: Actiniaria). Sci. Mar. 1996, 60, 339–351. [Google Scholar]
- Williams, R.B. Measurements of cnidae from sea anemones (Cnidaria: Actinaria), II: Further studies of differences amongst sample means and their taxonomic relevance. Sci. Mar. 1998, 62, 361–372. [Google Scholar] [CrossRef]
- Williams, R.B. Measurements of cnidae from sea anemones (Cnidaria: Actiniaria), III: Ranges and other measures of statistical dispersion, their interrelations and taxonomic relevance. Sci. Mar. 2000, 64, 49–68. [Google Scholar] [CrossRef]
- Häussermann, V. Identification and taxonomy of soft-bodied hexacorals exemplified by Chilean sea anemones; including guidelines for sampling, preservation and examination. J. Mar. Biol. Assoc. U. K. 2004, 84, 931–936. [Google Scholar] [CrossRef]
- Westfall, J.A. Nematocyst of the sea anemone Metridium. Am. Zool. 1965, 5, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Westfall, J.; Landers, D.; McCallum, J. Ultrastructure of neuro-spirocyte synapses in the sea anemone Aiptasia pallida (Cnidaria, Anthozoa, Zoantharia). J. Morphol. 1999, 241, 165–173. [Google Scholar] [CrossRef]
- Rifkin, J.F. A study of the spirocytes from the Ceriantharia and Actiniaria (Cnidaria: Anthozoa). Cell Tissue Res. 1991, 266, 365–373. [Google Scholar] [CrossRef]
- Williams, R.B. A redescription of the brackish-water sea anemone Nematostella vectensis Stephenson, with an appraisal of congeneric species. J. Nat. Hist. 1975, 9, 51–64. [Google Scholar] [CrossRef]
- Babonis, L.S.; Martindale, M.Q.; Ryan, J.F. Do novel genes drive morphological novelty? An investigation of the nematosomes in the sea anemone Nemastostella vectensis. BMC Evol. Biol. 2016, 16, 114. [Google Scholar] [CrossRef] [PubMed]
- Carlgren, O. The actinian fauna of the Gulf of California. Proc. U. S. Natl. Mus. 1951, 101, 415–449. [Google Scholar] [CrossRef]
- Stampar, S.N.; Maronna, M.M.; Kitahara, M.V.; Reimer, J.D.; Beneti, J.S.; Morandini, A.C. Ceriantharia in current systematics: Life cycles, morphology and genetics. In The Cnidaria, Past, Present and Future; Goffredo, S., Dubinsky, Z., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Stampar, S.N.; Maronna, M.M.; Vermeij, M.J.A.; Silveira, F.L.D.; Morandini, A.C. Evolutionary diversification of Banded tube-dwelling anemones (Cnidaria; Ceriantharia; Isarachnanthus) in the Atlantic Ocean. PLoS ONE 2012, 7, e41091. [Google Scholar] [CrossRef] [PubMed]
- Kise, H.; Fujii, T.; Masucci, G.D.; Biondi, P.; Reimer, J.D. Three new species and the molecular phylogeny of Antipathozoanthus from the Indo-Pacific Ocean (Anthozoa, Hexacorallia, Zoantharia). Zookeys 2017, 725, 97–122. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.; Fautin, D.G.; Cappola, V.A. Systematics of the hexacorallia (Cnidaria: Anthozoa). Zool. J. Linn. Soc. 2003, 139, 419–437. [Google Scholar] [CrossRef]
- Westfall, J.A.; Hand, C. Fine structure of nematocysts in a sea anemone. In Electron Microscopy, Proceedings of the 5th International Congress, 29 August–5 September; Sydney, S., Breese, S.S., Jr., Eds.; Academic Press: Philadelphia, PA, USA, 1962. [Google Scholar]
- Mariscal, R.N. Cnidaria: Cnidae. Biology of the integument. In Invertebrates; Bereiter-Hahn, J., Matoltsy, A.J., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 1, pp. 57–68. [Google Scholar]
- Reft, A.J.; Westfall, J.A.; Fautin, D.G. Formation of the apical flaps in nematocysts of sea anemones (Cnidaria: Actiniaria). Biol. Bull. 2009, 217, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Reft, A.J.; Daly, M. Morphology, distribution, and evolution of apical structure of nematocysts in hexacorallia. J. Morphol. 2012, 273, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.N.; McLean, R.B. The form and function of cnidarian spirocysts: 2. Ultrastructure of the capsule tip and wall and mechanism of discharge. Cell Tissue Res. 1976, 169, 313–321. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).