A Review of Northern Fur Seal (Callorhinus ursinus) Literature to Direct Future Health Monitoring Initiatives
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Disease Classification
3.1.1. Infectious Disease
Viruses
Bacteria
Fungi
Metazoan Parasites
Protozoan Parasites
3.1.2. Non-Infectious Disease
Neoplasia
Toxins and Contaminants
Trauma: Anthropogenic
Trauma Non-Anthropogenic/Unknown Source
Other
3.2. Health Classification
4. Discussion
“Absence of evidence is not evidence of absence.”- Carl Sagan
- General characteristics of the condition in question (e.g., susceptible hosts, reservoirs, speed of spread, virulence, pathogenicity, and immune response);
- Animal health impacts (e.g., morbidity, mortality, reproductive consequences, and welfare considerations);
- Public health impacts (e.g., transmissibility to humans, severity of human disease, opportunities for human protection, food safety and security, bio/agroterrorism potential, spread amongst humans, and economic consequences);
- Regulatory impacts (e.g., local, federal, or international trade consequences);
- Mitigation (e.g., diagnosis, prevention, and treatment).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelatt, T.; Ream, R.; Johnson, D. Callorhinus Ursinus. The IUCN Red List of Threatened Species 2015. Available online: https://www.iucnredlist.org/species/3590/45224953 (accessed on 5 May 2022).
- Fisheries, N. Northern Fur Seal. Available online: https://www.fisheries.noaa.gov/species/northern-fur-seal#:~:text=Male%20northern%20fur%20seals%20can,territories%20before%20the%20females%20arrive (accessed on 5 May 2022).
- Pacifici, M.; Santini, L.; Di Marco, M.; Baisero, D.; Francucci, L.; Grottolo Marasini, G.; Visconti, P.; Rondinini, C. Generation length for mammals. Nat. Conserv. 2013, 5, 5734. [Google Scholar] [CrossRef] [Green Version]
- Testa, J.W. Fur Seal Investigations, 2015–2016. 2018. Available online: https://doi.org/10.7289/V5/TM-AFSC-375 (accessed on 5 May 2022).
- Towell, R.G.; Ream, R.R.; York, A.E. Decline in northern fur seal (Callorhinus ursinus) pup production on the Pribilof Islands. Mar. Mammal Sci. 2006, 22, 486–491. [Google Scholar] [CrossRef]
- NMFS, Department of Commerce. North Pacific Fur Seal; Pribilof Island Population; Designation as Depleted. Fed. Reg. 1988, 53, 17888–17899. Available online: https://archives.federalregister.gov/issue_slice/1988/5/18/17881-17909.pdf#page=8 (accessed on 5 May 2022).
- Ryser-Degiorgis, M.-P. Wildlife health investigations: Needs, challenges and recommendations. BMC Vet. Res. 2013, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagre, A.C.; Patyk, K.A.; Nol, P.; Atwood, T.C.; Hueffer, K.; Duncan, C.G. A Review of Infectious Agents in Polar Bears (Ursus maritimus) and Their Long-Term Ecological Relevance. EcoHealth 2015, 12, 528–539. [Google Scholar] [CrossRef]
- Simeone, C.A.; Gulland, F.M.D.; Norris, T.; Rowles, T.K. A Systematic Review of Changes in Marine Mammal Health in North America, 1972–2012: The Need for a Novel Integrated Approach. PLoS ONE 2015, 10, e0142105. [Google Scholar] [CrossRef] [Green Version]
- Stephen, C. Toward a Modernized Definition of Wildlife Health. J. Wildl. Dis. 2014, 50, 427–430. [Google Scholar] [CrossRef]
- Wittrock, J.; Duncan, C.; Stephen, C. A Determinants of Health Conceptural Model for Fish and Wildlife Health. J. Wildl. Dis. 2019, 55, 285–297. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Spraker, T.R.; Lander, M.E. Causes of Mortality in northern Fur Seals (Callorhinus ursinus), St. Paul Island, Pribilof Islands, Alaska, 1986–2006. J. Wildl. Dis. 2010, 46, 450–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, J.A.; Roletto, J.; Morgan, L.E.; Smith, D.M.; Gage, L.J. Findings in pinnipeds stranded along the central and northern California coast, 1984–1990. J. Wildl. Dis. 1993, 29, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.W.; Vedros, N.A.; Akers, T.G.; Gilmartin, W.G. Hazards of disease transfer from marine mammals to land mammals: Review and recent findings. J. Am. Vet. Med. Assoc. 1978, 173, 1131–1133. [Google Scholar] [PubMed]
- Smith, A.W.; Prato, C.M.; Gilmartin, W.G.; Brown, R.J.; Keyes, M.C. A Preliminary Report on Potentially Pathogenic Microbiological Agents Recently Isolated from Pinnipeds. J. Wildl. Dis. 1974, 10, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.W.; Skilling, D.E. Viruses and virus diseases of marine mammals. J. Am. Vet. Med. Assoc. 1979, 175, 918–920. [Google Scholar]
- Barlough, J.E.; Matson, D.O.; Skilling, D.E.; Berke, T.; Berry, E.S.; Brown, R.F.; Smith, A.W. Isolation of Reptilian Calicivirus Crotalus Type 1 From Feral Pinnipeds. J. Wildl. Dis. 1998, 34, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, J.C.; Madin, S.H.; Skilling, D.E. Isolation of San Miguel Sea Lion Virus from Samples of an animal food product produced from northern fur seal (Callorhinus ursinus) carcasses. Am. J. Vet. Res. 1978, 39, 137–139. [Google Scholar]
- Smith, A.W.; Prato, C.M.; Skilling, D.E. Characterization of two new serotypes of San Miguel sea lion virus. Intervirology 1977, 8, 30–36. [Google Scholar] [CrossRef]
- Smith, A.W.; Skilling, D.E.; Latham, A.B. Isolation and identification of five new serotypes of calicivirus from marine mammals. Am. J. Vet. Res. 1981, 42, 693–694. [Google Scholar]
- Prato, C.M.; Akers, T.G.; Smith, A.W. Serological evidence of calicivirus transmission between marine and terrestrial mammals. Nature 1974, 249, 255–256. [Google Scholar] [CrossRef]
- Hadlow, W.J.; Cheville, N.F.; Jellison, W.L. Occurrence of pox in a northern fur seal on the Pribilof Islands in 1951. J. Wildl. Dis. 1980, 16, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, C.; Goldstein, T.; Hearne, C.; Gelatt, T.; Spraker, T. Novel polyomaviral infection in the placenta of a northern fur seal (Callorhinus ursinus) on the Pribilof Islands, Alaska, USA. J. Wildl. Dis. 2013, 49, 163–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Hinojosa, G.; Gulland, F.M.D.; DeLong, R.; Gelatt, T.; Archer, L.; Wellehan, J.F.X. A Novel Gammaherpesvirus in Northern Fur Seals (Callorhinus ursinus) Is Closely Related to the California Sea Lion (Zalophus californianus) Carcinoma-Associated Otarine Herpesvirus-1. J. Wildl. Dis. 2016, 52, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.; Kersh, G.; Spraker, T.; Patyk, K.; Fitzpatrick, K.; Massung, R.; Gelatt, T. Coxiella burnetii in Northern Fur Seal (Callorhinus ursinus) Placentas from St. Paul Island, Alaska. Vector-Borne Zoonotic Dis. 2012, 12, 192–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, C.; Savage, K.; Williams, M.; Dickerson, B.; Kondas, A.V.; Fitzpatrick, K.A.; Guerrero, J.L.; Spraker, T.; Kersh, G.J. Multiple strains of Coxiella burnetii are present in the environment of St. Paul Island, Alaska. Transbound. Emerg. Dis. 2013, 60, 345–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, E.; Ehrhart, E.J.; Charles, B.; Spraker, T.; Gelatt, T.; Duncan, C. Apoptosis in normal and Coxiella burnetii-infected placentas from Alaskan northern fur seals (Callorhinus ursinus). Vet. Pathol 2013, 50, 622–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, C.; Dickerson, B.; Pabilonia, K.; Miller, A.; Gelatt, T. Prevalence of Coxiella burnetii and Brucella spp. in tissues from subsistence harvested northern fur seals (Callorhinus ursinus) of St. Paul Island, Alaska. Acta Vet. Scand. 2014, 56, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minor, C.; Kersh, G.J.; Gelatt, T.; Kondas, A.V.; Pabilonia, K.L.; Weller, C.B.; Dickerson, B.R.; Duncan, C.G. Coxiella burnetii in northern fur seals and Steller sea lions of Alaska. J. Wildl. Dis. 2013, 49, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Duncan, C.G.; Tiller, R.; Mathis, D.; Stoddard, R.; Kersh, G.J.; Dickerson, B.; Gelatt, T. Brucella placentitis and seroprevalence in northern fur seals (Callorhinus ursinus) of the Pribilof Islands, Alaska. J. Vet. Diagn. Investig. 2014, 26, 507–512. [Google Scholar] [CrossRef] [Green Version]
- Nymo, I.H.; Rødven, R.; Beckmen, K.; Larsen, A.K.; Tryland, M.; Quakenbush, L.; Godfroid, J. Brucella Antibodies in Alaskan True Seals and Eared Seals—Two Different Stories. Front. Vet. Sci. 2018, 5, 8. [Google Scholar] [CrossRef] [Green Version]
- Gilmartin, W.G.; Vainik, P.M.; Neill, V.M. Salmonellae in feral pinnipeds off the Southern California coast. J. Wildl. Dis. 1979, 15, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Stroud, R.K.; Roelke, M.E. Salmonella meningoencephalomyelitis in a northern fur seal (Callorhinus ursinus). J. Wildl. Dis. 1980, 16, 15–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.W.; Brown, R.J.; Skilling, D.E.; Bray, H.L.; Keyes, M.C. Naturally-occurring leptospirosis in northern fur seals (Callorhinus ursinus). J. Wildl. Dis. 1977, 13, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suer, L.; Vedros, N.A. Erysipelothrix rhusiopathiae. I. Isolation and characterization from pinnipeds and bite/ abrasion wounds in humans. Dis. Aquat. Org. 1988, 5, 1–5. [Google Scholar] [CrossRef]
- Hansen, M.J.; Bertelsen, M.F.; Christensen, H.; Bisgaard, M.; Bojesen, A.M. Occurrence of Pasteurellaceae bacteria in the oral cavity of selected marine mammal species. J. Zoo Wildl. Med. 2012, 43, 828–835. [Google Scholar] [CrossRef]
- Vedros, N.A.; Quinlivan, J.; Cranford, R. Bacterial and fungal flora of wild northern fur seals (Callorhinus ursinus). J. Wildl. Dis. 1982, 18, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Vanpelt, R.W.; Ohata, C.A. Hemolytic Escherichia coli as Cause of Acute Enterotoxemia in a Captive Northern Fur Seal. Vet. Med. Small Anim. Clin. 1974, 69, 1251–1254. [Google Scholar]
- Dunn, J.L.; Buck, J.D.; Spotte, S. Candidiasis in captive pinnipeds. J. Am. Vet. Med. Assoc. 1984, 185, 1328–1330. [Google Scholar]
- Kuzmina, T.A.; Kuzmin, Y.; Dzeverin, I.; Lisitsyna, O.I.; Spraker, T.R.; Korol, E.M.; Kuchta, R. Review of metazoan parasites of the northern fur seal (Callorhinus ursinus) and the analysis of the gastrointestinal helminth community of the population on St. Paul Island, Alaska. Parasitol. Res. 2021, 120, 117–132. [Google Scholar] [CrossRef]
- Lyons, E.T.; Spraker, T.R.; De Long, R.L.; Ionita, M.; Melin, S.R.; Nadler, S.A.; Tolliver, S.C. Review of research on hookworms (Uncinaria lucasi Stiles, 1901) in northern fur seals (Callorhinus ursinus Linnaeus, 1758). Parasitol. Res. 2011, 109, 257–265. [Google Scholar] [CrossRef]
- Kuzmina, T.A.; Lyons, E.T.; Spraker, T.R. Anisakids (Nematoda: Anisakidae) from stomachs of northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska: Parasitological and pathological analysis. Parasitol. Res. 2014, 113, 4463–4470. [Google Scholar] [CrossRef] [PubMed]
- Holshuh, H.J.; Sherrod, A.E.; Taylor, C.R.; Andrews, B.F.; Howard, E.B. Toxoplasmosis in a feral northern fur seal. J. Am. Vet. Med. Assoc. 1985, 187, 1229–1230. [Google Scholar] [PubMed]
- Brown, R.J.; Smith, A.W.; Keyes, M.C. Sarcocystis in the northern fur seal. J. Wildl. Dis. 1974, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.J.; Smith, A.W.; Keyes, M.C. Renal fibrosarcoma in the northern fur seal. J. Wildl. Dis. 1975, 11, 23–25. [Google Scholar] [CrossRef]
- Stedham, M.A.; Casey, H.W.; Keyes, M.C. Lymphosarcoma in an infant northern fur seal (Callorhinus ursinus). J. Wildl. Dis. 1977, 13, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, K.A.; Robertson, A.; Frame, E.R.; Colegrove, K.M.; Nance, S.; Baugh, K.A.; Wiedenhoft, H.; Gulland, F.M.D. Clinical signs and histopathology associated with domoic acid poisoning in northern fur seals (Callorhinus ursinus) and comparison of toxin detection methods. Harmful Algae 2010, 9, 374–383. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Quakenbush, L.; Frame, E.; Huntington, K.B.; Sheffield, G.; Stimmelmayr, R.; Bryan, A.; Kendrick, P.; Ziel, H.; Goldstein, T.; et al. Prevalence of algal toxins in Alaskan marine mammals foraging in a changing arctic and subarctic environment. Harmful Algae 2016, 55, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Beckmen, K.B.; Duffy, L.K.; Zhang, X.; Pitcher, K.W. Mercury concentrations in the fur of Steller sea lions and northern fur seals from Alaska. Mar. Pollut. Bull. 2002, 44, 1130–1135. [Google Scholar] [CrossRef]
- Noda, K.; Ichihashi, H.; Loughlin, T.R.; Baba, N.; Kiyota, M.; Tatsukawa, R. Distribution of heavy metals in muscle, liver and kidney of northern fur seal (Callorhinus ursinus) caught off Sanriku, Japan and from the Pribilof Islands, Alaska. Environ. Pollut. 1995, 90, 51–59. [Google Scholar] [CrossRef]
- Goldblatt, C.J.; Anthony, R.G. Heavy Metals in Northern Fur Seals (Callorhinus ursinus) from the Pribilof Islands, Alaska. J. Environ. Qual. 1983, 12, 478–482. [Google Scholar] [CrossRef]
- Kim, K.C.; Chu, R.C.; Barron, G.P. Mercury in tissues and lice of Northern fur seals. Bull. Environ. Contam. Toxicol. 1974, 11, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Anas, R.E. Heavy metals in the northern fur seal, Callorhinus ursinus, and harbor seal, Phoca vitulina richardi. Fish. Bull. 1974, 72, 133–137. [Google Scholar]
- Zeisler, R.; Demiralp, R.; Koster, B.J.; Becker, P.R.; Burow, M.; Ostapczuk, P.; Wise, S.A. Determination of inorganic constituents in marine mammal tissues. Sci. Total Environ. 1993, 139–140, 365–386. [Google Scholar] [CrossRef]
- Saeki, K.; Nakajima, M.; Noda, K.; Loughlin, T.R.; Baba, N.; Kiyota, M.; Tatsukawa, R.; Calkins, D.G. Vanadium Accumulation in Pinnipeds. Arch. Environ. Contam. Toxicol. 1999, 36, 81–86. [Google Scholar] [CrossRef]
- Arai, T.; Ikemoto, T.; Hokura, A.; Terada, Y.; Kunito, T.; Tanabe, S.; Nakai, I. Chemical Forms of Mercury and Cadmium Accumulated in Marine Mammals and Seabirds as Determined by XAFS Analysis. Environ. Sci. Technol. 2004, 38, 6468–6474. [Google Scholar] [CrossRef]
- Fujihara, J.; Kunito, T.; Kubota, R.; Tanabe, S. Arsenic accumulation in livers of pinnipeds, seabirds and sea turtles: Subcellular distribution and interaction between arsenobetaine and glycine betaine. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 287–296. [Google Scholar] [CrossRef]
- Saeki, K.; Nakajima, M.; Loughlin, T.R.; Calkins, D.C.; Baba, N.; Kiyota, M.; Tatsukawa, R. Accumulation of silver in the liver of three species of pinnipeds. Environ. Pollut. 2001, 112, 19–25. [Google Scholar] [CrossRef]
- Donohue, M.J.; Masura, J.; Gelatt, T.; Ream, R.; Baker, J.D.; Faulhaber, K.; Lerner, D.T. Evaluating exposure of northern fur seals, Callorhinus ursinus, to microplastic pollution through fecal analysis. Mar. Pollut. Bull. 2019, 138, 213–221. [Google Scholar] [CrossRef]
- Wang, D.; Shelver, W.L.; Atkinson, S.; Mellish, J.A.; Li, Q.X. Tissue distribution of polychlorinated biphenyls and organochlorine pesticides and potential toxicity to Alaskan northern fur seals assessed using PCBs congener specific mode of action schemes. Arch. Environ. Contam. Toxicol. 2010, 58, 478–488. [Google Scholar] [CrossRef]
- Mössner, S.; Barudio, I.; Spraker, T.S.; Antonelis, G.; Early, G.; Geraci, J.R.; Becker, P.R.; Ballschmiter, K. Determination of HCHs, PCBs, and DDTs in brain tissues of marine mammals off different age. Fresenius J. Anal. Chem. 1994, 349, 708–716. [Google Scholar] [CrossRef]
- Norstrom, R.J.; Muir, D.C. Chlorinated hydrocarbon contaminants in arctic marine mammals. Sci. Total Environ. 1994, 154, 107–128. [Google Scholar] [CrossRef]
- Schantz, M.M.; Koster, B.J.; Wise, S.A.; Becker, P.R. Determination of PCBs and chlorinated hydrocarbons in marine mammal tissues. Sci. Total Environ. 1993, 139–140, 323–345. [Google Scholar] [CrossRef]
- Beckmen, K.B.; Blake, J.E.; Ylitalo, G.M.; Stott, J.L.; O’Hara, T.M. Organochlorine contaminant exposure and associations with hematological and humoral immune functional assays with dam age as a factor in free-ranging northern fur seal pups (Callorhinus ursinus). Mar. Pollut. Bull. 2003, 46, 594–606. [Google Scholar] [CrossRef]
- Loughlin, T.R.; Castellini, M.A.; Ylitalo, G. Spatial aspects of organochlorine contamination in northern fur seal tissues. Mar. Pollut. Bull. 2002, 44, 1024–1034. [Google Scholar] [CrossRef]
- Beckmen, K.B.; Ylitalo, G.M.; Towell, R.G.; Krahn, M.M.; O’Hara, T.M.; Blake, J.E. Factors affecting organochlorine contaminant concentrations in milk and blood of northern fur seal (Callorhinus ursinus) dams and pups from St. George Island, Alaska. Sci. Total Environ. 1999, 231, 183–200. [Google Scholar] [CrossRef]
- Mössner, S.; Spraker, T.R.; Becker, P.R.; Ballschmiter, K. Ratios of enantiomers of alpha-HCH and determination of alpha-, beta-, and gamma-HCH isomers in brain and other tissues of neonatal Northern fur seals (Callorhinus ursinus). Chemosphere 1992, 24, 1171–1180. [Google Scholar] [CrossRef]
- Reiner, J.L.; Becker, P.R.; Gribble, M.O.; Lynch, J.M.; Moors, A.J.; Ness, J.; Peterson, D.; Pugh, R.S.; Ragland, T.; Rimmer, C.; et al. Organohalogen Contaminants and Vitamins in Northern Fur Seals (Callorhinus ursinus) Collected During Subsistence Hunts in Alaska. Arch. Environ. Contam. Toxicol. 2016, 70, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Nomiyama, K.; Kanbara, C.; Ochiai, M.; Eguchi, A.; Mizukawa, H.; Isobe, T.; Matsuishi, T.; Yamada, T.K.; Tanabe, S. Halogenated phenolic contaminants in the blood of marine mammals from Japanese coastal waters. Mar. Environ. Res. 2014, 93, 15–22. [Google Scholar] [CrossRef]
- Tanabe, S.; Sung, J.-K.; Choi, D.-Y.; Baba, N.; Kiyota, M.; Yoshida, K.; Tatsukawa, R. Persistent organochlorine residues in northern fur seal from the Pacific coast of Japan since 1971. Environ. Pollut. 1994, 85, 305–314. [Google Scholar] [CrossRef]
- Kajiwara, N.; Ueno, D.; Takahashi, A.; Baba, N.; Tanabe, S. Polybrominated diphenyl ethers and organochlorines in archived northern fur seal samples from the Pacific coast of Japan, 1972–1998. Environ. Sci. Technol. 2004, 38, 3804–3809. [Google Scholar] [CrossRef]
- Iwata, H.; Tanabe, S.; Iida, T.; Baba, N.; Ludwig, J.P.; Tatsukawa, R. Enantioselective Accumulation of α-Hexachlorocyclohexane in Northern Fur Seals and Double-Crested Cormorants: Effects of Biological and Ecological Factors in the Higher Trophic Levels. Environ. Sci. Technol. 1998, 32, 2244–2249. [Google Scholar] [CrossRef]
- Ruedig, E.; Duncan, C.; Dickerson, B.; Williams, M.; Gelatt, T.; Bell, J.; Johnson, T.E. Fukushima derived radiocesium in subsistence-consumed northern fur seal and wild celery. J. Environ. Radioact. 2016, 152, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kuzin, A.E.; Trukhin, A.M. Entanglement of northern fur seals (Callorhinus ursinus) in marine debris on Tyuleniy Island (Sea of Okhotsk) in 1998–2013. Mar. Pollut. Bull. 2019, 143, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hanni, K.D.; Pyle, P. Entanglement of Pinnipeds in Synthetic Materials at South-east Farallon Island, California, 1976–1998. Mar. Pollut. Bull. 2000, 40, 1076–1081. [Google Scholar] [CrossRef]
- Kiyota, M.; Baba, N. Entanglement in marine debris among adult female northern fur seals at St. Paul Island, Alaska in 1991–1999. Bull.-Natl. Res. Inst. Far Seas Fish. 2001, 38, 13–20. [Google Scholar]
- Stewart, B.S.; Yochem, P.K. Entanglement of pinnipeds in synthetic debris and fishing net and line fragments at San Nicolas and San Miguel Islands, California, 1978–1986. Mar. Pollut. Bull. 1987, 18, 336–339. [Google Scholar] [CrossRef]
- Feldkamp, D.M.; Costa, D.P.; Dekrey, G.K. Energetic and Behavioral Effects of Net Entanglement on Juvenile Northern Fur Seals, Callorhinus ursinus. Fish. Bull. 1989, 87, 85–94. [Google Scholar]
- Jortner, B.S. Neuropathologic Observations of Head Trauma in the Northern Fur Seal. J. Wildl. Dis. 1974, 10, 121–129. [Google Scholar] [CrossRef]
- Frasca, S., Jr.; Van Kruiningen, H.J.; Dunn, J.L.; St Aubin, D.J. Gastric intramural hematoma and hemoperitoneum in a captive northern fur seal. J. Wildl. Dis. 2000, 36, 565–569. [Google Scholar] [CrossRef] [Green Version]
- Frasca, S., Jr.; Dunn, J.L.; Van Kruiningen, H.J. Acute gastric dilatation with volvulus in a northern fur seal (Callorhinus ursinus). J. Wildl. Dis. 1996, 32, 548–551. [Google Scholar] [CrossRef] [Green Version]
- Stoskopf, M.K.; Zimmerman, S.; Hirst, L.W.; Green, R. Ocular anterior segment disease in northern fur seals. J. Am. Vet. Med. Assoc. 1985, 187, 1141–1144. [Google Scholar] [PubMed]
- Miller, S.; Colitz, C.M.H.; St. Leger, J.; Dubielzig, R. A retrospective survey of the ocular histopathology of the pinniped eye with emphasis on corneal disease. Vet. Ophthalmol. 2013, 16, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Colitz, C.M.H.; Renner, M.S.; Manire, C.A.; Doescher, B.; Schmitt, T.L.; Osborn, S.D.; Croft, L.; Olds, J.; Gehring, E.; Mergl, J.; et al. Characterization of progressive keratitis in Otariids. Vet. Ophthalmol. 2010, 13, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Aalderink, M.T.; Nguyen, H.P.; Kass, P.H.; Arzi, B.; Verstraete, F.J. Dental and Temporomandibular Joint Pathology of the Northern Fur Seal (Callorhinus ursinus). J. Comp. Pathol. 2015, 152, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Short, J.W.; Geiger, H.J.; Fritz, L.W.; Warrenchuk, J.J. First-Year Survival of Northern Fur Seals (Callorhinus ursinus) Can Be Explained by Pollock (Gadus chalcogrammus) Catches in the Eastern Bering Sea. J. Mar. Sci. Eng. 2021, 9, 975. [Google Scholar] [CrossRef]
- Kuhn, C.E.; Baker, J.D.; Towell, R.G.; Ream, R.R. Evidence of localized resource depletion following a natural colonization event by a large marine predator. J. Anim. Ecol. 2014, 83, 1169–1177. [Google Scholar] [CrossRef]
- Eberhardt, L.L.; Siniff, D.B. Population Dynamics and Marine Mammal Management Policies. J. Fish. Res. Board Can. 1977, 34, 183–190. [Google Scholar] [CrossRef]
- Leighton, F.A. Surveillance of wild animal diseases in Europe. Rev. Sci. Et Tech. Int. Off. Epizoot. 1995, 14, 819–830. [Google Scholar]
- Cooper, J.E. Diagnostic pathology of selected diseases in wildlife. Rev. Sci. Tech. 2002, 21, 77–89. [Google Scholar] [CrossRef]
- Küker, S.; Faverjon, C.; Furrer, L.; Berezowski, J.; Posthaus, H.; Rinaldi, F.; Vial, F. The value of necropsy reports for animal health surveillance. BMC Vet. Res. 2018, 14, 191. [Google Scholar] [CrossRef] [Green Version]
- McAloose, D.; Colegrove, K.M.; Newton, A.L. Chapter 1—Wildlife Necropsy. In Pathology of Wildlife and Zoo Animals; Terio, K.A., McAloose, D., Leger, J.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–20. [Google Scholar]
- Colegrove, K.M.; Burek-Huntington, K.A.; Roe, W.; Siebert, U. Pinnipediae. Pathol. Wildl. Zoo Anim. 2018, 4, 569–592. [Google Scholar] [CrossRef]
- Yano, K.; Fowler, C. Population Changes in Northern Fur Seal Rookeries at Reef Rookery on St. Paul Island of the Pribilof Islands, Alaska. Available online: https://apps-afsc.fisheries.noaa.gov/pubs/posters/pdfs/pYano02_n-fur-seal-aerial.pdf (accessed on 15 April 2022).
- Yano, K.M.; Tingg, J.; Fowler, C. Northern Fur Seal Rookery Photo Archive: Aerial and Ground-Level Photos, Pribilof Islands, Alaska, 1895–2006; Alaska Fisheries Science Center, NOAA, National Marine Fisheries Service: Seattle, WA, USA, 2009; p. 62.
- Patyk, K.A.; Duncan, C.; Nol, P.; Sonne, C.; Laidre, K.; Obbard, M.; Wiig, Ø.; Aars, J.; Regehr, E.; Gustafson, L.L.; et al. Establishing a definition of polar bear (Ursus maritimus) health: A guide to research and management activities. Sci. Total Environ. 2015, 514, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, G. How can infectious diseases be prioritized in public health? A standardized prioritization scheme for discussion. EMBO Rep. 2008, 9 (Suppl. S1), S22–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, D.; Scudamore, J.; Charlier, J.; Delavergne, M. DISCONTOOLS: A database to identify research gaps on vaccines, pharmaceuticals and diagnostics for the control of infectious diseases of animals. BMC Vet. Res. 2016, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Humblet, M.-F.; Vandeputte, S.; Albert, A.; Gosset, C.; Kirschvink, N.; Haubruge, E.; Fecher-Bourgeois, F.; Pastoret, P.-P.; Saegerman, C. Multidisciplinary and evidence-based method for prioritizing diseases of food-producing animals and zoonoses. Emerg. Infect. Dis. 2012, 18, e1. [Google Scholar] [CrossRef]
- Cardoen, S.; Van Huffel, X.; Berkvens, D.; Quoilin, S.; Ducoffre, G.; Saegerman, C.; Speybroeck, N.; Imberechts, H.; Herman, L.; Ducatelle, R.; et al. Evidence-based semiquantitative methodology for prioritization of foodborne zoonoses. Foodborne Pathog. Dis. 2009, 6, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, J.; Simpson, H.; Langstaff, I. Development of methodology to prioritise wildlife pathogens for surveillance. Prev. Vet. Med. 2007, 81, 194–210. [Google Scholar] [CrossRef]
- Ng, V.; Sargeant, J.M. A stakeholder-informed approach to the identification of criteria for the prioritization of zoonoses in Canada. PLoS ONE 2012, 7, e29752. [Google Scholar] [CrossRef]
- Waltzek, T.B.; Cortés-Hinojosa, G.; Wellehan, J.F.X., Jr.; Gray, G.C. Marine mammal zoonoses: A review of disease manifestations. Zoonoses Public Health 2012, 59, 521–535. [Google Scholar] [CrossRef]
- Hunt, T.D.; Ziccardi, M.H.; Gulland, F.M.D.; Yochem, P.K.; Hird, D.W.; Rowles, T.; Mazet, J.A.K. Health risks for marine mammal workers. Dis. Aquat. Org. 2008, 81, 81–92. [Google Scholar] [CrossRef]
- Parker, N.R.; Barralet, J.H.; Bell, A.M. Q fever. Lancet 2006, 367, 679–688. [Google Scholar] [CrossRef]
- Kersh, G.J.; Fitzpatrick, K.; Pletnikoff, K.; Brubaker, M.; Bruce, M.; Parkinson, A. Prevalence of serum antibodies to Coxiella burnetii in Alaska Native Persons from the Pribilof Islands. Zoonoses Public Health 2020, 67, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Grigg, M.E. Recent epidemiologic and clinical importance of Toxoplasma gondii infections in marine mammals: 2009–2020. Vet. Parasitol. 2020, 288, 109296. [Google Scholar] [CrossRef] [PubMed]
- Rea, L.D.; Castellini, J.M.; Avery, J.P.; Fadely, B.S.; Burkanov, V.N.; Rehberg, M.J.; O’Hara, T.M. Regional variations and drivers of mercury and selenium concentrations in Steller sea lions. Sci. Total Environ. 2020, 744, 140787. [Google Scholar] [CrossRef]
- Gulland, F.M.; Koski, M.; Lowenstine, L.J.; Colagross, A.; Morgan, L.; Spraker, T. Leptospirosis in California sea lions (Zalophus californianus) stranded along the central California coast, 1981–1994. J. Wildl. Dis. 1996, 32, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, M.M.; Kashinsky, L.; Rotstein, D.S.; Colegrove, K.M.; Haman, K.H.; Magargal, S.L.; Sweeny, A.R.; Kaufman, A.C.; Grigg, M.E.; Littnan, C.L. Protozoal-related mortalities in endangered Hawaiian monk seals Neomonachus schauinslandi. Dis. Aquat. Org. 2016, 121, 85–95. [Google Scholar] [CrossRef]
- Ng, V.; Sargeant, J.M. A quantitative and novel approach to the prioritization of zoonotic diseases in North America: A public perspective. PLoS ONE 2012, 7, e48519. [Google Scholar] [CrossRef]
- Veltre, D.W.; Veltre, M.J. The Northern Fur Seal: A Subsistence and Commercial Resource for Aleuts of the Aleutian and Pribilof Islands, Alaska. Études Inuit Stud. 1987, 11, 51–72. [Google Scholar]
- Ostertag, S.K.; Loseto, L.L.; Snow, K.; Lam, J.; Hynes, K.; Gillman, D.V. “That’s how we know they’re healthy”: The inclusion of traditional ecological knowledge in beluga health monitoring in the Inuvialuit Settlement Region. Arct. Sci. 2018, 4, 292–320. [Google Scholar] [CrossRef] [Green Version]
- Breton-Honeyman, K.; Furgal, C.M.; Hammill, M.O. Systematic Review and Critique of the Contributions of Traditional Ecological Knowledge of Beluga Whales in the Marine Mammal Literature. Arctic 2016, 69, 37–46. [Google Scholar] [CrossRef]
- Wyllie de Echeverria, V.R.; Thornton, T.F. Using traditional ecological knowledge to understand and adapt to climate and biodiversity change on the Pacific coast of North America. Ambio 2019, 48, 1447–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntington, H.P.; Braem, N.M.; Brown, C.L.; Hunn, E.; Krieg, T.M.; Lestenkof, P.; Noongwook, G.; Sepez, J.; Sigler, M.F.; Wiese, F.K.; et al. Local and traditional knowledge regarding the Bering Sea ecosystem: Selected results from five indigenous communities. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2013, 94, 323–332. [Google Scholar] [CrossRef]
- Peacock, S.J.; Mavrot, F.; Tomaselli, M.; Hanke, A.; Fenton, H.; Nathoo, R.; Aleuy, O.A.; Di Francesco, J.; Aguilar, X.F.; Jutha, N.; et al. Linking co-monitoring to co-management: Bringing together local, traditional, and scientific knowledge in a wildlife status assessment framework. Arct. Sci. 2020, 6, 247–266. [Google Scholar] [CrossRef]
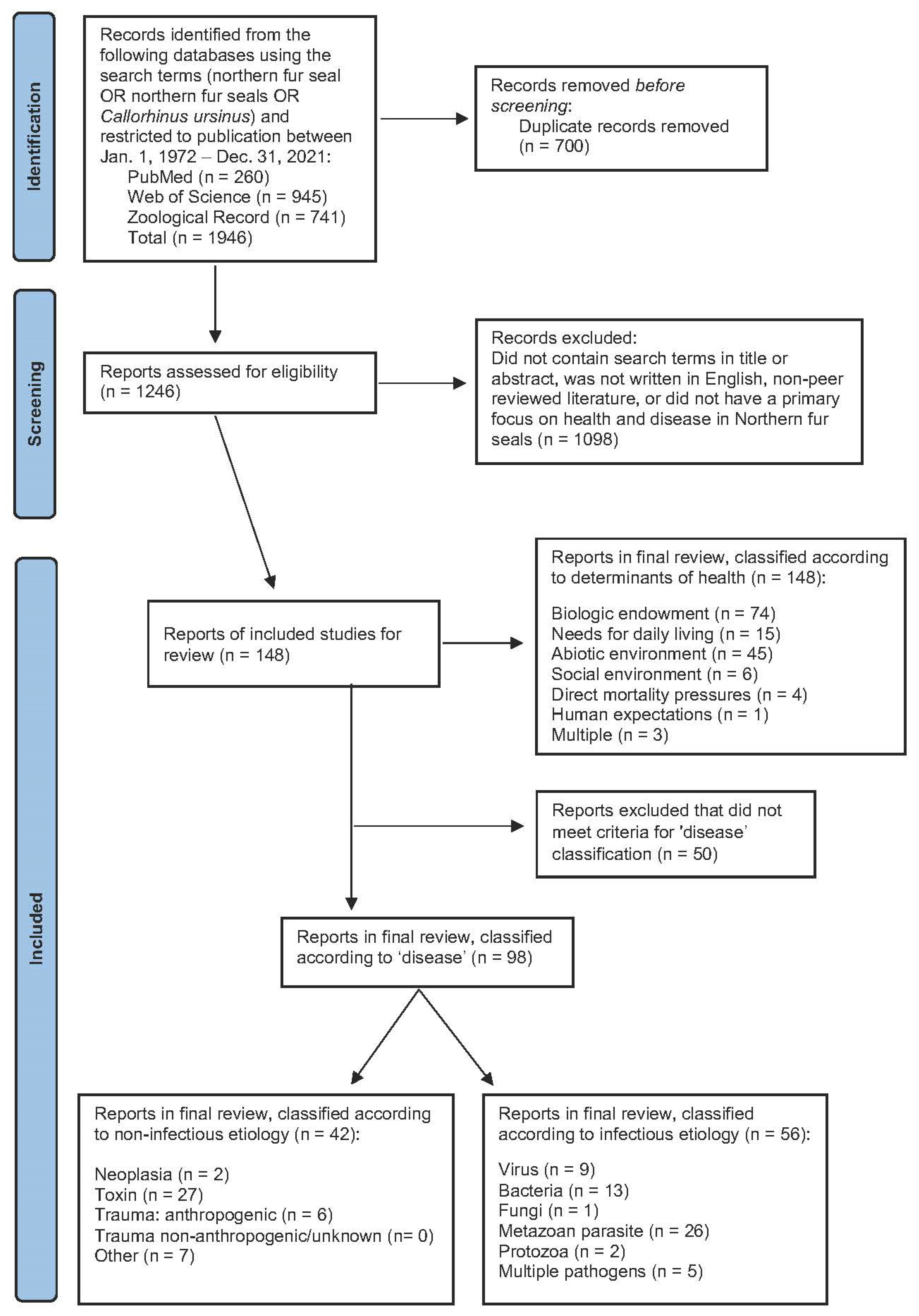
| Contaminant | US | Japan |
|---|---|---|
| Heavy metals (e.g., mercury, cadmium, arsenic, silver, vanadium) | [51,52,53,54,55,56,57] | [52,57,58,59,60] |
| Microplastics | [61] | |
| Persistent organic pollutants (e.g., PCB, DDT, PBDEs) | [62,63,64,65,66,67,68,69,70] | [71,72,73,74] |
| Radiocesium | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, V.; Patyk, K.; Simeone, C.; Johnson, V.; Vega, J.; Savage, K.; Duncan, C. A Review of Northern Fur Seal (Callorhinus ursinus) Literature to Direct Future Health Monitoring Initiatives. Oceans 2022, 3, 303-318. https://doi.org/10.3390/oceans3030021
Cortés V, Patyk K, Simeone C, Johnson V, Vega J, Savage K, Duncan C. A Review of Northern Fur Seal (Callorhinus ursinus) Literature to Direct Future Health Monitoring Initiatives. Oceans. 2022; 3(3):303-318. https://doi.org/10.3390/oceans3030021
Chicago/Turabian StyleCortés, Valerie, Kelly Patyk, Claire Simeone, Valerie Johnson, Johanna Vega, Kate Savage, and Colleen Duncan. 2022. "A Review of Northern Fur Seal (Callorhinus ursinus) Literature to Direct Future Health Monitoring Initiatives" Oceans 3, no. 3: 303-318. https://doi.org/10.3390/oceans3030021